Abstract
In this study, the effects of borage seed oil (BO) and matcha tea (MT) on the physicochemical properties, oxidative stability, color, and tenderness of vacuum-packed lamb meatloaf during 14 days of refrigerated storage were evaluated. No significant effect of the plant additives was observed on cooking yield (66%) or pH (≈5.95). Samples with added oil had a higher fat content, which contributed to hydrolytic and oxidative changes that were already evident immediately after production. The addition of MT, as well as the combined use of both BO and MT, effectively limited these changes. After 14 days, TBARS values in these samples decreased by up to 80% compared to the control. Neither BO nor MT affected lightness (L*) or yellowness (b*), whereas matcha, being naturally green, reduced redness (a*) by about 50%. However, no significant differences in total color (ΔE* < 4) were observed between control and experimental samples. The addition of BO also had a significant effect on the texture of meatloaves, which showed a 15% reduction in shear force after 14 days of storage. In conclusion, the combined application of BO and MT effectively limited fat oxidation while maintaining desirable color, tenderness, and overall physicochemical quality of meatloaves comparable to the control.
1. Introduction
Lamb meat is highly valued in global markets not only for its palatability but also for its rich nutritional profile. It is a source of high-quality protein and essential amino acids, as well as heme iron and B vitamins [1]. Being meat from a young animal, lamb contains a favorable balance of saturated and unsaturated fatty acids, including conjugated linoleic acid (CLA) and omega-3 polyunsaturated fatty acids (PUFAs), which have been associated with cardiovascular and metabolic health benefits [2,3]. The composition of muscle fibers in lamb meat affects its culinary quality attributes. Lamb meat exhibits a higher proportion of oxidative fibers, which significantly enhances both the sensory and nutritional properties of the meat by modulating lipid metabolism, amino acid availability, and flavor development [4]. Lamb meat products, especially from native breeds [5], represent a suitable raw material for processed meat products, particularly when enriched with plant-based sources of bioactive compounds during processing.
Considering the current trend toward clean-label foods, ongoing research focuses on identifying and characterizing plant-derived antioxidant compounds, such as plant oils rich in polyunsaturated fatty acids (PUFAs) and teas abundant in catechins [6]. Borage (Borago officinalis L.) is an annual herb primarily cultivated for medicinal purposes. Oil is produced from its seeds [7], which is rich in bioactive fatty acids, including gamma-linolenic acid (26–38%), linoleic acid (35–38%), and oleic acid (16–20%), along with smaller amounts of palmitic acid (10–11%), stearic acid (3.5–4.5%), eicosenoic acid (3.5–5.5%), and erucic acid (1.5–3.5%) [8]. Borage seeds also contain high levels of flavonoids with anti-inflammatory and antioxidant properties; tannins with antibacterial activity; saponins, which enhance nutrient absorption from the intestines into the bloodstream, stimulate the secretion of gastric, bile, and intestinal juices, may influence cholesterol levels, and promote fat digestion; and mineral salts such as iron, zinc, magnesium, calcium, copper, manganese, silicon, and potassium. Due to the high content of these bioactive compounds, borage seed oil is used in the treatment of various disorders, including multiple sclerosis, diabetes, cardiovascular diseases, arthritis, and eczema [9]. Although borage seed oil is edible, it is rarely used as a culinary oil [10] and is more commonly applied in cosmetology and medicine. Meanwhile, in scientific research, water extracts of borage are most frequently used [11,12,13,14,15,16].
Matcha (Camellia sinensis L.) is a finely powdered green tea produced from fresh tea leaves grown in the shade, steamed or hot-air dried, and processed by grinding [17]. Matcha retains nearly all the nutritional and health-promoting properties of green tea, and its powdered form broadens its potential applications. Green tea is widely recognized for its antioxidant properties. The beneficial effects of green tea extracts are attributed to the presence of polyphenols, polysaccharides, vitamins B, C, and E, and amino acids [18]. The most important group of bioactive compounds are polyphenols (phenolic acids, stilbenes, flavonoids, and lignans), comprising approximately 30% of the dry leaf mass [19]. Among the flavonoids (catechins) are epicatechin, epicatechin gallate, epigallocatechin, and epigallocatechin gallate, with the latter being the most abundant and biologically active component of matcha [20,21]. Its characteristic chemical composition and unique flavor distinguish it from other teas [22]. Furthermore, green tea exhibits notable health-promoting effects, including anticancer activity, cardiovascular protection, anti-obesity and anti-diabetic effects, oral and bone health benefits, and enhancement of burn and wound healing [23,24,25].
The combined use of both plant-based additives—borage seed oil (BO) and matcha tea (MT)—may exert a synergistic effect, which motivated the present study. The aim was to evaluate the impact of these natural additives—borage seed oil (Borago officinalis L.) and matcha tea (Camellia sinensis L.)—on the physicochemical properties, oxidative stability, color, and tenderness of vacuum-packed lamb meatloaf during 14 days of storage at 4 °C.
2. Materials and Methods
2.1. Materials
The research material consisted of lamb meatloaves. The meat was obtained from lambs of the native Polish Świniarka breed, which were raised according to organic farming standards [26] in the Lublin region, Poland. From the second week of life, the lambs received meadow hay and crushed oats along with their mother’s milk, and from the fifth week, hydrated pulp. At 8 weeks of age, the lambs had an average body weight of 15.7 kg. On day 90, they were weaned and fed exclusively with meadow hay, supplemented with fresh forage (a mixture of grasses and alfalfa) and crushed oats, modified every two weeks. At four months of age, they grazed on xerothermic grasslands within a Natura 2000 protected area. Five lambs were selected for the study. Animal care complied with the Guide for the Care and Use of Laboratory Animals [27], Directive 2010/63/EU [28], and Directive 1998/58/EC [29]. At seven months of age, the lambs had an average body weight of 29 kg and were slaughtered based on farming efficiency and meat processing requirements. Transport and slaughter were carried out by a certified company at a small abattoir supervised by the Veterinary Inspection, in accordance with EU regulations. Carcasses were held at 10–15 °C for 4 h and subsequently chilled at 3–4 °C for 20 h. Meat from the deboned carcasses and lamb legs was vacuum-packed and transported at 4–6 °C for use in the present experiment.
The plant-based additives originated from the southwestern region of Türkiye. Borage seed oil (Borago officinalis L.) 100% pure (Polente Natural Hodan, Istanbul, Türkiye), cold-pressed, was purchased from a local pharmacy in Mugla (Türkiye). A product with a high content of polyunsaturated fatty acids was selected for the experiment. According to the manufacturer’s declaration, 100 g of the oil contains 27 g of monounsaturated fatty acids, including 18 g of oleic acid (C18:1), and 57 g of polyunsaturated fatty acids, including 37 g of linoleic acid (C18:2) and 20 g of gamma-linolenic acid (C18:3n-6). Matcha tea 100% (Camellia sinensis L.) (Şölen Çay Tic. Ltd. Şti., Sürmene, Turkey) was purchased from an organic food store in Fethiye (Turkey).
2.2. Lamb Meatloaf Preparation
The meat was ground using a meat grinder (KU2-3EK, Mesko-AGD, Skarżysko-Kamienna, Poland) with an 8 mm plate. All ingredients (Table 1) were mixed in a universal mixer (KU2-3EK, Mesko-AGD, Skarżysko-Kamienna, Poland) equipped with an R4 paddle (100 rpm for 3 min). Plant additives were incorporated into the mixture during mixing, resulting in three experimental lamb meatloaves: BO + MT—with borage seed oil (0.4 g/kg of meat) and matcha powder (0.05 g/kg of meat), BO—with borage seed oil (0.4 g/kg of meat), MT—with matcha powder (0.05 g/kg of meat), and CON—control meatloaf was prepared without plant additives. The concentrations of the plant additives were selected based on our own pilot studies, the results of which have not yet been published. Each portion of the mixture (approximately 300 ± 10 g) was placed in aluminum baking trays and baked in a convection oven (XF 135 UNOX S.p.A. Padova, Italy) at 160 °C until the internal temperature at the geometric center reached 72 °C. After cooling, the samples were vacuum-packed in polyethylene bags and stored in a refrigerator at 4 °C. The product was analyzed at three storage times: 24 h, 7 days, and 14 days. The experiment was conducted twice, one week apart, under identical conditions. Analyses were performed on replicates.

Table 1.
Samples of lamb meatloaf.
2.3. Lamb Meatloaf Preparation for Analysis
The samples were ground twice using a 3 mm mesh in a grinder (Zelmer, Rzeszów, Poland). The ground bulk was thoroughly mixed, and then samples were collected for all assays.
2.4. Physicochemical Properties of Vacuum-Packed Lamb Meatloaf
Cooking yield was calculated based on the difference in weight before and after heat treatment. Moisture content was determined using ISO method [30]. Fat content was determined during the FFA test (described in Section 2.5). For pH measurement, a digital CPC-501 pH meter (Elmetron, Zabrze, Poland) equipped with a temperature sensor and ERH-111 pH electrode (Hydromet, Gliwice, Poland) was used. The pH measurement was performed on a homogenate of the meatloaf and distilled water (1:5 ratio) prepared just before the measurement [31].
2.5. Oxidative Stability and Hydrolysis of Vacuum-Packed Lamb Meatloaf
The content of free fatty acids (FFA) in meatloaf was determined according to the method described by Koniecko [32] with modifications by Malik and Sharma [33]. A homogenate prepared from 10 g of minced meatloaf, 60 mL of chloroform, and 10 g of anhydrous sodium sulfate was filtered through Whatman No. 1 filter paper. Twenty mL of the filtrate were dried in an oven (SPU-200, ZUT Colector, Cracow, Poland) to determine the fat content. The remaining filtrate was titrated with 0.1 M ethanolic potassium hydroxide solution against 0.2% phenolphthalein until a light pink color appeared. The quantity of potassium hydroxide consumed during the titration was recorded. FFA as percentage of oleic acid was calculated as follows:
FFA (% oleic acid) = (2.82 ⋅ mL 0.1 N potassium hydroxide)/mass of fat (g)
The peroxide value was determined according to the procedure described by Koniecko [32], with Wagh et al. [34] modifications. A 5 g portion of the sample was homogenized with 30 mL of chloroform in the presence of anhydrous sodium sulfate for 2 min. The mixture was filtered through Whatman No. 1 filter paper, and a 25 mL aliquot of the filtrate was transferred into a 250 mL conical flask. Subsequently, 30 mL of glacial acetic acid and 2 mL of saturated potassium iodide solution were added, and the mixture was allowed to stand for 2 min with occasional swirling. Thereafter, 100 mL of distilled water and 2 mL of freshly prepared 1% starch solution were added. The contents were titrated immediately with 0.1 N sodium thiosulfate solution until the end point was reached (disappearance of the color in the non-aqueous layer). The PV as milliequivalents of peroxide per kg was calculated as follows:
PV (mEq/kg) = (10 ⋅ mL 0.1 N sodium thiosulfate)/sample weight (g)
Lipid oxidation (TBARS) was investigated using 2-thiobarbituric acid (TBA), according to Pikul et al. [35] with slight modifications. Four grams of meatloaf were homogenized with 12 mL of cold perchloric acid (4%) and 200 μL of an alcohol solution (0.01%) of BHT at 1300 g for 2 min using a homogenizer (T25 Basic Ultra-Turrax, IKA, Staufen, Germany). Then, it was filtered through Whatman No.1 filter paper. Subsequently, 650 μL of filtrate and TBA solution (0.02 mol/L in distilled water) were mixed. A mixture of 650 μL of 4% cold perchloric acid and 650 μL of TBA solution was used as the reference sample. The heating part of the experiment was performed for 20 min at 100 °C. Then, spectrophotometrically (U-5100 UV-Vis, Hitachi High Technologies America Inc., Schaumburg, IL, USA) the absorbance at 532 nm was measured. The TBARS value as mg malondialdehyde per kg was calculated as follows:
TBARS (mg MDA/kg) = 5.5 ∙ Absorbance at 532 nm
2.6. Color Parameters CIE L*a*b* of Vacuum-Packed Lamb Meatloaf
The color of lamb meatloaves was measured using an spectrophotometer (Color 8200, X-Rite Inc., Grand Rapids, MI, USA) with a port (Ø 12 mm) and the D65 illuminant, and standard observer 10. The device was calibrated, and color measurements were performed at room temperature at fifteen different locations of sample. The color was expressed in the CIE L*a*b* space, where L*—lightness, a*—redness, and b*—yellowness [36].
The total color difference (ΔE*) was calculated based on the ΔL*, Δa*, and Δb* results for each sample with the plant additive compared to the control sample (CON), for three time intervals of 1, 7, and 14 days. The formula used for the calculation was:
ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2
ΔE* values below 2 indicate a color difference that is barely perceptible. Values in the range of 2–3.5 suggest that an inexperienced observer would notice the difference, while values above 3.5 indicate a clearly perceptible and significant color difference [36].
2.7. Tenderness of Vacuum-Packed Lamb Meatloaf
Textural characteristics were carried out using Warner–Bratzler Shear Force test [37]. The Warner–Bratzler ‘V’ slot blade was attached to the universal testing machine TA.XT2 (Stable Micro System Corporation, Godalming, UK) and the shearing speed was 2 mm/s. The load cell capacity used in this study was 50 kg. The sample diameter was about 10 mm. To assess the tenderness of the meatloaf samples two parameters were measured: the maximum shear force (N) and work of shearing (N∙mm)—the total shear work necessary to cut through the sample. At least ten technical replicates were used for each sample.
2.8. Statistical Analysis
All calculations and comparisons were performed using Statistica version 13.3 (Dell, Inc., Round Rock, TX, USA). Data were analyzed using ANOVA for factorial designs. A two-way ANOVA was applied for statistical comparisons between the treatment (type of plant additive), storage time, and the measured parameters. Tukey’s test was used for post hoc comparisons at a 95% confidence level (α = 0.05). Differences were considered statistically significant at p > 0.05. Results are presented as mean ± standard deviation. Principal Component Analysis (PCA) was used to evaluate and classify the main variables of all samples. The experiment was conducted twice, with analyses performed in at least three replicates.
3. Results
3.1. Physicochemical Properties of Vacuum-Packed Lamb Meatloaf
In the cooking performance assessment, the results indicated that neither the 0.5% addition of matcha tea powder nor the 4% addition of borage seed oil had an effect on cooking yield or moisture content (Table 2). However, the addition of 4% oil clearly increased the fat content in the samples containing it. The pH values of all lamb meatloaves remained stable (ranging from 5.92 to 5.98) and did not change during 14 days of refrigerated storage in vacuum-packed conditions.

Table 2.
Physicochemical properties of vacuum-packed lamb meatloaf (mean ± standard deviation).
3.2. Oxidative Stability and Hydrolysis of Vacuum-Packed Lamb Meatloaf
Hydrolytic changes contribute to the deterioration of the shelf life of meat products, which is why selecting appropriately high-quality raw meat is important (in this study, organically raised lamb was used). Furthermore, the use of additives with antioxidant potential (matcha), along with proper storage conditions (exclusion of air and light, and low temperature), limits the development of hydrolytic and oxidative processes, thereby helping to preserve the quality of the meat product [38]. The content of free fatty acids (FFA) reflects the extent of fat molecule decomposition, and both the addition of plant-based ingredients and storage time influenced the amount of oleic acid measured by the FFA method. In products stored for 14 days, FFA increased by approximately 33% (from 1.8% to 2.7% oleic acid) (Table 3). Matcha (MT) alone significantly reduced (p < 0.05) the FFA concentration in fresh meatloaf (1.5%) compared to the control sample (1.8% oleic acid). However, MT combined with BO was less effective at inhibiting fat hydrolysis, and the FFA content in the BO + MT sample was approximately one-third higher than in the MT-only sample.

Table 3.
Oxidative stability and hydrolysis of vacuum-packed lamb meatloaf (mean ± standard deviation).
Peroxide value (PV) is an indicator of the formation of primary lipid oxidation products and reflects the extent of fat oxidation in meat. PV analysis is based on determining the amount of iodine released from potassium iodide under the influence of peroxides present in the tested fat. In this study, changes in PV were observed (Table 3). Overall, PV in stored meatloaves increased by approximately 13% (from 7 meq O2/kg on day 1 to 8 meq O2/kg on day 14). Initially, PV decreased by 0.6 meq O2/kg after 7 days of storage, followed by a significant (p < 0.05) increase, reaching an average of 8.3 meq O2/kg in the oil-containing samples (BO and BO + MT). The smallest changes (3%) were observed in the control sample, which already had the highest initial peroxide content (7.1 meq O2/kg) on day 1. In meatloaf with added matcha tea (MT), changes in PV were more subtle (6.8 meq O2/kg on day 1, 6.6 meq O2/kg on day 7, and 8 meq O2/kg on day 14), indicating that the addition of MT-only effectively limited peroxide formation during the 7-day storage period.
TBARS represent a group of various chemical compounds, including malondialdehyde. When these compounds react with 2-thiobarbituric acid (TBA) at elevated temperatures, colored complexes are formed. TBARS are quantified by measuring the intensity of these complexes; the greater the amount of lipid-derived aldehydes in the sample, the more intense the color. TBA reacts particularly readily with substances containing carbonyl groups. The maximum consumer-acceptable TBARS level has been established at 2 mg per 1 kg of product [26], and this threshold was not exceeded in the present study (Table 3). The addition of matcha, which is rich in antioxidants significantly (p < 0.05) stabilized lipid oxidation in meatloaf. In samples treated with BO and the control, TBARS levels increased with prolonged storage. In contrast, in meatloaves containing matcha (MT and BO + MT), TBARS values after 7 and 14 days of storage remained as low as in the freshly prepared product, approximately 0.5–0.7 mg/kg. For comparison, the control sample reached 2 mg/kg. These results confirm the effectiveness of tea, including matcha, as a natural antioxidant in meat product technology.
3.3. Color Parameters CIE L*a*b* of Vacuum-Packed Lamb Meatloaf
Analysis of the results from vacuum-packed samples stored at refrigeration for 24 h, 7 days, and 14 days showed no significant (p < 0.05) effect of the plant-based additives on the lightness (L*) and yellowness (b*) parameters (Table 4). Although not statistically significant (p > 0.05), the BO sample was the brightest (highest L* value). In each of the storage periods, this sample had slightly higher L* values than the others. Conversely, the addition of MT caused a darkening of the color. This sample exhibited lower, but not statistically significant (p > 0.05), L* values across all storage periods. The addition of plant-based ingredients, particularly MT, reduced (p < 0.05) the intensity of red color (a*) compared to the control (CON). With extended storage time, a* values decreased in both CON and plant-supplemented samples. However, the most pronounced (p < 0.05) decline in a* over time was observed in the BO sample. In the other samples, a significant decrease occurred only after 14 days. Analysis of the b* parameter indicated that plant additives affected yellowness compared to CON. Higher values correspond to greater yellow color saturation. The highest values were observed in the BO + MT sample, while the lowest were recorded in CON. The addition of both plant ingredients caused a significant (p < 0.05) increase in yellow saturation throughout the storage period compared to CON. For the MT sample, storage time significantly reduced b*, whereas in the other samples, a decline in yellowness was also observed, but it was not significant (p > 0.05). Overall, no clear trend in b* changes over time was observed, although the highest b* values occurred in all samples after 7 days of storage, with only minor differences between day 1 and day 14. The total color difference (ΔE*) of lamb meatloaf was calculated depending on the plant additive used. Values above 3.5 indicate a clearly perceptible color difference. In this study, only one such case was observed (ΔE* = 3.96) between the MT and CON samples after 14 days of storage. In the remaining cases, the lowest ΔE* values were recorded for meatloaf with the addition of borage seed oil (BO), ranging from 0.58 to 1.35. For samples with matcha, ΔE* values ranged from 3.11 to 3.33 (BO + MT) and from 1.35 to 3.96 (MT). Importantly, no clear differences were found between the control and experimental samples, which indicates that the product with plant additives does not visually deviate from the reference lamb meatloaf.

Table 4.
Color parameters CIE L*a*b* of vacuum-packed lamb meatloaf (mean ± standard deviation).
3.4. Tenderness of Vacuum-Packed Lamb Meatloaf
Comparison of Warner–Bratzler shear force results for meat products revealed a significant (p < 0.05) effect of plant-based additives on this parameter (Table 5). Samples with plant additives required less cutting force than the control (CON). Among them, the addition of borage seed oil (BO) resulted in the greatest reduction in sample hardness. A significant (p < 0.05) effect of plant additives on decreasing shear force was observed, especially in samples containing BO, throughout the study period.

Table 5.
Tenderness of vacuum-packed lamb meatloaf (mean ± standard deviation).
The addition of BO and/or MT significantly reduced the work required to cut the samples. On days 1 and 7, CON required the greatest cutting work, whereas during the same periods, cutting the BO samples required approximately half the work. After 14 days of storage, the work needed to cut the samples was similar across all treatments. Statistical analysis (p < 0.05) confirmed a significant effect of plant additives on reducing cutting work.
4. Discussion
4.1. Physicochemical Properties of Vacuum-Packed Lamb Meatloaf
Consumption of meat and meat products has beneficial effects on the human body due to the presence of high-quality protein, vitamins, minerals, and other valuable components. However, meat products are perishable and thus require appropriate processing. Thermal treatment is necessary to ensure desirable sensory characteristics, texture, and microbiological safety. This process, however, leads to losses of nutrients and bioactive compounds due to water and volatile compound evaporation, juice leakage, solubilization of components in water, or fat rendering. The extent of these losses depends on the type of meat, as well as the method and duration of processing. Analysis of technological properties, such as cooking yield, allows for the assessment of the impact of different processing methods on meat [39]. Roldan et al. [40] demonstrated that temperature significantly affects the thermal loss of lamb tenderloins, with lower mass loss occurring at 60 °C compared to 80 °C. The baking method used in our study for minced meat (160 °C until reaching an internal temperature of 72 °C) resulted in cooking losses of 32–35%, regardless of the plant-based additive used. The lack of significant differences in cooking yield also corresponded with no differences in moisture content (Table 2). Meanwhile, Kavusan et al. [39] investigated the addition of flaxseed oil emulsion to poultry sausage and observed a decrease in yield after thermal processing, attributing this to a reduced water-binding capacity of proteins in the presence of plant fat. However, Moawad et al. [41], in studies on poultry sausage with green tea extract, reported an increase in yield by 2 percentage points compared to the control (CON). Regarding fat content, it is evident that the addition of oil to the meat filling increased the fat content in samples with BO (Table 2).
The pH value of meat varies depending on the type of raw material, with meat used for sausage production exhibiting a slightly acidic reaction. The acidity level depends not only on the raw material but also on the quality of additional ingredients in the formulation and the storage time of the products [42]. Our study did not show that BO and/or MT affected the pH of vacuum-packed lamb meatloaf stored under refrigeration (Table 2). Similarly, Purnamayanti et al. [43], who added 1–2% green tea leaf powder to lamb sausage stored for 14 days under refrigeration, did not observe a significant effect on pH. However, they noted that in many studies, the pH of meat products decreases when green tea extract is added, mainly due to an increase in lactic acid bacteria [44]. The authors did not observe, when measuring bacterial counts, that green tea powder had the same effect as the extract. Based on this, we also assumed that the addition of MT did not influence the growth of lactic acid bacteria, which could have lowered the pH during storage. Furthermore, plant-based additives with antioxidant and antimicrobial properties, including BO and MT, stabilize the pH of meat products, protecting them from adverse changes [41,45].
4.2. Oxidative Stability and Hydrolysis of Vacuum-Packed Lamb Meatloaf
Negative changes in the quality of meat and meat products mainly result from oxidative processes affecting proteins, lipids, and myoglobin autoxidation. These processes lead to the loss of sensory attributes, reduction in nutritional value due to depletion of vitamins and polyunsaturated fatty acids, and the formation of toxic oxidation products [46]. Therefore, monitoring the hydrolytic and oxidative stability of lipids in meat products through the determination of free fatty acids (FFA), TBARS, and peroxide value (PV) is crucial for evaluating meat product quality [42].
Analysis of free fatty acids in meat products allows the assessment of both the rate of oxidative changes and the influence of storage conditions and plant-based additives on lipid stability and product quality. The formation of free fatty acids in meat results from hydrolytic processes, primarily enzymatic lipid degradation [47]. These acids are among the first reagents involved in lipid peroxidation in meat, making their determination an important marker of the rate of oxidative changes in meat products [48]. Literature emphasizes that free fatty acid content increases with prolonged storage of meat products [49]. In the present study, a gradual increase in FFA (by approximately one-third) was observed over 14 days of storage (Table 3), likely due to vacuum packaging of the samples. Mir and Masoodi [50] indicated that vacuum packaging limits the increase in free fatty acids compared to aerobic storage. The level of FFA also depends on the composition of the raw material, particularly the presence of compounds with antioxidant activity. Moreover, spraying chicken breast with an aqueous green tea extract, even during 12 days of aerobic storage, significantly (p > 0.05) reduced FFA content compared to the control [49]. Conversely, the introduction of BO, rich in polyunsaturated fatty acids, increased FFA content due to the high susceptibility of oil components to hydrolytic changes.
Peroxide value (PV) determination is a widely used indicator for assessing the degree of oxidative changes in meat products, reflecting the concentration of primary lipid peroxidation products [42]. The rate of lipid peroxidation depends on the chemical structure of fatty acids and the proportion of different fatty acid groups in the meat, with unsaturated fatty acids being the most prone to oxidation. Additionally, the degree of meat fragmentation influences the oxidation rate, as disruption of muscle membrane integrity increases susceptibility to oxidative processes [51]. Authors [49,52] report an increase in peroxide concentration in meat and meat products during storage. The effectiveness of plant-based antioxidants depends on the type of component, level of addition, and chemical properties of the active compounds, such as chemical structure, polarity, and interactions with other ingredients [43]. Kavusan et al. [39] showed that plant oils, such as black cumin and flaxseed oil, exhibit high oxidative instability, resulting in elevated PVs. However, in our study (Table 3), the addition of BO led to a decrease in PV after 7 days of meatloaf storage, which may be attributed to the antioxidant activity of phenolic compounds present in borage seeds. These compounds, present both in aqueous extracts [53] and borage seed oil, can quench reactive oxygen species, limiting oxidation reactions. The reduction and stabilization of PV in MT-containing samples is unsurprising, as matcha tea contains natural antioxidants that delay and limit oxidation through both phenolic compounds and the antioxidant properties of caffeine [54,55] and catechins. These compounds effectively reduce peroxide formation in meat products [45,56,57]. Nain et al. [58] found that green tea extract increased the oxidative stability of oils rich in docosahexaenoic acid (DHA) by reducing the formation of peroxides and secondary oxidation products. Therefore, from a practical standpoint, developing a commercial formulation containing both BO and MT would be advantageous. Storage time also affects PV, as primary oxidation products are unstable and gradually convert into secondary lipid or protein peroxidation products [59]. The variability of PV during storage highlights the importance of monitoring oxidative stability to maintain meat quality.
TBARS analysis is a widely used tool to assess lipid oxidation in meat products. This test measures malondialdehyde (MDA), a secondary lipid peroxidation product, expressed in mg MDA/kg product [48]. Low MDA content indicates initial signs of meat rancidity, whereas exceeding 2 mg MDA/kg product may result in undesirable sensory changes [26]. The value of this indicator largely depends on thermal processing—high temperatures, such as grilling, significantly increase MDA concentration compared to cooking at lower temperatures due to intensified lipid oxidation [60]. Technological factors, such as meat grinding and the addition of curing salt, can also accelerate MDA formation [61]. However, plant-based additives significantly limit lipid oxidation. Our study shows that the addition of BO reduced MDA levels in the product compared to CON (Table 5). Other studies [39,62] indicate that plant oils rich in unsaturated fatty acids lower TBARS values, despite the susceptibility of these fatty acids to oxidation. As far as we know, BO has not been previously used in studies on lipid oxidation in meat products. Nevertheless, the literature reports that aqueous extracts of borage seeds reduce lipid oxidation in various meat and fish products [11,13,15,16]. The addition of MT reduced MDA content by more than 70% compared to CON (Table 3). Green tea is rich in polyphenols [49]. The antioxidant activity of phenolic compounds is closely related to the hydroxyl group attached to the aromatic ring, which can donate electrons with hydrogen atoms to neutralize free radicals. This mechanism blocks further degradation of highly reactive oxidizing species, preventing the formation of lipid oxidation products such as ketones and aldehydes. As a result, low MDA levels are observed in the final product, as also reported in other studies [11,43,49]. Storage time of meat products also affects TBARS values [39,57,59]. With refrigerated storage extended to 14 days, an increase in MDA was observed in CON and, to a lesser extent, in the BO trial, indicating intensified lipid oxidation. The highest MDA values after 14 days of storage were 2.27 and 1.80 mg/kg product, respectively. In samples with MT, MDA levels remained low and stable. The presence of plant antioxidants effectively limits the rate of oxidative changes occurring during refrigerated storage [46].
4.3. Color Parameters CIE L*a*b* of Vacuum-Packed Lamb Meatloaf
The color of meat and meat products is one of the most important quality indicators. Subjective color evaluation influences the perception of tenderness, palatability, and freshness of the product. Meat color depends on the content and form of heme pigments, the rate of myoglobin transformations in contact with oxygen, the physicochemical properties of muscle tissue, as well as water and fat content [61]. Additionally, color is influenced by the formulation, meat proportion, amount of added water, and the presence of plant-based ingredients. Color values also depend on the thermal processing method, processing temperature, and storage duration [63]. Higher temperatures above 70 °C accelerate protein denaturation and aggregation, resulting in more intense light scattering and reduced meat lightness [39]. The conversion of myoglobin to brown-colored forms can be enhanced by the presence of salts, which exhibit pro-oxidative activity by detaching iron atoms from heme pigment molecules [64].
The physicochemical properties of plant-based additives determine the hue of meat products—dark and intensely colored components cause greater changes in color parameters than lighter components. Measurement of color parameters (Table 5) indicated that the addition of BO did not significantly affect the lightness of lamb products. Meanwhile, other authors [62,65] reported an increase in L* values with higher oil levels. This increase in lightness was attributed to the better dispersion of plant oil compared to animal fat, allowing incident light to scatter more effectively, thus enhancing the lightness effect. Furthermore, smaller oil droplets have a larger surface area, which may lead to greater light reflection. Our results may not have confirmed these observations due to the low level of added oil, whereas other studies used 5–30% oil. In contrast, powdered green tea imparts a darker color to products, as confirmed by Bellés et al. [11], who stored raw lamb legs sprayed with aqueous extracts of matcha or borage for 13 days, and Jamróz et al. [66], who used green tea extracts in edible films prepared from furcellaran-gelatin blends. Wereńska-Sudnik et al. [59] and Bellés et al. [11] demonstrated that product lightness tends to decrease during storage. Our study, similarly to Uzlasir et al. [62], who used pumpkin seed oil as a partial animal fat replacer in Bologna sausages, did not confirm these observations.
Samples with the addition of MT were characterized by significantly lower redness values (a*) compared to CON (p < 0.05) (Table 5). Chlorophyll and carotenoids present in tea extracts impart a yellow-green pigmentation to the products. The reduced redness observed with the addition of oil, also reported by Uzlasir et al. [62] and Ambrosiadis et al. [67], is likely due to the distribution of the oil phase within the myofibrillar protein matrix, which increases the surface area of fat droplets. They noted that the inclusion of plant-based components, such as green tea or pumpkin seed oil, results in meat discoloration, shifting the hue toward green tones. Red color saturation also decreased over storage time, regardless of the additives used. The chromaticity of the yellow–blue axis (b*) is influenced by the formulation and storage duration. No significant effect of adding 4% BO and/or 0.5% MT on the b* parameter was observed. However, other studies [62,68] demonstrated that higher oil levels (>5%) increased yellowness (b*), likely due to the yellow color of the oil [69]. Literature reports also confirm the effect of green tea addition on meat product yellowness. However, Akcan et al. [70] and Jamróz et al. [66] emphasized that this was a result of acidic pH acting on chlorophyll in matcha tea, which degrades to pheophytin and pheophorbide. Consequently, this intensifies olive-green or yellow tones, leading to increased yellowness in samples with green tea addition. Nevertheless, color is a key quality attribute of lamb meat valued by consumers at the point of purchase. Therefore, the protective effect stabilizing color parameters, provided by the proposed addition of 4% BO and 0.5% MT, may positively influence consumer purchasing decisions.
4.4. Tenderness of Vacuum-Packed Lamb Meatloaf
Tenderness along with flavor and aroma, is one of the key indicators of meat quality. It is defined as the ease with which a bite can be bitten off, chewed, and swallowed. Tenderness is inversely related to the instrumentally measured maximum shear force. According to Novaković and Tomasević [71], this method provides a better overall assessment of product texture than the TPA (Texture Profile Analysis) test. Among the quality attributes of meat products, tenderness is the least stable. It depends on multiple factors, including the structure of muscle fibers, especially myofibrils, and the content of connective tissue, which varies depending on the animal species, age, sex, and feeding system [72]. Postmortem processes and meat aging also have a significant impact [73]. Additionally, tenderness is influenced by the method of thermal processing, temperature, and duration [47]. During thermal processing, collagen dissolves, increasing tenderness, whereas myofibrillar proteins denature, causing meat contraction and increased firmness [74]. Processing at temperatures above 70 °C reduces meat tenderness [63].
The addition of borage seed oil, or the combination of borage seed oil and matcha, significantly increased the tenderness of lamb meatloaf. Samples containing only matcha and the control were significantly harder, i.e., less tender (Table 5). Similar effects were observed by Uzlasir et al. [62] when adding plant oil to finely minced beef sausage. They reported that higher pumpkin seed oil content resulted in lower maximum shear force and reduced work of shear. However, Youssef and Barbut [68] showed that the hardness of minced meat products increases with higher protein content. In our study, no modifications were applied to alter protein content. Based on the results of other studies [43,75], it was assumed that protein content was the same across all samples. Tenderness is also affected by moisture content [76]. However, our results indicate that the addition of BO and/or MT did not significantly change the moisture content. Similarly, Purnamayanti et al. [43], who used 1–2% powdered green tea in lamb sausage, observed no effect on moisture content or tenderness. Likewise, Cegiełka [77] and Serdaroglu et al. [78] did not report significant differences in moisture content in meat products with added plant oils. In the authors’ opinion, the increased tenderness of samples containing plant oils can be attributed to their physicochemical properties. Plant fats have a significantly lower melting point than animal fats due to their higher content of unsaturated fatty acids. As a result, when incorporated into comminuted meat products, these oils form a softer and less cohesive matrix at room or refrigerated temperatures, contributing to a more tender texture compared to products containing primarily animal fats, which are richer in saturated fatty acids and solidify more readily. Additionally, the presence of these oils may interact with water and protein components, further influencing the product’s structural characteristics and overall mouthfeel. Moreover, the addition of matcha powder, due to its hygroscopic properties, may also enhance in the absorption and retention of water during storage, which affects the texture and juiciness of the product.
4.5. Statistical Analysis-Principal Component Analysis
To evaluate the impact of borage seed oil (BO) and matcha tea (MT) on the physicochemical properties, oxidative stability, color, and tenderness of vacuum-packed lamb meatloaf during 14 days of storage at 4 °C, Principal Component Analysis (PCA) was conducted. The first two principal components accounted for 85.49% of the total variance (Figure 1).
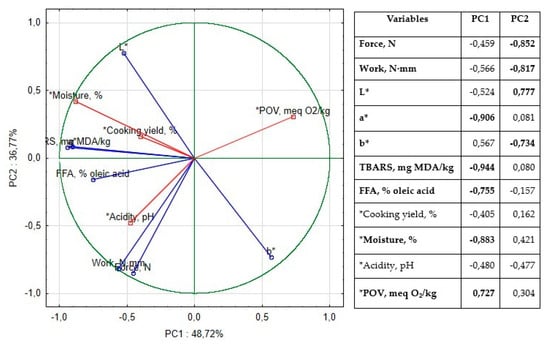
Figure 1.
Loadings for the two principal components and table of correlations of variables to factors in principal component analysis based on factor loadings. Correlations above 0.700 were marked in bold.
The first component (PC1) explained 48.77% of the variability, with TBARS, redness (a*), moisture, and FFA showing strong negative correlations, while peroxide value (PV) exhibited a strong positive correlation. The most influential contributions to PC1 were from the CON sample (64.87%) and the BO + MT sample (12.52%). The second component (PC2), explaining 36.77% of the variance, was positively associated with lightness (L*) and negatively associated with yellowness (b*) and tenderness. The BO sample had the highest contribution to PC2 (89.49%). These results suggest that the combined use of BO and MT influences oxidative stability, color parameters, and texture, highlighting their potential synergistic effects in lamb meatloaf during refrigerated storage.
5. Conclusions
The results of this study indicate a beneficial combination of plant oils and powdered green tea in meat products, suggesting a promising direction for further research aimed at improving the quality and stability of processed meat. Lamb, as a high-quality raw material, possesses a favorable nutritional profile and delicate flavor, making it an excellent base for the development of innovative meat products enriched with natural plant-based additives. The innovative combination of plant-derived additives, such as borage seed oil and matcha tea, in meat products ensures high oxidative stability. The synergistic action of phenolic compounds and oil components limits oxidation processes, enhancing product quality and safety. Instrumental measurement of color parameters showed no significant differences in color between control and experimental samples, while the addition of oil increased the tenderness of the lamb meatloaf. These findings may be relevant for the food industry, as they suggest that incorporating natural plant antioxidants into meat formulations can improve product stability and quality, meet consumer demand for clean-label foods, and open new opportunities for developing functional meat products with extended shelf life and additional health benefits. Further research should focus on in-depth analysis of bioactive properties and consumer studies.
Author Contributions
Conceptualization, A.L. and M.M.; methodology, A.L., J.L., N.Ö. and M.M.; formal analysis, A.L. and J.L.; investigation, A.L., J.L., N.Ö. and M.M.; resources, A.L., J.L., N.Ö. and M.M.; data curation, A.L., J.L., N.Ö. and M.M.; writing—original draft preparation, A.L., J.L., N.Ö. and M.M.; writing—review and editing, A.L. and J.L.; visualization, J.L.; supervision, A.L. and J.L.; project administration, A.L. and J.L.; funding acquisition, A.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the IDUB program in the Food Science research area, which enabled the establishment of cooperation between the University of Life Sciences in Lublin (Poland) and Muğla Sıtkı Koçman University (Türkiye).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Andrzej Junkuszew from the Department of Animal Breeding and Agricultural Consulting, University of Life Sciences in Lublin (Poland) for providing lamb meat for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BO + MT | lamb meatloaf with 4% borage seed oil and 0.5% matcha tea powder |
| BHT | butylated hydroxytoluene |
| BO | lamb meatloaf with 4% borage seed oil |
| CLA | conjugated linoleic acid |
| CON | lamb meatloaf without plant additives |
| FFA | free fatty acids |
| MT | lamb meatloaf with 0.5% matcha tea powder |
| PCA | principal component analysis |
| PV | peroxide value |
| PUFA | polyunsaturated fatty acids |
| TBA | 2-thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
| TPA | texture profile analysis |
References
- Stewart, S.M.; Polkinghorne, R.; Pethick, D.W.; Pannier, L. Carcass assessment and value in the Australian beef and sheep meat industry. Anim. Front. 2024, 14, 5–14. [Google Scholar] [CrossRef]
- Chikwandha, O.; Vahmani, P.; Muchenje, V.; Dugan, M.; Mapiye, C. Nutritional enhancement of sheep meat fatty acid profile for human health and wellbeing. Food Res. Int. 2017, 104, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Van Le, H.; Nguyen, D.; Nguyen, Q.; Malau-Aduli, B.; Nichols, P.; Malau-Aduli, A. Fatty acid profiles of muscle, liver, heart and kidney of Australian prime lambs fed different polyunsaturated fatty acids enriched pellets in a feedlot system. Sci. Rep. 2019, 9, 1238. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, Y.; Han, X.; Tan, D.; Chen, J.; Lai, C.; Yang, X.; Shan, X.; Silva, L.H.P.; Jiang, H. Effects of muscle fiber composition on meat quality, flavor characteristics, and nutritional traits in lamb. Foods 2025, 14, 2309. [Google Scholar] [CrossRef]
- Latoch, A.; Stasiak, D.M.; Libera, J.; Junkuszew, A. Assessment of suitability and lipid quality indicators of lamb meat of Polish native breeds. Ann. Anim. Sci. 2023, 23, 897–908. [Google Scholar] [CrossRef]
- Selani, M.M.; Herrero, A.M.; Ruiz-Capillas, C. Plant antioxidants in dry fermented meat products with a healthier lipid profile. Foods 2022, 11, 3558. [Google Scholar] [CrossRef]
- Akbar, S. Borago officinalis L. (Boraginaceae). In Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Akbar, S., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 445–450. [Google Scholar]
- Abu-Qaoud, H.; Shawarb, N.; Hussen, F.; Jaradat, N.; Shtaya, M. Comparison of qualitative, quantitative analysis and antioxidant potential between wild and cultivated Borago officinalis leaves from Palestine. Pak. J. Pharm. Sci. 2018, 31, 953–959. [Google Scholar]
- Shin, J.A.; Sun, M.; Jeong, J.-M. Borage oil treated with immobilized lipase inhibits melanogenesis. Lipids 2020, 55, 649–659. [Google Scholar] [CrossRef]
- Foster, R.H.; Hardy, G.; Alany, R.G. Borage oil in the treatment of atopic dermatitis. Nutrition 2010, 26, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Bellés, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. Effect of borage and green tea aqueous extracts on the quality of lamb leg chops displayed under retail conditions. Meat Sci. 2017, 129, 153–160. [Google Scholar] [CrossRef] [PubMed]
- García-Íñiguez de Ciriano, M.; García-Herreros, C.; Larequi, E.; Valencia, I.; Ansorena, D.; Astiasarán, I. Use of natural antioxidants from lyophilized water extracts of Borago officinalis in dry fermented sausages enriched in ω-3 PUFA. Meat Sci. 2009, 83, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.C.; Gómez-Guillén, M.; Pérez-Mateos, P.; Montero, P.; Márquez-Ruiz, G. Evaluation of lipid oxidation in horse mackerel patties covered with borage-containing film during frozen storage. Food Chem. 2011, 124, 1393–1403. [Google Scholar] [CrossRef]
- Hasdemir, Ö.; Kesbiç, O.S.; Cravana, C.; Fazio, F. Antioxidant performance of Borago officinalis leaf essential oil and protective effect on thermal oxidation of fish oil. Sustainability 2023, 15, 10227. [Google Scholar] [CrossRef]
- Martínez, L.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Antioxidant effect of rosemary, borage, green tea, pu-erh tea and ascorbic acid on fresh pork sausages packaged in a modified atmosphere: Influence of the presence of sodium chloride. J. Sci. Food Agric. 2006, 86, 1298–1307. [Google Scholar] [CrossRef]
- Sánchez-Escalante, A.; Djenane, D.; Torrescano, G.; Beltran, J.A.; Roncalés, P. Antioxidant action of borage, rosemary, oregano, and ascorbic acid in beef patties packaged in modified atmosphere. J. Food Sci. 2003, 68, 339–344. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kochman, J.; Kwiatkowska, A.; Kałduńska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant Properties and Nutritional Composition of Matcha Green Tea. Foods 2020, 9, 483. [Google Scholar] [CrossRef]
- Sarwa, K.K.; Rudrapal, M.; Debnath, M. Extraction of green tea leaves: The use of different methods, their optimization and comparative evaluation. Biosci. Biotechnol. Res. Asia 2013, 10, 383–386. [Google Scholar] [CrossRef]
- Thi Anh Dao, D.; Van Thanh, H.; Viet Ha, D.; Duc Nguyen, V. Optimization of spray-drying process to manufacture green tea powder and its characteristics. Food Sci. Nutr. 2021, 9, 6566–6574. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health benefits and chemical composition of Matcha green tea: A review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Qu, F.; Wang, P.; Gao, J.; Zhang, X.; Hu, J. Characterization of the key aroma compounds of Shandong Matcha using HS-SPME-GC/MS and SAFE-GC/MS. Foods 2022, 11, 2964. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Sehgal, A. Antioxidant activity of different forms of green tea: Loose leaf, bagged and Matcha. Curr. Res. Nutr. Food Sci. 2018, 6, 1. Available online: http://www.foodandnutritionjournal.org/?p=5061 (accessed on 4 August 2025). [CrossRef]
- Gupta, D.; Bhaskar, D.; Gupta, R.; Karim, B.; Jain, A.; Dalai, D. Green tea: A review on its natural antioxidant therapy and cariostatic benefits. Biol. Sci. Pharm. Res. 2014, 2, 8–12. Available online: https://journalissues.org/wp-content/uploads/sites/6/2014/01/Gupta-et-al.pdf (accessed on 4 August 2025).
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef]
- Alghamdi, A.I. Antibacterial activity of green tea leaves extracts against specific bacterial strains. J. King Saud Univ. Sci. 2023, 35, 102650. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EU) 2018/848 of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Union 2018, L150, 1–92. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32018R0848 (accessed on 30 July 2025).
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33. Available online: https://eur-lex.europa.eu/eli/dir/2010/63/oj/eng (accessed on 4 August 2025).
- Council of the European Union. Council Directive 98/58/EC of 20 July 1998 concerning the protection of animals kept for farming purposes. Off. J. Eur. Union 1998, L221, 23–27. Available online: https://eur-lex.europa.eu/eli/dir/1998/58/oj/eng (accessed on 17 July 2025).
- ISO 1442; Meat and Meat Products—Determination of moisture content—Reference Method. ISO: Geneva, Switzerland, 2000.
- ISO 2917; Meat and Meat Products—Measurement of pH—Reference Method. ISO: Geneva, Switzerland, 1999.
- Koniecko, E.S. Handbook for Meat Chemists; Avery Publishing Group, Inc.: Wayne, NJ, USA, 1979. [Google Scholar]
- Malik, A.H.; Sharma, B.D. Use of hurdle techniques to maintain the quality of vacuum packed buffalo meat during ambient storage temperatures. Afr. J. Food Sci. 2011, 5, 626–636. Available online: https://scholar.google.pl/scholar?cluster=5472541562775014173&hl=pl&as_sdt=0,5 (accessed on 23 July 2025).
- Wagh, R.V.; Chatli, M.K.; Ruusunen, M.; Puolanne, E.; Ertbjerg, P. Effect of various phyto-extracts on physico-chemical, colour, and oxidative stability of pork frankfurters. Asian Australas. J. Anim. Sci. 2015, 28, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Pikul, J.; Leszczyński, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 37, 1309–1315. [Google Scholar] [CrossRef]
- Commission Internationale de l’Éclairage (CIE). Supplement No. 2 to CIE Publication No. 15 Colorimetry; Bureau Central de la CIE: Paris, France, 1987. [Google Scholar]
- De Huidobro, F.R.; Miguel, E.; Blázquez, B.; Onega, E. A comparison between two methods (Warner–Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Sci. 2005, 69, 527–536. [Google Scholar] [CrossRef]
- Dominguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Kavusan, H.S.; Serdaroglu, M.; Nacak, B.; Ipek, G. An approach to manufacture of fresh chicken sausages incorporated with black cumin and flaxseed oil in water gelled emulsion. Food Sci. Anim. Resour. 2020, 40, 426–443. [Google Scholar] [CrossRef]
- Roldan, M.; Antequera, T.; Hernandez, A.; Ruiz, J. Physicochemical and microbiological changes during the refrigerated storage of lamb sous-vide cooked at different combinations of time and temperature. Food Sci. Technol. Int. 2015, 21, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Moawad, R.K.; Mohamed, O.S.S.; Abdelmaguid, N.M. Shelf-life evaluation of raw chicken sausage incorporated with green tea and clove powder extracts at refrigerated storage. Plant Arch. 2020, 20, 8821–8830. Available online: https://www.plantarchives.org/20-2/8821-8830%20(6621).pdf (accessed on 4 August 2025).
- Aksu, M.I. The effect of α-tocopherol, storage time and storage temperature on peroxide value, free fatty acids and pH of kavurma, a cooked meat product. J. Muscle Foods 2007, 18, 370–379. [Google Scholar] [CrossRef]
- Purnamayanti, L.; Jamhari, J.; Hanim, C.; Irawan, A. Physicochemical properties, oxidative stability and sensory quality of lamb sausage added with green tea leaves (Camellia sinensis) powder. Trop. Anim. Sci. J. 2020, 43, 57–63. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Effect of added citrus fibre and spice essential oils on quality characteristics and shelf-life of mortadella. Meat Sci. 2010, 85, 568–576. [Google Scholar] [CrossRef]
- Nath, P.M.; Kumar, V.; Kumar Praveen, P.; Ganguly, S. A comparative study of green tea extract and rosemary extract on quality characteristics of chevon patties. Int. J. Sci. Environ. Technol. 2016, 5, 1680–1688. Available online: https://www.researchgate.net/profile/Subha-Ganguly/publication/303698366_A_COMPARATIVE_STUDY_OF_GREEN_TEA_EXTRACT_AND_ROSEMARY_EXTRACT_ON_QUALITY_CHARACTERISTICS_OF_CHEVON_PATTIES/links/574e558c08ae061b33038cab/A-COMPARATIVE-STUDY-OF-GREEN-TEA-EXTRACT-AND-ROSEMARY-EXTRACT-ON-QUALITY-CHARACTERISTICS-OF-CHEVON-PATTIES.pdf (accessed on 23 July 2025).
- Salejda, A.M.; Krasnowska, G.; Tril, U. Attempt to utilize antioxidant properties of green tea extract in the production of model meat products. Żywność Nauka Technol. Jakość 2011, 78, 107–118. Available online: https://wydawnictwo.pttz.org/wp-content/uploads/2015/02/107_118_Salejda.pdf (accessed on 23 July 2025). (In Polish) [CrossRef]
- Jezek, F.; Kamenik, J.; Macharacova, B.; Bogdanovicova, K.; Bednar, J. Cooking of meat: Effect on texture, cooking loss and microbiological quality—A review. Acta Vet. Brno 2019, 88, 487–496. [Google Scholar] [CrossRef]
- Reitznerova, A.; Sulekova, M.; Nagy, J.; Marcincak, S.; Semojon, B.; Certik, M.; Klempova, T. Lipid peroxidation process in meat and meat products: A comparison study of malondialdehyde determination between modified 2-thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules 2017, 22, 1988. [Google Scholar] [CrossRef] [PubMed]
- Alheeti, M.Y.Y.; Al-Jugifi, W.I.; Alhadithi, H.J.M. Use of aqueous extract of matcha tea as an antioxidant in poultry meat storage. IOP Conf. Ser. Earth Environ. Sci. 2023, 1252, 012135. [Google Scholar] [CrossRef]
- Mir, S.A.; Masoodi, F.A. Effect of packaging on lipid oxidation, sensory and color attributes of value added mutton meatballs during refrigeration. J. Nutr. Health Food Eng. 2017, 7, 301–309. [Google Scholar] [CrossRef]
- Amaral, A.B.; Silva, M.V.; Silva Lannes, S.C. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Milon, M.; Kabir, M.H.; Hossain, M.A.; Rahman, M.; Azad, M.A.K.; Hashem, M.A. Value added beef meatballs using turmeric (Curcuma longa) powder as a source of natural antioxidant. Int. J. Nat. Soc. Sci. 2016, 3, 52–61. Available online: https://www.researchgate.net/profile/Md-Abul-Hashem-2/publication/318892558_Value_added_beef_meatballs_using_turmeric_Curcuma_longa_powder_as_a_source_of_natural_antioxidant_ARTICLE_INFO_ABSTRACT/links/5983c87aaca272a947c72f64/Value-added-beef-meatballs-using-turmeric-Curcuma-longa-powder-as-a-source-of-natural-antioxidant-ARTICLE-INFO-ABSTRACT.pdf (accessed on 23 July 2025).
- Wettasinghe, M.; Shahidi, F. Evening primrose meal: A source of natural antioxidants and scavenger of hydrogen peroxide and oxygen-derived free radicals. J. Agric. Food Chem. 1999, 47, 1801–1812. [Google Scholar] [CrossRef]
- Horie, H.; Ema, K.; Sumikawa, O. Chemical components of Matcha and powdered green tea. J. Cook. Sci. Jpn. 2017, 50, 182–188. Available online: https://www.jstage.jst.go.jp/article/cookeryscience/50/5/50_182/_pdf/-char/ja?trk=public_post_comment-text (accessed on 23 July 2025).
- Alnori, H.M.; Saeed, O.A.; Alnoori, M.A.; Leo, T.K.; Sani, U.M. Green tea extract improved minced mutton quality during chilled storage. Food Res. 2022, 6, 200–205. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Şen, D.B.; Kılıç, B. Effects of edible coatings containing acai powder and matcha extracts on shelf life and quality parameters of cooked meatballs. Meat Sci. 2021, 179, 108547. [Google Scholar] [CrossRef]
- Nain, C.W.; Berdal, G.; Thao, P.T.P.; Mignolet, E.; Buchet, M.; Page, M.; Larondelle, Y. Green tea extract enhances the oxidative stability of DHA-rich oil. Antioxidants 2021, 10, 982. [Google Scholar] [CrossRef]
- Wereńska-Sudnik, M.; Chełmecka, I.; Wołoszyn, J.; Okruszek, A.; Haraf, G.; Orkusz, A. Effect of green tea powder added on quality of offal products stored under refrigeration. Żywność Nauka Technol. Jakość 2016, 108, 60–71. (In Polish) [Google Scholar] [CrossRef]
- Klinhom, P.; Klinhom, J.; Methawiwat, S. Effect of different cooking method on cooking loss and lipid oxidation in buffalo meat. Appl. Mech. Mater. 2016, 885, 70–74. [Google Scholar] [CrossRef]
- Moawad, R.K.; Abozeid, W.M.; Nadir, A.S. Effect of nitrite level and tea catechins on residual nitrite and quality indices of raw-cured sausage. J. Appl. Sci. Res. 2012, 8, 815–822. [Google Scholar]
- Uzlasir, T.; Aktas, N.; Gercekaslan, K.E. Pumpkin seed oil as a partial animal fat replacer in Bologna-type sausages. Food Sci. Anim. Resour. 2020, 40, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Gok, V.; Uzun, T.; Tomar, O.; Caglar, M.Y.; Caglar, A. The effect of cooking methods on some quality characteristics of gluteus medius. Food Sci. Technol. 2019, 39, 999–1004. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Narsaiah, K.; Borah, A. The effect of salt, extract of kinnow and pomegranate fruit by-products on colour and oxidative stability of raw chicken patties during refrigerated storage. J. Food Sci. Technol. 2011, 48, 472–477. [Google Scholar] [CrossRef]
- Kang, Z.L.; Zhu, D.; Li, B.; Ma, H.J.; Song, Z.J. Effect of pre-emulsified sesame oil on physico-chemical and rheological properties of pork batters. Food Sci. Technol. 2019, 37, 620–626. [Google Scholar] [CrossRef][Green Version]
- Jamróz, E.; Kulawik, P.; Krzysciak, P.; Talaga-Cwiertnia, K.; Juszczak, L. Intelligent and active furcellaran-gelatin films containing green or pu-erh tea extracts: Characterization, antioxidant and antimicrobial potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef]
- Ambrosiadis, J.; Vareltzis, K.P.; Georgakis, S.A. Physical, chemical and sensory characteristics of cooked meat emulsion style products containing vegetable oils. Int. J. Food Sci. Technol. 1996, 31, 189–194. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Effects of protein level and fat/oil on emulsion stability, texture, microstructure and color of meat batters. Meat Sci. 2009, 82, 228–233. [Google Scholar] [CrossRef]
- Aktas, N.; Gercekaslan, K.E.; Uzlasir, T. The effect of some pre-roasting treatments on quality characteristics of pumpkin seed oil. OCL 2018, 25, A202. [Google Scholar] [CrossRef]
- Akcan, T.; Estevez, M.; Serdaroglu, M. Antioxidant protection of cooked meatballs during frozen storage by whey protein edible films with phytochemicals from Laurus nobilis L. and Salvia officinalis L. LWT-Food Sci. Technol. 2017, 77, 323–331. [Google Scholar] [CrossRef]
- Novaković, S.; Tomasević, I. A comparison between Warner–Bratzler shear force measurement and texture profile analysis of meat and meat products: A review. IOP Conf. Ser. Earth Environ. Sci. 2017, 85, 012063. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.A. Applied and emerging methods for meat tenderization: A comparative perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 841–859. [Google Scholar] [CrossRef]
- Bai, Y.; Ren, C.; Wu, S.; Hou, C.; Li, X.; Zhang, D. Co-regulation of very fast chilling treatment and the follow-up storage temperature on meat tenderness through glycolysis. Foods 2025, 14, 2932. [Google Scholar] [CrossRef]
- Wyrwisz, J.; Półtorak, A.; Poławska, E.; Pierzchała, M.; Jóźwik, A.; Zalewska, M.; Zaremba, R.; Wierzbicka, A. The impact of heat treatment methods on the physical properties and cooking yield of selected muscles from Limousine breed cattle. Anim. Sci. Pap. Rep. 2012, 30, 339–351. Available online: https://www.researchgate.net/profile/Mariusz-Pierzchala/publication/259219549_The_impact_of_heat_treatment_methods_on_the_physical_properties_and_cooking_yield_of_selected_muscles_from_Limousine_breed_cattle/links/56fe417308aee995dde72877/The-impact-of-heat-treatment-methods-on-the-physical-properties-and-cooking-yield-of-selected-muscles-from-Limousine-breed-cattle.pdf (accessed on 24 July 2025).
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, D.W. Factors affecting the water holding capacity of red meat products: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2008, 48, 137–159. [Google Scholar] [CrossRef]
- Cegiełka, A. Applying plant oils and fibre preparations to produce chicken meat burgers. Żywność Nauka Technol. Jakość 2012, 82, 88–100. Available online: https://wydawnictwo.pttz.org/wp-content/uploads/2015/02/08_Cegielka.pdf (accessed on 20 July 2025). (In Polish) [CrossRef]
- Serdaroglu, M.; Kavusan, S.; Ipek, G.; Ozturk, B. Evaluation of the quality of beef patties formulated with dried pumpkin pulp and seed. Korean J. Food Sci. Anim. Resour. 2018, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).