Effects of Lacticaseibacillus casei LK-1 Fermentation on Physicochemical Properties, Chemical Compositions, Antioxidant Activities and Volatile Profiles of Pineapple Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Inoculum
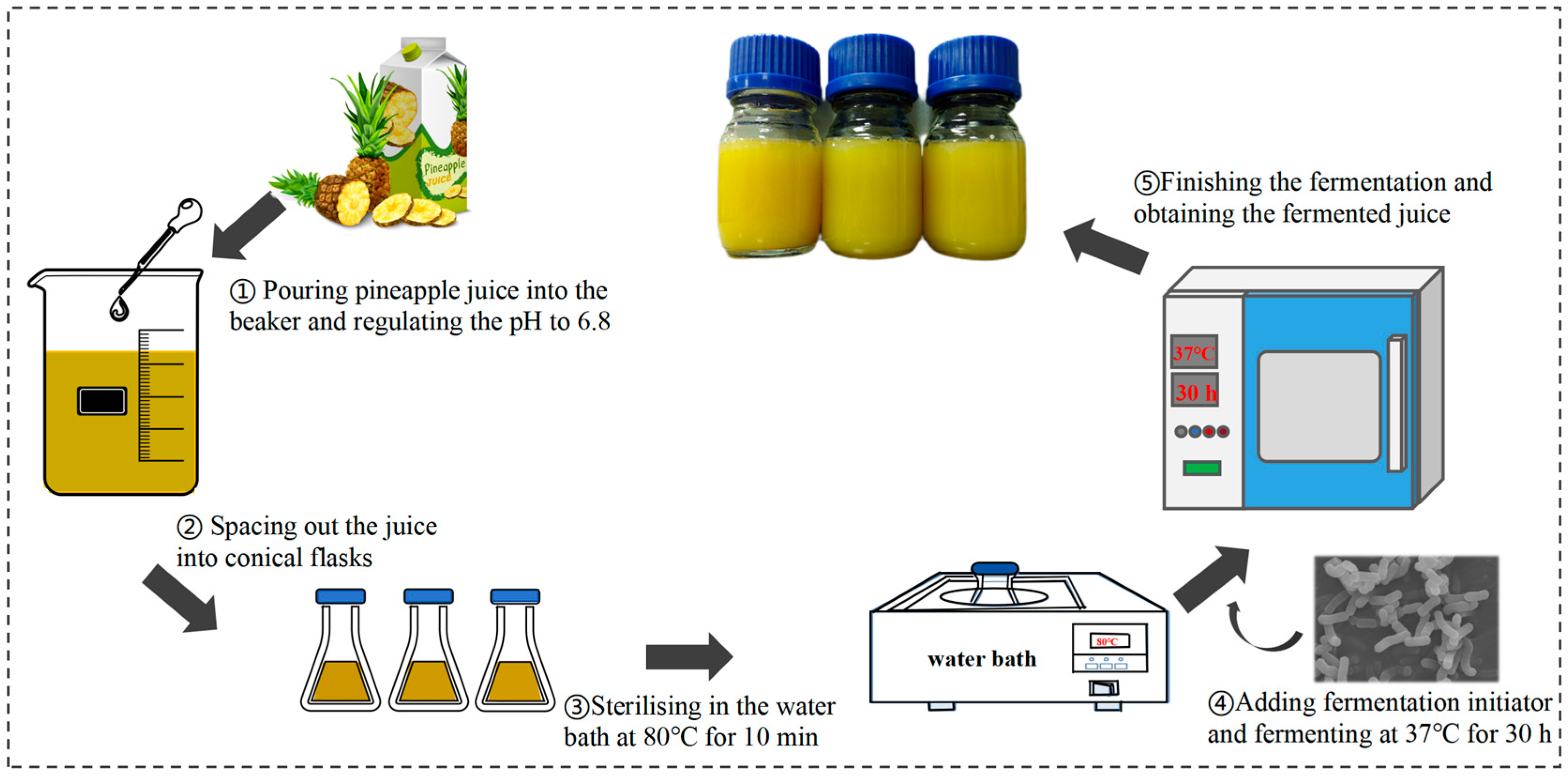
2.3. Preparation of Fermented Pineapple Juice
2.4. Enumeration of Microorganisms
2.5. Determination of pH, TSS, and Color
2.6. Determination of Total Flavonoid Content (TFC) and Total Phenolic Content (TPC)
2.7. Determination of Carbohydrates and Organic Acids
2.8. Determination of FAAs
2.9. Determination of Antioxidant Activity
2.10. Volatiles Analysis
2.11. Statistical Analysis
3. Results and Discussion
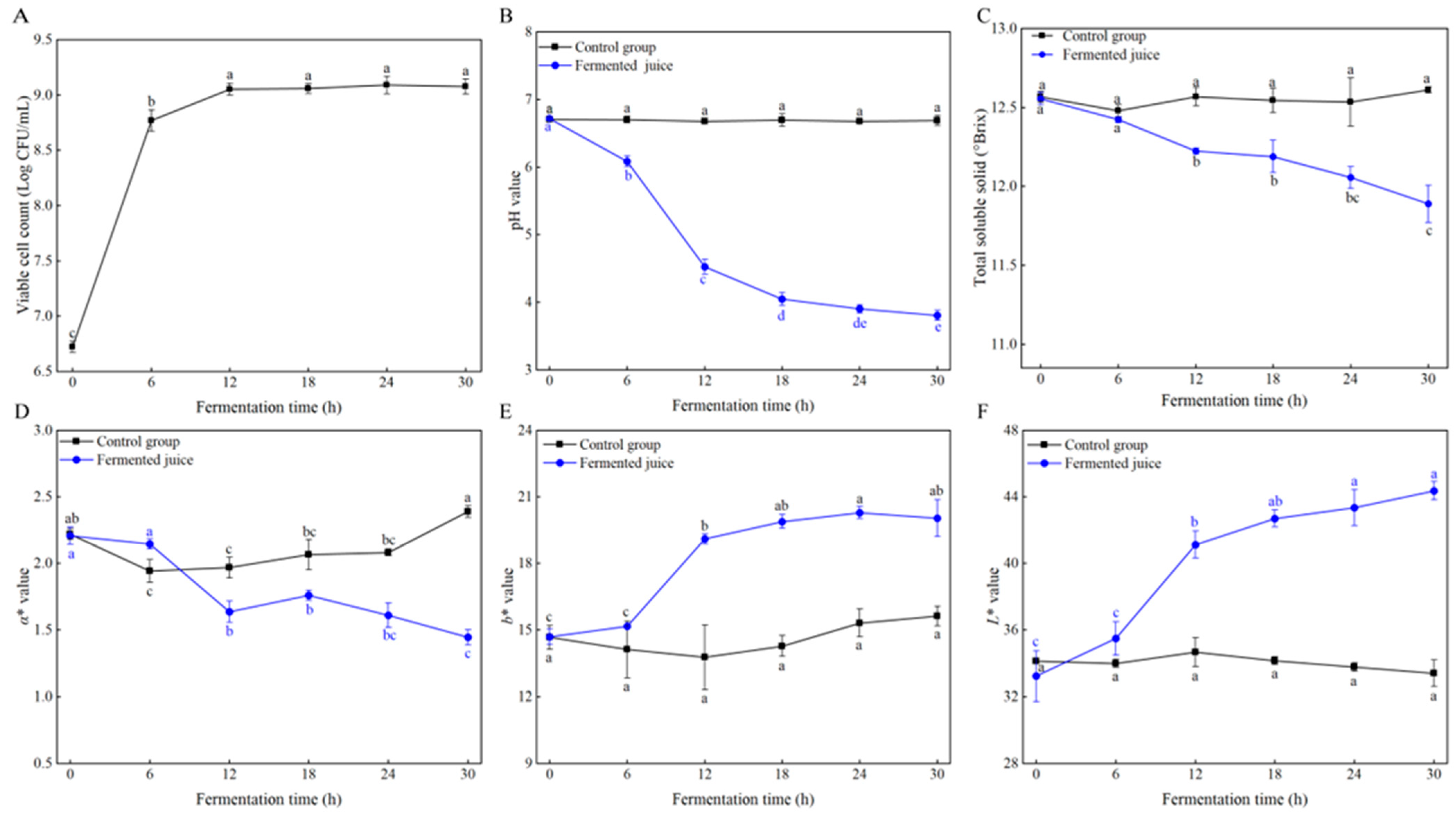
3.1. Changes in Viable Cell Counts During Fermentation
3.2. Changes in Physicochemical Properties During Fermentation
3.3. Changes in Total Phenolics and Flavonoids During Fermentation
3.4. Changes in Carbohydrates During Fermentation
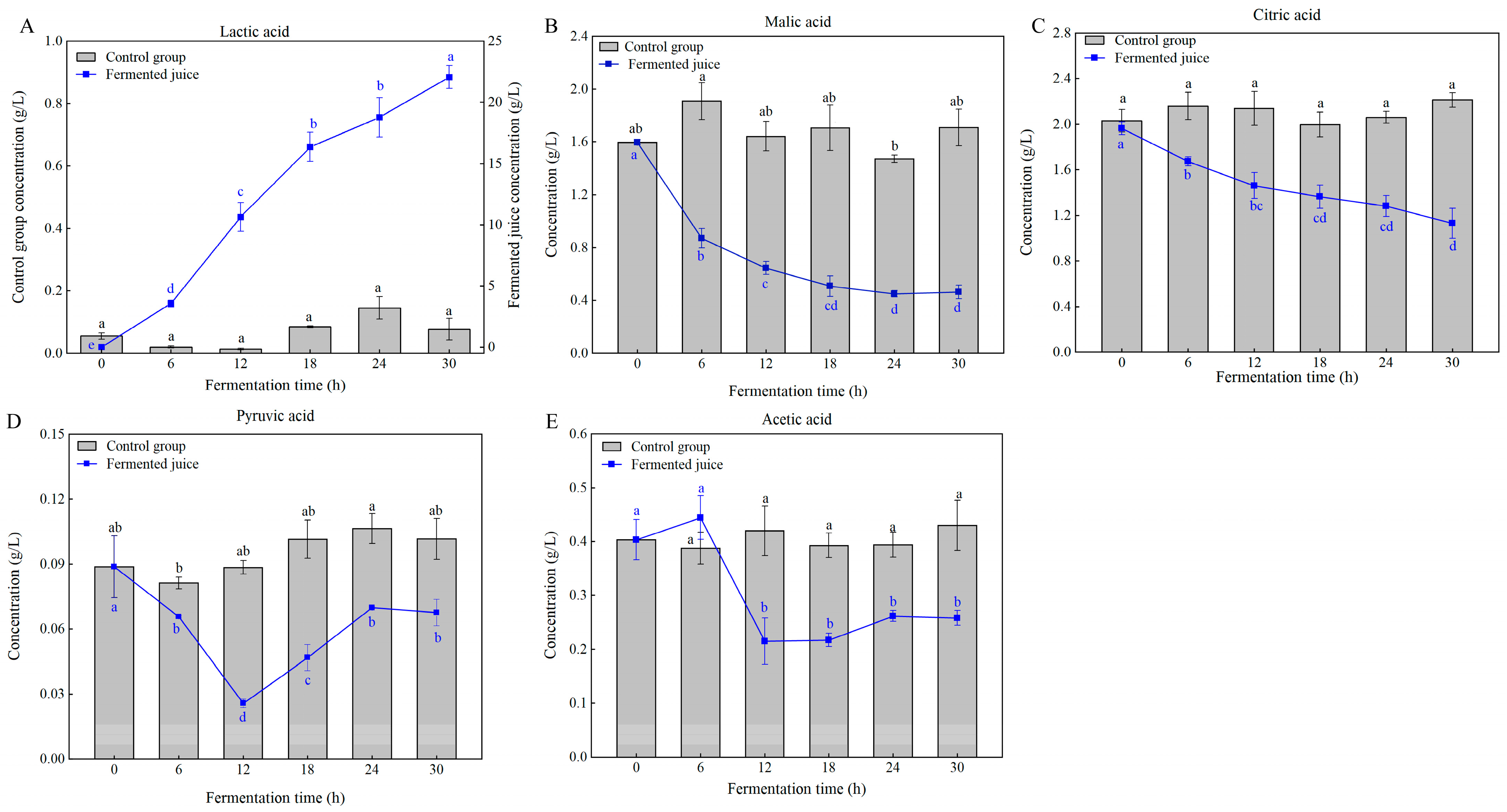
3.5. Changes in Organic Acids During Fermentation
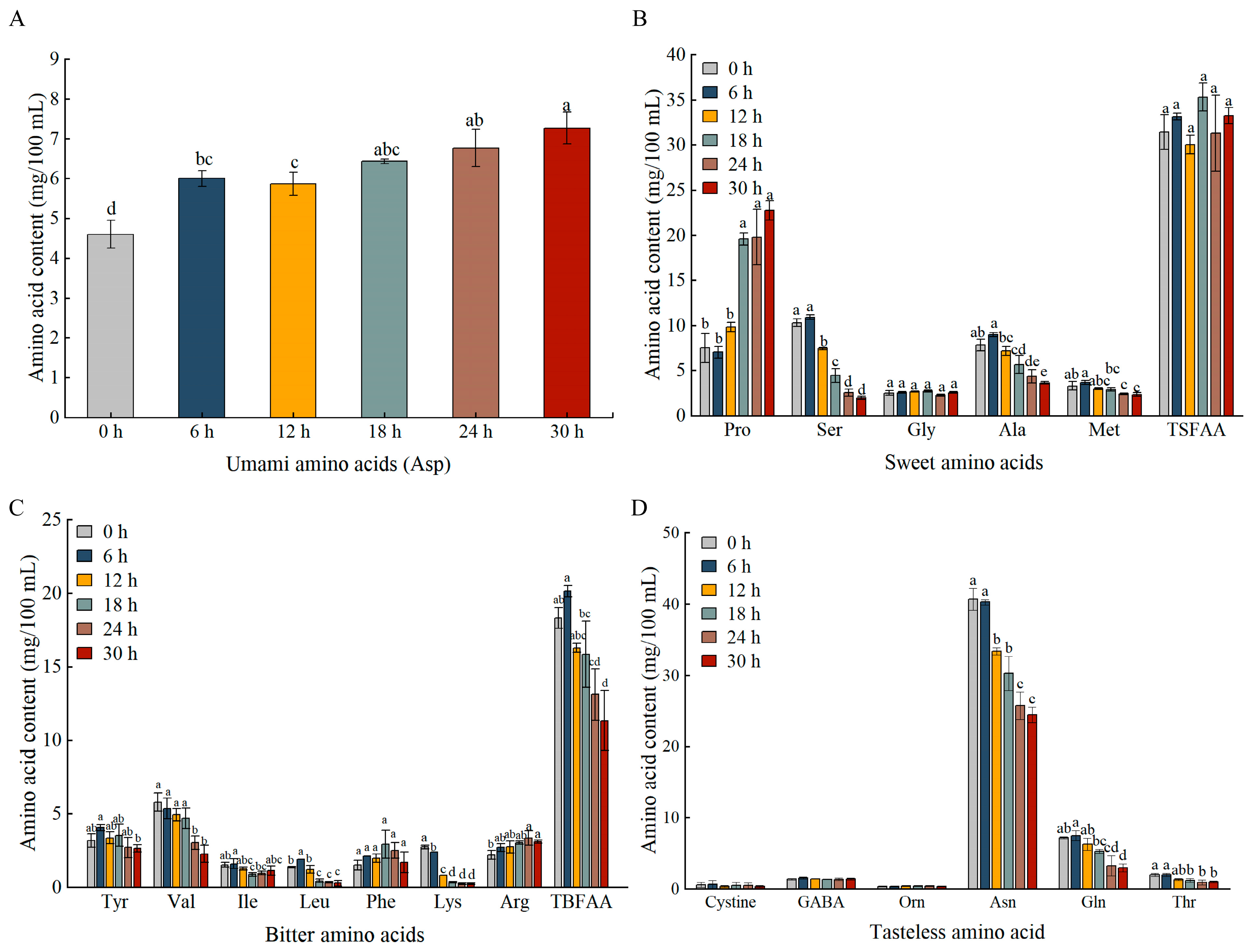
3.6. Changes in FAAs During Fermentation
3.7. Changes in Antioxidant Activity During Fermentation
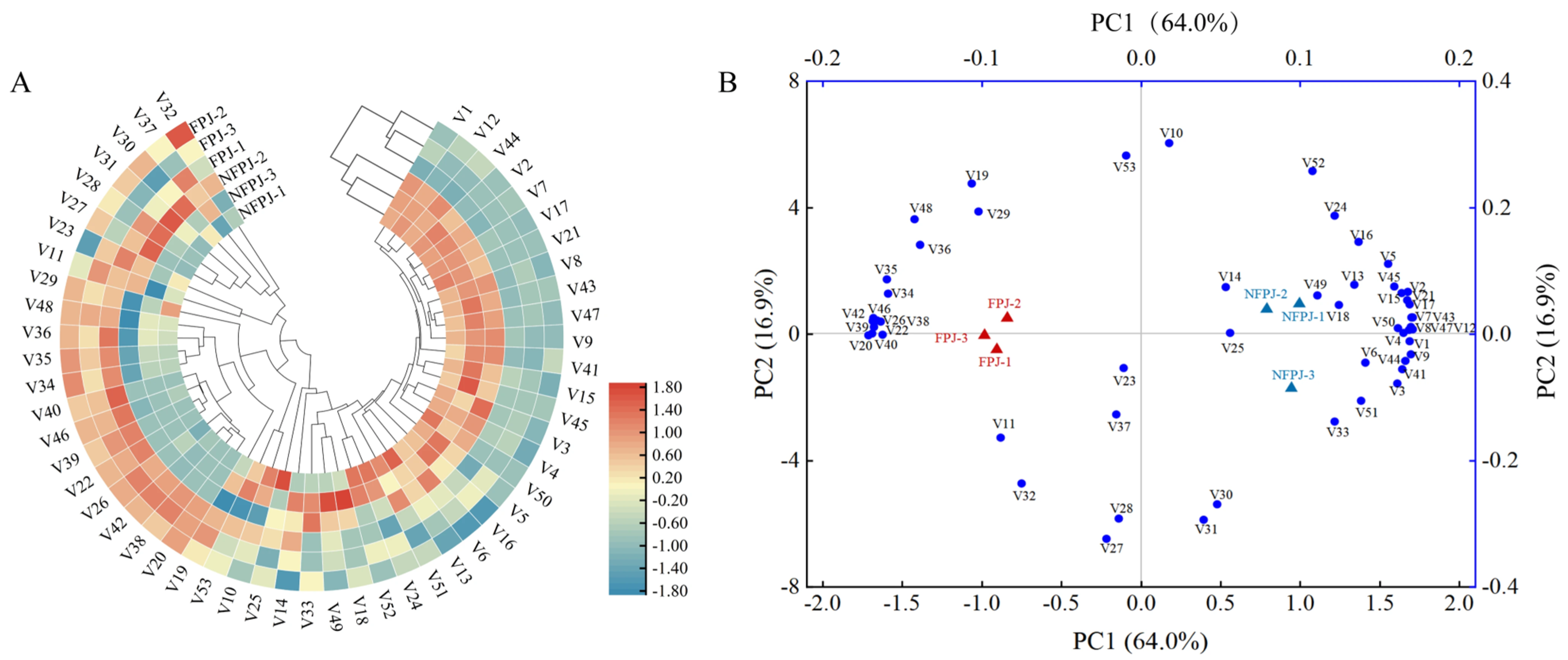
3.8. Volatile Profiles in Pineapple Juice After Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Liu, Y. Effects of elevated temperature postharvest on color aspect, physiochemical characteristics, and aroma components of pineapple fruits. J. Food Sci. 2014, 79, C2409–C2414. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Bujna, E.; Fekete, N.; Tran, A.T.M.; Rezessy-Szabo, J.M.; Prasad, R.; Nguyen, Q.D. Probiotic beverage from pineapple juice fermented with Lactobacillus and Bifidobacterium strains. Front. Nutr. 2019, 6, 54. [Google Scholar] [CrossRef]
- Del Juncal-Guzmán, D.; Hernández-Maldonado, L.M.; Sánchez-Burgos, J.A.; González-Aguilar, G.A.; Ruiz-Valdiviezo, V.M.; Tovar, J.; Sáyago-Ayerdi, S.G. In vitro gastrointestinal digestion and colonic fermentation of phenolic compounds in UV-C irradiated pineapple (Ananas comosus) snack-bars. LWT-Food Sci. Technol. 2021, 138, 110636. [Google Scholar] [CrossRef]
- Haiyan, X.; Lingxing, F.; Yuan, D.; Lihua, C.; Yiyi, L.; Liujun, L.; Mengyuan, L.; Xinyang, J.; Fang, W.; Zhihong, S. Change of phytochemicals and bioactive substances in Lactobacillus fermented Citrus juice during the fermentation process. LWT-Food Sci. Technol. 2023, 180, 114715. [Google Scholar]
- Cirlini, M.; Ricci, A.; Galaverna, G.; Lazzi, C. Application of lactic acid fermentation to elderberry juice: Changes in acidic and glucidic fractions. LWT-Food Sci. Technol. 2020, 118, 108779. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial effects of tomato juice fermented by Lactobacillus Plantarum and Lactobacillus Casei: Antioxidation, antimicrobial effect, and volatile profiles. Molecules 2018, 23, 2366. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Jin, Z.; Gu, Z.; Rao, J.; Chen, B. Physicochemical property changes and aroma differences of fermented yellow pea flours: Role of lactobacilli and fermentation time. Food Funct. 2021, 12, 6950–6963. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced antioxidant activity for apple juice fermented with Lactobacillus plantarum ATCC14917. Molecules 2019, 24, 51. [Google Scholar] [CrossRef]
- Liao, L.K.; Wei, X.Y.; Gong, X.; Li, J.H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus casei LK-1 by spray drying related to its stability and in vitro digestion. LWT-Food Sci. Technol. 2017, 82, 82–89. [Google Scholar]
- Yang, L.; Peng, S.; Ma, J.; Zhang, C.; Wu, G.; Huang, X.; Li, J.; Liao, L. Fermentation characteristics of Lactobacillus casei LK-1 and its application in pineapple juice. Sci. Technol. Food Ind. 2024, 45, 199–207. [Google Scholar]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Xiao, L.; Li, X.; Hu, M. Effect of fermentation parameters and their optimization on the phytochemical properties of lactic-acid-fermented mulberry juice. J. Food Meas. Charact. 2017, 11, 1462–1473. [Google Scholar]
- Wu, P.; Ma, G.; Li, N.; Deng, Q.; Yin, Y.; Huang, R. Investigation of in vitro and in vivo antioxidant activities of flavonoids rich extract from the berries of Rhodomyrtus tomentosa (Ait.) Hassk. Food Chem. 2015, 173, 194–202. [Google Scholar]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef]
- Shangguan, L.; Liu, Z.; Zhang, H.; Yang, Q.; Zhang, X.; Yao, L.; Li, P.; Chen, X.; Dai, J. Improved umami taste of the enzymatic hydrolysate of soybean protein isolate by Corynebacterium glutamicum P-45 fermentation. Food Biosci. 2024, 58, 103565. [Google Scholar]
- Pontonio, E.; Montemurro, M.; Pinto, D.; Marzani, B.; Trani, A.; Ferrara, G.; Mazzeo, A.; Gobbetti, M.; Rizzello, C.G. Lactic acid fermentation of pomegranate juice as a tool to improve antioxidant activity. Front. Microbiol. 2019, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bi, S.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Comprehensive investigation on volatile and non-volatile metabolites in broccoli juices fermented by animal- and plant-derived Pediococcus pentosaceus. Food Chem. 2021, 341, 128118. [Google Scholar] [PubMed]
- Costa, K.K.F.D.; Soares Júnior, M.S.; Rosa, S.I.R.; Caliari, M.; Pimentel, T.C. Changes of probiotic fermented drink obtained from soy and rice byproducts during cold storage. LWT-Food Sci. Technol. 2017, 78, 23–30. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: Process optimisation and product stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef]
- Guo, W.; Chen, M.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H. Effects of Lacticaseibacillus casei fermentation on the bioactive compounds, volatile and non-volatile compounds, and physiological properties of barley beverage. Food Biosci. 2023, 53, 102695. [Google Scholar] [CrossRef]
- Managa, M.G.; Akinola, S.A.; Remize, F.; Garcia, C.; Sivakumar, D. Physicochemical parameters and bioaccessibility of lactic acid bacteria fermented chayote leaf (Sechium edule) and pineapple (Ananas comosus) smoothies. Front. Nutr. 2021, 8, 649189. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4, 21. [Google Scholar] [CrossRef]
- Lu, C.; Li, Y.; Wang, J.; Qu, J.; Chen, Y.; Chen, X.; Huang, H.; Dai, S. Flower color classification and correlation between color space values with pigments in potted multiflora chrysanthemum. Sci. Hortic. 2021, 283, 110082. [Google Scholar] [CrossRef]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of total polyphenols, proffle anthocyanins, color analysis, carotenoids and tocols in pigmented maize. LWT-Food Sci. Technol. 2021, 144, 111257. [Google Scholar]
- Zhu, Y.; Jiang, J.; Yue, Y.; Feng, Z.; Chen, J.; Ye, X. Influence of mixed probiotics on the the bioactive composition, antioxidant activity and appearance of fermented red bayberry pomace. LWT-Food Sci. Technol. 2020, 133, 110076. [Google Scholar]
- Wang, L.; Zhang, H.; Lei, H. Phenolics profile, antioxidant activity and flavor volatiles of pear juice: Influence of lactic acid fermentation using three Lactobacillus strains in monoculture and binary mixture. Foods 2022, 11, 11. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Razola-Díaz, M.D.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of lactic acid bacteria fermentation on phenolic compounds and antioxidant activity of avocado Leaf extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef]
- Parhi, P.; Song, K.P.; Choo, W.S. Effect of inulin and fructooligosaccharide supplementation on the growth and survival of Lactobacillus casei in model sugar systems. J. Food Process. Preserv. 2021, 45, e15228. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Ren, Y.; Wang, Y.; Wang, X.; Yue, T.; Wang, Z.; Gao, Z. Preparation, model construction and efficacy lipid-lowering evaluation of kiwifruit juice fermented by probiotics. Food Biosci. 2022, 47, 101710. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2020, 340, 127922. [Google Scholar] [PubMed]
- Liu, Y.; Sheng, J.; Li, J.; Zhang, P.; Tang, F.; Shan, C. Influence of lactic acid bacteria on physicochemical indexes, sensory and flavor characteristics of fermented sea buckthorn juice. Food Biosci. 2022, 46, 101519. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [PubMed]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and storage characteristics of “Fuji” apple juice using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial growth, metabolism of bioactives and in vitro bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, N.; Li, J.; Xu, R.; Wang, T.; Guo, L.; Ma, M.; Fan, M.; Wei, X. Selenium-enriched Lactobacillus plantarum improves the antioxidant activity and flavor properties of fermented Pleurotus eryngii. Food Chem. 2021, 345, 128770. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: Chemical composition, antioxidant activity and sensorial properties. LWT-Food Sci. Technol. 2020, 131, 109803. [Google Scholar]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Hadinejad, M.; Emam-Djomeh, Z.; Mirzapour, M. Effect of fermentation of pomegranate juice by Lactobacillus plantarum and Lactobacillus acidophilus on the antioxidant activity and metabolism of sugars, organic acids and phenolic compounds. Food Biotechnol. 2013, 27, 1–13. [Google Scholar]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Marulo, S.; De Caro, S.; Nitride, C.; Di Renzo, T.; Di Stasio, L.; Ferranti, P.; Mamone, M. Bioactive peptides released by lactic acid bacteria fermented pistachio beverages. Food Biosci. 2024, 59, 103988. [Google Scholar] [CrossRef]
- Liang, J.R.; Deng, H.; Hu, C.Y.; Zhao, P.T.; Meng, Y.H. Vitality, fermentation, aroma profile, and digestive tolerance of the newly selected Lactiplantibacillus plantarum and Lacticaseibacillus paracasei in fermented apple juice. Front. Nutr. 2022, 9, 1045347. [Google Scholar] [CrossRef]
- Alves Filho, E.D.G.; Soares Rodrigues, T.H.; Narciso Fernandes, F.A.; Fernandes Pereira, A.L.; Narain, N.; De Brito, E.S.; Rodrigues, S. Chemometric evaluation of the volatile profile of probiotic melon and probiotic cashew juice. Food Res. Int. 2017, 99, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Dan, T.; Chen, H.; Li, T.; Tian, J.; Ren, W.; Zhang, H.; Sun, T. Influence of Lactobacillus plantarum P-8 on fermented milk flavor and storage stability. Front. Microbiol. 2019, 9, 3133. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Simmons, T.R.; Cordell, W.T.; Hernández Lozada, N.J.; Breckner, C.J.; Chen, X.; Jindra, M.A.; Pfleger, B.F. Metabolic engineering of β-oxidation to leverage thioesterases for production of 2-heptanone, 2-nonanone and 2-undecanone. Metab. Eng. 2020, 61, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, G.; Zhao, Y.; Chen, B.; Li, W.; Guo, Z.; He, S.; Koris, A.; Zhu, X.; Mu, Z.; et al. The Fermentation Mechanism of Pea Protein Yogurt and Its Bean Odour Removal Method. Foods 2025, 14, 3363. [Google Scholar] [CrossRef]
- Dan, T.; Ren, W.; Liu, Y.; Tian, J.; Chen, H.; Li, T.; Liu, W. Volatile flavor compounds profile and fermentation characteristics of milk fermented by Lactobacillus delbrueckii subsp. bulgaricus. Front. Microbiol. 2019, 10, 02183. [Google Scholar]
- Siroli, L.; Camprini, L.; Pisano, M.B.; Patrignani, F.; Lanciotti, R. Volatile molecule profiles and anti-listeria monocytogenes activity of Nisin producers Lactococcus lactis strains in vegetable drinks. Front. Microbiol. 2019, 10, 00563. [Google Scholar] [CrossRef]
- Fei, Z.; Xie, D.; Wang, M.; Zhang, Y.; Zhang, H.; Du, Q.; Jin, P. Enhanced biotransformation of bioactive components and volatile compounds of bamboo (Phyllostachys glauca McClure) leaf juice fermented by probiotic Streptococcus thermophiles. LWT-Food Sci. Technol. 2023, 173, 114363. [Google Scholar]



| Free Amino Acid (mg/100 mL) | Fermentation Time (h) | |||||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 30 | |
| Cysteine (Cys) | 0.57 ± 0.35 a | 0.63 ± 0.59 a | 0.37 ± 0.15 a | 0.48 ± 0.46 a | 0.47 ± 0.38 a | 0.33 ± 0.15 a |
| Gamma-aminobutyric acid (GABA) | 1.40 ± 0.10 a | 1.63 ± 0.26 a | 1.43 ± 0.16 a | 1.40 ± 0.10 a | 1.30 ± 0.10 a | 1.20 ± 0.30 a |
| Ornithine (Orn) | 0.37 ± 0.06 a | 0.37 ± 0.06 a | 0.43 ± 0.06 a | 0.43 ± 0.06 a | 0.43 ± 0.06 a | 0.37 ± 0.06 a |
| Arginine (Arg) | 2.20 ± 0.30 b | 2.70 ± 0.26 ab | 2.73 ± 0.40 ab | 3.03 ± 0.12 ab | 3.33 ± 0.49 a | 3.10 ± 0.10 a |
| Aspartic Acid (Asp) | 4.60 ± 0.30 d | 6.00 ± 0.20 bc | 5.87 ± 0.29 c | 6.43 ± 0.06 abc | 6.77 ± 0.47 ab | 7.27 ± 0.40 a |
| Proline (Pro) | 7.50 ± 1.61 b | 7.03 ± 0.67 b | 9.83 ± 0.50 b | 19.57 ± 0.67 a | 19.77 ± 3.07 a | 22.73 ± 1.06 a |
| Tyrosine (Tyr) | 3.17 ± 0.46 ab | 4.07 ± 0.21 a | 3.37 ± 0.42 ab | 3.53 ± 0.76 ab | 2.70 ± 0.69 ab | 2.63 ± 0.25 b |
| Serine (Ser) | 10.29 ± 0.42 a | 10.90 ± 0.26 a | 7.43 ± 0.15 b | 4.43 ± 0.76 c | 2.53 ± 0.40 d | 1.97 ± 0.15 d |
| Asparagine (Asn) | 40.67 ± 1.53 a | 40.23 ± 0.38 a | 33.37 ± 0.47 b | 30.23 ± 2.40 b | 25.73 ± 1.93 c | 24.50 ± 1.06 c |
| Glutamine (Gln) | 7.19 ± 0.16 ab | 7.47 ± 0.71 a | 6.27 ± 0.85 ab | 5.30 ± 0.30 bc | 3.23 ± 1.42 cd | 2.97 ± 0.57 d |
| Glycine (Gly) | 2.50 ± 0.26 ab | 2.57 ± 0.06 ab | 2.63 ± 0.06 a | 2.70 ± 0.10 a | 2.27 ± 0.12 b | 2.57 ± 0.17 ab |
| Alanine (Ala) | 7.83 ± 0.65 ab | 8.97 ± 0.21 a | 7.17 ± 0.50 bc | 5.67 ± 1.00 cd | 4.33 ± 0.75 de | 3.63 ± 0.15 e |
| Valine (Val) | 5.80 ± 0.62 a | 5.37 ± 0.71 a | 4.93 ± 0.42 a | 4.70 ± 0.70 a | 3.03 ± 0.47 b | 2.27 ± 0.59 b |
| Methionine (Met) | 3.30 ± 0.50 ab | 3.67 ± 0.21 a | 2.97 ± 0.12 abc | 2.90 ± 0.20 bc | 2.40 ± 0.10 c | 2.33 ± 0.21 c |
| Isoleucine (Ile) | 1.53 ± 0.15 ab | 1.60 ± 0.35 a | 1.27 ± 0.12 abc | 0.87 ± 0.12 c | 0.97 ± 0.12 bc | 1.13 ± 0.32 abc |
| Leucine (Leu) | 1.37 ± 0.06 b | 1.90 ± 0.00 a | 1.20 ± 0.26 b | 0.43 ± 0.12 c | 0.33 ± 0.06 c | 0.27 ± 0.21 c |
| Threonine (Thr) | 1.97 ± 0.23 a | 1.97 ± 0.21 a | 1.33 ± 0.15 ab | 1.20 ± 0.30 b | 0.90 ± 0.35 b | 0.97 ± 0.15 b |
| Phenylalanine (Phe) | 1.50 ± 0.35 a | 2.10 ± 0.00 a | 1.97 ± 0.29 a | 2.93 ± 0.95 a | 2.50 ± 0.52 a | 1.70 ± 0.70 a |
| Lysine (Lys) | 2.73 ± 0.12 a | 2.40 ± 0.00 b | 0.80 ± 0.00 c | 0.33 ± 0.06 d | 0.23 ± 0.06 d | 0.23 ± 0.06 d |
| Volatile Compounds | Non-Fermented Pineapple Juice (ug/100 mL) | Fermented Pineapple Juice (ug/100 mL) |
|---|---|---|
| Esters | ||
| [V1] Methyl 2-methylbutyrate | 344.16 ± 8.18 a | 225.10 ± 22.49 a |
| [V2] Ethyl butyrate | 99.26 ± 11.20 a | 39.70 ± 1.72 b |
| [V3] Ethyl 2-methylbutyrate | 252.02 ± 10.04 a | 217.33 ± 3.91 b |
| [V4] Isoamyl acetate | 103.08 ± 1.35 a | 85.60 ± 4.17 b |
| [V5] Ethyl valerate | 5.24 ± 0.76 a | 2.74 ± 0.87 b |
| [V6] Methyl caproate | 899.04 ± 15.19 a | 844.70 ± 28.60 b |
| [V7] Methyl (E)-hex-3-enoate | 4.68 ± 0.25 a | 1.64 ± 0.21 b |
| [V8] Ethyl caproate | 829.13 ± 25.84 a | 549.96 ± 34.92 b |
| [V9] Ethyl 3-hexenoate | 26.20 ± 1.64 a | 13.67 ± 0.97 b |
| [V10] Methyl 3-(methylthio)propionate | 345.28 ± 46.18 a | 337.19 ± 19.66 a |
| [V11] tert-Butyl propionate | 1.45 ± 0.22 a | 1.61 ± 0.10 a |
| [V12] Methyl 2-methylacetoacetate | 9.39 ± 0.19 a | 5.75 ± 0.53 b |
| [V13] Ethyl 3-methylthiopropionate | 224.28 ± 13.10 a | 193.64 ± 13.16 b |
| [V14] 4-Octenoic acid methyl ester | 33.00 ± 5.47 a | 29.96 ± 7.09 a |
| [V15] Methyl caprylate | 80.18 ± 6.39 a | 52.37 ± 4.94 b |
| [V16] Ethyl (Z)-oct-4-enoate | 20.29 ± 2.19 a | 14.83 ± 3.02 b |
| [V17] Ethyl caprylate | 129.25 ± 7.42 a | 63.75 ± 3.77 b |
| [V18] Methyl 3-acetoxyhexanoate | 11.18 ± 3.87 a | 6.88 ± 0.94 a |
| [V19] Methyl (Z)-4-decenoate | 4.55 ± 0.83 a | 5.38 ± 0.29 a |
| [V20] 1-Octen-3-yl-acetate | 0 | 6.73 ± 0.04 |
| [V21] Decanoic acid, ethyl ester | 20.50 ± 3.22 | 0 |
| [V22] Heptanoic acid, ethyl ester | 0 | 7.93 ± 1.32 |
| Terpenoids | ||
| [V23] beta-(Z)-Ocimene | 5.76 ± 0.25 a | 5.79 ± 0.49 a |
| [V24] (E)-3,7-dimethylocta-1,3,6-triene | 39.97 ± 3.43 a | 34.88 ± 2.77 a |
| [V25] Caryophyllene | 9.18 ± 0.92 a | 8.64 ± 0.54 a |
| [V26] Guaiene | 3.09 ± 0.31 b | 5.04 ± 0.25 a |
| [V27] Copaen | 105.62 ± 20.09 a | 108.93 ± 9.06 a |
| [V28] beta-ylangene | 11.26 ± 0.72 a | 11.33 ± 0.54 a |
| [V29] Germacrene D | 2.50 ± 0.36 a | 2.79 ± 0.058 a |
| [V30] (+)-Sativene | 13.26 ± 0.63 a | 12.92 ± 0.65 a |
| [V31] (−)-Clovene | 5.43 ± 0.32 a | 5.34 ± 0.19 a |
| [V32] Valencene | 24.58 ± 1.07 a | 25.52 ± 1.21 a |
| [V33] β-Selinene | 4.71 ± 0.54 a | 4.08 ± 0.28 a |
| [V34] α-Muurolene | 34.21 ± 2.42 b | 42.83 ± 2.37 a |
| [V35] delta-Cadinene | 10.23 ± 1.25 b | 14.15 ± 0.76 a |
| [V36] Guaiazulene | 1.32 ± 0.26 a | 1.78 ± 0.17 a |
| [V37] (−)-Aristolene | 82.61 ± 5.59 a | 83.59 ± 5.04 a |
| Ketones | ||
| [V38] 2-Heptanone | 0 | 13.72 ± 2.96 |
| [V39] 2-Nonanone | 0 | 102.50 ± 19.67 |
| [V40] 2-Undecanone | 0 | 24.67 ± 7.97 |
| [V41] 2,5-Dimethyl-4-methoxy-3(2H)-furanone | 20.20 ± 2.03 a | 12.21 ± 1.47 b |
| Acid | ||
| [V42] Hexanoic acid | 1.11 ± 0.12 b | 2.87 ± 0.25 a |
| Aldehyde | ||
| [V43] 2-Furaldehyde | 16.40 ± 1.09 a | 10.27 ± 0.42 b |
| Alcohols | ||
| [V44] trans-Geraniol | 10.32 ± 0.25 a | 7.33 ± 0.68 b |
| [V45] 1,6-Hexanediol | 1.61 ± 0.64 | 0 |
| [V46] 1,2,3-Butanetriol | 7.07 ± 2.27 b | 27.48 ± 4.10 a |
| Others | ||
| [V47] (3E,5E)-2,6-Dimethyl-1,3,5,7-octatetrene | 22.99 ± 1.54 a | 14.80 ± 0.40 b |
| [V48] (E,Z)-undeca-1,3,5-triene | 28.10 ± 3.32 b | 33.64 ± 0.26 a |
| [V49] 6-Butyl-1,4-cycloheptadiene | 26.64 ± 3.95 a | 22.86 ± 1.74 a |
| [V50] 3,7-Dimethyldecane | 10.21 ± 1.69 a | 3.25 ± 0.18 b |
| [V51] n-Butyl ether | 2.45 ± 0.12 a | 2.14 ± 0.16 b |
| [V52] 1,3-Di-tert-butylbenzene | 24.39 ± 1.63 a | 22.73 ± 0.66 a |
| [V53] 3,5-di-tert-butylphenol | 3.50 ± 1.27 a | 3.56 ± 0.40 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Yang, L.; Zhou, W.; Yuan, Y.; Liao, L.; Huang, X.; Zhang, C.; Gong, X.; Li, J. Effects of Lacticaseibacillus casei LK-1 Fermentation on Physicochemical Properties, Chemical Compositions, Antioxidant Activities and Volatile Profiles of Pineapple Juice. Foods 2025, 14, 3474. https://doi.org/10.3390/foods14203474
Peng S, Yang L, Zhou W, Yuan Y, Liao L, Huang X, Zhang C, Gong X, Li J. Effects of Lacticaseibacillus casei LK-1 Fermentation on Physicochemical Properties, Chemical Compositions, Antioxidant Activities and Volatile Profiles of Pineapple Juice. Foods. 2025; 14(20):3474. https://doi.org/10.3390/foods14203474
Chicago/Turabian StylePeng, Shaodan, Lian Yang, Wei Zhou, Yuan Yuan, Liangkun Liao, Xiaobing Huang, Chenghui Zhang, Xiao Gong, and Jihua Li. 2025. "Effects of Lacticaseibacillus casei LK-1 Fermentation on Physicochemical Properties, Chemical Compositions, Antioxidant Activities and Volatile Profiles of Pineapple Juice" Foods 14, no. 20: 3474. https://doi.org/10.3390/foods14203474
APA StylePeng, S., Yang, L., Zhou, W., Yuan, Y., Liao, L., Huang, X., Zhang, C., Gong, X., & Li, J. (2025). Effects of Lacticaseibacillus casei LK-1 Fermentation on Physicochemical Properties, Chemical Compositions, Antioxidant Activities and Volatile Profiles of Pineapple Juice. Foods, 14(20), 3474. https://doi.org/10.3390/foods14203474







