A New β-Galactosidase from Pseudomonas tritici SWRI145 for Efficient Bioproduction of Galactooligosaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid, Strains, and Chemicals
2.2. Molecular Cloning of Pstr β-Gal
2.3. Three-Dimensional Modeling of Pstr β-Galactosidase
2.4. Protein Overexpression and Purification of Pstr β-Galactosidase
2.5. Hydrolytic Activity Assay of Pstr β-Galactosidase
2.6. Effects of Temperature, pH and Metal Ions
2.7. Optimization of GOS Bioproduction
2.8. Analytical Methods
3. Results and Discussion
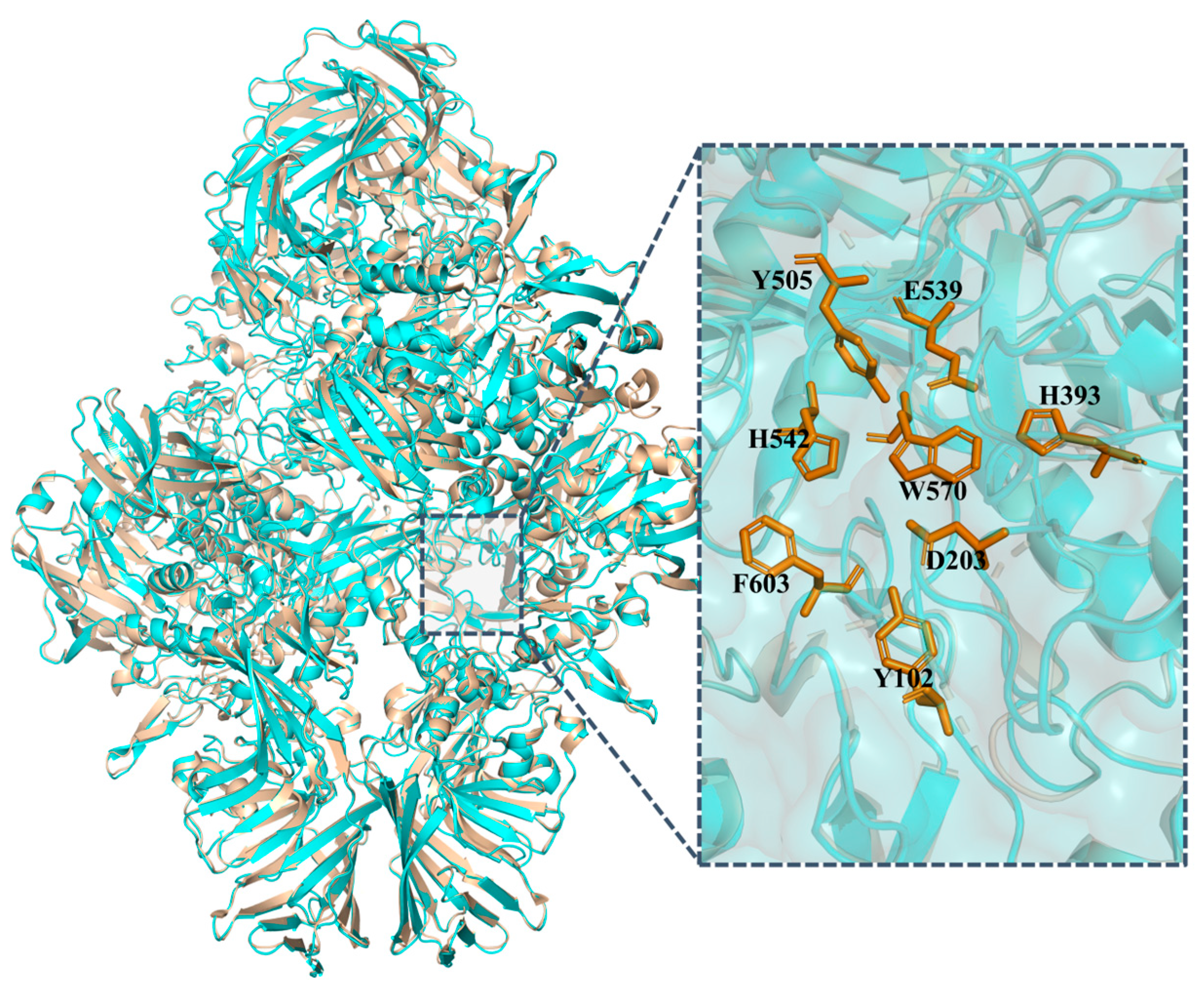
3.1. Amino Acid Sequence and Structure Analysis
3.2. Overexpression and Purification
3.3. Biochemical Characterization of Pstr β-Galactosidase
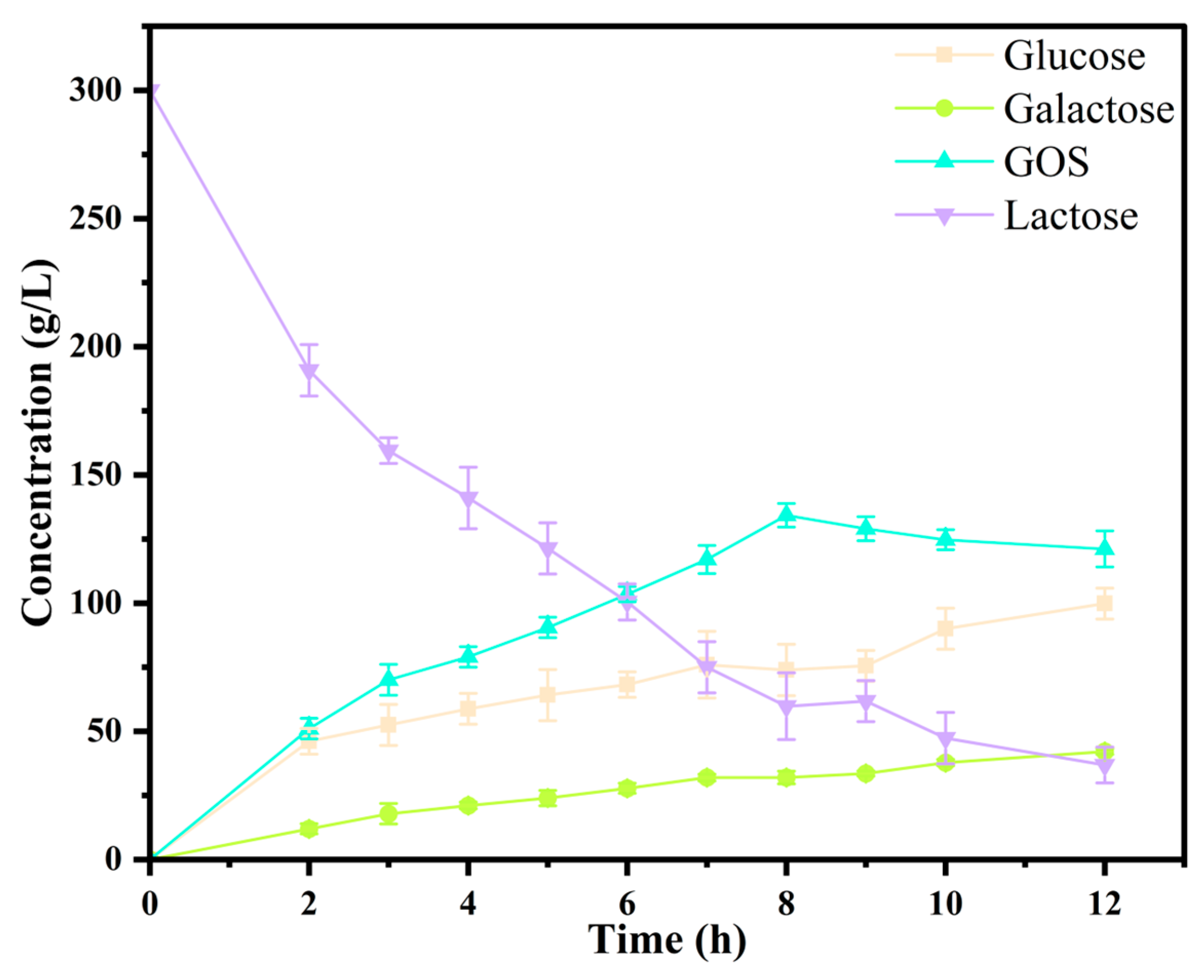
3.4. Bioconversion of GOS from Lactose
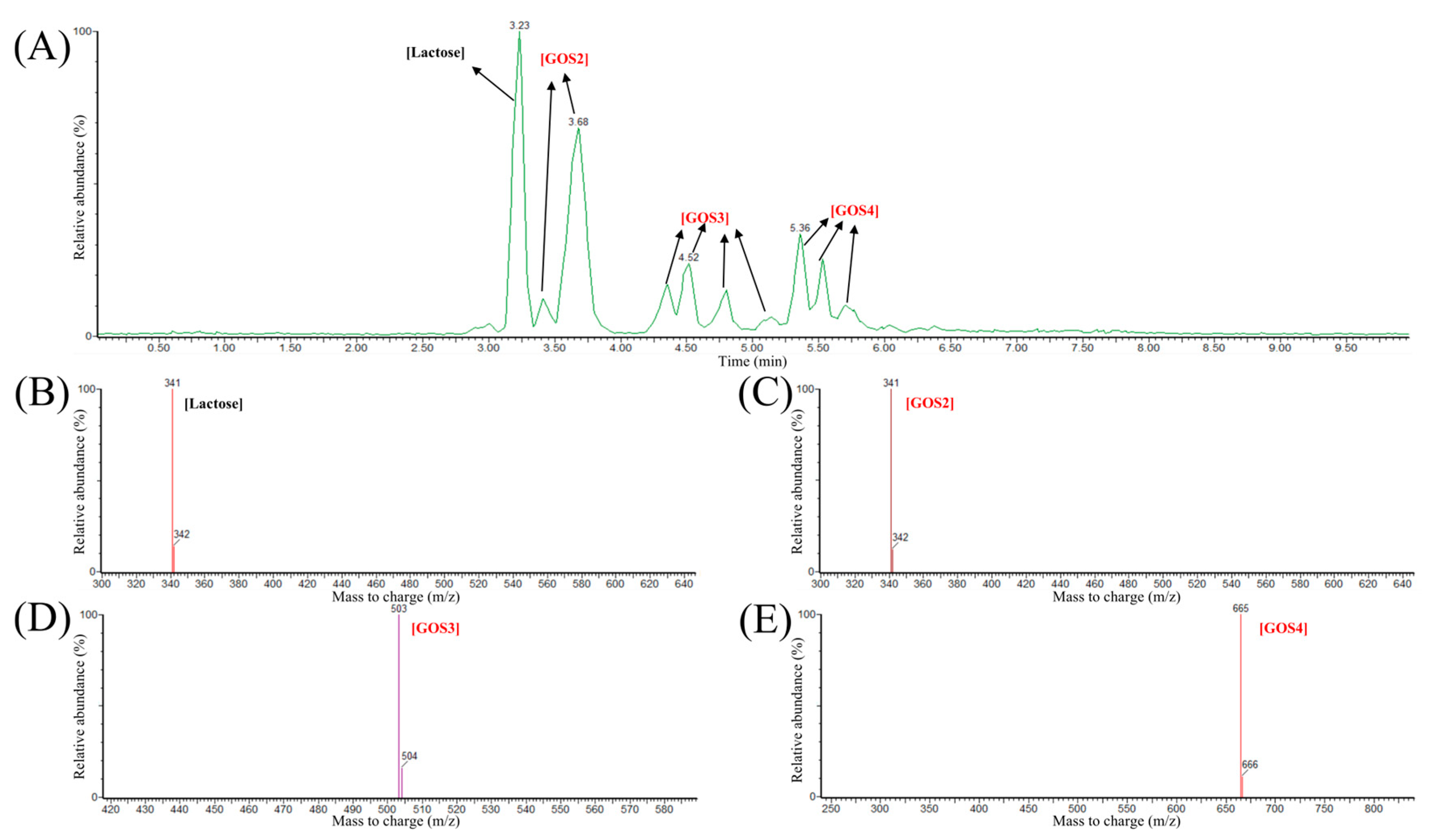
3.5. In Vitro GOS Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Souza, A.F.C.; Gabardo, S.; Coelho, R.D.J.S. Galactooligosaccharides: Physiological benefits, production strategies, and industrial application. J. Biotechnol. 2022, 359, 116–129. [Google Scholar] [CrossRef]
- Yang, F.; Wei, J.; Lu, Y.; Sun, Y.; Wang, Q.; Zhang, R. Galacto-oligosaccharides modulate gut microbiota dysbiosis and intestinal permeability in rats with alcohol withdrawal syndrome. J. Funct. Foods 2019, 60, 103423. [Google Scholar] [CrossRef]
- Lammerts Van Bueren, A.; Mulder, M.; Leeuwen, S.V.; Dijkhuizen, L. Prebiotic galactooligosaccharides activate mucin and pectic galactan utilization pathways in the human gut symbiont Bacteroides thetaiotaomicron. Sci. Rep. 2017, 7, 40478. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.; Tomar, S.; Ali, B.; Singh, R.; Singh, A. Galactooligosaccharides reduce infection caused by Listeria monocytogenes and modulate IgG and IgA levels in mice. Int. Dairy J. 2015, 41, 58–63. [Google Scholar] [CrossRef]
- Ignatova, I.; Arsov, A.; Petrova, P.; Petrov, K. Prebiotic effects of α-and β-galactooligosaccharides: The structure-function relation. Molecules 2025, 30, 803. [Google Scholar] [PubMed]
- Wu, Y.; Zhang, X.; Liu, X.; Zhao, Z.; Tao, S.; Xu, Q.; Zhao, J.; Dai, Z.; Zhang, G.; Han, D. Galactooligosaccharides and Limosilactobacillus reuteri synergistically alleviate gut inflammation and barrier dysfunction by enriching Bacteroides acidifaciens for pentadecanoic acid biosynthesis. Nat. Commun. 2024, 15, 9291. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Pelaez, C.; et al. Safety of the extension of use of galacto-oligosaccharides as a novel food pursuant to regulation (EU) 2015/2283. Efsa J. 2021, 19, e06844. [Google Scholar]
- GRAS Notice 000236; Generally Recognized as Safe (GRAS) Determination for the Use of Galacto-Oligosaccharides (GOS) in Foods and Term Infant Formulas. ENVIRON International Corporation: Arlington, VA, USA, 2007.
- Ambrogi, V.; Bottacini, F.; Cao, L.; Kuipers, B.; Schoterman, M.; van Sinderen, D. Galacto-oligosaccharides as infant prebiotics: Production, application, bioactive activities and future perspectives. Cri. Rev. Food Sci. Nutr. 2023, 63, 753–766. [Google Scholar]
- Balthazar, C.; Silva, H.; Celeguini, R.; Santos, R.; Pastore, G.; Junior, C.C.; Freitas, M.; Nogueira, L.; Silva, M.; Cruz, A. Effect of galactooligosaccharide addition on the physical, optical, and sensory acceptance of vanilla ice cream. J. Dairy Sci. 2015, 98, 4266–4272. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Rasouli Pirouzian, H. Optimization of prebiotic sucrose-free milk chocolate formulation by mixture design. J. Food Sci. Technol. 2021, 58, 244–254. [Google Scholar]
- Chaiwong, N.; Seesuriyachan, P.; Rachtanapun, P.; Gavahian, M.; Bangar, S.P.; Khaneghah, A.M.; Wangtueai, S.; Leksawasdi, N.; Jantanasakulwong, K.; Chailangka, A. Enhancing solubility, emulsion properties, and antioxidant activity of whey protein powder via wet-heating conjugated with galactooligosaccharides. J. Agric. Food Res. 2025, 19, 101666. [Google Scholar] [CrossRef]
- Prasad, L.N.; Sherkat, F.; Shah, N.P. Influence of galactooligosaccharides and modified waxy maize starch on some attributes of yogurt. J. Food Sci. 2013, 78, M77–M83. [Google Scholar] [CrossRef]
- Seijo, M.; Bonanno, M.S.; Vénica, C.I.; Marotte, C.; Pita Martín de Portela, M.L.; Bergamini, C.V.; Wolf, I.V.; Perotti, M.C.; Zeni, S.N. A yoghurt containing galactooligosaccharides and having low-lactose level improves calcium absorption and retention during growth: Experimental study. Int. J. Food Sci. Technol. 2022, 57, 48–56. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Pi, Y.; Han, D.; Feng, C.; Zhao, J.; Chen, L.; Che, D.; Bao, H.; Xie, Z. Maternal galactooligosaccharides supplementation programmed immune defense, microbial colonization and intestinal development in piglets. Food Funct. 2021, 12, 7260–7270. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.J.; Flaujac Lafontaine, G.M.; Connerton, P.L.; Liang, L.; Asiani, K.; Fish, N.M.; Connerton, I.F. Galacto-oligosaccharides modulate the juvenile gut microbiome and innate immunity to improve broiler chicken performance. Msystems 2020, 5, e00827-19. [Google Scholar] [CrossRef] [PubMed]
- Rentas, M.F.; Pedreira, R.S.; Perini, M.P.; Risolia, L.W.; Zafalon, R.V.A.; Alvarenga, I.C.; Vendramini, T.H.A.; Balieiro, J.C.C.; Pontieri, C.F.F.; Brunetto, M.A. Galactoligosaccharide and a prebiotic blend improve colonic health and immunity of adult dogs. PLoS ONE 2020, 15, e0238006. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Mahmood, S.; Liaqat, H.; Mushtaq, A.; Khan, S.; Amin, S.; Qi, X. Recent advances in the production, analysis, and application of galacto-oligosaccharides. Food Rev. Int. 2023, 39, 5814–5843. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Ma, C.; Ouyang, J.; Zheng, Z. β-Galactosidase: A traditional enzyme given multiple roles through protein engineering. Crit. Rev. Food Sci. Nutr. 2025, 65, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Bultema, J.B.; Dijkhuizen, L.; Van Leeuwen, S.S. Reaction kinetics and galactooligosaccharide product profiles of the β-galactosidases from Bacillus circulans, Kluyveromyces lactis and Aspergillus oryzae. Food Chem. 2017, 225, 230–238. [Google Scholar] [CrossRef]
- Frenzel, M.; Zerge, K.; Clawin-Rädecker, I.; Lorenzen, P.C. Comparison of the galacto-oligosaccharide forming activity of different β-galactosidases. LWT-Food Sci. Technol. 2015, 60, 1068–1071. [Google Scholar] [CrossRef]
- Arsov, A.; Ivanov, I.; Tsigoriyna, L.; Petrov, K.; Petrova, P. In vitro production of galactooligosaccharides by a novel β-galactosidase of Lactobacillus bulgaricus. Int. J. Mol. Sci. 2022, 23, 14308. [Google Scholar] [CrossRef]
- Girard, L.; Lood, C.; Höfte, M.; Vandamme, P.; Rokni-Zadeh, H.; Van Noort, V.; Lavigne, R.; De Mot, R. The ever-expanding pseudomonas genus: Description of 43 new species and partition of the Pseudomonas putida Group. Microorganisms 2021, 9, 1766. [Google Scholar] [CrossRef]
- Compton, S.J.; Jones, C.G. Mechanism of dye response and interference in the Bradford protein assay. Anal. Biochem. 1985, 151, 369–374. [Google Scholar] [CrossRef]
- Lutz-Wahl, S.; Mozer, H.; Kussler, A.; Schulz, A.; Seitl, I.; Fischer, L. A new β-galactosidase from Paenibacillus wynnii with potential for industrial applications. J. Dairy Sci. 2024, 107, 3429–3442. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, J.; Liu, L.; Huang, J. Recombinant β-galactosidase derived from Enterobacter cloacae Zjut HJ2001 for efficient biotransformation of galactooligosaccharides. Biochem. Eng. J. 2024, 212, 109514. [Google Scholar] [CrossRef]
- Hon, J.; Borko, S.; Stourac, J.; Prokop, Z.; Zendulka, J.; Bednar, D.; Martinek, T.; Damborsky, J. EnzymeMiner: Automated mining of soluble enzymes with diverse structures, catalytic properties and stabilities. Nucleic Acids Res. 2020, 48, W104–W109. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, A.; Aguerrebere, C.; Falconieri, V.; Banerjee, S.; Earl, L.A.; Zhu, X.; Grigorieff, N.; Milne, J.L.; Sapiro, G.; Wu, X. Atomic resolution cryo-EM structure of β-galactosidase. Structure 2018, 26, 848–856. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Alghamdi, S.A.; ur Rehman, K.; Zaman, U.; Alshareef, S.A.; Zghab, I.; Alanazi, A.N.; Nasr, S.; Khan, S.U.; Alissa, M.; Alqasem, A.A. β-Galactosidase isolated from Ranunculus arvensis seeds to synthesize trisaccharide: Kinetics and thermodynamic properties. Food Biosci. 2024, 59, 103943. [Google Scholar] [CrossRef]
- Chen, J.; Ni, D.; Xu, W.; Zhang, W.; Mu, W. Recent advances in discovery, protein engineering, and heterologous production of ketose 3-epimerase for rare sugar biosynthesis. Trends Food Sci. Technol. 2024, 149, 104552. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, H.; Wang, X.; Dai, Q.; Liu, X.; Ni, D.; Zhu, Y.; Xu, W.; Mu, W. Rational design for thermostability improvement of a novel PL-31 family alginate lyase from Paenibacillus sp. YN15. Int. J. Biol. Macromol. 2023, 253, 126919. [Google Scholar] [CrossRef]
- Zerva, A.; Limnaios, A.; Kritikou, A.S.; Thomaidis, N.S.; Taoukis, P.; Topakas, E. A novel thermophile β-galactosidase from Thermothielavioides terrestris producing galactooligosaccharides from acid whey. New Biotechnol. 2021, 63, 45–53. [Google Scholar] [CrossRef]
- Vera, C.; Córdova, A.; Aburto, C.; Guerrero, C.; Suárez, S.; Illanes, A. Synthesis and purification of galacto-oligosaccharides: State of the art. World J. Microbiol. Biotechnol. 2016, 32, 197. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.; Kjaer, K.; Yde, C.; Wichmann, J.; Jensen, H.; Kortman, G.; Dellomonaco, C.; Ewert, J. β-galactosidase from Bifidobacterium bifidum for improved in situ synthesis of GOS-oligomers with prebiotic effects. Int. Dairy J. 2025, 163, 106164. [Google Scholar] [CrossRef]
- Benjamins, E.; Boxem, L.; KleinJan-Noeverman, J.; Broekhuis, T. Assessment of repetitive batch-wise synthesis of galacto-oligosaccharides from lactose slurry using immobilised β-galactosidase from Bacillus circulans. Int. Dairy J. 2014, 38, 160–168. [Google Scholar] [CrossRef]
- Ten Kate, G.; Sanders, P.; Dijkhuizen, L.; van Leeuwen, S. Kinetics and products of Thermotoga maritima β-glucosidase with lactose and cellobiose. Appl. Microbiol. Biotechnol. 2024, 108, 349. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, D.; Liu, J.; Jin, Z.; Mchunu, N.P.; Singh, S.; Wang, Z. Enhancing β-galactosidase performance for galactooligosaccharides preparation via strategic glucose re-tunneling. Int. J. Mol. Sci. 2024, 25, 12316. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, S.; Chen, S.; Wu, D.; Chen, J.; Wu, J. Enhancing the production of galacto-oligosaccharides by mutagenesis of Sulfolobus solfataricus β-galactosidase. Food Chem. 2013, 138, 1588–1595. [Google Scholar] [CrossRef]
- Logtenberg, M.; Donners, K.; Vink, J.; van Leeuwen, S.; de Waard, P.; de Vos, P.; Schols, H. Touching the high complexity of prebiotic vivinal galacto-oligosaccharides using porous graphitic carbon ultra-high-performance liquid chromatography coupled to mass spectrometry. J. Agric. Food Chem. 2020, 68, 7800–7808. [Google Scholar] [CrossRef]


| Metal Ion (1 mM) | Relative Activity (%) |
|---|---|
| None | 100 ± 3.6 |
| K+ | 75.6 ± 2.1 |
| Na+ | 59.4 ± 1.3 |
| Mg2+ | 125.1 ± 4.7 |
| Ca2+ | 60.7 ± 1.2 |
| Mn2+ | 45.2 ± 1.7 |
| Co2+ | 3.1 ± 0.3 |
| Ni2+ | 2.4 ± 0.7 |
| Cu2+ | 0.6 ± 0.1 |
| Zn2+ | 1 ± 0.3 |
| Fe3+ | 71.2 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Cheng, Z.; Zhang, Y.; Petrova, P.; Petrov, K.; Zhang, W.; Mu, W. A New β-Galactosidase from Pseudomonas tritici SWRI145 for Efficient Bioproduction of Galactooligosaccharides. Foods 2025, 14, 3125. https://doi.org/10.3390/foods14173125
Jin X, Cheng Z, Zhang Y, Petrova P, Petrov K, Zhang W, Mu W. A New β-Galactosidase from Pseudomonas tritici SWRI145 for Efficient Bioproduction of Galactooligosaccharides. Foods. 2025; 14(17):3125. https://doi.org/10.3390/foods14173125
Chicago/Turabian StyleJin, Xiangpeng, Zhuo Cheng, Yulei Zhang, Penka Petrova, Kaloyan Petrov, Wenli Zhang, and Wanmeng Mu. 2025. "A New β-Galactosidase from Pseudomonas tritici SWRI145 for Efficient Bioproduction of Galactooligosaccharides" Foods 14, no. 17: 3125. https://doi.org/10.3390/foods14173125
APA StyleJin, X., Cheng, Z., Zhang, Y., Petrova, P., Petrov, K., Zhang, W., & Mu, W. (2025). A New β-Galactosidase from Pseudomonas tritici SWRI145 for Efficient Bioproduction of Galactooligosaccharides. Foods, 14(17), 3125. https://doi.org/10.3390/foods14173125







