Valorization of Dairy By-Products, Sweet Whey, and Acid Whey, in the Production of Fermented Black Carrot Juice: A Comparative Study of the Phytochemical, Physicochemical, Microbiological, and Sensorial Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fermentation Method
2.3. Determination of Titratable Acidity, pH, Turbidity, and Color Parameters
2.4. Determination of Total Monomeric Anthocyanin Content
2.5. Determination of Individual Phenolic Compounds
2.6. Determination of Organic Acids and Ethanol Content
2.7. Determination of Lactic Acid Bacteria (LAB) and Yeast Counts
2.8. Sensorial Evaluation
2.9. Statistical Analysis
3. Results
3.1. Physicochemical and Phytochemical Properties of Samples
3.2. Microbiological Properties of Fermented Black Carrot Juice Samples
3.3. Sensorial Properties of Fermented Black Carrot Juice Samples
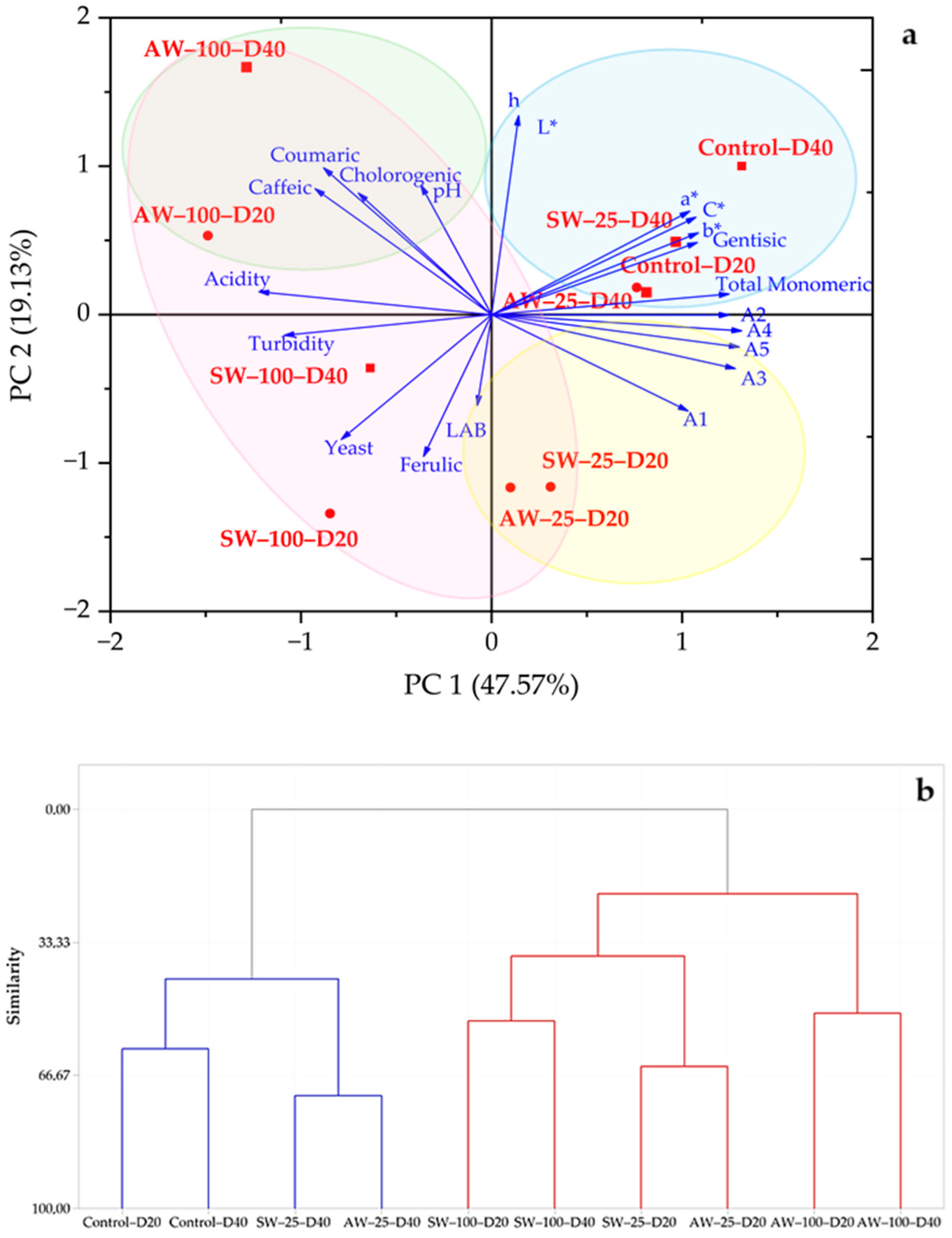
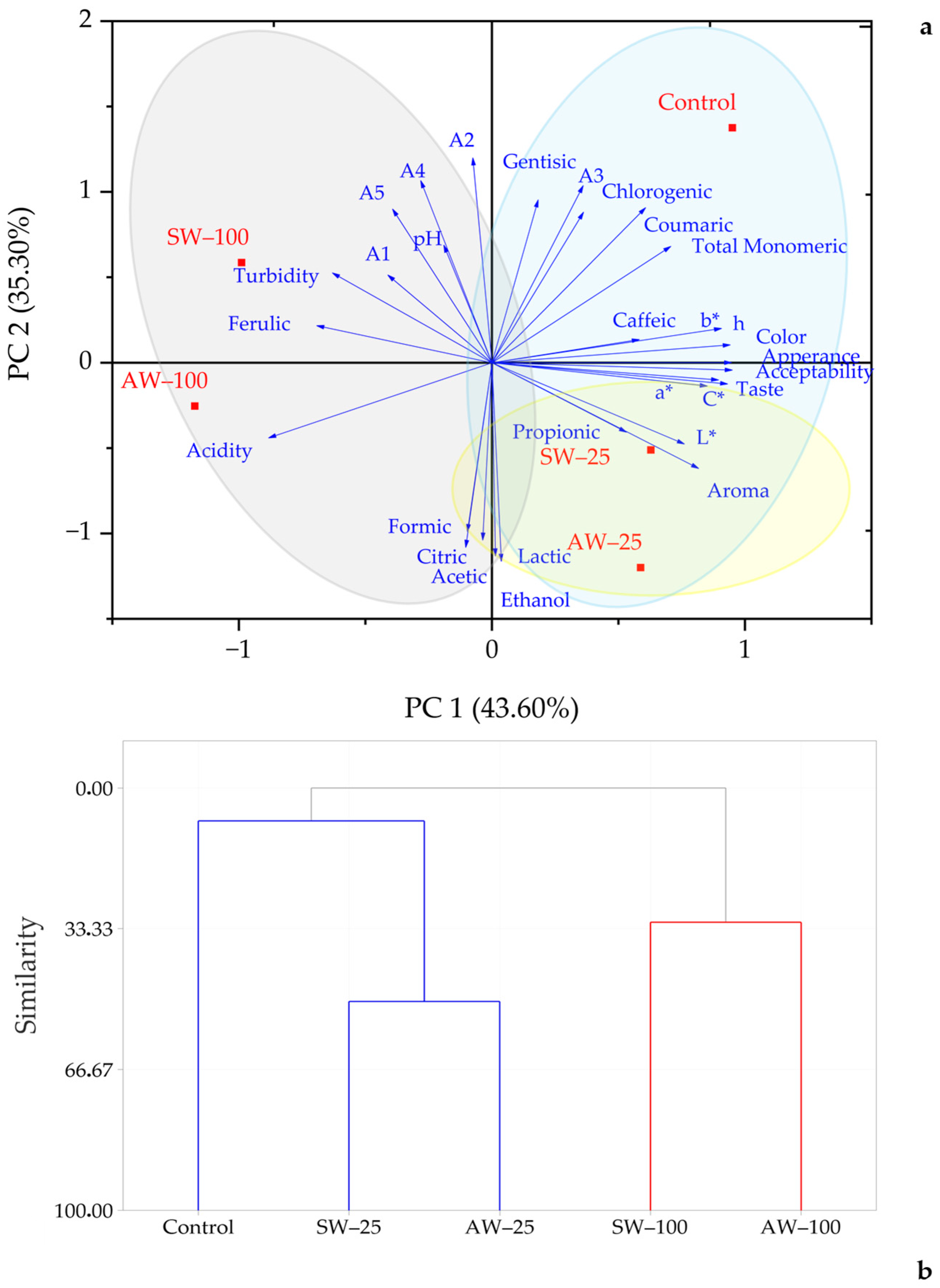
3.4. Principle Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ketterings, Q.; Czymmek, K.; Gami, S.; Godwin, G.; Ganoe, K. Guidelines for Land Application of Acid Whey. Available online: http://nmsp.cals.cornell.edu/publications/files/AcidWheyGuidelines2017.pdf (accessed on 23 March 2023).
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent Advances in Whey Processing and Valorisation: Technological and Environmental Perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 23 October 2024).
- Tsakali, E.; Petrotos, K.; D’Allessandro, A.; Goulas, P. A Review on Whey Composition and the Methods Used for its Utilization for Food and Pharmaceutical Products. In Proceedings of the 6th International Conference on Simulation and Modeling in the Food and Bio-Industry, Bragança, Portugal, 24–26 June 2010; pp. 195–201. [Google Scholar]
- Khurana, S.; Kankarwal, P.; Saini, J.; Panghal, P.; Panghal, A.; Chhikara, N. Potential of Whey for Production of Value-Added Products Using Microbial Fermentations. In Whey Valorization: Innovations, Technological Advancements and Sustainable Exploitation; Poonia, A., Petkoska, A.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 115–137. [Google Scholar]
- Ramos, O.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible Films and Coatings from Whey Proteins: A Review on Formulation, and on Mechanical and Bioactive Properties. Crit. Rev. Food Sci. Nutr. 2012, 52, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Roy, S.; Malik, B. Valorisation of Whey for Development of Different Types of Food Products Including Fermented Beverages. In Whey Valorization: Innovations, Technological Advancements and Sustainable Exploitation; Springer: Berlin/Heidelberg, Germany, 2023; pp. 211–238. [Google Scholar]
- Suomalainen, T.; Lagström, H.; Mättö, J.; Saarela, M.; Arvilommi, H.; Laitinen, I.; Ouwehand, A.C.; Salminen, S. Influence of Whey-Based Fruit Juice Containing Lactobacillus rhamnosus on Intestinal Well-Being and Humoral Immune Response in Healthy Adults. LWT-Food Sci. Technol. 2006, 39, 788–795. [Google Scholar] [CrossRef]
- Gallardo-Escamilla, F.; Kelly, A.; Delahunty, C. Mouthfeel and Flavour of Fermented Whey With Added Hydrocolloids. Int. Dairy J. 2007, 17, 308–315. [Google Scholar] [CrossRef]
- AbdulAlim, T.S.; Zayan, A.F.; Campelo, P.H.; Bakry, A.M. Development of New Functional Fermented Product: Mulberry-Whey Beverage. J. Nutr. Food Technol. 2018, 1, 64–69. [Google Scholar] [CrossRef]
- Erten, H.; Tanguler, H.; Canbaş, A. A traditional Turkish Lactic Acid Fermented Beverage: Shalgam (Salgam). Food Rev. Int. 2008, 24, 352–359. [Google Scholar] [CrossRef]
- Akbulut, M.; Çoklar, H.; Bulut, A.N.; Hosseini, S.R. Evaluation of black grape pomace, a fruit juice by-product, in shalgam juice production: Effect on phenolic compounds, anthocyanins, resveratrol, tannin, and in vitro antioxidant activity. Food Sci. Nutr. 2024, 12, 4372–4384. [Google Scholar] [CrossRef]
- Nie, Q.; Feng, L.; Hu, J.; Wang, S.; Chen, H.; Huang, X.; Nie, S.; Xiong, T.; Xie, M. Effect of Fermentation and Sterilization on Anthocyanins in Blueberry. J. Sci. Food Agric. 2017, 97, 1459–1466. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhu, Y.; Ren, M.; Tian, C. Influence of Acetaldehyde Induction on Monomeric and Polymeric Polyphenols in Wine Using the Polyphenol/Protein-Binding Model. S. Afr. J. Enol. Vitic. 2018, 39, 149–156. [Google Scholar] [CrossRef]
- Fulcrand, H.; Atanasova, V.; Salas, E.; Cheynier, V. The fate of Anthocyanins in Wine: Are There Determining Factors? In Red Wine Color; Waterhouse, A.L., Kennedy, J.A., Eds.; ACS Publications: Washington, DC, USA, 2004. [Google Scholar]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of Polyphenol-Protein Interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, S.; Li, Z.; Chou, S.; Shu, C.; Chen, Y.; Chen, W.; Yang, S.; Yang, Y.; Tian, J. An Updated Review on the Stability of Anthocyanins Regarding the Interaction with Food Proteins and Polysaccharides. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4378–4401. [Google Scholar] [CrossRef]
- Rocha, J.C.G.; Viana, K.W.C.; Mendonca, A.C.; Neves, N.A.; Carvalho, A.F.; Minim, V.P.R.; Barros, F.A.R.; Stringheta, P.C. Protein Beverages Containing Anthocyanins of Jabuticaba. Food Sci. Technol. Int. 2018, 39, 112–119. [Google Scholar] [CrossRef]
- Lugo-Zarate, L.; Delgado-Olivares, L.; Cruz-Cansino, N.d.S.; González-Olivares, L.G.; Castrejón-Jiménez, N.S.; Estrada-Luna, D.; Jiménez-Osorio, A.S. Blackberry Juice Fermented with Two Consortia of Lactic Acid Bacteria and Isolated Whey: Physicochemical and Antioxidant Properties during Storage. Int. J. Mol. Sci. 2024, 25, 8882. [Google Scholar] [CrossRef]
- Rejdlová, A.; Salek, R.N.; Míšková, Z.; Lorencová, E.; Kůrová, V.; Adámek, R.; Sumczynski, D. Physical Characterization of a Novel Carrot Juice Whey-Enriched Beverage Fermented with Milk or Water Kefir Starter Cultures. Foods 2023, 12, 3368. [Google Scholar] [CrossRef]
- Silva, V.L.; Silva, A.C.; Costa-Lima, B.R.; Carneiro, C.S.; Conte-Junior, C.A. Stability of Polyphenols from Blueberry (Vaccinium corymbosum L.) in Fermented Dairy Beverage. J. Food Process. Preserv. 2017, 41, e13305. [Google Scholar] [CrossRef]
- Alwohaibi, A.A.; Ali, A.A.; Sakr, S.S.; Mohamed Ahmed, I.A.; Alhomaid, R.M.; Alsaleem, K.A.; Aladhadh, M.; Barakat, H.; Hassan, M.F. Valorization of Different Dairy By-Products to Produce a Functional Dairy–Millet Beverage Fermented with Lactobacillus paracasei as an Adjunct Culture. Fermentation 2023, 9, 927. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, W.; Liu, X.; Shen, W.; Gu, R.; Tang, C. The Antioxidant Activity and Protection of Probiotic Bacteria in the in vitro Gastrointestinal Digestion of a Blueberry Juice and Whey Protein Fermentation System. Fermentation 2023, 9, 335. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Yu, Q.; Zhou, J.; Lu, M.; Gu, R.; Huang, Y. Structural and Compositional Changes of Whey Protein and Blueberry Juice Fermented Using Lactobacillus plantarum or Lactobacillus casei During Fermentation. RSC Adv. 2021, 11, 26291–26302. [Google Scholar]
- Rizzolo, A.; Cortellino, G. Ricotta Cheese Whey-Fruit-Based Beverages: Pasteurization Effects on Antioxidant Composition and Color. Beverages 2017, 3, 15. [Google Scholar] [CrossRef]
- Güven, N.; Yetim, H.; Cankurt, H. Physicochemical and Sensory Properties of Low Salt Turnip Juice Produced by Using Black Carrot Juice and Whey. Eur. J. Sci. Technol. 2019, 599–610. [Google Scholar] [CrossRef]
- Ulucan, E.; Çoklar, H.; Akbulut, M. Application of Ultrasound to Extend the Shelf Life of Shalgam Juice: Changes in Various Physicochemical, Nutritional, and Microbiological Properties. J. Food Process. Preserv. 2022, 46, e16501. [Google Scholar] [CrossRef]
- Akbulut, M.; Tekce, N.; Coklar, H. Evaluation of Grape Pomace in Production of Shalgam Juice: Effects on Some Physicochemical, Microbiological and Sensorial Properties. Braz. Arch. Biol. Technol. 2024, 67, e24230582. [Google Scholar] [CrossRef]
- Tangüler, H. The Effect of Using Different Size Purple Carrots and Lactobacillus Plantarum on the Properties of Fermented Shalgam (Şalgam). TURJAF 2021, 9, 1759–1766. [Google Scholar] [CrossRef]
- Agirman, B.; Settanni, L.; Erten, H. Effect of Different Mineral Salt Mixtures and Dough Extraction Procedure on the Physical, Chemical and Microbiological Composition of Şalgam: A black Carrot Fermented Beverage. Food Chem. 2021, 344, 128618. [Google Scholar] [CrossRef] [PubMed]
- Tanguler, H.; Erten, H. Chemical and Microbiological Characteristics of Shalgam (Şalgam): A Traditional Turkish Lactic Acid Fermented Beverage. J. Food Qual. 2012, 35, 298–306. [Google Scholar] [CrossRef]
- Kahve, H.I.; Akbulut, M.; Coklar, H. Identification and Technological Characterization of Endogenous Yeast Isolated from Fermented Black Carrot Juice, Shalgam. LWT-Food Sci. Technol. 2022, 154, 112823. [Google Scholar] [CrossRef]
- Ekinci, F.Y.; Baser, G.M.; Özcan, E.; Üstündağ, Ö.G.; Korachi, M.; Sofu, A.; Blumberg, J.B.; Chen, C.-Y.O. Characterization of Chemical, Biological, and Antiproliferative Properties of Fermented Black Carrot Juice, Shalgam. Eur. Food Res. Technol. 2016, 242, 1355–1368. [Google Scholar] [CrossRef]
- Mullin, W.; Emmons, D. Determination of Organic Acids and Sugars in Cheese, Milk and Whey by High Performance Liquid Chromatography. Food Res. Int. 1997, 30, 147–151. [Google Scholar] [CrossRef]
- Nursiwi, A.; Nurhartadi, E.; Utami, R.; Sari, A.; Laksono, P.; Aprilia, E. Characteristic of Fermented Whey Beverage with Addition of Tomato Juice (Lycopersicum esculentum). IOP Conf. Ser. Mater. Sci. Eng. 2017, 193, 012009. [Google Scholar] [CrossRef]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An Overview of Plant-Autochthonous Microorganisms and Fermented Vegetable Foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Mavropoulou, I.; Kosikowski, F. Free Amino Acids and Soluble Peptides of Whey Powders. J. Dairy Sci. 1973, 56, 1135–1138. [Google Scholar] [CrossRef]
- Ribeiro-Filho, N.; Ayed, C.; Akepach, P. The Buffering Capacity of Single Amino Acids in Brewing Wort. Int. J. Food Sci. Technol. 2023, 58, 1611–1620. [Google Scholar] [CrossRef]
- Karam, N.; Belarbi, A. Detection of Polygalacturonases and Pectin Esterases in Lactic Acid Bacteria. World J. Microbiol. Biotechnol. 1995, 11, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.; Goh, K.K.; Waterland, M.R.; Huffman, L.M.; Weeks, M.; Matia-Merino, L. Complexation of Anthocyanin-Bound Blackcurrant Pectin and Whey Protein: Effect of pH and Heat Treatment. Molecules 2022, 27, 4202. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Heras, J.M.; Callejo, M.J.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Yeast Influence on the Formation of Stable Pigments in Red Winemaking. Food Chem. 2016, 197, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J. Adsorption of Anthocyanins by Yeast Cell Walls During the Fermentation of Red Wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef]
- Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An overview. Beverages 2020, 6, 19. [Google Scholar] [CrossRef]
- Rodrigues, A.; Ricardo-da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of Commercial Mannoproteins on Wine Colour and Tannins Stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, J.; Zhang, L. Interaction of Preheated Whey Protein Isolate with Rose Anthocyanin Extracts in Beverage Model System: Influence on Color Stability, Astringency and Mechanism. Food Chem. 2023, 412, 135507. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of Whey Protein Isolate on the Stability and Antioxidant Capacity of Blueberry Anthocyanins: A Mechanistic and in vitro Simulation Study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on Protein–Phenolic Interactions and Associated Changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Alminderej, F.M.; Ammar, C.; El-Ghoul, Y. Functionalization, Characterization and Microbiological Performance of New Biocompatible Cellulosic Dressing Grafted Chitosan and Polysaccharide Extract. Cellulose 2021, 28, 9821–9835. [Google Scholar] [CrossRef]
- Durell, S.R.; Ben-Naim, A. Temperature Dependence of Hydrophobic and Hydrophilic Forces and Interactions. J. Phys. Chem. B. 2021, 125, 13137–13146. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, I.; Nie, R.; Ge, Z.; Li, K.; Li, C. Understanding the Shielding Effects of Whey Protein on Mulberry Anthocyanins: Insights from Multispectral and Molecular Modelling Investigations. Int. J. Biol. Macromol. 2018, 119, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Oliveira, G.; Lila, M.A. Protein-Binding Approaches for Improving Bioaccessibility and Bioavailability of Anthocyanins. Compr. Rev. Food Sci. Food Saf. 2023, 22, 333–354. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Krishna, T.C.; Chawla, P.; Dyduch-Siemińska, M.; Klepacka, J.; Kaushik, R. A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics. Int. J. Mol. Sci. 2021, 22, 13548. [Google Scholar] [CrossRef]
- Tazeddinova, D.; Toshev, A.D.; Abylgazinova, A.; Rahman, M.; Matin, M.; Bakri, M.B.; Ayan, O. A Review of Polyphenol and Whey Protein-Based Conjugates. BioResources 2022, 17, 6997. [Google Scholar] [CrossRef]
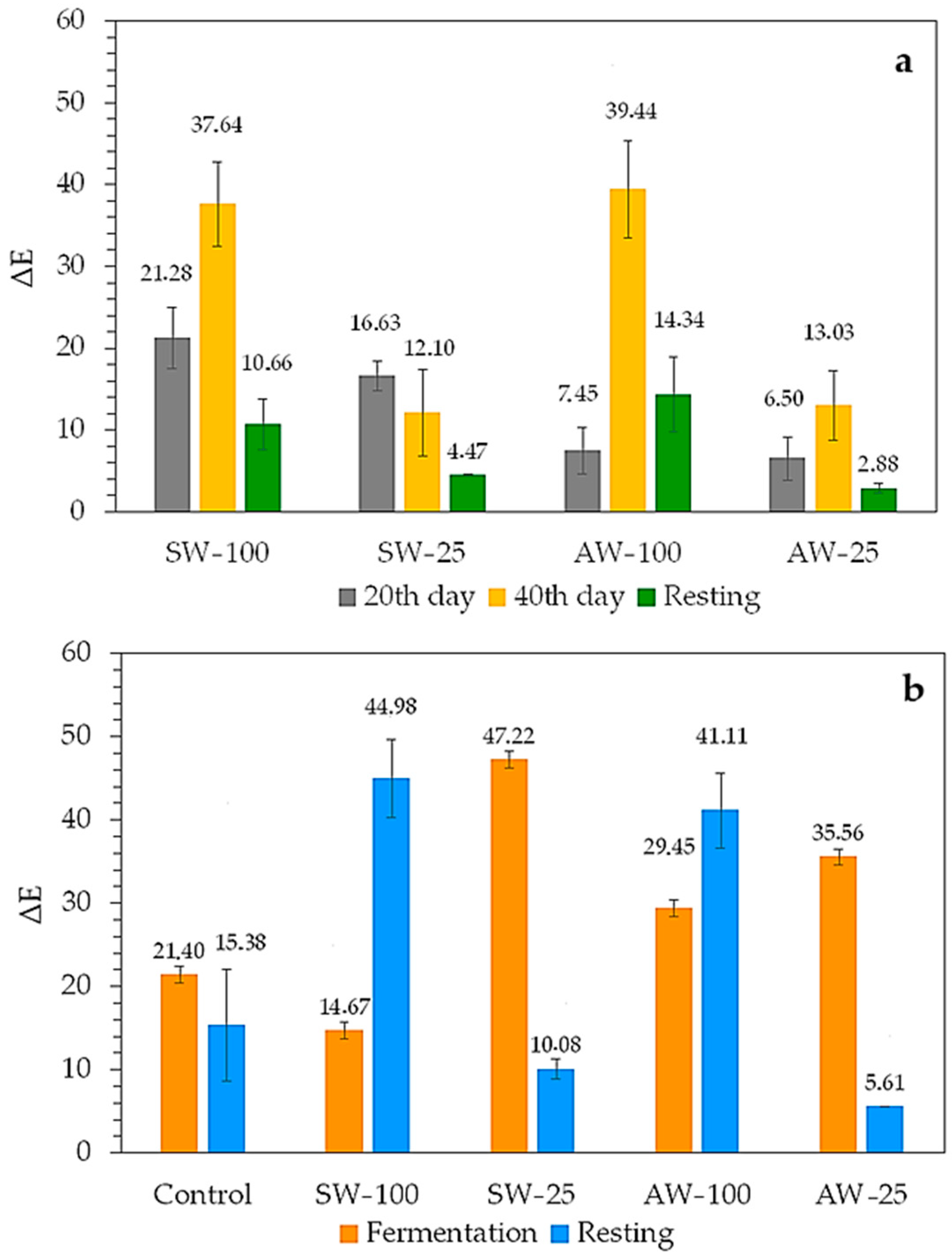

| Samples | FP (Day) | pH | Titratable Acidity (g LA/100 mL) | Turbidity (NTU) | TMA (mg CGE/L) |
|---|---|---|---|---|---|
| Control | 20 | 3.49 ± 0.04 | 0.349 ± 0.025 | 188.50 ± 6.50 d | 63.62 ± 2.50 |
| 40 | 3.46 ± 0.04 | 0.460 ± 0.042 | 120.50 ± 5.50 ef | 58.61 ± 0.67 | |
| SW-25 * | 20 | 3.12 ± 0.01 | 0.855 ± 0.015 | 285.00 ± 13.00 c | 38.16 ± 1.50 |
| 40 | 3.09 ± 0.02 | 0.889 ± 0.031 | 75.60 ± 4.40 fg | 38.28 ± 0.21 | |
| SW-100 | 20 | 3.29 ± 0.02 | 1.147 ± 0.073 | 294.00 ± 16.00 c | 11.86 ± 0.33 |
| 40 | 3.22 ± 0.03 | 1.215 ± 0.114 | 153.00 ± 4.00 de | 12.69 ± 0.25 | |
| AW-25 | 20 | 3.26 ± 0.03 | 0.801 ± 0.047 | 375.00 ± 14.00 b | 34.11 ± 0.21 |
| 40 | 3.22 ± 0.01 | 0.938 ± 0.052 | 50.40 ± 1.79 g | 35.49 ± 0.58 | |
| AW-100 | 20 | 3.47 ± 0.01 | 1.426 ± 0.088 | 513.00 ± 7.00 a | 12.15 ± 1.04 |
| 40 | 3.45 ± 0.04 | 1.404 ± 0.104 | 377.00 ± 12.00 b | 11.06 ± 0.71 |
| Samples | pH | Titratable Acidity (g LA/100 mL) | Turbidity (NTU) | TMA (mg CGE/L) |
|---|---|---|---|---|
| Control | 3.46 ± 0.03 a | 0.490 ± 0.020 c | 63.30 ± 11.03 | 65.13 ± 0.88 a |
| SW-25 * | 3.08 ± 0.06 c | 0.860 ± 0.040 b | 54.20 ± 3.50 | 59.91 ± 0.35 b |
| SW-100 | 3.24 ± 0.02 bc | 1.160 ± 0.010 a | 61.70 ± 8.50 | 52.46 ± 0.43 c |
| AW-25 | 3.22 ± 0.01 c | 0.880 ± 0.020 b | 54.05 ± 4.35 | 53.16 ± 0.85 c |
| AW-100 | 3.43 ± 0.03 ab | 1.370 ± 0.070 a | 71.60 ± 2.40 | 52.81 ± 0.54 c |
| Samples | FP (Day) | L* | a* | b* | C* | h |
|---|---|---|---|---|---|---|
| Control | 20 | 17.60 ± 1.17 de | 41.22 ± 0.86 b | 27.85 ± 1.45 b | 49.75 ± 1.52 b | 34.02 ± 0.83 abc |
| 40 | 29.46 ± 4.05 bc | 53.83 ± 5.59 a | 40.37 ± 4.78 a | 67.29 ± 7.34 a | 36.83 ± 0.40 ab | |
| SW-25 * | 20 | 10.32 ± 0.54 e | 31.37 ± 0.63 bc | 16.61 ± 0.51 c | 35.50 ± 0.50 bc | 27.90 ± 0.92 de |
| 40 | 33.74 ± 0.39 b | 55.56 ± 0.44 a | 49.70 ± 0.70 a | 69.70 ± 0.58 a | 37.14 ± 0.77 a | |
| SW-100 | 20 | 9.11 ± 1.09 e | 28.22 ± 1.88 c | 13.38 ± 1.53 c | 31.23 ± 1.34 c | 25.37 ± 0.75 ef |
| 40 | 23.76 ± 2.18 cd | 28.70 ± 2.09 c | 13.51 ± 2.19 c | 31.72 ± 1.92 c | 34.20 ± 0.83 abc | |
| AW-25 | 20 | 16.32 ± 0.69 de | 36.47 ± 0.62 bc | 23.92 ± 0.58 bc | 43.61 ± 0.52 bc | 33.26 ± 0.47 bc |
| 40 | 37.99 ± 1.83 ab | 59.29 ± 1.16 a | 40.29 ± 1.11 a | 71.69 ± 1.25 a | 34.20 ± 0.52 abc | |
| AW-100 | 20 | 18.36 ± 1.21 de | 36.99 ± 1.19 bc | 22.58 ± 1.34 bc | 43.34 ± 1.21 bc | 31.41 ± 0.64 cd |
| 40 | 45.85 ± 1.44 a | 32.51 ± 1.52 bc | 13.03 ± 1.47 c | 35.03 ± 1.57 c | 21.85 ± 0.36 f |
| Samples | L* | a* | b* | C* | h |
|---|---|---|---|---|---|
| Control | 38.65 ± 1.53 | 64.33 ± 2.47 | 49.77 ± 9.84 | 81.34 ± 7.71 | 37.73 ± 4.89 |
| SW-25 * | 38.00 ± 0.52 | 62.43 ± 3.69 | 52.81 ± 3.85 | 81.77 ± 3.59 | 40.23 ± 4.69 |
| SW-100 | 34.19 ± 0.62 | 56.89 ± 3.29 | 43.96 ± 5.80 | 71.89 ± 2.85 | 37.69 ± 1.26 |
| AW-25 | 40.05 ± 1.11 | 63.55 ± 2.06 | 48.50 ± 4.87 | 79.94 ± 7.33 | 37.35 ± 5.21 |
| AW-100 | 36.75 ± 8.26 | 60.00 ± 6.66 | 41.56 ± 5.73 | 72.98 ± 2.96 | 34.71 ± 3.54 |
| Samples | FP (Day) | Gentisic | Chlorogenic | Caffeic | p-Coumaric | Ferulic |
|---|---|---|---|---|---|---|
| Control | 20 | 1.04 ± 0.12 | 59.56 ± 2.44 | 4.49 ± 0.52 | 0.66 ± 0.05 | nd |
| 40 | 1.10 ± 0.02 | 49.22 ± 1.45 | 3.97 ± 0.19 | 0.64 ± 0.07 | nd | |
| SW-25 | 20 | 0.53 ± 0.06 | 64.90 ± 0.94 | 4.61 ± 1.06 | 0.54 ± 0.07 | 0.08 ± 0.04 |
| 40 | 0.54 ± 0.09 | 57.43 ± 1.00 | 4.31 ± 0.16 | 0.52 ± 0.08 | 0.07 ± 0.01 | |
| SW-100 | 20 | 0.17 ± 0.10 | 46.37 ± 1.34 | 3.95 ± 0.61 | 0.56 ± 0.12 | 0.19 ± 0.02 |
| 40 | 0.18 ± 0.03 | 44.59 ± 0.50 | 3.63 ± 0.37 | 0.51 ± 0.02 | 0.14 ± 0.02 | |
| AW-25 | 20 | 0.38 ± 0.08 | 44.14 ± 1.86 | 2.15 ± 0.60 | 0.45 ± 0.14 | 0.78 ± 0.07 |
| 40 | 0.35 ± 0.04 | 44.59 ± 0.53 | 1.79 ± 0.21 | 0.42 ± 0.09 | 0.81 ± 0.08 | |
| AW-100 | 20 | 0.29 ± 0.03 | 81.71 ± 0.57 | 9.64 ± 0.83 | 1.02 ± 0.48 | 0.08 ± 0.00 |
| 40 | 0.31 ± 0.08 | 77.02 ± 1.14 | 8.03 ± 0.47 | 0.94 ± 0.17 | 0.07 ± 0.03 |
| Samples | Gentisic | Chlorogenic | Caffeic | p-Coumaric | Ferulic |
|---|---|---|---|---|---|
| Control | 2.11 ± 0.18 a | 70.33 ± 1.70 a | 4.43 ± 0.60 | 0.91 ± 0.12 | 0.11 ± 0.08 |
| SW-25 | 0.70 ± 0.11 b | 63.66 ± 1.23 a | 3.83 ± 0.24 | 0.64 ± 0.15 | 0.01 ± 0.00 |
| SW-100 | 1.98 ± 0.02 a | 67.09 ± 1.34 a | 4.31 ± 0.19 | 0.64 ± 0.11 | 0.12 ± 0.06 |
| AW-25 | 0.99 ± 0.07 b | 50.19 ± 1.84 b | 4.70 ± 0.31 | 0.55 ± 0.07 | 0.08 ± 0.02 |
| AW-100 | 0.41 ± 0.15 b | 45.24 ± 0.85 b | 2.75 ± 0.38 | 0.42 ± 0.15 | 0.25 ± 0.07 |
| Samples | FP (Day) | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|---|
| Control | 20 | 1.70 ± 0.02 | 24.69 ± 1.43 | 4.28 ± 0.69 | 27.31 ± 1.19 | 11.00 ± 0.78 |
| 40 | 1.54 ± 0.16 | 23.28 ± 0.75 | 5.14 ± 0.11 | 25.81 ± 0.29 | 12.65 ± 0.47 | |
| SW-25 | 20 | 3.43 ± 0.31 | 19.24 ± 0.79 | 4.73 ± 0.36 | 22.61 ± 4.37 | 13.14 ± 0.27 |
| 40 | 2.75 ± 0.95 | 17.18 ± 1.58 | 4.38 ± 0.41 | 22.84 ± 0.31 | 11.95 ± 0.45 | |
| SW-100 | 20 | 0.92 ± 0.18 | 2.90 ± 0.13 | 1.08 ± 0.04 | 12.33 ± 1.07 | 6.01 ± 0.70 |
| 40 | 0.80 ± 0.17 | 2.76 ± 0.28 | 0.98 ± 0.26 | 10.28 ± 1.37 | 5.51 ± 0.59 | |
| AW-25 | 20 | 2.43 ± 0.14 | 11.01 ± 1.03 | 4.71 ± 0.30 | 17.10 ± 0.89 | 7.93 ± 0.58 |
| 40 | 2.89 ± 0.90 | 10.38 ± 0.67 | 4.47 ± 0.51 | 18.98 ± 0.12 | 9.83 ± 0.52 | |
| AW-100 | 20 | 0.35 ± 0.07 | 0.94 ± 0.09 | 0.58 ± 0.11 | 7.46 ± 0.63 | 3.32 ± 0.71 |
| 40 | 0.32 ± 0.13 | 0.97 ± 0.15 | 0.56 ± 0.32 | 7.83 ± 0.37 | 3.79 ± 0.40 |
| Sample | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|
| Control | 6.30 ± 0.65 | 27.35 ± 0.73 a | 7.82 ± 0.33 a | 32.91 ± 0.07 a | 16.08 ± 1.32 ab |
| SW-25 | 7.23 ± 0.79 | 15.82 ± 0.63 bc | 5.06 ± 0.64 b | 23.39 ± 1.01 bc | 11.71 ± 0.41 b |
| SW-100 | 8.97 ± 0.04 | 24.44 ± 0.97 a | 4.55 ± 0.46 b | 36.13 ± 0.30 a | 20.01 ± 1.09 a |
| AW-25 | 5.25 ± 0.93 | 11.00 ± 0.90 c | 3.27 ± 0.44 b | 21.55 ± 0.75 c | 11.66 ± 0.74 b |
| AW-100 | 6.10 ± 0.82 | 17.26 ± 1.15 b | 4.67 ± 0.29 b | 25.76 ± 0.34 b | 13.21 ± 0.63 b |
| Organic Acids and Ethanol | Control | SW-25 | SW-100 | AW-25 | AW-100 |
|---|---|---|---|---|---|
| Citric | nd | 0.194 ± 0.014 a | 0.266 ± 0.054 a | 0.163 ± 0.027 ab | 0.286 ± 0.034 a |
| Lactic | 0.264 ± 0.026 d | 0.665 ± 0.015 c | 0.892 ± 0.029 b | 0.725 ± 0.035 bc | 1.290 ± 0.040 a |
| Formic | 0.003 ± 0.001 c | 0.028 ± 0.003 ab | 0.038 ± 0.002 a | 0.021 ± 0.002 b | 0.034 ± 0.002 a |
| Propionic | 0.087 ± 0.004 b | 0.039 ± 0.002 d | 0.056 ± 0.004 cd | 0.069 ± 0.002 bc | 0.113 ± 0.007 a |
| Acetic | 0.031 ± 0.006 b | 0.106 ± 0.014 ab | 0.136 ± 0.015 a | 0.084 ± 0.011 ab | 0.139 ± 0.021 a |
| Ethanol | 0.438 ± 0.048 d | 0.537 ± 0.043 cd | 0.728 ± 0.024 bc | 0.854 ± 0.034 b | 1.158 ± 0.061 a |
| Samples | Fermentation Period (Day) | Lactic Acid Bacteria (Log CFU/mL) | Yeast Counts (Log CFU/mL) |
|---|---|---|---|
| Control | 20 | 6.43 ± 0.08 c | 6.70 ± 0.15 |
| 40 | 4.08 ± 0.06 e | 4.07 ± 0.07 | |
| SW-25 | 20 | 7.53 ± 0.13 b | 7.37 ± 0.07 |
| 40 | 5.44 ± 0.05 d | 4.44 ± 0.17 | |
| SW-100 | 20 | 8.04 ± 0.06 a | 8.10 ± 0.08 |
| 40 | <1 | 4.18 ± 0.09 | |
| AW-25 | 20 | 6.43 ± 0.10 c | 7.50 ± 0.08 |
| 40 | 4.42 ± 0.09 e | 4.21 ± 0.22 | |
| AW-100 | 20 | 8.08 ± 0.07 a | 8.11 ± 0.09 |
| 40 | 3.43 ± 0.07 f | 4.17 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çoklar, H.; Akbulut, M.; Aygun, A.; Akbulut, M.T. Valorization of Dairy By-Products, Sweet Whey, and Acid Whey, in the Production of Fermented Black Carrot Juice: A Comparative Study of the Phytochemical, Physicochemical, Microbiological, and Sensorial Aspects. Foods 2025, 14, 218. https://doi.org/10.3390/foods14020218
Çoklar H, Akbulut M, Aygun A, Akbulut MT. Valorization of Dairy By-Products, Sweet Whey, and Acid Whey, in the Production of Fermented Black Carrot Juice: A Comparative Study of the Phytochemical, Physicochemical, Microbiological, and Sensorial Aspects. Foods. 2025; 14(2):218. https://doi.org/10.3390/foods14020218
Chicago/Turabian StyleÇoklar, Hacer, Mehmet Akbulut, Ali Aygun, and Muhammed Talha Akbulut. 2025. "Valorization of Dairy By-Products, Sweet Whey, and Acid Whey, in the Production of Fermented Black Carrot Juice: A Comparative Study of the Phytochemical, Physicochemical, Microbiological, and Sensorial Aspects" Foods 14, no. 2: 218. https://doi.org/10.3390/foods14020218
APA StyleÇoklar, H., Akbulut, M., Aygun, A., & Akbulut, M. T. (2025). Valorization of Dairy By-Products, Sweet Whey, and Acid Whey, in the Production of Fermented Black Carrot Juice: A Comparative Study of the Phytochemical, Physicochemical, Microbiological, and Sensorial Aspects. Foods, 14(2), 218. https://doi.org/10.3390/foods14020218





