The Effect of Lipids on the Structure and Function of Egg Proteins in Response to Pasteurization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lipids and Proteins Solution
2.3. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis
2.4. Circular Dichroism Spectrum
2.5. Ultraviolet Spectrum
2.6. Fluorescence Spectrum
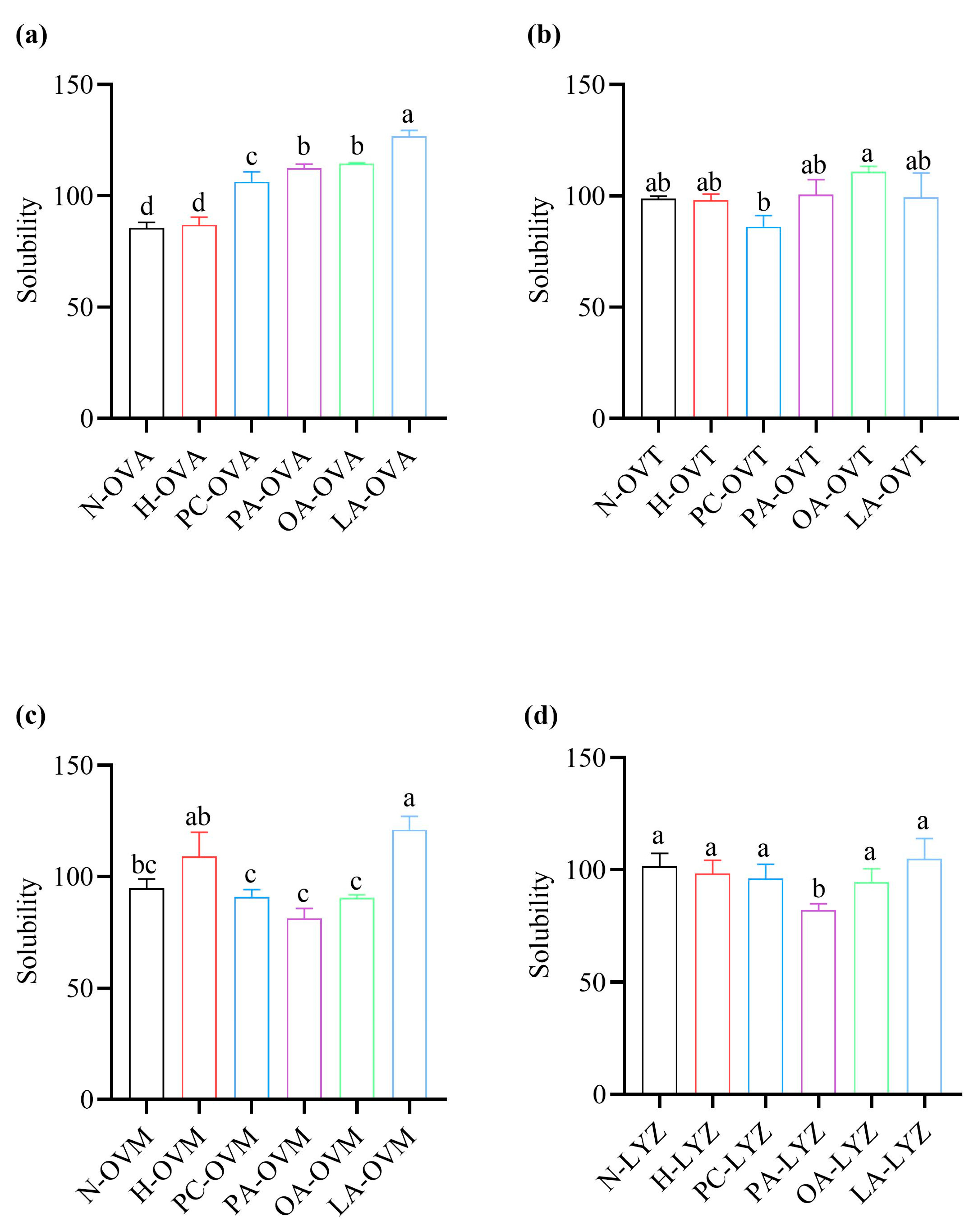
2.7. Solubility
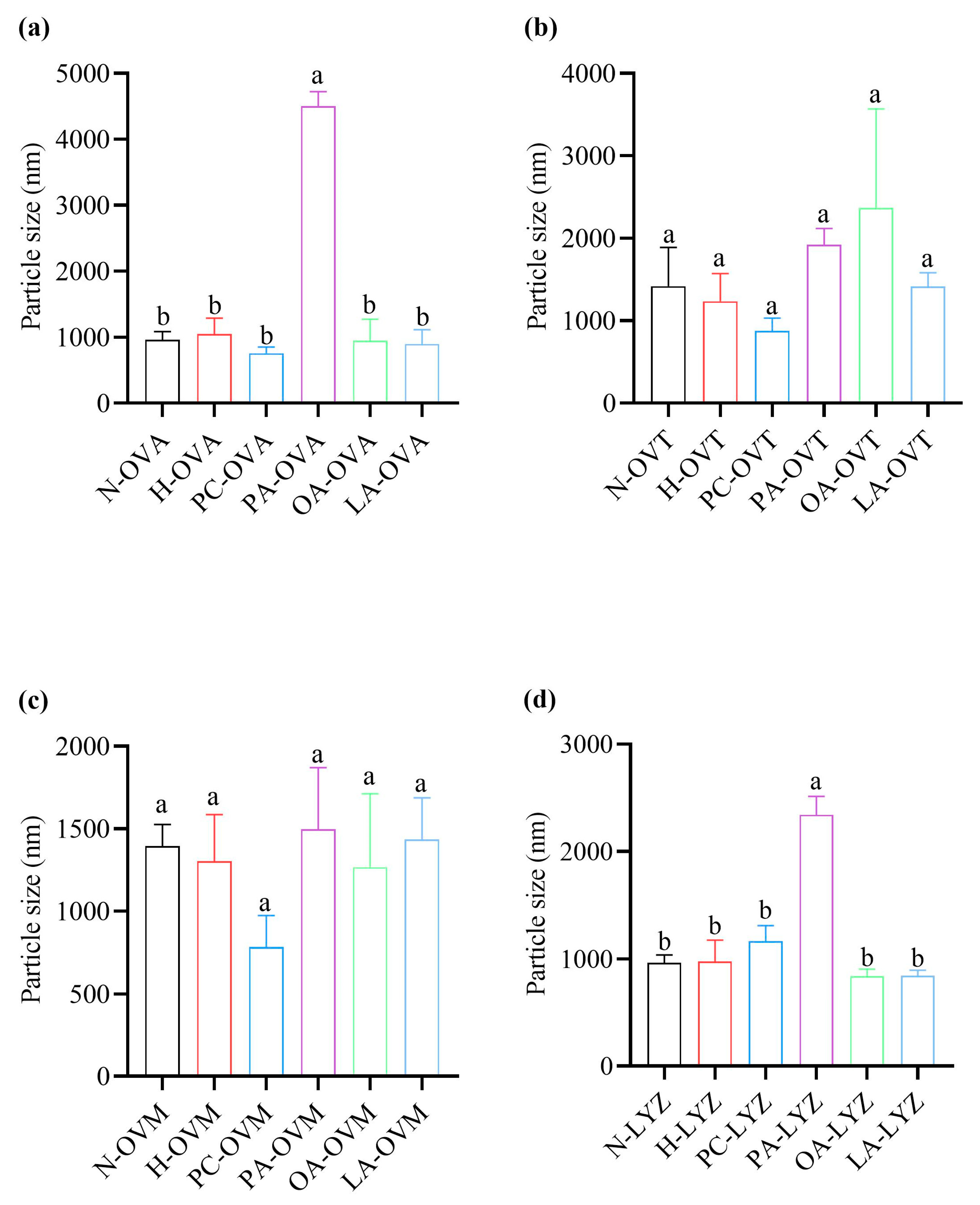
2.8. Particle Size Distribution
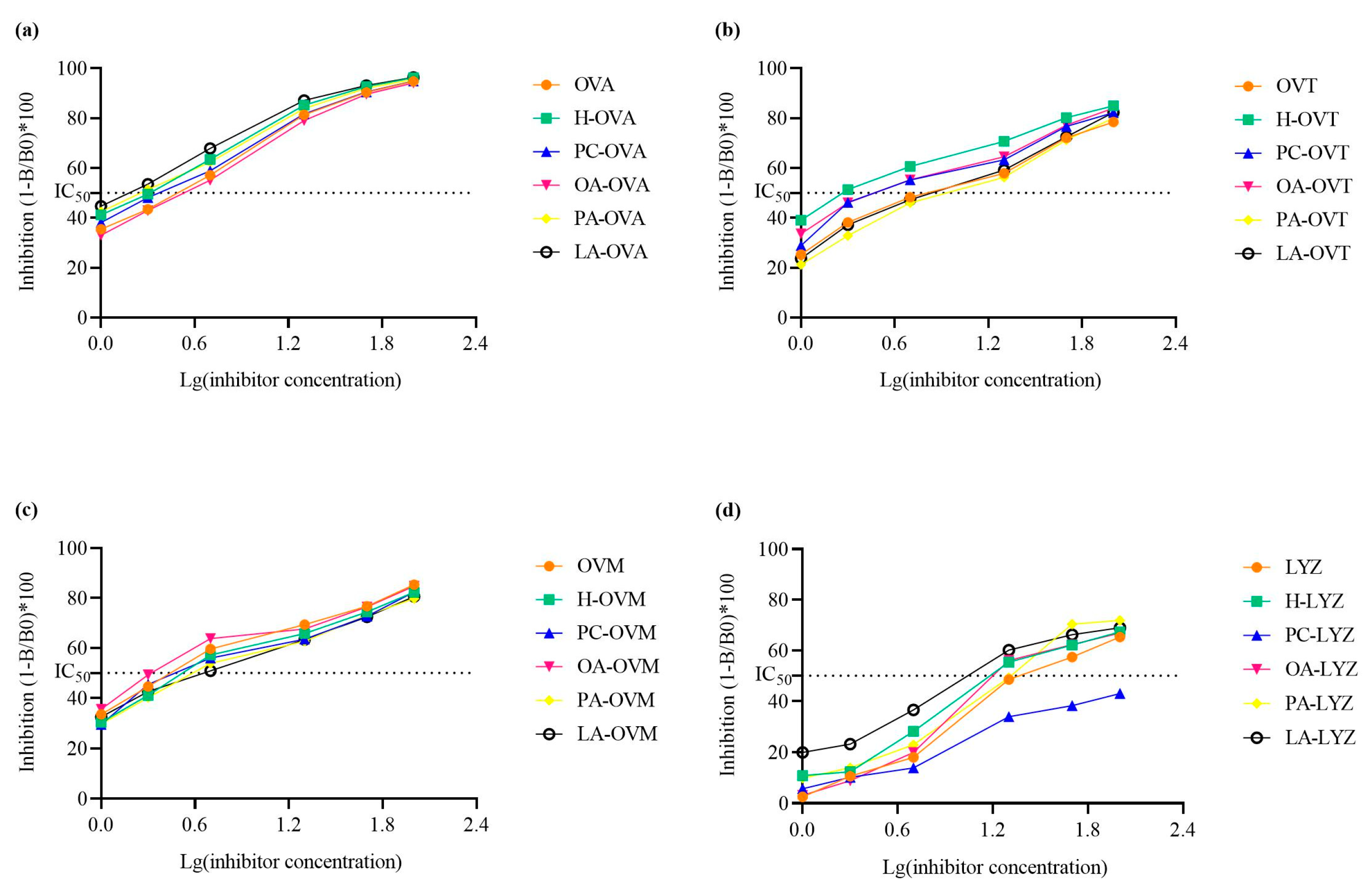
2.9. Immunoglobulin G Binding Capacity Assessment
2.10. Statistical Analysis
3. Results
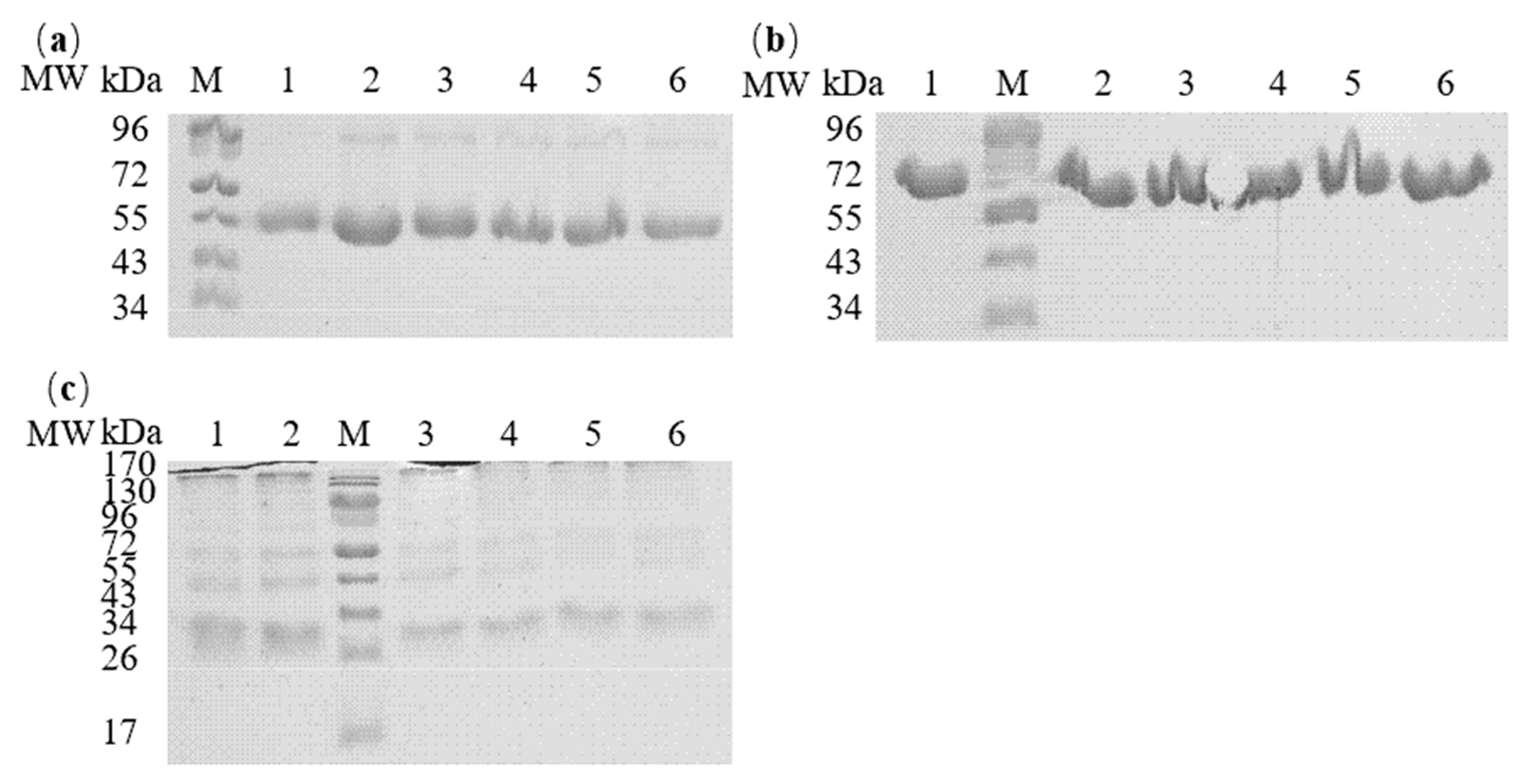
3.1. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis Analysis of the Egg Proteins
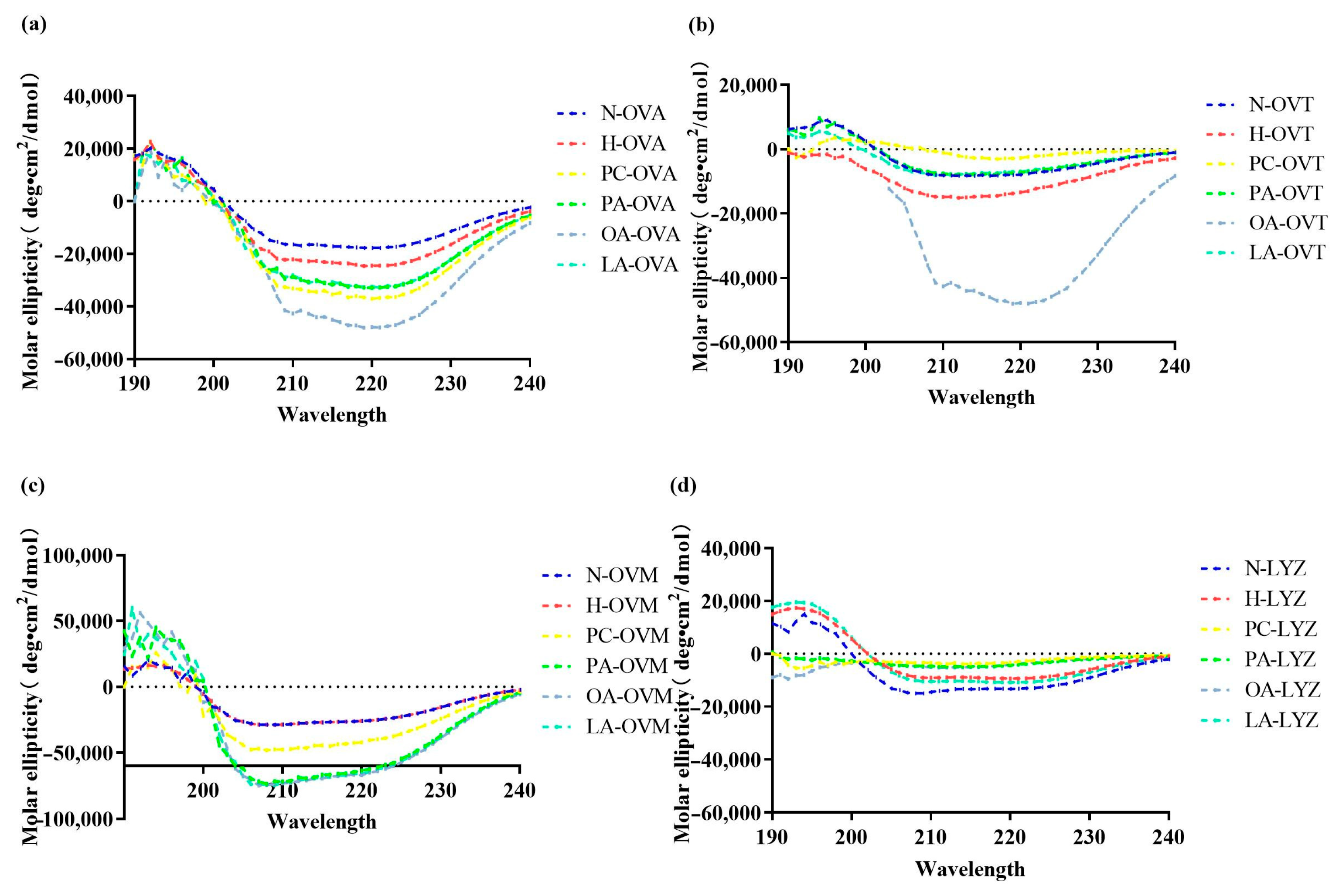
3.2. Secondary Structure Analysis of the Egg Proteins
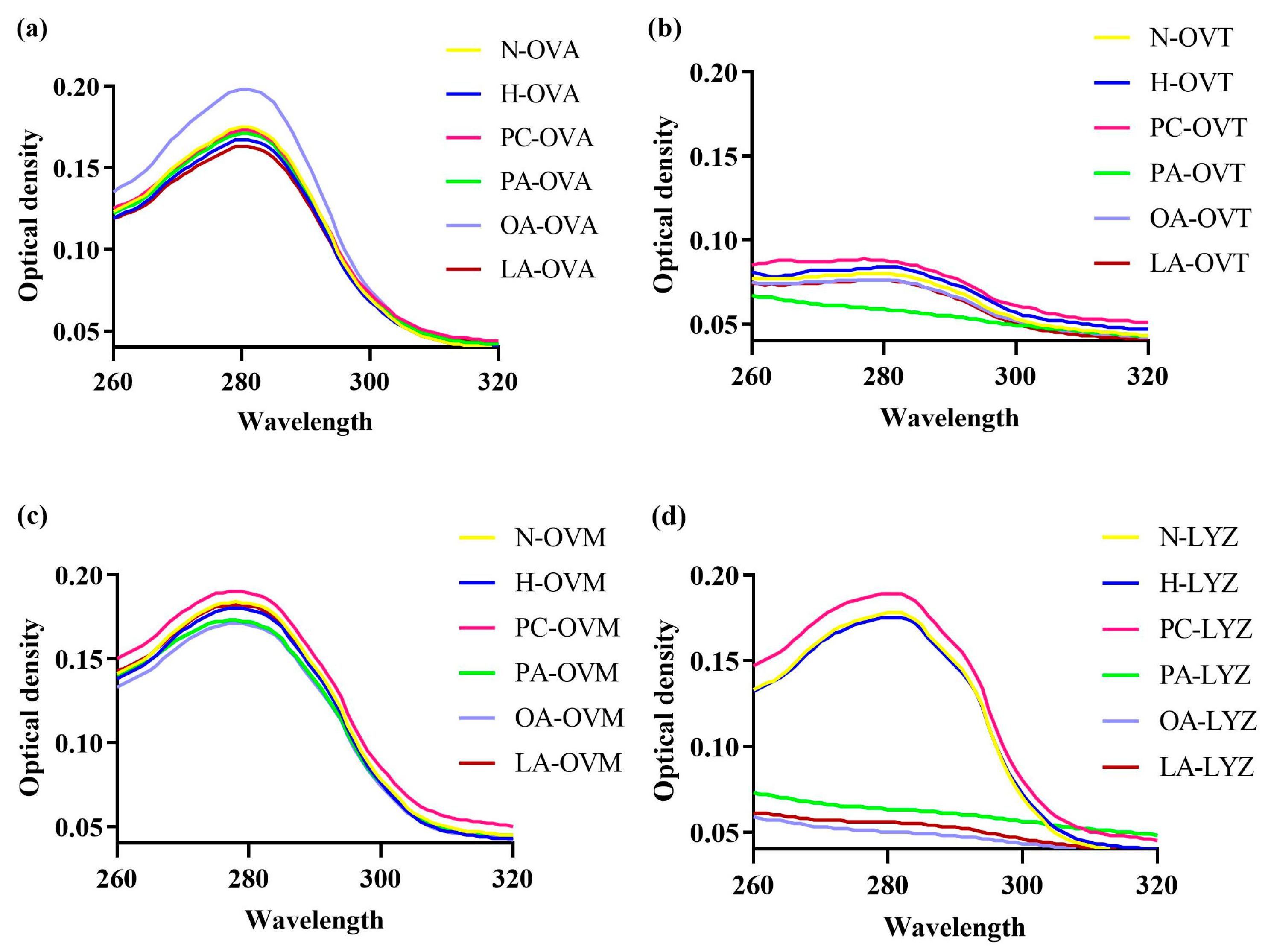
3.3. Impact on Protein Tertiary Structure
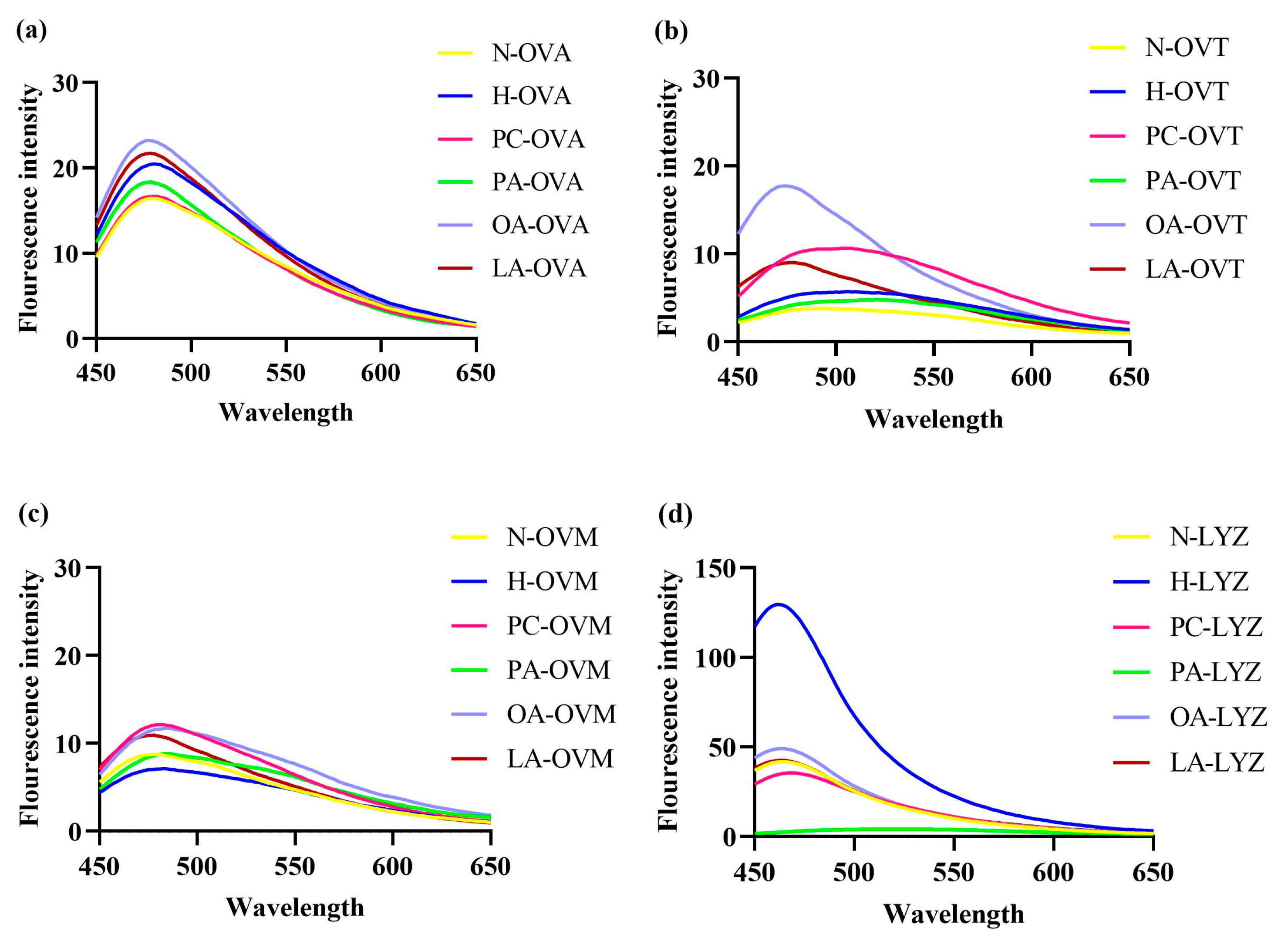
3.4. Alterations in Surface Hydrophobicity
3.5. Differential Effects on Protein Solubility
3.6. Impact on Particle Size Distribution
3.7. Modulation of Protein Antigenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kudre, T.G.; Kumar, B.S.; Kanwate, B.W.; Sakhare, P.Z. Comparative Study on Physicochemical and Functional Properties of Egg Powders from Japanese Quail and White Leghorn Chicken. Int. J. Food Prop. 2018, 21, 956–971. [Google Scholar] [CrossRef]
- Ginka, A.A.; Vasko, T.G.; Zhana, Y.P.; Veselina, N.B.; Ivelina, N.B.; Dimo, S.P.; Petar, B.P. Comparative Analysis of Nutrient Content and Energy of Eggs from Different Chicken Genotypes. J. Sci. Food Agric. 2019, 99, 5890–5898. [Google Scholar] [CrossRef]
- Jun, S.; Yaoyao, M.; Hui, J.; Obadi, M.; Bin, X. Effects of Single- and Dual-Frequency Ultrasound on the Functionality of Egg White Protein. J. Food Eng. 2020, 277, 109902–109909. [Google Scholar] [CrossRef]
- Shinn, S.E.; Proctor, A.; Gilley, A.D.; Cho, S.; Martin, E.; Anthony, N.B. Effect of Feeding CLA on Plasma and Granules Fatty Acid Composition of Eggs and Prepared Mayonnaise Quality. Food Chem. 2016, 197, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Stnciuc, N.; Banu, I.; Turturic, M.; Aprodu, I. ph and Heat Induced Structural Changes of Chicken Ovalbumin in Relation with Antigenic Properties. Int. J. Biol. Macromol. 2016, 93, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhou, Z. Ellagic Acid Can Act as a Chaperone and Suppress the Heat-Induced Amyloid-Like Aggregation of Ovalbumin. Food Hydrocoll. 2020, 100, 105408–105417. [Google Scholar] [CrossRef]
- Iwashita, K.; Handa, A.; Shiraki, K. Co-Aggregation of Ovotransferrin and Lysozyme. Food Hydrocoll. 2019, 89, 416–424. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, W.; Wu, Y.; Chen, H.; Tong, P.; Gao, J. Pasteurization Induced Protein Interaction Decreased the Potential Allergenicity of Ovalbumin and Ovomucoid in Egg White. J. Sci. Food Agric. 2022, 102, 6835–6847. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, Y.; Wu, Y.; Guo, L.; Ma, Q.; Qiu, Z.; Zhang, B. Novel Lysozyme–Mannooligosaccharide Conjugate with Improved Antimicrobial Activity: Preparation and Characterization. J. Food Meas. Charact. 2020, 14, 2529–2537. [Google Scholar] [CrossRef]
- Sang, S.; Chen, Y.; Zhu, X.; Narsimhan, G.; Hu, Q.; Jin, Z.; Xu, X. Effect of Egg Yolk Lipids on Structure and Properties of Wheat Starch in Steamed Bread. J. Cereal Sci. 2019, 86, 77–85. [Google Scholar] [CrossRef]
- Salminen, H.; Bischoff, S.; Weiss, J. Formation and Stability of Emulsions Stabilized by Quillaja Saponin–Egg Lecithin Mixtures. J. Food Sci. 2020, 85, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Moreno, L.; Shamir, R. Palmitic Acid and Health: Introduction. Crit. Rev. Food Sci. Nutr. 2015, 56, 1941–1942. [Google Scholar] [CrossRef]
- Mishra, S.; Chattopadhyay, A.; Naaz, S.; Ghosh, A.K.; Das, A.R.; Bandyopadhyay, D. Oleic Acid Ameliorates Adrenaline Induced Dysfunction of Rat Heart Mitochondria by Binding with Adrenaline: An Isothermal Titration Calorimetry Study. Life Sci. 2019, 218, 96–111. [Google Scholar] [CrossRef]
- Kurniawan, J.; Suga, K.; Kuhl, T.L. Interaction Forces and Membrane Charge Tunability: Oleic Acid Containing Membranes in Different ph Conditions. BBA Biomembr. 2017, 1859, 211–217. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef]
- Uysal, R.S.; Boyaci, I.H.; Sumnu, G. Determination of Pasteurization Treatment of Liquid Whole Egg by Measuring Physical and Rheological Properties of Cake Cream. J. Food Process Eng. 2019, 42, 13167–13174. [Google Scholar] [CrossRef]
- Pei, J.; Pei, S.; Wang, W.; Li, S.; Youravong, W.; Li, Z. Athermal forward Osmosis Process for the Concentration of Liquid Egg White: Process Performance and Improved Physicochemical Property of Protein. Food Chem. 2020, 312, 126032–126040. [Google Scholar] [CrossRef]
- Kang, I.-B.; Kim, D.-H.; Jeong, D.; Park, J.-H.; Lim, H.-W.; Seo, K.-H. Heat Resistance of Salmonella Enteritidis in Four Different Liquid Egg Products and the Performance and Equivalent Conditions of Ministry of Food and Drug Safety of South Korea and US Department of Agriculture Protocols. Food Control 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, Y.; Zhang, X.; Mourad, F.K.; Jin, H.; Shu, D.; Wang, Z.; Cai, Z. Effect of Egg Yolk Lipoproteins on Foaming Properties and Interfacial Behavior of Egg White: Insights into Implications for Aerated Food Production. Colloid. Surf. A 2023, 679, 132558–132568. [Google Scholar] [CrossRef]
- Cong, J.; Cui, J.; Zhang, H.; Dzah, C.S.; He, Y.; Duan, Y. Binding Affinity, Antioxidative Capacity and in Vitro Digestion of Complexes of Grape Seed Procyanidins and Pork, Chicken and Fish Protein. Food Res. Int. 2020, 136, 109530–109539. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Dopierała, K.; Prochaska, K. Lipid-Protein Interactions in Langmuir Monolayers under Dynamically Varied Conditions. J. Phys. Chem. B 2019, 124, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Zhang, M.; Tian, M.; Jiang, L.; Guo, H.Y.; Ren, F.Z. Bovine Lactoferrin Binds Oleic Acid to Form an Anti-Tumor Complex Similar to HAMLET. BBA Biomembr. 2014, 1841, 535–543. [Google Scholar] [CrossRef]
- Rizzuti, B.; Bartucci, R.; Guzzi, R. Effects of Polar Head Nature and Tail Length of Single-Chain Lipids on the Conformational Stability of Β-Lactoglobulin. J. Phys. Chem. B 2020, 124, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zheng, Y.; Wu, Y.; Chen, H.; Tong, P.; Gao, J. Double Enzyme Hydrolysis for Producing Antioxidant Peptide from Egg White: Optimization, Evaluation, and Potential Allergenicity. J. Food Biochem. 2019, 44, e13113–e13124. [Google Scholar] [CrossRef]
- Brunelle, J.L.; Green, R. One-Dimensional SDS-Polyacrylamide Gel Electrophoresis (1D SDS-PAGE). Methods Enzymol. 2014, 541, 151–159. [Google Scholar] [CrossRef]
- Colón, W. Analysis of Protein Structure by Solution Optical Spectroscopy. Methods Enzymol. 1999, 309, 605–632. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-pasdar, N.; Li-chan, E.C.Y. Comparison of Protein Surface Hydrophobicity Measured at Various ph Values Using Three Different Fluorescent Probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef]
- Qi, X.; Xu, D.; Zhu, J.; Wang, S.; Peng, J.; Gao, W.; Cao, Y. Interaction of Ovalbumin with Lutein Dipalmitate and Their Effects on the Color Stability of Marigold Lutein Esters Extracts. Food Chem. 2022, 372, 131211–131219. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Ye, Z.T.; Cai, X.P.; Wang, L.X.; Cao, Z. Biophysical Study on the Interaction of Ceftriaxone Sodium with Bovine Serum Albumin Using Spectroscopic Methods. J. Biochem. Mol. Toxicol. 2012, 26, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Li, Y.; Dong, L.; Liu, Y.; Liu, L.; Farag, M.A.; Liu, L. Interaction and Binding Mechanism of Ovalbumin with Cereal Phenolic Acids: Improved Structure, Antioxidant Activity, Emulsifying and Digestion Properties for Potential Targeted Delivery Systems. Food Res. Int. 2024, 175, 113726–113741. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Z.; Xue, C. The Past and Future of Ovotransferrin: Physicochemical Properties, Assembly and Applications. Trends Food Sci. Technol. 2021, 116, 47–62. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, X.; Ma, B.; Fu, X.; Ren, H.; Ma, M. Ultrasonic Pretreatment Enhanced the Glycation of Ovotransferrin and Improved Its Antibacterial Activity. Food Chem. 2021, 346, 128905–128911. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Ji, S.; Xia, M.; Fu, X.; Huang, X. Mechanistic Studies of Polyphenols Reducing the Trypsin Inhibitory Activity of Ovomucoid: Structure, Conformation, and Interactions. Food Chem. 2023, 408, 135063–135070. [Google Scholar] [CrossRef]
- Chaturvedi, S.K.; Alam, P.; Khan, J.M.; Siddiqui, M.K.; Kalaiarasan, P.; Subbarao, N.; Ahmad, Z.; Khan, R.H. Biophysical Insight into the Anti-Amyloidogenic Behavior of Taurine. Int. J. Biol. Macromol. 2015, 80, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.-S.; Park, K.-M.; Yu, H.; Park, J.-Y.; Chang, P.-S. Effect of Intense Pulsed Light on the Deactivation of Lipase: Enzyme-Deactivation Kinetics and Tertiary Structural Changes by Fragmentation. Enzym. Microb. Technol. 2019, 124, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, L.; Feng, S.; Liu, F.; Luo, Y. Mechanistic Study on the Nanocomplexation between Curcumin and Protein Hydrolysates from Great Northern Bean (Phaseolus vulgaris L.) for Delivery Applications in Functional Foods. LWT-Food Sci. Technol. 2021, 139, 110572–110581. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; Xu, Z.; Zhao, L.; Wang, Y.; Liao, X. Understanding the ph-Dependent Interaction of Anthocyanin with Two Food-Derived Transferrins. Food Chem. 2023, 410, 135473–135782. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Al-lohedan, H.A.; Bhati, R.; Muthukumaran, J. Interaction of the Lysozyme with Anticoagulant Drug Warfarin: Spectroscopic and Computational Analyses. Heliyon 2024, 10, e30818–e30833. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Modification of Ovotransferrin by Maillard Reaction: Consequences for Structure, Fibrillation and Emulsifying Property of Fibrils. Food Hydrocoll. 2019, 97, 105186–105196. [Google Scholar] [CrossRef]
- Pan, X.; Fang, Y.; Wang, L.; Shi, Y.; Xie, M.; Xia, J.; Pei, F.; Li, P.; Xiong, W.; Shen, X.; et al. Covalent Interaction between Rice Protein Hydrolysates and Chlorogenic Acid: Improving the Stability of Oil-In-Water Emulsions. J. Agric. Food Chem. 2019, 67, 4023–4030. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and Conformational Changes to Soy Proteins Accompanying Anthocyanins: Focus on Covalent and Non-Covalent Interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Tu, Z.C.; Wang, H.; Zhang, L.; Xu, S.S.; Niu, C.D.; Yao, H.L.; Kaltashov, I.A. Mechanism of Reduction in IgG and IgE Binding of β-Lactoglobulin Induced by Ultrasound Pretreatment Combined with Dry-State Glycation: A Study Using Conventional Spectrometry and High Resolution Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 8018–8027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Tu, Z.-C.; Wang, H.; Hu, Y.-M.; Du, P.-C.; Yang, Y.-P. Mechanism of the Effect of 2, 2′-Azobis (2-Amidinopropane) Dihydrochloride Simulated Lipid Oxidation on the IgG/IgE Binding Ability of Ovalbumin. Food Chem. 2020, 327, 127037–127045. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Gao, J.; Chen, H.; Li, X.; Zhang, Y.; Jian, S.; Wichers, H.; Wu, Z.; Yang, A.; Liu, F. Effect of Heat Treatment on the Potential Allergenicity and Conformational Structure of Egg Allergen Ovotransferrin. Food Chem. 2012, 131, 603–610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Shi, Q.; Wang, Z.; Chen, X.; Min, F.; Meng, X.; Tong, P.; Wu, Y.; Chen, H. The Effect of Lipids on the Structure and Function of Egg Proteins in Response to Pasteurization. Foods 2025, 14, 219. https://doi.org/10.3390/foods14020219
Yang H, Shi Q, Wang Z, Chen X, Min F, Meng X, Tong P, Wu Y, Chen H. The Effect of Lipids on the Structure and Function of Egg Proteins in Response to Pasteurization. Foods. 2025; 14(2):219. https://doi.org/10.3390/foods14020219
Chicago/Turabian StyleYang, Hao, Qiang Shi, Zhongliang Wang, Xiao Chen, Fangfang Min, Xuanyi Meng, Ping Tong, Yong Wu, and Hongbing Chen. 2025. "The Effect of Lipids on the Structure and Function of Egg Proteins in Response to Pasteurization" Foods 14, no. 2: 219. https://doi.org/10.3390/foods14020219
APA StyleYang, H., Shi, Q., Wang, Z., Chen, X., Min, F., Meng, X., Tong, P., Wu, Y., & Chen, H. (2025). The Effect of Lipids on the Structure and Function of Egg Proteins in Response to Pasteurization. Foods, 14(2), 219. https://doi.org/10.3390/foods14020219




