Optimized Alkaline Extraction and Functional Characterization of Carrageenan from Eucheuma perplexum Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Raw Material, Preparation, and Drying of E. perplexum
2.2. Determination of Dried E. perplexum, Water Activity, and Moisture Content After Drying
2.3. Optimization of Carrageenan Extraction from E. perplexum
Extraction and Recovery of Crude Carrageenan
2.4. Characterization of Carrageenan Extract
2.4.1. Carrageenan Yield and Carbohydrate Content
2.4.2. Emulsifying Properties
2.4.3. Color Properties
2.4.4. Sulfate Content
2.4.5. Viscosity
2.4.6. ATR-FTIR Analysis
2.5. Statistical Analysis
3. Results and Discussion
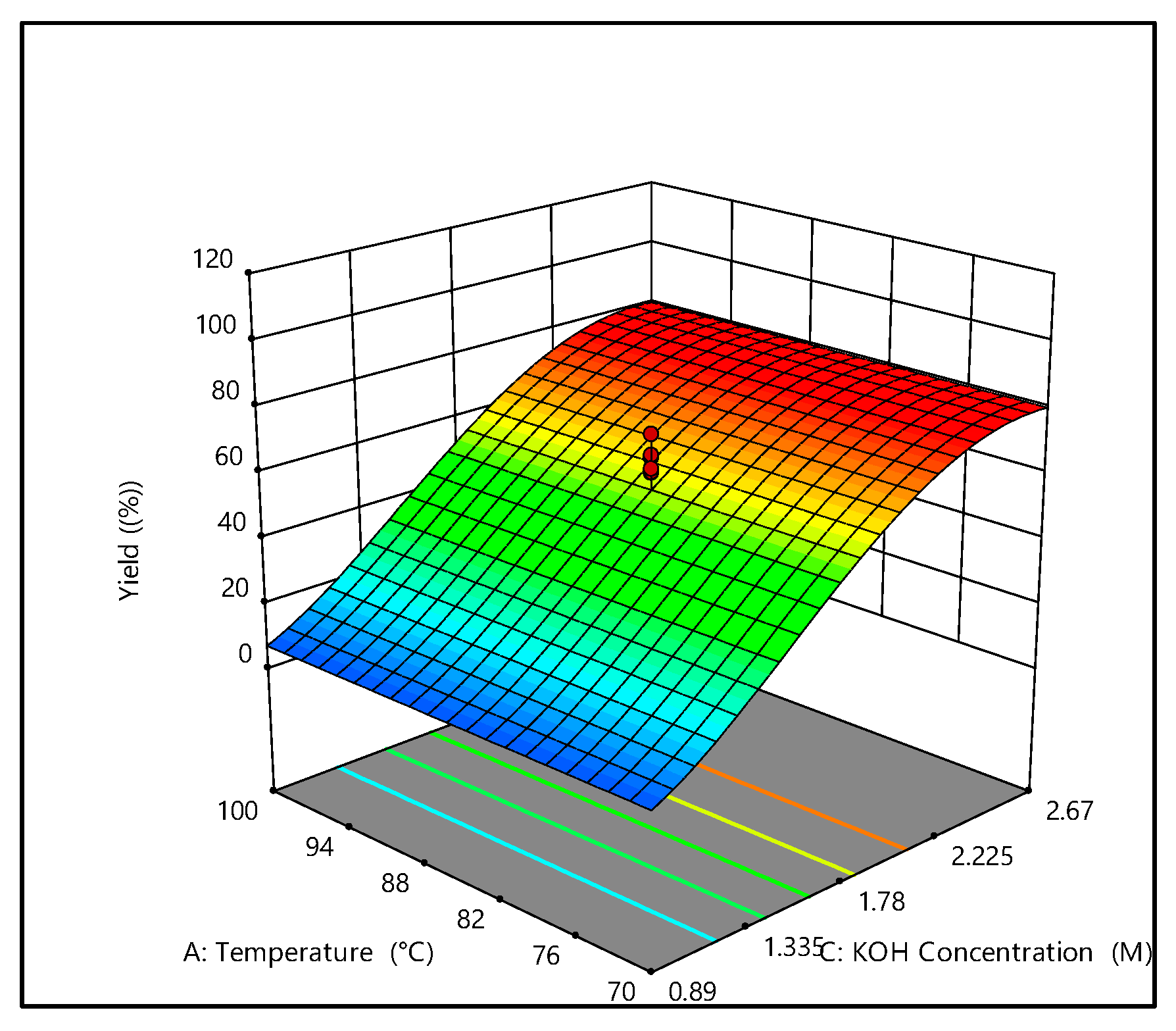
3.1. Optimization of Carrageenan Extraction
3.1.1. Carrageenan Yield and Properties
3.1.2. Effect of Extraction Conditions on the Yield of Carrageenan
3.2. Characterization of Carrageenan
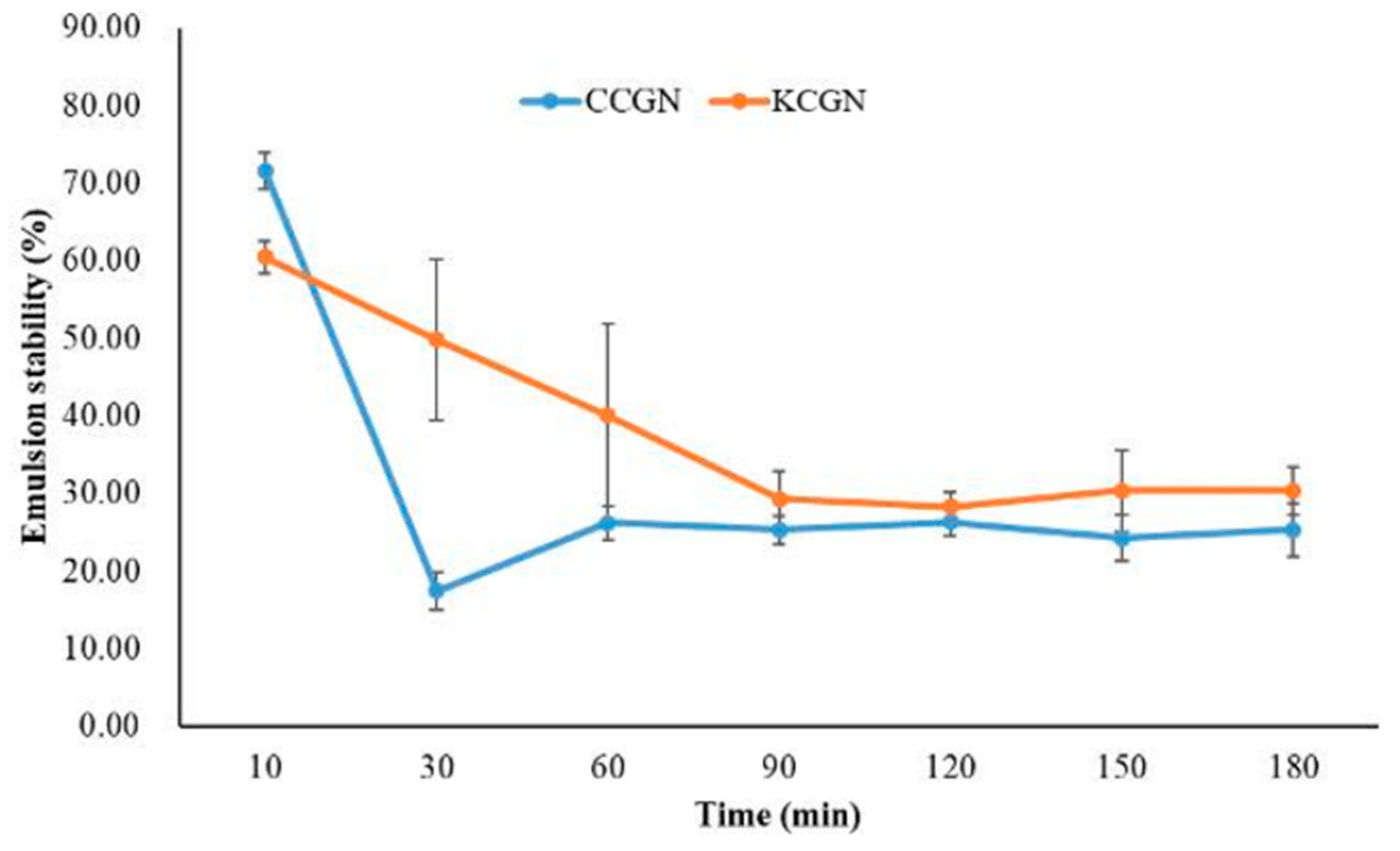
3.2.1. Emulsifying Properties
3.2.2. Color Properties
3.2.3. Physiochemical Properties, Including Sulfate, Total Carbohydrate Contents, and Viscosity for Carrageenan Extraction from E. perplexum
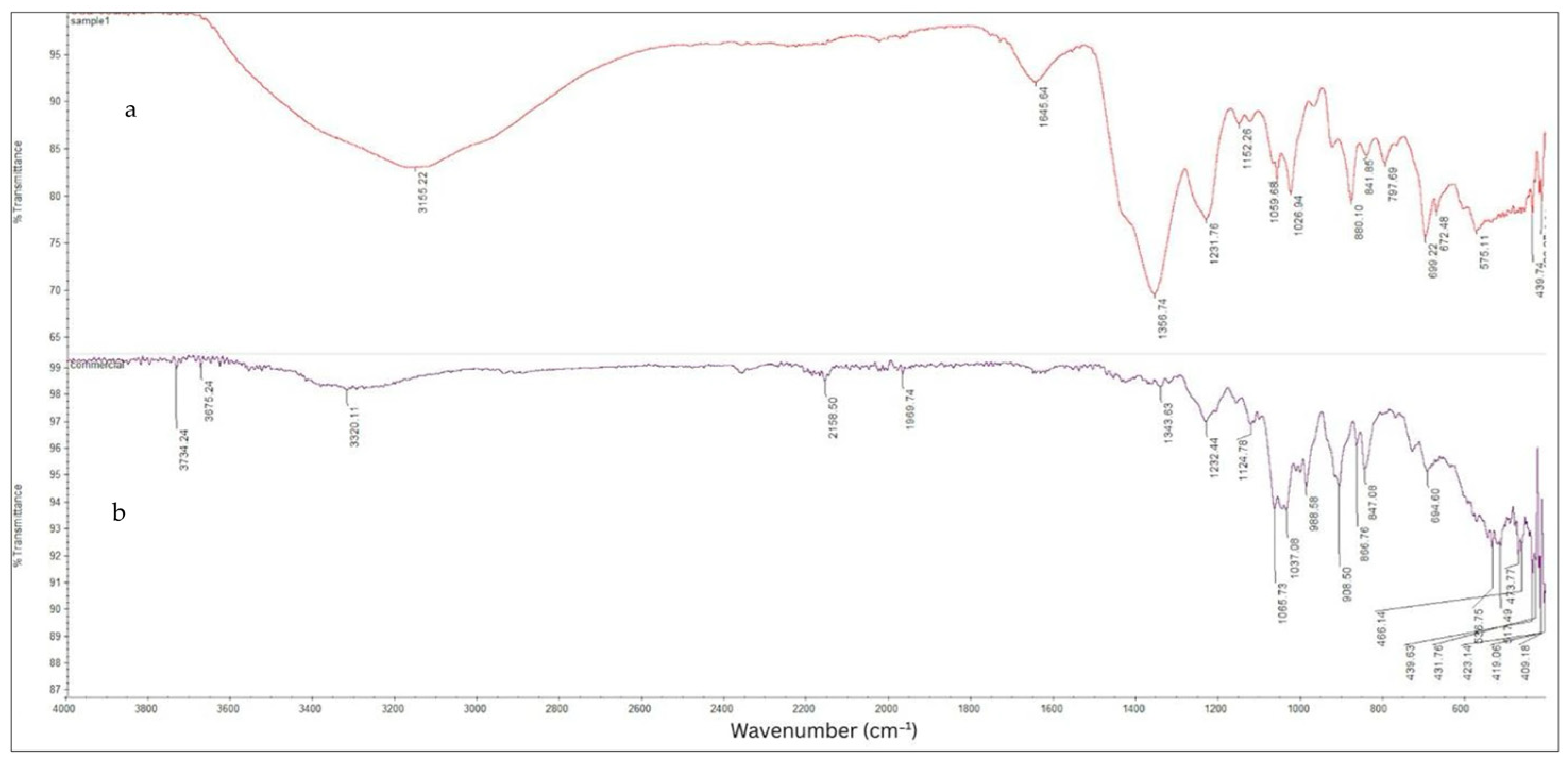
3.2.4. ATF-FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azevedo, G.; Torres, M.D.; Sousa-Pinto, I.; Hilliou, L. Effect of pre-extraction alkali treatment on the chemical structure and gelling properties of hybrid carrageenan from Chondrus crispus and Ahnfeltiopsis devoniensis. Food Hydrocoll. 2015, 50, 150–158. [Google Scholar] [CrossRef]
- Tahiluddin, A.B.; Carpio, R.M.; delos Reyes, F.L.; Cabrera, E.C.; Serrano, A.E. Effects of rainwater on carrageenan yield and quality of Kappaphycus. Aquat. Bot. 2025, 187, 103694. [Google Scholar]
- Cai, J.; Hishamunda, N.; Ridler, N. The Global Status of Seaweed Production, Trade and Utilization. FAO Fish. Aquac. Circ. 2021, 1229, 1–52. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/3/cc0461en/cc0461en.pdf (accessed on 20 August 2023).
- Research and Markets. Carrageenan Market by Type, Application, and Region—Global Forecast to 2030; Research and Markets: Dublin, Ireland, 2022; Available online: https://www.researchandmarkets.com/reports/5598652 (accessed on 5 August 2025).
- Bureau of Fisheries and Aquatic Resources (BFAR). Carrageenan Remains on the US National List of Allowed Substances; BFAR/NAST: Manila, Philippines, 2019. Available online: https://transactions.nast.ph/ (accessed on 16 August 2025).
- Philippine Council for Agriculture and Fisheries (PCAF). Policy Brief: The Philippine Carrageenan Industry; Department of Agriculture: Quezon City, Philippines, 2022. Available online: https://www.pcaf.da.gov.ph (accessed on 5 August 2025).
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Fazita, M.R.N.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Falshaw, R.; Bixler, H.J.; Johndro, K. Structure and performance of commercial kappa-2 carrageenan extracts. Food Hydrocoll. 2001, 15, 441–451. [Google Scholar] [CrossRef]
- Basmal, J.; Suryaningrum, D.; Yeni, Y. Pengaruh konsentrasi larutan potasium hidroksida terhadap karagenan kertas. J. Penelit. Perikan. Indones. 2005, 11, 1–9. [Google Scholar]
- Distantina, S.; Wiratni; Fahrurrozi, M.; Rochmadi. Carrageenan properties extracted from Eucheuma cottonii, Indonesia. Int. J. Chem. Mol. Eng. 2011, 5, 501–505. [Google Scholar]
- Webber, V.; De Carvalho, S.M.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the extraction of carrageenan from Kappaphycus alvarezii using response surface methodology. Cienc. Tecnol. Aliment. 2012, 32, 812–818. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Montaño, M.N.E. Enhancement of carrageenan gel quality in Eucheuma denticulatum with postharvest treatment in low-nutrient conditions. Bot. Mar. 2014, 57, 217–223. [Google Scholar] [CrossRef]
- Hasizah, A.; Mahendradatta, M.; Laga, A.; Metusalach, M.; Supratomo, A.; Waris, A.; Salengke, S. A novel ohmic-based technology for seaweed processing. Int. Food Res. J. 2018, 25, 1341–1348. [Google Scholar]
- El-Sersy, N.A.; Ebrahim, H.A.H.; Abou-Elela, G.M. Response surface methodology as a tool for optimizing the production of antimicrobial agents from Bacillus licheniformis SN2. Curr. Res. Biotechnol. 2010, 3, 1–14. [Google Scholar] [CrossRef]
- Elumalai, S.; Thangavelu, V. Simultaneous saccharification and fermentation of pretreated sugarcane bagasse using cellulase and Saccharomyces cerevisiae: Kinetics and modeling. Chem. Eng. Res. Bull. 2010, 14, 29–35. [Google Scholar] [CrossRef]
- Fan, X.; Wang, X.; Chen, F. Biodiesel production from crude cottonseed oil: An optimization process using response surface methodology. Open Fuels Energy Sci. J. 2011, 4, 1–8. [Google Scholar]
- Bono, A.; Anisuzzaman, S.M.; Ding, O.W. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan from Kappaphycus alvarezii. J. King Saud Univ. Eng. Sci. 2014, 26, 3–9. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Han, D.; Row, K.H. Optimization of enzymatic extraction of polysaccharides from marine algae by response surface methodology. Korean J. Chem. Eng. 2012, 29, 650–656. [Google Scholar] [CrossRef]
- Mustapha, S.; Chandar, H.; Abidin, Z.Z.; Saghravani, R.; Harun, M.Y. Production of semi-refined carrageenan from Eucheuma cottonii. J. Sci. Ind. Res. 2011, 70, 865–870. [Google Scholar]
- Liao, J.L.; Ding, Z.N.; Hsu, Y.H.; Chang, J.S. Physiological and molecular insights into color variation and light-intensity adaptation in Eucheuma perplexum with applications for selective cultivation. Algal Res. 2025, 89, 104067. [Google Scholar] [CrossRef]
- Nielsen, S.S. Moisture content determination. In Food Analysis Laboratory Manual; Springer International Publishing: Cham, Switzerland, 2017; pp. 105–115. [Google Scholar]
- Hasizah, A.; Mahendradatta, M.; Laga, A.; Metusalach, M.; Salengke, S. Extraction of carrageenan from Eucheuma spinosum using ohmic heating: Optimization of extraction conditions using response surface methodology. Food Sci. Technol. Camp. 2021, 41, 928–937. [Google Scholar] [CrossRef]
- Nurmiah, S.; Syarief, R.; Sukarno; Peranginangin, R.; Nurtama, B.; Jaswir, I. Production of refined carrageenan from Kappaphycus alvarezii on pilot plant scale: Optimization of water extraction using response surface methodology. Int. Food Res. J. 2017, 24, S522–S528. [Google Scholar]
- Firdayanti, L.; Yanti, R.; Rahayu, E.S.; Hidayat, C. Carrageenan extraction from red seaweed (Kappaphycopsis cottonii) using the bead mill method. Algal Res. 2023, 69, 102906. [Google Scholar] [CrossRef]
- Deans, C.A.; Sword, G.A.; Lenhart, P.A.; Burkness, E.; Hutchison, W.D.; Behmer, S.T. Quantifying plant soluble protein and digestible carbohydrate content, using corn (Zea mays) as an exemplar. J. Vis. Exp. 2018, e58164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F. Comparative study of surface-active properties and antimicrobial activities of disaccharide monoesters. PLoS ONE 2014, 9, e114845. [Google Scholar] [CrossRef] [PubMed]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- JECFA. Processed Eucheuma cottonii. Available online: www.marinalg.org (accessed on 17 May 2025).
- Torres, M.D.; Florez-Fernandez, N.; Dominguez, H. Ultrasound-Assisted water extraction of Mastocarpus stellatus carrageenan with adequate mechanical and antiproliferative properties. Mar. Drugs 2021, 19, 280. [Google Scholar] [CrossRef]
- Ponthier, E.; Domínguez, H.; Torres, M.D. The microwave-assisted extraction sway on the features of antioxidant compounds and gelling biopolymers from Mastocarpus stellatus. Algal Res. 2020, 51, 102081. [Google Scholar] [CrossRef]
- Kumari, K.S.; Babu, I.S.; Rao, G.H. Process optimization for citric acid production from raw glycerol using response surface methodology. Indian J. Biotechnol. 2008, 7, 496–501. [Google Scholar]
- Koocheki, A.; Taherian, A.R.; Razavi, S.M.A.; Bostan, A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocoll. 2009, 23, 2369–2379. [Google Scholar] [CrossRef]
- Mendes, M.; Cotas, J.; Gutiérrez, I.B.; Gonçalves, A.M.M.; Critchley, A.T.; Hinaloc, L.A.R.; Roleda, M.Y.; Pereira, L. Advanced extraction techniques and physicochemical properties of carrageenan from a novel Kappaphycus alvarezii cultivar. Mar. Drugs 2024, 22, 491. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Mishra, P.C.; Jayasankar, R.; Seema, C. Yield and quality of carrageenan from Kappaphycus alvarezii subjected to different physical and chemical treatments. Seaweed Res. Util. 2006, 28, 113–117. [Google Scholar]
- Setyopratomo, P.; Sapei, L. Rheological behavior and antioxidant activity of carrageenan extracted from Eucheuma cottonii using alkaline solution. AIP Conf. Proc. 2022, 2470, 040002. [Google Scholar]
- Naseri, A.; Jacobsen, C.; Sejberg, J.J.P.; Pedersen, T.E.; Larsen, J.; Hansen, K.M.; Holdt, S.L. Multi-extraction and quality of protein and carrageenan from commercial Eucheuma denticulatum. Foods 2020, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Murano, E. Agars from three species of Gracilaria (Rhodophyta) from Yucatán Peninsula. Bioresour. Technol. 2005, 96, 295–302. [Google Scholar] [CrossRef]
- Truus, K.; Vaher, M.; Taure, I. Algal biomass from Fucus vesiculosus (Phaeophyta): Investigation of themineral and alginate components. Proc. Est. Acad. Sci. Chem. 2001, 50, 95–103. [Google Scholar]
- Wangtueai, S.; Noomhorm, A. Processing optimization and characterization of gelatin from lizardfish (Saurida spp.) scales. LWT-Food Sci. Technol. 2009, 42, 825–834. [Google Scholar]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribereau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea, and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Gbadamosi, S.O.; Abiose, S.H.; Aluko, R.E. Amino acid profile, protein digestibility, thermal and functional properties of Conophor nut (Tetracarpidium conophorum) defatted flour, protein concentrate, and isolates. Int. J. Food Sci. Technol. 2012, 47, 731–739. [Google Scholar] [CrossRef]
- Martin-del-Campo, A.; Fermin-Jimenez, J.A.; Fernandez-Escamilla, V.V.; Escalante-Garcia, Z.Y.; Macias-Rodriguez, M.A.; Estrada-Giron, Y. Improved extraction of carrageenan from red seaweed (Chondracanthus canaliculatus) using ultrasound-assisted methods and evaluation of the yield, physicochemical properties, and functional groups. Food Sci. Biotechnol. 2021, 30, 901–910. [Google Scholar] [CrossRef]
- Paz-Cedeno, F.R.; Solórzano-Chávez, E.G.; de Oliveira, L.E.; Gelli, V.C.; Monti, R.; de Oliveira, S.C.; Masarin, F. Sequential Enzymatic and Mild-Acid Hydrolysis of By-Product of Carrageenan Process from Kappaphycus alvarezii. BioEnergy Res. 2019, 12, 419–432. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- Diharmi, A.; Fardiaz, D.; Andarwulan, N. Chemical and minerals composition of dried seaweed Eucheuma spinosum collected from Indonesia Coastal Sea Regions. Int. J. Oceans Oceanogr. 2019, 13, 65–71. [Google Scholar]
- Jumaah, F.N.; Mobarak, N.N.; Ahmad, A.; Ghani, M.A.; Rahman, M.Y.A. Derivative of iota-carrageenan as solid polymer electrolyte. Ionics 2015, 21, 1311–1320. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ramli, N.; Ahmad, A.; Rahman, M.Y.A. Chemical interaction and conductivity of carboxymethyl κ-carrageenan-based green polymer electrolyte. Solid State Ionics 2012, 224, 51–57. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Azamar, J.A.; Robledo, D. Preliminary characterization of carrageenan from the red seaweed Halymenia floresii. J. Aquat. Food Prod. Technol. 2011, 20, 73–83. [Google Scholar] [CrossRef]
- Tranquilan-Aranilla, C.; Nagasawa, N.; Bayquen, A.; Dela Rosa, A. Synthesis and characterization of carboxymethyl derivatives of kappa-carrageenan. Carbohydr. Polym. 2012, 87, 1810–1816. [Google Scholar] [CrossRef]
| Independent Variable | Symbol | Coded Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | +α | ||
| Extraction temperature (°C) | X1 | 59.77 | 70 | 85 | 100 | 110.23 |
| Extraction time (h) | X2 | 1.32 | 2 | 3 | 4 | 4.68 |
| KOH (M) | X3 | 0.28 | 0.89 | 1.78 | 2.67 | 3.28 |
| Runs | Independent Variables | Response Values (Y) | ||
|---|---|---|---|---|
| Temperature, °C | Time, h | KOH, M | Yield, % | |
| 1 | 100 | 2 | 2.67 | 66.87 |
| 2 | 70 | 4 | 2.67 | 50.03 |
| 3 | 110.22 | 3 | 1.78 | -* |
| 4 | 85 | 4.68 | 1.78 | 74.40 |
| 5 | 85 | 3 | 1.78 | 65.20 |
| 6 | 85 | 3 | 3.28 | 77.49 |
| 7 | 85 | 1.32 | 1.78 | 61.94 |
| 8 | 100 | 2 | 0.89 | 0.00 |
| 9 | 100 | 4 | 0.89 | 0.00 |
| 10 | 85 | 3 | 1.78 | 60.64 |
| 11 | 85 | 3 | 1.78 | 72.32 |
| 12 | 85 | 3 | 1.78 | 61.87 |
| 13 | 85 | 3 | 1.78 | 66.02 |
| 14 | 100 | 4 | 2.67 | 64.31 |
| 15 | 85 | 3 | 1.78 | 60.86 |
| 16 | 70 | 2 | 2.67 | 60.67 |
| 17 | 85 | 3 | 0.28 | 0.00 |
| 18 | 70 | 4 | 0.89 | 0 |
| 19 | 70 | 2 | 0.89 | 0 |
| 20 | 59.77 | 3 | 1.78 | 66.03 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 363.74 | 4 | 90.94 | 3.72 | 0.0419 | significant |
| B-Time | 0.3832 | 1 | 0.3832 | 0.0157 | 0.9029 | |
| C-KOH Concentration | 77.41 | 1 | 77.41 | 3.17 | 0.1055 | |
| BC | 114.96 | 1 | 114.96 | 4.70 | 0.0553 | |
| C2 | 241.26 | 1 | 241.26 | 9.87 | 0.0105 | |
| Residual | 244.49 | 10 | 24.45 | |||
| Lack of Fit | 145.47 | 5 | 29.09 | 1.47 | 0.3416 | not significant |
| Pure Error | 99.02 | 5 | 19.80 | |||
| Cor Total | 608.23 | 14 |
| Runs | Parameters | |||
|---|---|---|---|---|
| L* | a* | b* | Total Color Difference (ΔE) | |
| 1 | 94.90 ± 0.91 a | −11.81 ± 1.06 a | 36.11 ± 6.74 c | 10.86 ± 6.74 ab |
| 2 | 93.44 ± 1.10 ab | −12.17 ± 0.80 abc | 41.44 ± 2.35 bc | 5.41 ± 2.40 abcd |
| 3 | 94.67 ± 1.07 a | −11.93 ± 0.59 ab | 37.05 ± 6.06 c | 9.90 ± 6.06 abc |
| 4 | 90.88 ± 1.90 abc | −13.88 ± 0.31 def | 46.54 ± 0.53 ab | 2.41 ± 0.89 cd |
| 5 | 90.67 ± 0.80 abc | −14.60 ± 0.41 ef | 48.81 ± 1.17 ab | 3.72 ± 0.82 bcd |
| 6 | 91.72 ± 3.29 ab | −12.17 ± 0.38 abc | 46.42 ± 2.96 ab | 0.00 ± 0.00 d (Control) |
| 7 | 88.84 ± 5.85 abc | −13.51 ± 0.68 cdef | 48.36 ± 2.23 ab | 5.15 ± 4.56 abcd |
| 10 | 84.70 ± 3.44c | −14.50 ± 1.18 ef | 47.17 ± 1.14 ab | 7.64 ± 3.19 abcd |
| 11 | 88.58 ± 4.27 abc | −14.77 ± 0.55 ef | 46.91 ± 1.69 ab | 5.23 ± 2.47 abcd |
| 12 | 87.57 ± 3.27 bc | −14.27 ± 0.57 ef | 48.97 ± 0.87 ab | 5.57 ± 2.79 abcd |
| 13 | 84.91 ± 5.90 c | −13.42 ± 0.81 bcde | 50.18 ± 0.76 a | 8.53 ± 4.49 abc |
| 14 | 94.27 ± 3.82 ab | −11.24 ± 1.37 a | 36.75 ± 11.80 c | 12.35 ± 8.86 a |
| 15 | 88.73 ± 4.32 abc | −15.04 ± 0.33 f | 46.20 ± 1.90 ab | 5.35 ± 2.46 abcd |
| 16 | 87.53 ± 3.95 bc | −12.45 ± 1.10 abcd | 51.47 ± 1.25 a | 7.35 ± 1.72 abcd |
| 20 | 91.14 ± 1.98 abc | −14.50 ± 0.99 ef | 48.43 ± 1.95 ab | 3.68 ± 1.80 bcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daet, I.P.; Chen, T.-Y.; Nuñal, S.N.; Peralta, J.P.; Simora, R.M.C.; Lee, M.C.; Chang, J.-S.; Traifalgar, R.F.M. Optimized Alkaline Extraction and Functional Characterization of Carrageenan from Eucheuma perplexum Using Response Surface Methodology. Foods 2025, 14, 3496. https://doi.org/10.3390/foods14203496
Daet IP, Chen T-Y, Nuñal SN, Peralta JP, Simora RMC, Lee MC, Chang J-S, Traifalgar RFM. Optimized Alkaline Extraction and Functional Characterization of Carrageenan from Eucheuma perplexum Using Response Surface Methodology. Foods. 2025; 14(20):3496. https://doi.org/10.3390/foods14203496
Chicago/Turabian StyleDaet, Irene P., Tai-Yuan Chen, Sharon N. Nuñal, Jose P. Peralta, Rhoda Mae C. Simora, Meng Chou Lee, Jui-Sheng Chang, and Rex Ferdinand M. Traifalgar. 2025. "Optimized Alkaline Extraction and Functional Characterization of Carrageenan from Eucheuma perplexum Using Response Surface Methodology" Foods 14, no. 20: 3496. https://doi.org/10.3390/foods14203496
APA StyleDaet, I. P., Chen, T.-Y., Nuñal, S. N., Peralta, J. P., Simora, R. M. C., Lee, M. C., Chang, J.-S., & Traifalgar, R. F. M. (2025). Optimized Alkaline Extraction and Functional Characterization of Carrageenan from Eucheuma perplexum Using Response Surface Methodology. Foods, 14(20), 3496. https://doi.org/10.3390/foods14203496








