Microbial Production of N-Acetylneuraminic Acid Using Metabolically Engineered Escherichia coli and Bacillus subtilis: Advances and Perspectives
Abstract
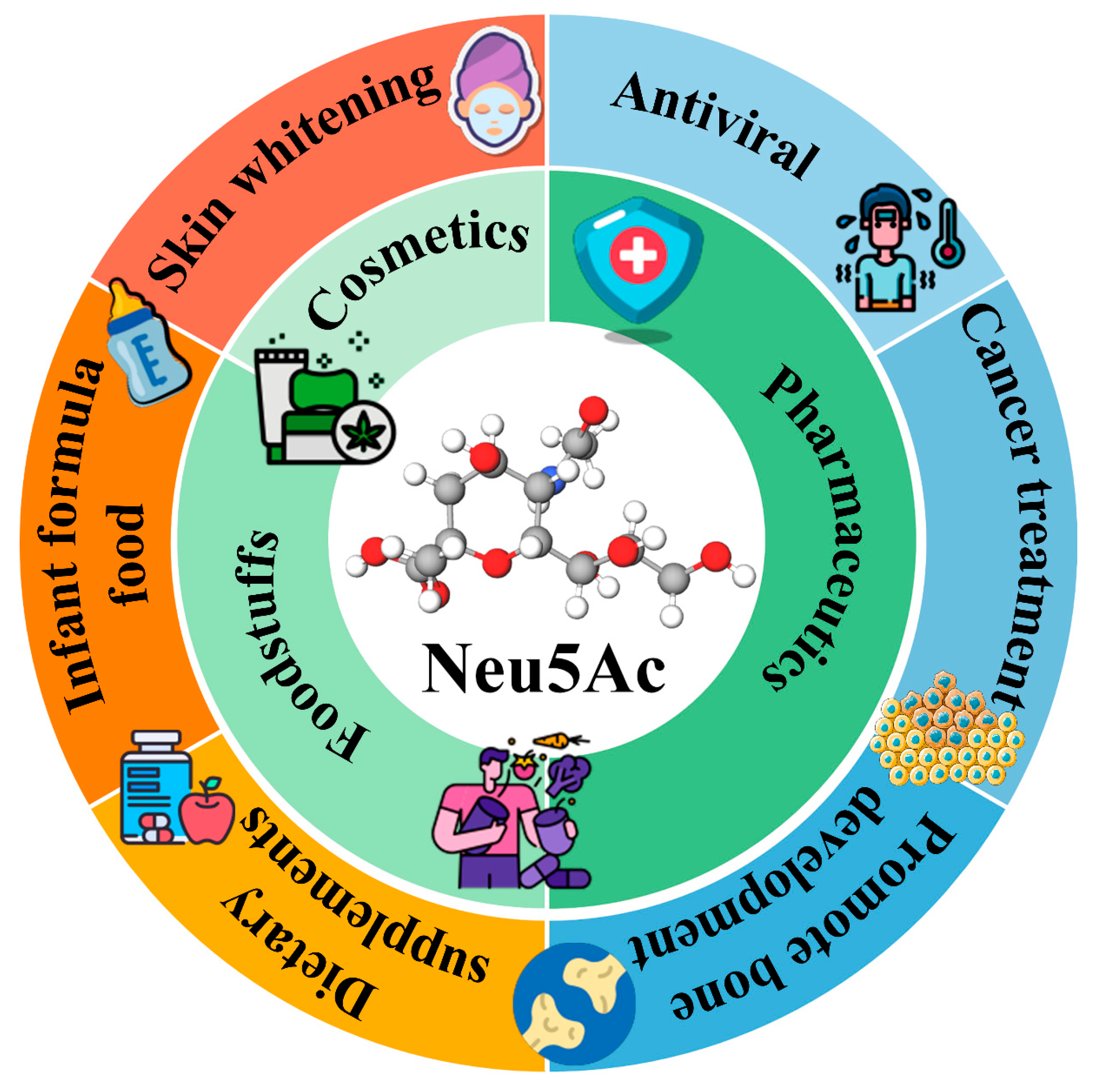
1. Introduction
2. Biosynthetic Pathway, Regulation Mechanisms, and Production of Neu5Ac
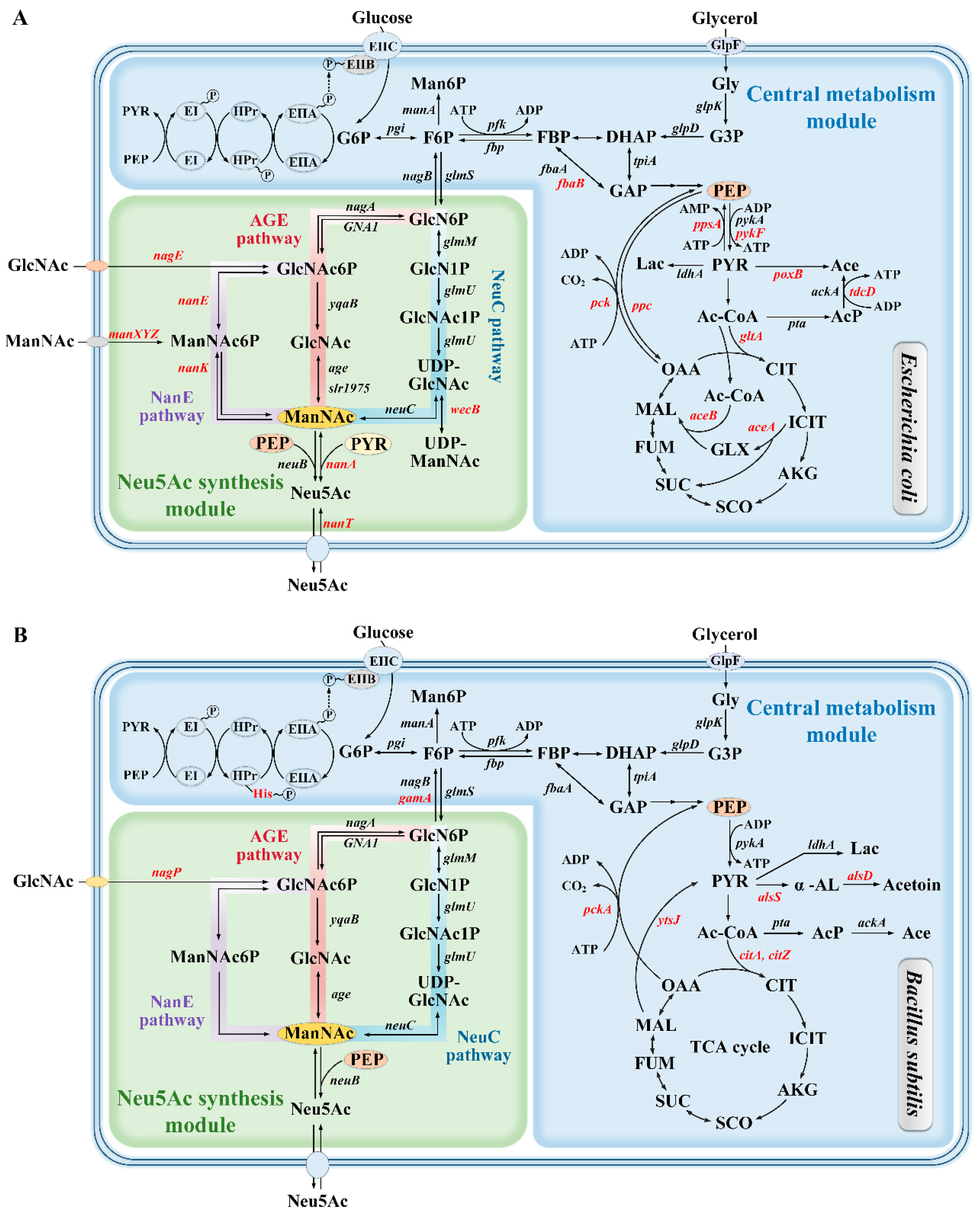
2.1. Biosynthetic Pathway of Neu5Ac
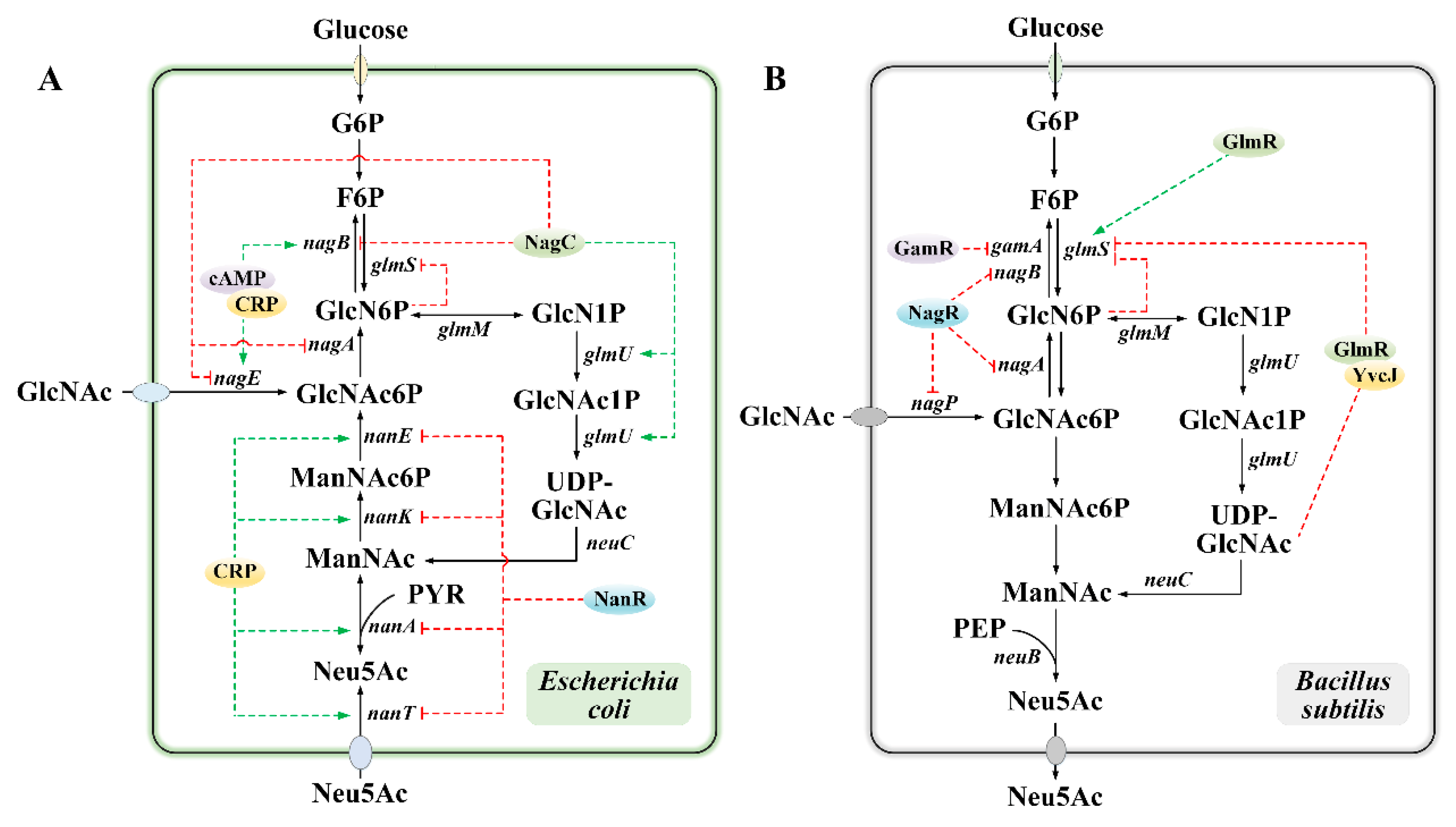
2.2. Regulation of Neu5Ac Synthesis in Microbial Systems
2.3. Production Methods and Purification Challenges of Neu5Ac
3. Strategies in the Construction of Cell Factories for Neu5Ac Production
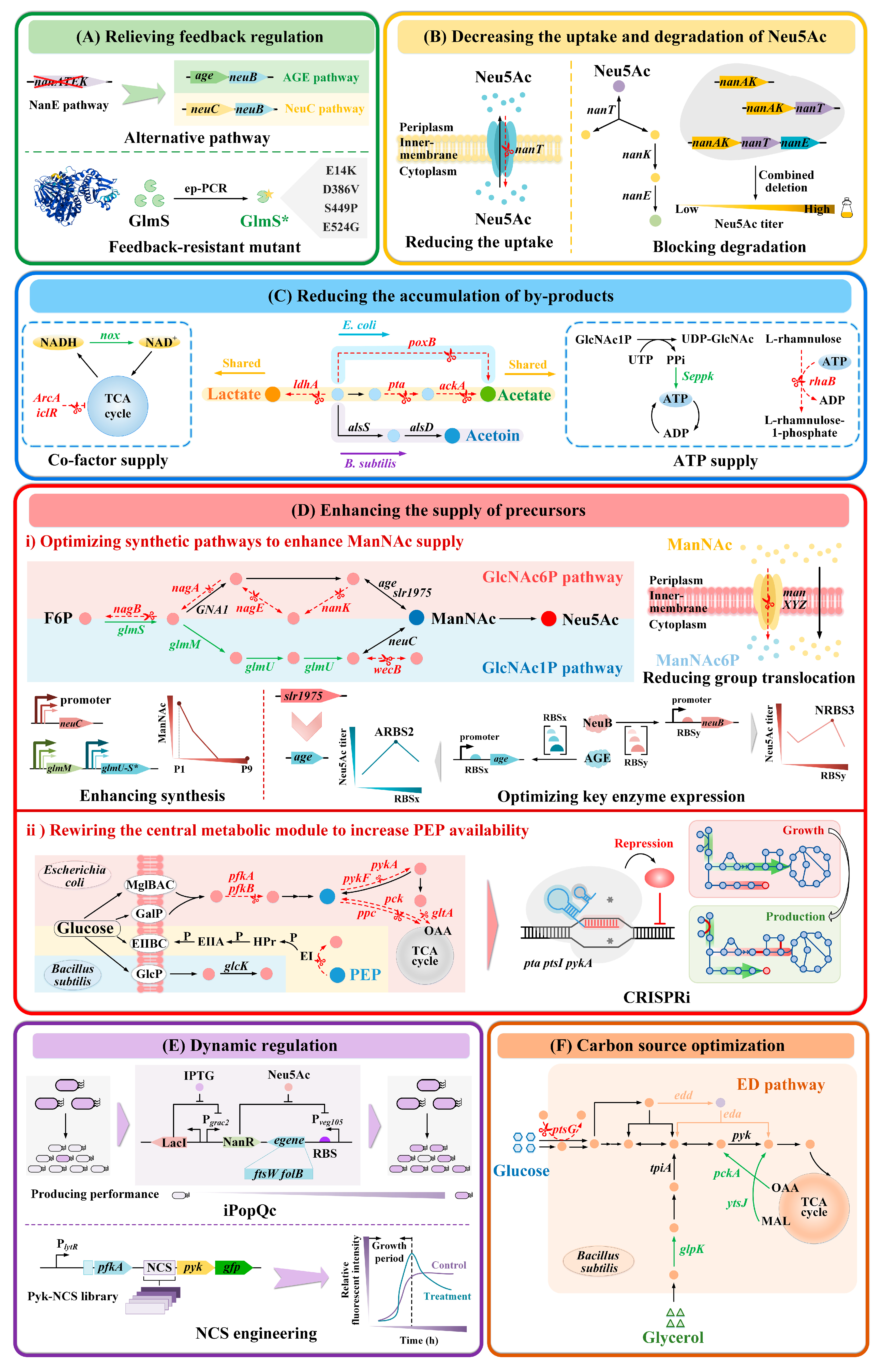
3.1. Rational Metabolic Engineering Strategies
3.1.1. Relieving Feedback Regulation
3.1.2. Decreasing the Uptake and Degradation of Neu5Ac
3.1.3. Reducing the Accumulation of By-Products
3.1.4. Enhancing the Supply of Precursors
Optimizing Synthetic Pathways to Enhance ManNAc Supply
Rewiring the Central Metabolic Module to Increase PEP Availability
3.1.5. Dynamic Regulation
3.1.6. Carbon Source Optimization
Single Carbon Source
Mixed Carbon Sources
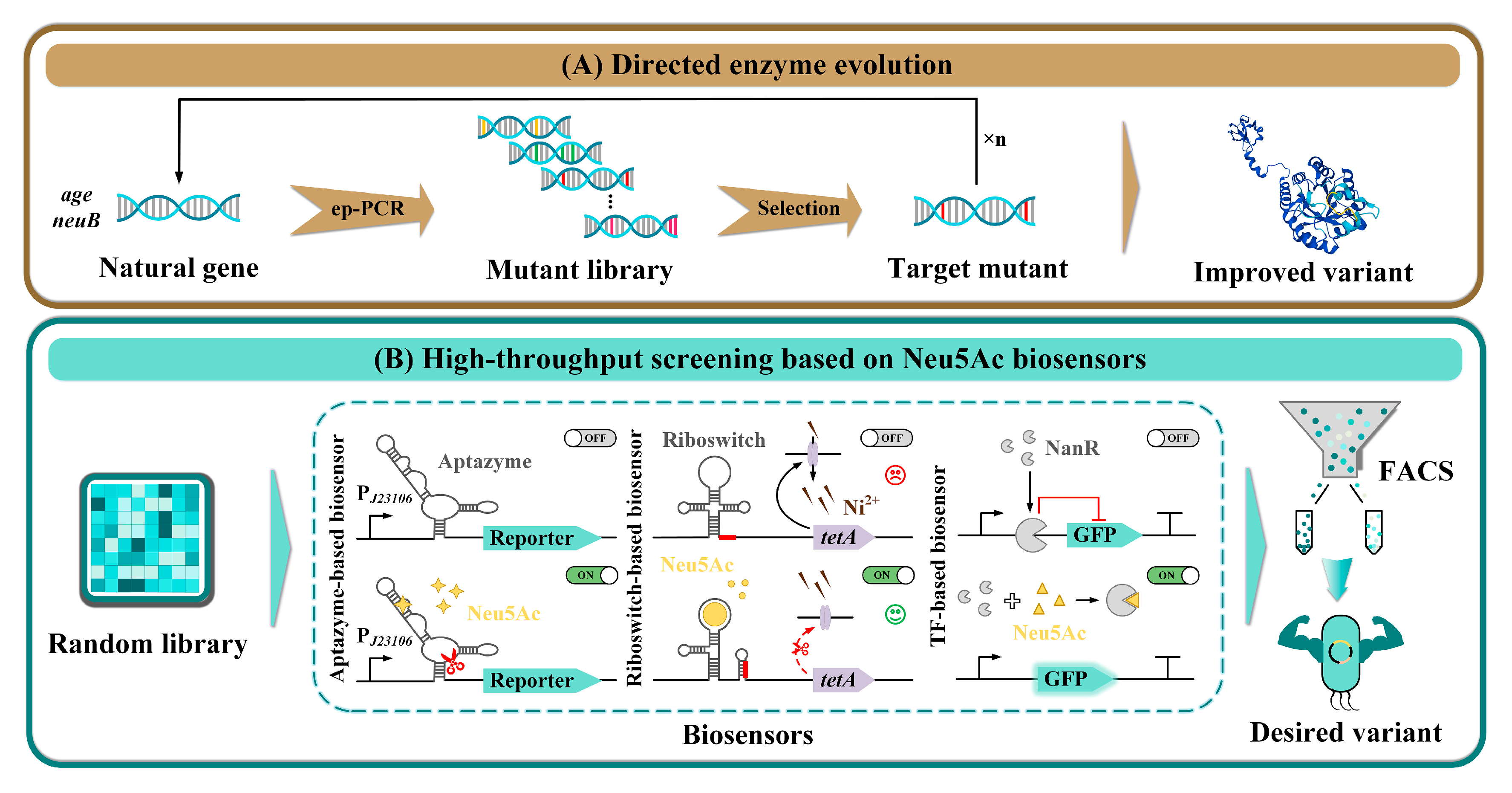
3.2. Irrational Strategies
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traving, C.; Schauer, R. Structure, Function and Metabolism of Sialic Acids. Cell. Mol. Life Sci. CLMS 1998, 54, 1330–1349. [Google Scholar] [CrossRef]
- Yang, H.; Lu, L.; Chen, X. An Overview and Future Prospects of Sialic Acids. Biotechnol. Adv. 2021, 46, 107678. [Google Scholar] [CrossRef]
- Haghani, A.; Mehrbod, P.; Safi, N.; Kadir, F.A.A.; Omar, A.R.; Ideris, A. Edible Bird’s Nest Modulate Intracellular Molecular Pathways of Influenza A Virus Infected Cells. BMC Complement. Altern. Med. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Pawluczyk, I.Z.A.; Najafabadi, M.G.; Brown, J.R.; Bevington, A.; Topham, P.S. Sialic Acid Supplementation Ameliorates Puromycin Aminonucleoside Nephrosis in Rats. Lab. Investig. 2015, 95, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Ofek, I.; Hasty, D.L.; Sharon, N. Anti-Adhesion Therapy of Bacterial Diseases: Prospects and Problems. FEMS Immunol. Med Microbiol. 2003, 38, 181–191. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Ismail, N.; Ideris, A.; Abdullah, M.A. High Fat Diet-Induced Inflammation and Oxidative Stress are Attenuated by N-acetylneuraminic Acid in Rats. J. Biomed. Sci. 2015, 22, 96. [Google Scholar] [CrossRef]
- Liu, F.; Simpson, A.B.; D’Costa, E.; Bunn, F.S.; van Leeuwen, S.S. Sialic Acid, the Secret Gift for the Brain. Crit. Rev. Food Sci. Nutr. 2022, 63, 9875–9894. [Google Scholar] [CrossRef]
- van Karnebeek, C.D.M.; Bonafé, L.; Wen, X.-Y.; Tarailo-Graovac, M.; Balzano, S.; Royer-Bertrand, B.; Ashikov, A.; Garavelli, L.; Mammi, I.; Turolla, L.; et al. NANS-mediated Synthesis of Sialic Acid is Required for Brain and Skeletal Development. Nat. Genet. 2016, 48, 777–784. [Google Scholar] [CrossRef]
- Chan, G.K.L.; Wong, Z.C.F.; Lam, K.Y.C.; Cheng, L.K.W.; Zhang, L.M.; Lin, H.; Dong, T.T.; Tsim, K.W.K. Edible Bird’s Nest, an Asian Health Food Supplement, Possesses Skin Lightening Activities: Identification of N-Acetylneuraminic Acid as Active Ingredient. J. Cosmet. Dermatol. Sci. Appl. 2015, 05, 262–274. [Google Scholar] [CrossRef]
- Chen, X.; Varki, A. Advances in the Biology and Chemistry of Sialic Acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Synthetic N-acetyl-d-Neuraminic Acid as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04918. [Google Scholar] [CrossRef]
- Magano, J. Synthetic Approaches to the Neuraminidase Inhibitors Zanamivir (Relenza) and Oseltamivir Phosphate (Tamiflu) for the Treatment of Influenza. Chem. Rev. 2009, 109, 4398–4438. [Google Scholar] [CrossRef]
- Ghosh, S. Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Global Growth Insights. Sialic Acid Market Size, Share, Growth, and Industry Analysis, By Types (Solid, Liquid), Applications (Pharmaceutical, Food & Preservatives, Cosmetics, Other) and Regional Insights and Forecast to 2033. 2025. Available online: https://www.globalgrowthinsights.com/market-reports/sialic-acid-market-115728 (accessed on 3 October 2025).
- Martinsson, A.; Raal, A.; Svennerholm, L. Isolation of N-acetylsialic acid from Normal Liver. Biochim. et Biophys. Acta 1957, 23, 652. [Google Scholar] [CrossRef]
- Zhao, L.; Tian, R.; Shen, Q.; Liu, Y.; Liu, L.; Li, J.; Du, G. Pathway Engineering of Bacillus subtilis for Enhanced N-Acetylneuraminic Acid Production via Whole-Cell Biocatalysis. Biotechnol. J. 2019, 14, e1800682. [Google Scholar] [CrossRef]
- Cheng, J.; Zhuang, W.; Tang, C.; Chen, Y.; Wu, J.; Guo, T.; Ying, H. Efficient Immobilization of AGE and NAL Enzymes Onto Functional Amino Resin as Recyclable and High-Performance Biocatalyst. Bioprocess Biosyst. Eng. 2016, 40, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, X.; Lu, L.; Govender, A.; Yang, H.; Shen, W. Enhanced Production of N-acetyl-d-neuraminic Acid by Whole-cell Bio-catalysis of Escherichia coli. J. Mol. Catal. B: Enzym. 2016, 125, 42–48. [Google Scholar] [CrossRef]
- Mo, Y.; Li, X.; Li, Q.; Han, Y.; Su, T.; Zhao, P.; Qiao, L.; Xiang, M.; Li, F.; Guo, X.; et al. Rational Design of N-Acetylglucosamine-2-epimerase and N-Acetylneuraminic Lyase for Efficient N-Acetylneuraminic Acid Biosynthesis. J. Agric. Food Chem. 2025, 73, 5320–5327. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-X.; Zhang, Z.-J.; Liu, W.-F.; Dong, Z.-Y.; Tao, Y. Enhanced Production of N-acetyl-d-neuraminic Acid by Multi-Approach Whole-Cell Biocatalyst. Appl. Microbiol. Biotechnol. 2013, 97, 4775–4784. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Lv, X.; Liu, L.; Li, J.; Du, G.; Wang, M.; Liu, Y. Engineering of Synthetic Multiplexed Pathways for High-Level N-Acetylneuraminic Acid Bioproduction. J. Agric. Food Chem. 2021, 69, 14868–14877. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, Y.; Wang, H.; Zhang, J.; Xu, W.; Mu, W. Efficient Production of N-Acetylneuraminic Acid in Escherichia coli Based on the UDP-N-Acetylglucosamine Biosynthetic Pathway. J. Agric. Food Chem. 2023, 71, 10701–10709. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tanaka, T.; Kondo, A. Engineering Metabolic Pathways in Escherichia coli for Constructing a “Microbial Chassis” for Biochemical Production. Bioresour. Technol. 2017, 245 Pt B, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, D.; Zhou, B.; Li, Z.; Liu, G.; Li, H.; Hu, X.; Wang, X. Fine-Regulating the Carbon Flux of l-Isoleucine Producing Corynebacterium glutamicum WM001 for Efficient l-Threonine Production. ACS Synth. Biol. 2024, 13, 3446–3460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Du, G.; Chen, J.; Liu, L. Metabolic Engineering of Bacillus subtilis Fueled by Systems Biology: Recent Advances and Future Directions. Biotechnol. Adv. 2017, 35, 20–30. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Li, J.; Du, G.; Chen, J. Synthetic Biology Toolbox and Chassis Development in Bacillus subtilis. Trends Biotechnol. 2019, 37, 548–562. [Google Scholar] [CrossRef]
- Sun, C.; Yi, J.; Zhang, Y.; Chen, X.; Cheng, Z.; Hou, Z.; Tan, M.; Gao, J.; Chen, Y.; Wu, H.; et al. Metabolic Engineering of Escherichia coli for Highly Efficient N-acetylneuraminic Acid Production. Bioresour. Technol. 2025, 439, 133343. [Google Scholar] [CrossRef]
- Dauner, M.; Bailey, J.E.; Sauer, U. Metabolic Flux Analysis with a Comprehensive Isotopomer Model in Bacillus subtilis. Biotechnol. Bioeng. 2001, 76, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Buffing, M.F.; Link, H.; Christodoulou, D.; Sauer, U. Capacity for Instantaneous Catabolism of Preferred and non-Preferred Carbon Sources in Escherichia coli and Bacillus subtilis. Sci. Rep. 2018, 8, 11760. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Liu, H.; Wang, Y.; Zheng, Z.; Zhang, Y.; Tan, T. Engineering Corynebacterium glutamicum for the Efficient Production of N-acetylglucosamine. Bioresour. Technol. 2023, 390, 129865. [Google Scholar] [CrossRef]
- Uhde, A.; Brühl, N.; Goldbeck, O.; Matano, C.; Gurow, O.; Rückert, C.; Marin, K.; Wendisch, V.F.; Krämer, R.; Seibold, G.M. Transcription of Sialic Acid Catabolism Genes in Corynebacterium glutamicum Is Subject to Catabolite Repression and Control by the Transcriptional Repressor NanR. J. Bacteriol. 2016, 198, 2204–2218. [Google Scholar] [CrossRef]
- Yang, P.; Wang, J.; Pang, Q.; Zhang, F.; Wang, J.; Wang, Q.; Qi, Q. Pathway Optimization and Key Enzyme Evolution of N-acetylneuraminate Biosynthesis Using an in vivo Aptazyme-based Biosensor. Metab. Eng. 2017, 43, 21–28. [Google Scholar] [CrossRef]
- Hao, Y.; Pan, X.; You, J.; Li, G.; Xu, M.; Rao, Z. Microbial Production of Branched Chain Amino Acids: Advances and Perspectives. Bioresour. Technol. 2024, 397, 130502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, P.; Liu, H.; Fang, H. Production of Pyrimidine Nucleosides in Microbial Systems via Metabolic Engineering: Theoretical Analysis Research and Prospects. Biotechnol. Adv. 2024, 75, 108419. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Han, H.; Xu, Y.; Liu, X.; Qi, Q.; Wang, Q. Exploring Amino Sugar and Phosphoenolpyruvate Metabolism to Improve Escherichia coli N-Acetylneuraminic Acid Production. J. Agric. Food Chem. 2020, 68, 11758–11764. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Liu, Y.; Chen, J.; Li, J.; Liu, L.; Du, G.; Chen, J. Synthetic N-Terminal Coding Sequences for Fine-Tuning Gene Expression and Metabolic Engineering in Bacillus subtilis. Metab. Eng. 2019, 55, 131–141. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, R.; Lv, X.; Li, J.; Liu, L.; Du, G.; Chen, J.; Liu, Y. Inducible Population Quality Control of Engineered Bacillus subtilis for Improved N-Acetylneuraminic Acid Biosynthesis. ACS Synth. Biol. 2021, 10, 2197–2209. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, J.; Ma, W.; Wu, Y.; Lv, X.; Liu, L.; Li, J.; Du, G.; Liu, Y. Biosensor-Assisted Multitarget Gene Fine-Tuning for N-Acetylneuraminic Acid Production in Escherichia coli with Sole Carbon Source Glucose. J. Agric. Food Chem. 2025, 73, 9793–9806. [Google Scholar] [CrossRef]
- Pang, Q.; Han, H.; Liu, X.; Wang, Z.; Liang, Q.; Hou, J.; Qi, Q.; Wang, Q. In vivo Evolutionary Engineering of Riboswitch with High-Threshold for N-acetylneuraminic Acid Production. Metab. Eng. 2020, 59, 36–43. [Google Scholar] [CrossRef]
- Liu, C.; Lv, X.; Li, J.; Liu, L.; Du, G.; Liu, Y. Metabolic Engineering of Escherichia coli for Increased Bioproduction of N-Acetylneuraminic Acid. J. Agric. Food Chem. 2022, 70, 15859–15868. [Google Scholar] [CrossRef]
- Blencke, H.-M.; Homuth, G.; Ludwig, H.; Mäder, U.; Hecker, M.; Stülke, J. Transcriptional Profiling of Gene Expression in Response to Glucose in Bacillus subtilis: Regulation of the Central Metabolic Pathways. Metab. Eng. 2003, 5, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Millard, P.; Smallbone, K.; Mendes, P. Metabolic Regulation is Sufficient for Global and Robust Coordination of Glucose Uptake, Catabolism, Energy Production and Growth in Escherichia coli. PLOS Comput. Biol. 2017, 13, e1005396. [Google Scholar] [CrossRef]
- Durnin, G.; Clomburg, J.; Yeates, Z.; Alvarez, P.J.; Zygourakis, K.; Campbell, P.; Gonzalez, R. Understanding and Harnessing the Microaerobic Metabolism of Glycerol in Escherichia coli. Biotechnol. Bioeng. 2009, 103, 148–161. [Google Scholar] [CrossRef]
- Park, S.J.; McCabe, J.; Turna, J.; Gunsalus, R.P. Regulation of the Citrate Synthase (gltA) gene of Escherichia coli in Response to Anaerobiosis and Carbon Supply: Role of the arcA Gene Product. J. Bacteriol. 1994, 176, 5086–5092. [Google Scholar] [CrossRef]
- Jin, S.; Sonenshein, A.L. Transcriptional Regulation of Bacillus subtilis Citrate Synthase Genes. J. Bacteriol. 1994, 176, 4680–4690. [Google Scholar] [CrossRef]
- Tan, Z.; Zhu, X.; Chen, J.; Li, Q.; Zhang, X. Activating Phosphoenolpyruvate Carboxylase and Phosphoenolpyruvate Carboxykinase in Combination for Improvement of Succinate Production. Appl. Environ. Microbiol. 2013, 79, 4838–4844. [Google Scholar] [CrossRef]
- Kwon, Y.-D.; Lee, S.Y.; Kim, P. A Physiology Study of Escherichia coli Overexpressing Phosphoenolpyruvate Carboxykinase. Biosci. Biotechnol. Biochem. 2014, 72, 1138–1141. [Google Scholar] [CrossRef]
- Peng, L.; Shimizu, K. Global Metabolic Regulation Analysis for Escherichia coli K12 Based on Protein Expression by 2-dimensional Electrophoresis and Enzyme Activity Measurement. Appl. Microbiol. Biotechnol. 2003, 61, 163–178. [Google Scholar] [CrossRef]
- Shin, B.-S.; Choi, S.-K.; Park, S.-H. Regulation of the Bacillus subtilis Phosphotransacetylase Gene. J. Biochem. 1999, 126, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.; Merino, E.; Bolivar, F.; Gosset, G.; Martinez, A. Metabolic Engineering of Bacillus subtilis for Ethanol Production: Lactate Dehydrogenase Plays a Key Role in Fermentative Metabolism. Appl. Environ. Microbiol. 2007, 73, 5190–5198. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Cerezo, S.; Pastor, J.M.; Renilla, S.; Bernal, V.; Iborra, J.L.; Cánovas, M. An Insight into the Role of Phosphotransacetylase (pta) and the acetate/acetyl-CoA Node in Escherichia coli. Microb. Cell Factories 2009, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Frädrich, C.; March, A.; Fiege, K.; Hartmann, A.; Jahn, D.; Härtig, E. The Transcription Factor AlsR Binds and Regulates the Promoter of the alsSD Operon Responsible for Acetoin Formation in Bacillus subtilis. J. Bacteriol. 2011, 194, 1100–1112. [Google Scholar] [CrossRef]
- Lerondel, G.; Doan, T.; Zamboni, N.; Sauer, U.; Aymerich, S. YtsJ Has the Major Physiological Role of the Four Paralogous Malic Enzyme Isoforms in Bacillus subtilis. J. Bacteriol. 2006, 188, 4727–4736. [Google Scholar] [CrossRef]
- Kalamorz, F.; Reichenbach, B.; März, W.; Rak, B.; Görke, B. Feedback Control of Glucosamine-6-phosphate Synthase GlmS Expression Depends on the Small RNA GlmZ and Involves the Novel Protein YhbJ in Escherichia coli. Mol. Microbiol. 2007, 65, 1518–1533. [Google Scholar] [CrossRef]
- Ma, G.; Jiang, X.; Yang, B.; Li, L.; Liu, R.; Meng, Q.; Li, J.; Xie, L.; Guo, H.; Liu, S.; et al. Development of a High-Efficiency N-acetylneuraminic Acid Production Platform Through Multi-Pathway Synergistic Engineering. Trends Biotechnol. 2025. [Google Scholar] [CrossRef]
- Urban, J.H.; Papenfort, K.; Thomsen, J.; Schmitz, R.A.; Vogel, J. A Conserved Small RNA Promotes Discoordinate Expression of the glmUS Operon mRNA to Activate GlmS Synthesis. J. Mol. Biol. 2007, 373, 521–528. [Google Scholar] [CrossRef]
- Reichenbach, B.; Maes, A.; Kalamorz, F.; Hajnsdorf, E.; Görke, B. The Small RNA GlmY Acts Upstream of the sRNA GlmZ in the Activation of glmS Expression and is Subject to Regulation by Polyadenylation in Escherichia coli. Nucleic Acids Res. 2008, 36, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Winkler, W.C.; Nahvi, A.; Roth, A.; Collins, J.A.; Breaker, R.R. Control of Gene Expression by a Natural Metabolite-Responsive Ribozyme. Nature 2004, 428, 281–286. [Google Scholar] [CrossRef]
- Barrick, J.E.; Breaker, R.R. The Distributions, Mechanisms, and Structures of Metabolite-Binding Riboswitches. Genome Biol. 2007, 8, R239. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Irnov, I.; Baker, S.; Winkler, W.C. Mechanism of mRNA Destabilization by the glmS Ribozyme. Genes Dev. 2007, 21, 3356–3368. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Wu, Q.; Chandrangsu, P.; Helmann, J.D. A metabolic Checkpoint Protein GlmR is Important for Diverting Carbon into Peptidoglycan Biosynthesis in Bacillus subtilis. PLOS Genet. 2018, 14, e1007689. [Google Scholar] [CrossRef]
- Foulquier, E.; Pompeo, F.; Byrne, D.; Fierobe, H.-P.; Galinier, A. Uridine Diphosphate N-acetylglucosamine Orchestrates the Interaction of GlmR with Either YvcJ or GlmS in Bacillus subtilis. Sci. Rep. 2020, 10, 15938. [Google Scholar] [CrossRef]
- Plumbridge, J.A. Induction of the Nag Regulon of Escherichia coli by N-acetylglucosamine and Glucosamine: Role of the Cyclic AMP-Catabolite Activator Protein Complex in Expression of the Regulon. J. Bacteriol. 1990, 172, 2728–2735. [Google Scholar] [CrossRef]
- Plumbridge, J.; Kolb, A. DNA Loop Formation between Nag Repressor Molecules Bound to its Two Operator Sites is Necessary for Repression of the nag Regulon of Escherichia coli in vivo. Mol. Microbiol. 1993, 10, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Plumbridge, J. Co-ordinated Regulation of Amino Sugar Biosynthesis and Degradation: The NagC Repressor Acts as both an Activator and a Repressor for the Transcription of the glmUS Operon and Requires Two Separated NagC Binding Sites. EMBO J. 1995, 14, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Gaugué, I.; Oberto, J.; Putzer, H.; Plumbridge, J. The Use of Amino Sugars by Bacillus subtilis: Presence of a Unique Operon for the Catabolism of Glucosamine. PLoS ONE 2013, 8, e63025. [Google Scholar] [CrossRef]
- Gaugué, I.; Oberto, J.; Plumbridge, J. Regulation of Amino Sugar Utilization in Bacillus subtilis by the GntR Family Regulators, NagR and GamR. Mol. Microbiol. 2014, 92, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Vimr, E.R.; Kalivoda, K.A.; Deszo, E.L.; Steenbergen, S.M. Diversity of Microbial Sialic Acid Metabolism. Microbiol. Mol. Biol. Rev. 2004, 68, 132–153. [Google Scholar] [CrossRef]
- Vimr, E.R. Unified Theory of Bacterial Sialometabolism: How and Why Bacteria Metabolize Host Sialic Acids. ISRN Microbiol. 2013, 2013, 816713. [Google Scholar] [CrossRef]
- Kalivoda, K.A.; Steenbergen, S.M.; Vimr, E.R.; Plumbridge, J. Regulation of Sialic Acid Catabolism by the DNA Binding Protein NanR in Escherichia coli. J. Bacteriol. 2003, 185, 4806–4815. [Google Scholar] [CrossRef]
- Kalivoda, K.A.; Steenbergen, S.M.; Vimr, E.R. Control of the Escherichia coli Sialoregulon by Transcriptional Repressor NanR. J. Bacteriol. 2013, 195, 4689–4701. [Google Scholar] [CrossRef]
- Bloemendal, V.R.L.J.; Moons, S.J.; Heming, J.J.A.; Chayoua, M.; Niesink, O.; Van Hest, J.C.M.; Boltje, T.J.; Rutjes, F.P.J.T.; Bloemendal, V.R.L.J.; Moons, S.; et al. Chemoenzymatic Synthesis of Sialic Acid Derivatives Using Immobilized N-Acetylneuraminate Lyase in a Continuous Flow Reactor. Adv. Synth. Catal. 2019, 361, 2443–2447. [Google Scholar] [CrossRef]
- Dong, X.; Han, Z.; Zou, D.; Guo, A.; Ye, C.; Wei, X. Dual-Cell Co-Catalytic System Engineering Enhances N-acetylneuraminic Acid Synthesis by Bacillus amyloliquefaciens. Bioresour. Technol. 2025, 436, 132969. [Google Scholar] [CrossRef]
- Schneier, M.; Razdan, S.; Miller, A.M.; Briceno, M.E.; Barua, S. Current Technologies to Endotoxin Detection and Removal for Biopharmaceutical Purification. Biotechnol. Bioeng. 2020, 117, 2588–2609. [Google Scholar] [CrossRef]
- Lundgren, B.R.; Boddy, C.N. Sialic Acid and N-acyl Sialic Acid Analog Production by Fermentation of Metabolically and Genetically Engineered Escherichia coli. Org. Biomol. Chem. 2007, 5, 1903–1909. [Google Scholar] [CrossRef]
- Kang, J.; Gu, P.; Wang, Y.; Li, Y.; Yang, F.; Wang, Q.; Qi, Q. Engineering of an N-acetylneuraminic Acid Synthetic Pathway in Escherichia coli. Metab. Eng. 2012, 14, 623–629. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Liu, L.; Wang, M.; Li, J.; Du, G.; Chen, J. Modular Pathway Engineering of Key Carbon-precursor Supply-pathways for Improved N-acetylneuraminic Acid Production in Bacillus subtilis. Biotechnol. Bioeng. 2018, 115, 2217–2231. [Google Scholar] [CrossRef]
- Guo, H.; Tian, R.; Wang, C.; Zhao, R.; Lv, X.; Liu, L.; Liu, Y. Improved N-acetylneuraminic Acid Bioproduction by Optimizing Pathway for Reducing Intermediate Accumulation. Food Bioeng. 2022, 1, 205–211. [Google Scholar] [CrossRef]
- Milewski, S. Glucosamine-6-phosphate Synthase—The Multi-Facets Enzyme. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 2002, 1597, 173–192. [Google Scholar] [CrossRef]
- Deng, M.-D.; Grund, A.D.; Wassink, S.L.; Peng, S.S.; Nielsen, K.L.; Huckins, B.D.; Walsh, B.L.; Burlingame, R.P. Directed Evolution and Characterization of Escherichia coli Glucosamine Synthase. Biochimie 2006, 88, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Teplyakov, A.; Obmolova, G.; Badet-Denisot, M.-A.; Badet, B.; Polikarpov, I. Involvement of the C terminus in Intramolecular Nitrogen Channeling in Glucosamine 6-phosphate synthase: Evidence from a 1.6 å Crystal Structure of the Isomerase Domain. Structure 1998, 6, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.; Tang, J.M.; Wirth, C.; Peneff, C.M.; Eisenberg, B. Single-Channel Measurements of an N-acetylneuraminic Acid-Inducible Outer Membrane Channel in Escherichia coli. Eur. Biophys. J. 2012, 41, 259–271. [Google Scholar] [CrossRef]
- Bell, A.; Severi, E.; Lee, M.; Monaco, S.; Latousakis, D.; Angulo, J.; Thomas, G.H.; Naismith, J.H.; Juge, N. Uncovering a Novel Molecular Mechanism for Scavenging Sialic Acids in Bacteria. J. Biol. Chem. 2020, 295, 13724–13736. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Tsukada, Y.; Sugimori, T. Purification and Properties of N-Acetylneuraminate Lyase from Escherichia coli. J. Biochem. 1984, 96, 507–522. [Google Scholar] [CrossRef]
- Plumbridge, J.; Vimr, E. Convergent Pathways for Utilization of the Amino Sugars N-Acetylglucosamine, N-Acetylmannosamine, and N-Acetylneuraminic Acid by Escherichia coli. J. Bacteriol. 1999, 181, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, C.R.; Vadali, R.V.; Bennett, G.N.; San, K.-Y. Redistribution of Metabolic Fluxes in the Central Aerobic Metabolic Pathway of E. coli Mutant Strains with Deletion of the ackA-pta and poxB Pathways for the Synthesis of Isoamyl Acetate. Biotechnol. Prog. 2008, 21, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Peebo, K.; Valgepea, K.; Nahku, R.; Riis, G.; Õun, M.; Adamberg, K.; Vilu, R. Coordinated Activation of PTA-ACS and TCA Cycles Strongly Reduces Overflow Metabolism of Acetate in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 5131–5143. [Google Scholar] [CrossRef]
- Ma, W.; Liu, Y.; Shin, H.-D.; Li, J.; Chen, J.; Du, G.; Liu, L. Metabolic Engineering of Carbon Overflow Metabolism of Bacillus subtilis for Improved N-acetyl-glucosamine Production. Bioresour. Technol. 2017, 250, 642–649. [Google Scholar] [CrossRef]
- De Mey, M.; De Maeseneire, S.; Soetaert, W.; Vandamme, E. Minimizing Acetate Formation in E. coli Fermentations. J. Ind. Microbiol. Biotechnol. 2007, 34, 689–700. [Google Scholar] [CrossRef]
- Krivoruchko, A.; Zhang, Y.; Siewers, V.; Chen, Y.; Nielsen, J. Microbial acetyl-CoA Metabolism and Metabolic Engineering. Metab. Eng. 2015, 28, 28–42. [Google Scholar] [CrossRef]
- Moon, S.Y.; An, N.Y.; Oh, S.S.; Lee, J.Y. Coordinated Reprogramming of ATP Metabolism Strongly Enhances Adipic Acid Production in Escherichia coli. Metab. Eng. 2024, 86, 234–241. [Google Scholar] [CrossRef]
- Zou, S.; Zhang, B.; Han, Y.; Liu, J.; Zhao, K.; Xue, Y.; Zheng, Y. Design of a Cofactor Self-sufficient Whole-cell Biocatalyst for Enzymatic Asymmetric Reduction via Engineered Metabolic Pathways and Multi-enzyme Cascade. Biotechnol. J. 2024, 19, 2300744. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, Y.; Wang, Q.; Wang, X.; Li, Q.; Liu, W.; Zhao, Z.K. Non-natural Cofactor and Formate-Driven Reductive Carboxylation of Pyruvate. Angew. Chem. Int. Ed. Engl. 2019, 59, 3143–3146. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, N.; Malicdan, M.C.; Leoyklang, P.; Shrader, J.A.; Joe, G.; Slota, C.; Perreault, J.; Heiss, J.D.; Class, B.; Liu, C.-Y.; et al. Safety and Efficacy of N-acetylmannosamine (ManNAc) in Patients with GNE Myopathy: An Open-Label Phase 2 Study. Genet. Med. 2021, 23, 2067–2075. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, F.; Du, M.; Ma, C.; Qiu, J.; Gu, L.; He, X.; Xu, P. An Efficient Method for N-acetyl-d-neuraminic Acid Production Using Coupled Bacterial Cells with a Safe Temperature-induced System. Appl. Microbiol. Biotechnol. 2009, 86, 481–489. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, L.; Meng, J.; Luo, G.; Chen, G.; Wu, H.; Zhang, W.; Mu, W. Metabolic Engineering of Escherichia coli for Lacto-N-triose II Production with High Productivity. J. Agric. Food Chem. 2021, 69, 3702–3711. [Google Scholar] [CrossRef]
- Gosset, G. Improvement of Escherichia coli Production Strains by Modification of the Phosphoenolpyruvate: Sugar Phosphotransferase System. Microb. Cell Factories 2005, 4, 14. [Google Scholar] [CrossRef]
- Inaoka, T.; Ochi, K. Glucose Uptake Pathway-Specific Regulation of Synthesis of Neotrehalosadiamine, a Novel Autoinducer Produced in Bacillus subtilis. J. Bacteriol. 2007, 189, 65–75. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, D.; Tang, Y.; Hu, X.; Wang, X. Recent Advances on Engineering Escherichia coli and Corynebacterium glutamicum for Efficient Production of L-threonine and its Derivatives. Metab. Eng. 2025, 90, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Mo, Y.; Yuan, Y.; Su, T.; Qi, Q. A Rapid and Efficient Strategy for Combinatorial Repression of Multiple Genes in Escherichia coli. Microb. Cell Factories 2025, 24, 74. [Google Scholar] [CrossRef]
- Borujeni, A.E.; Cetnar, D.; Farasat, I.; Smith, A.; Lundgren, N.; Salis, H.M. Precise Quantification of Translation Inhibition by mRNA Structures that Overlap with the Ribosomal Footprint in N-Terminal Coding Sequences. Nucleic Acids Res. 2017, 45, 5437–5448. [Google Scholar] [CrossRef]
- Xiao, Y.; Bowen, C.H.; Liu, D.; Zhang, F. Exploiting Nongenetic Cell-to-cell Variation for Enhanced Biosynthesis. Nat. Chem. Biol. 2016, 12, 339–344. [Google Scholar] [CrossRef]
- Cheng, K.-K.; Lee, B.-S.; Masuda, T.; Ito, T.; Ikeda, K.; Hirayama, A.; Deng, L.; Dong, J.; Shimizu, K.; Soga, T.; et al. Global Metabolic Network Reorganization by Adaptive Mutations Allows Fast Growth of Escherichia coli on Glycerol. Nat. Commun. 2014, 5, 3233. [Google Scholar] [CrossRef]
- Liu, N.; Santala, S.; Stephanopoulos, G. Mixed Carbon Substrates: A Necessary Nuisance or a Missed Opportunity? Curr. Opin. Biotechnol. 2019, 62, 15–21. [Google Scholar] [CrossRef]
- Wang, X.; Xia, K.; Yang, X.; Tang, C. Growth Strategy of Microbes on Mixed Carbon Sources. Nat. Commun. 2019, 10, 1279. [Google Scholar] [CrossRef]
- Ma, Q.; Yi, J.; Tang, Y.; Geng, Z.; Zhang, C.; Sun, W.; Liu, Z.; Xiong, W.; Wu, H.; Xie, X. Co-Utilization of Carbon Sources in Microorganisms for the Bioproduction of Chemicals. Biotechnol. Adv. 2024, 73, 108380. [Google Scholar] [CrossRef]
- Meyer, F.M.; Gerwig, J.; Hammer, E.; Herzberg, C.; Commichau, F.M.; Völker, U.; Stülke, J. Physical Interactions Between Tricarboxylic Acid Cycle Enzymes in Bacillus subtilis: Evidence for a Metabolon. Metab. Eng. 2010, 13, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Darbon, E.; Servant, P.; Poncet, S.; Deutscher, J. Antitermination by GlpP, Catabolite Repression via CcpA and Inducer Exclusion Triggered by P~GlpK Dephosphorylation Control Bacillus subtilis glpFK Expression. Mol. Microbiol. 2002, 43, 1039–1052. [Google Scholar] [CrossRef]
- Ko, Y.-S.; Kim, J.W.; Lee, J.A.; Han, T.; Kim, G.B.; Park, J.E.; Lee, S.Y. Tools and Strategies of Systems Metabolic Engineering for the Development of Microbial Cell Factories for Chemical Production. Chem. Soc. Rev. 2020, 49, 4615–4636. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.; De Paepe, B.; De Wannemaeker, L.; Duchi, D.; Maertens, J.; Lammertyn, J.; De Mey, M. Development of N-acetylneuraminic Acid Responsive Biosensors Based on the Transcriptional Regulator NanR. Biotechnol. Bioeng. 2018, 115, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, Q.; Xu, Q.; Zhang, C.; Li, Y.; Fan, X.; Xie, X.; Chen, N. Systems Metabolic Engineering Strategies for the Production of Amino Acids. Synth. Syst. Biotechnol. 2017, 2, 87–96. [Google Scholar] [CrossRef]
- Goshisht, M.K. Machine Learning and Deep Learning in Synthetic Biology: Key Architectures, Applications, and Challenges. ACS Omega 2024, 9, 9921–9945. [Google Scholar] [CrossRef]
- Shimazaki, S.; Yamada, R.; Yamamoto, Y.; Matsumoto, T.; Ogino, H. Building a Machine-Learning Model to Predict Optimal Mevalonate Pathway Gene Expression Levels for Efficient Production of a Carotenoid in Yeast. Biotechnol. J. 2024, 19, 2300285. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhuang, M.; Fang, Y.; Hu, X.; Wang, X. Self-Regulated Efficient Production of L-threonine via an Artificial Quorum Sensing System in Engineered Escherichia coli. Microbiol. Res. 2024, 284, 127720. [Google Scholar] [CrossRef]
- Yang, H.; He, Y.; Zhou, S.; Deng, Y. Dynamic Regulation and Cofactor Engineering of Escherichia coli to Enhance Production of Glycolate from Corn Stover Hydrolysate. Bioresour. Technol. 2024, 398, 130531. [Google Scholar] [CrossRef]
- Sun, B.; Zou, K.; Zhao, Y.; Tang, Y.; Zhang, F.; Chen, W.; Tang, X.; Chang, C.; Zheng, Y. The Fermentation Optimization for Alkaline Protease Production by Bacillus subtilis BS-QR-052. Front. Microbiol. 2023, 14, 1301065. [Google Scholar] [CrossRef] [PubMed]
- Lefin, N.; Miranda, J.; Munhoz Costa, I.; Pedroso Reynaldo, A.; Monteiro, G.; Zamorano, M.; Pessoa, A., Jr.; Farias, J.G. Optimized Amino Acid-Enhanced Medium for Efficient L-Asparaginase II Production in E. coli: From Shake Flask to Bioreactor. Fermentation 2025, 11, 239. [Google Scholar] [CrossRef]
| Method | Cost | Yield | Scalability | Safety | Representative Reference |
|---|---|---|---|---|---|
| Chemical synthesis | High costs mainly result from complex reactions, pricey catalysts, and strict purification. | Involves multi-step reactions and stereochemical control, resulting in low yield. | Cumbersome protection steps and reaction conditions make it unsuitable for large-scale production. | High safety risks; eco-unfriendly. | [16] |
| Enzymatic synthesis | The preparation and purification of enzymes, along with the requirement for ATP addition, result in high costs. | High conversion rate, with the overall conversion rate of the two-step enzymatic catalytic reaction reaching up to 82%. | Immobilized enzyme technology offers the possibility of large-scale production, but enzyme stability and cost are major limiting factors. | Low biosafety risks; eco-friendly. | [72] |
| Whole-cell catalysis | Saves enzyme purification costs, but requires adding pricey and excess substrates (like pyruvate, GlcNAc), at medium cost. | High yield, GlcNAc conversion maxes at 77%, via Bacillus amyloliquefaciens dual-cell co-catalysis. | Shows good scalability potential, but costs are relatively high for large-scale industrial use. | Biosafety relies on host strain used; high operational safety. | [73] |
| Microbial fermentation (E. coli) | Often use cheap carbon sources (like glucose, glycerol) as raw materials, with low costs. | High-yield Neu5Ac production at 0.217 g/g glucose has been reported. | High scalability, since mature fermentation technology fits large-scale industrial output. | Biosafety risks exist, as E. coli contains endotoxins. | [27] |
| Microbial fermentation (B. subtilis) | Relatively high-yield Neu5Ac production at 0.049 g/g glucose has been reported. | Highest safety due to endotoxin-free B. subtilis (GRAS). | [21] |
| Strain | Construction Strategies | Cultivation | Carbon Sources | Titer (g/L) | Reference |
|---|---|---|---|---|---|
| E. coli MG1655 | ΔnanT, ΔnanA, (+)glmS, (+)neuB, (+)neuC | shake flask | glucose | 1.7 | [75] |
| E. coli DH5α | ΔnagAB, ΔnanATEK, ΔackA, ΔpoxB, ΔldhA, (+)GNA1, (+)slr1975, (+)glmS* | 5 L bioreactor | glucose | 7.85 | [76] |
| E. coli DH5α | ΔnagAB, ΔnanATEK, ΔackA, ΔpoxB, ΔldhA, (+)GNA1, (+)slr1975, (+)neuB*, (+)glmS*; Directed evolution of NeuB was performed using an Neu5Ac aptazyme-based biosensor. | two-stage fermentation | glucose | 8.31 | [32] |
| E. coli DH5α | ΔnagAB, ΔnanATEK, ΔackA, ΔpoxB, ΔldhA, (+)GNA1, (+)neuB, (+)glmS*, (+)age*; Directed evolution of AGE was performed using a Neu5Ac riboswitch-based biosensor. | shake flask | glucose | 14.32 | [39] |
| E. coli DH5α | ΔnagEBAC, ΔnanATEK, ΔackA, ΔpoxB, ΔldhA, ΔpykA, ΔptsG, (+)age, (+)neuB, (+)GNA1, (+)glmS* | shake flask | glucose | 16.7 | [35] |
| E. coli BL21(DE3) | ΔnagAB, ΔnanATEK, ΔmanXYZ, ΔpykA, ΔwecB, ΔmanA, (+)neuB, (+)neuC, (+)glmU, (+)glmM, (+)glmS* | 3 L bioreactor | glycerol | 23.46 | [40] |
| E. coli BL21(DE3) | ΔnagB, ΔnanA, ΔnanT, ΔnanK, (+)neuB, (+)neuC, (+)glmU, (+)glmM, (+)glmS* | 5 L bioreactor | glycerol | 46.92 | [22] |
| E. coli BL21(DE3) | ΔnagAB, ΔnanATEK, ΔmanXYZ, ΔpykA, ΔwecB, ΔmanA, ΔpoxB, ΔarcA, ΔiclR, ΔptsG, ΔpfkA, (+)neuB, (+)neuC, (+)glmU, (+)glmM, (+)glmS*, (+)nox, (+)glf, (+)Seppk; Combining the Neu5Ac TF-based biosensor with HTS by flow cytometry to synergistically optimize the expression levels of glmS, glmM and glmU. | 3 L bioreactor | glucose | 58.26 | [38] |
| E. coli BL21(DE3) | ΔnanATEK, ΔnagAB, Δzwf, ΔpfkA, ΔptsG, ΔpykA, ΔldhA, ΔpoxB, ΔadhE, ΔackA, ΔgldA, (+)galP, (+)glk, (+)glmS*, (+)GNA1, (+)age, (+)neuB, (+)glmM, (+)glmU, (+)neuC, (+)pck, (+)ppsA, (+)glpK* | 5 L bioreactor | glucose and glycerol | 70.4 | [55] |
| E. coli W3110 | ΔlacIZ::PxylF-T7RNAP, mlc*, ΔnagEBAC, ΔmanXYZ, ΔnanATEK, ΔptsG, ΔpykA, ΔpoxB, ΔackA, Δpta, ΔiclR, ΔrhaB, (+)glk, (+)glmS, (+)yqaB, (+)neuB | 5 L bioreactor | glucose | 77.12 | [27] |
| B. subtilis | ΔnagAB, ΔldhA, Δpta, Δpyk, ΔptsG, ΔgamA, ΔgamP, (+)glmS, (+)GNA1, (+)yqaB, (+)age, (+)neuB, (+)pckA, (+)ytsJ | shake flask | glucose and malic acid | 2.18 | [77] |
| B. subtilis | ΔnagAB, ΔgamA, ΔgamP, ΔnagP, ΔldhA, Δpta, ΔptsG, (+)glmS, (+)GNA1, (+)yqaB, (+)age, (+)neuB, (+)pyk, (+)pfkA | shake flask | glucose | 2.75 | [36] |
| B. subtilis | ΔnagAB, ΔldhA, Δpta, Δpyk, ΔptsG, ΔgamA, ΔgamP, (+)glmS, (+)GNA1, (+)yqaB, (+)age, (+)neuB, (+)pckA, (+)ytsJ, (+)nanR, (+)folB | 3 L bioreactor | glucose | 4.23 | [37] |
| B. subtilis | ΔnagAB, ΔldhA, Δpta, ΔptsG, ΔgamA, ΔgamP, ΔnagP, (+)glmS, (+)glmU, (+)glmM, (+)glpK, (+)neuB | 3 L bioreactor | glucose and glycerol | 21.8 | [78] |
| B. subtilis | ΔnagAB, ΔldhA, Δpta, ΔptsG, ΔgamA, ΔgamP, ΔnagP, (+)neuB, (+)neuC, (+)GNA1, (+)age, (+)nanE | 5 L bioreactor | glucose | 30.10 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, J.; Shi, Z.; Wu, H.; Ma, Q.; Xie, X. Microbial Production of N-Acetylneuraminic Acid Using Metabolically Engineered Escherichia coli and Bacillus subtilis: Advances and Perspectives. Foods 2025, 14, 3478. https://doi.org/10.3390/foods14203478
Dang J, Shi Z, Wu H, Ma Q, Xie X. Microbial Production of N-Acetylneuraminic Acid Using Metabolically Engineered Escherichia coli and Bacillus subtilis: Advances and Perspectives. Foods. 2025; 14(20):3478. https://doi.org/10.3390/foods14203478
Chicago/Turabian StyleDang, Jingru, Zhijie Shi, Heyun Wu, Qian Ma, and Xixian Xie. 2025. "Microbial Production of N-Acetylneuraminic Acid Using Metabolically Engineered Escherichia coli and Bacillus subtilis: Advances and Perspectives" Foods 14, no. 20: 3478. https://doi.org/10.3390/foods14203478
APA StyleDang, J., Shi, Z., Wu, H., Ma, Q., & Xie, X. (2025). Microbial Production of N-Acetylneuraminic Acid Using Metabolically Engineered Escherichia coli and Bacillus subtilis: Advances and Perspectives. Foods, 14(20), 3478. https://doi.org/10.3390/foods14203478






