Studies on Quality Deterioration and Metabolomic Changes in Oysters Induced by Spoilage Bacteria During Chilled Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and the Analysis of the Microbial Composition
2.1.1. Preparation of Shelled Oyster Meat
2.1.2. Microbiological Analysis of Oyster Spoilage
2.2. Isolation, Purification, and Identification of Spoilage Bacteria
2.3. Sterilized Treatment and Inoculation of Oyster Meat
2.4. Microbiological and Biochemical Analysis of Bacteria-Inoculated Samples
2.4.1. Determination of the Total Viable Count (TVC)
2.4.2. Determination of the TVB-N
2.4.3. Determination of pH
2.4.4. Determination of Thiobarbituric Acid Reactive Substances (TBARSs)
2.5. TCA-Soluble Peptides
2.6. SDS-PAGE of Oyster Protein
2.7. Determination of Weight Loss
2.8. LC-MS-Based Untargeted Metabolomics of Inoculated Samples
2.9. Statistical Analysis
3. Results and Discussion
3.1. Bacteriome Composition of Oyster Meat
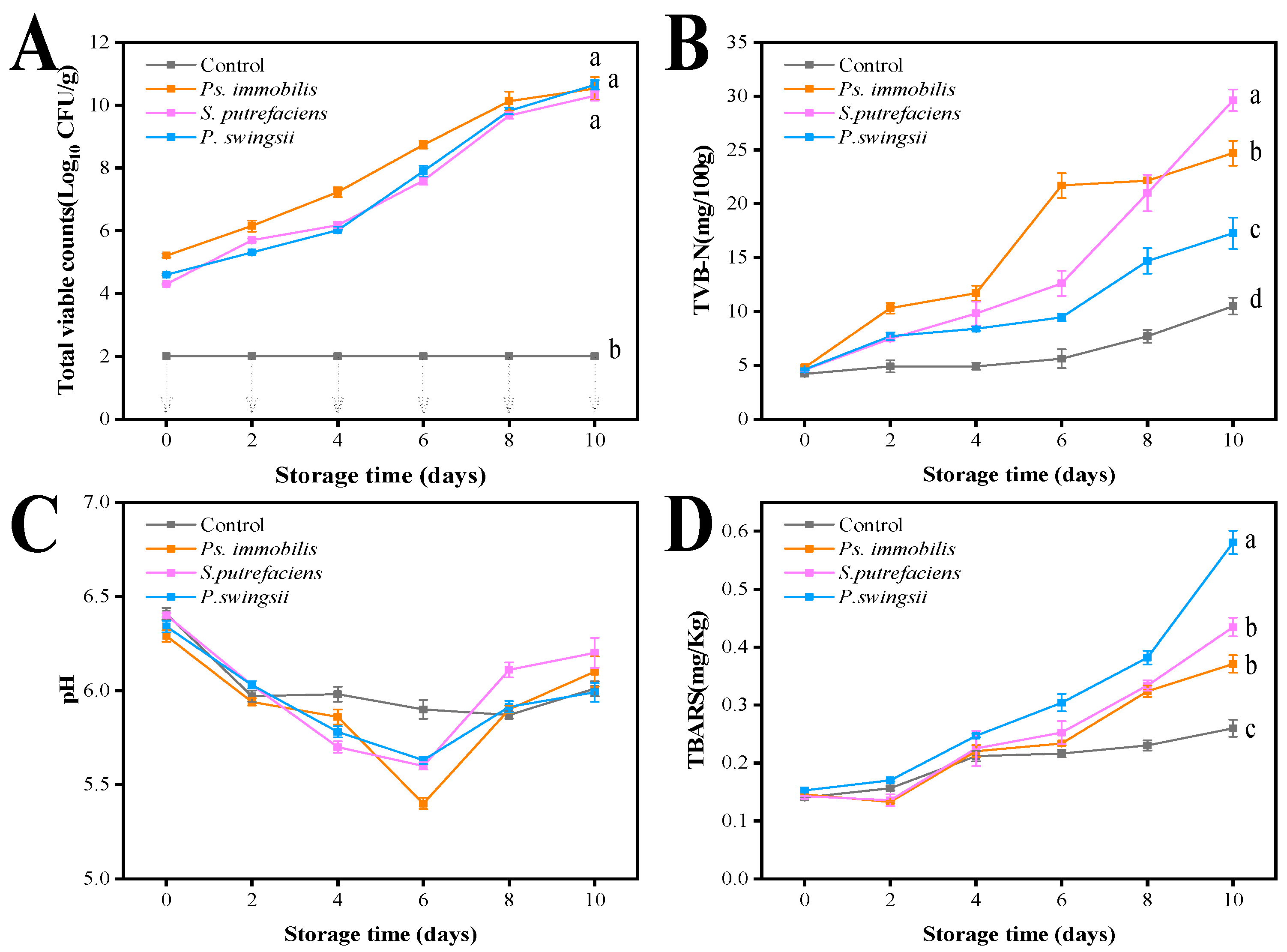
3.2. TVC
3.3. TVB-N
3.4. pH Value
3.5. TBARS
3.6. Analysis of the Role of Microorganisms in the Hydrolysis of Oyster Proteins
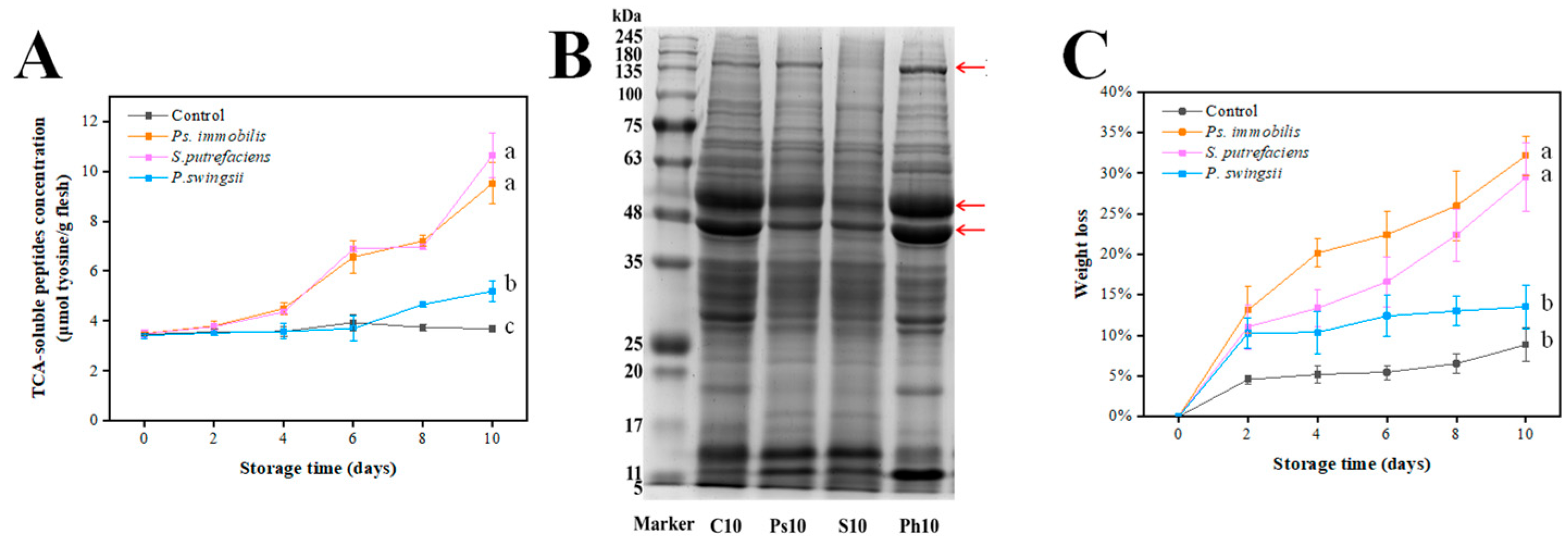
3.6.1. Changes in TCA-Soluble Peptides
3.6.2. Oyster Protein Degradation
3.6.3. Weight Loss
3.7. Metabolomics Analysis
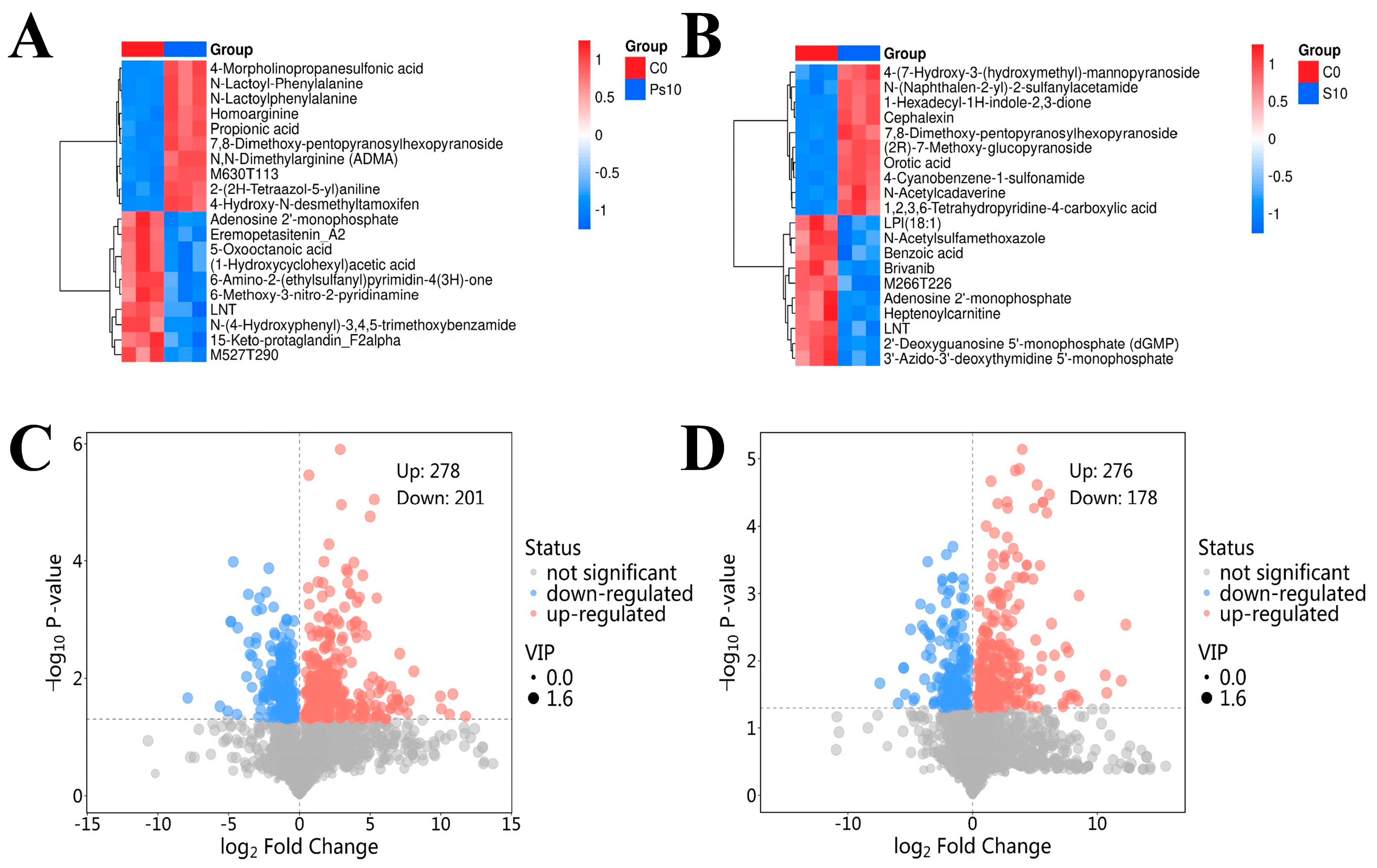
3.7.1. Analysis of Metabolic Metabolite of Oyster Spoilage
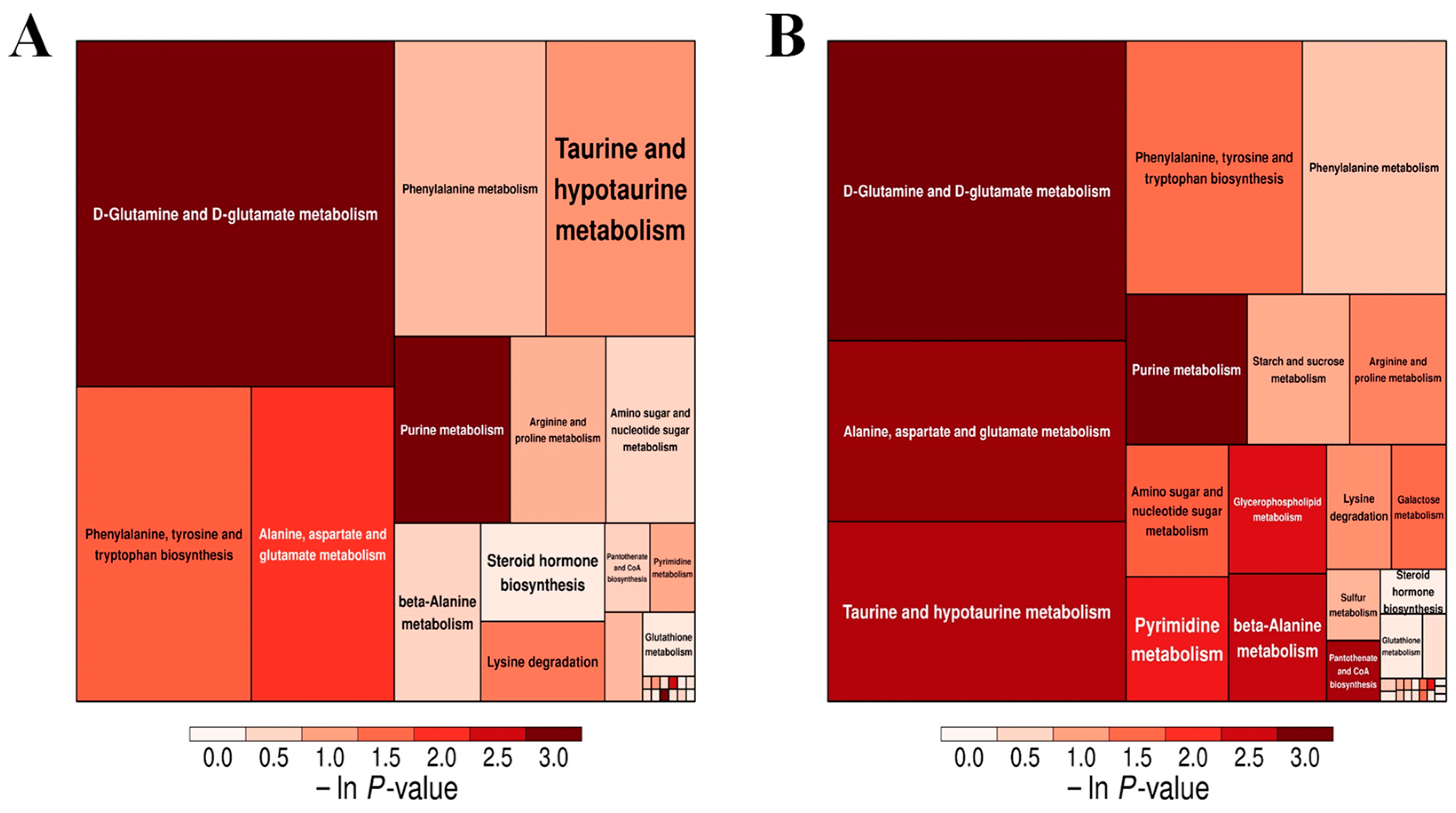
3.7.2. Analysis of Metabolic Pathways of Oyster Spoilage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Li, X.; Lou, X.; Shu, W.; Hai, Y.; Wen, X.; Yang, H. NMR-based metabolomics reveals the antibacterial effect of electrolysed water combined with citric acid on Aeromonas spp. in barramundi (Lates calcarifer) fillets. Food Res. Int. 2022, 162, 112046. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Boziaris, I.S. The evolution of knowledge on seafood spoilage microbiota from the 20th to the 21st century: Have we finished or just begun? Trends Food Sci. Technol. 2022, 120, 236–247. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Parlapani, F.F. Chapter 3—Specific Spoilage Organisms (SSOs) in Fish. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 61–98. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Bhagwat, P.K.; Ozogul, F.; Benjakul, S.; Pillai, S.; Annapure, U.S. Cold plasma for the preservation of aquatic food products: An overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4407–4425. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ge, L.; Jiang, X.; Xia, W.; Jiang, Q. Inhibitory effect of aqueous extract of Allium species on endogenous cathepsin activities and textural deterioration of ice-stored grass carp fillets. Food Bioprocess Technol. 2015, 8, 2171–2175. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Hu, S.; Shen, H. Effects of Chitosan Coatings Enriched with Different Antioxidants on Preservation of Grass Carp (Ctenopharyngodon idellus) During Cold Storage. J. Aquat. Food Prod. Technol. 2012, 21, 508–518. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yan, J.; Xie, J. Applications of Genomics, Metabolomics, Fourier Transform Infrared in the Evaluation of Spoilage Targets of Shewanella putrefaciens from Spoiled Bigeye Tuna. J. Agric. Food Chem. 2023, 71, 9558–9568. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Jia, S.; Zhang, J.; Li, K.; Luo, Y. The roles of bacteria in the biochemical changes of chill-stored bighead carp (Aristichthys nobilis): Proteins degradation, biogenic amines accumulation, volatiles production, and nucleotides catabolism. Food Chem. 2018, 255, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Hu, J.; Gao, B.; Zhang, B.; Li, P.; Shang, N. Effects of the interaction between Aeromonas sobria and Macrococcus caseolyticus on protein degradation of refrigerated sturgeon fillets: Novel perspective on fish spoilage. LWT 2023, 183, 114908. [Google Scholar] [CrossRef]
- Li, Y.; Jia, S.; Hong, H.; Zhang, L.; Zhuang, S.; Sun, X.; Liu, X.; Luo, Y. Assessment of bacterial contributions to the biochemical changes of chill-stored blunt snout bream (Megalobrama amblycephala) fillets: Protein degradation and volatile organic compounds accumulation. Food Microbiol. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, Y.; Jia, S.; Hong, H.; Liu, Y.; Luo, Y. Effects of pomegranate peel extract on quality and microbiota composition of bighead carp (Aristichthys nobilis) fillets during chilled storage. Food Microbiol. 2019, 82, 445–454. [Google Scholar] [CrossRef]
- Yang, Z.; Chu, Y.; Zhang, C.; Yan, J.; Xie, J. Changes in the physicochemical properties of grouper (Epinephelus coioides) fillets stored under vacuum packaging at chilly temperature contributing with the spoilage bacteria. Food Biosci. 2023, 55, 103017. [Google Scholar] [CrossRef]
- Zhuang, S.; Tan, Y.; Hong, H.; Li, D.; Zhang, L.; Luo, Y. Exploration of the roles of spoilage bacteria in degrading grass carp proteins during chilled storage: A combined metagenomic and metabolomic approach. Food Res. Int. 2022, 152, 110926. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, S.; Dou, H.; Zhu, W.; Zhao, D.; Wang, Y.; Wang, H.; Xia, X. Development of a colorimetric nanofibrous film sensor based on polycaprolactone (PCL) incorporated with alizarin for tracking shrimp freshness. Food Packag. Shelf Life 2024, 42, 101249. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Yang, X.; Lu, S.; Zhang, H.; Fan, Y. Stability protection of lutein emulsions by utilizing a functional conjugate of collagen and Lycium barbarum L. leaf flavonoid. Food Res. Int. 2024, 176, 113775. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Liu, Y.; Gao, S.; Tan, Y.; Hong, H.; Luo, Y. Mechanisms of fish protein degradation caused by grass carp spoilage bacteria: A bottom-up exploration from the molecular level, muscle microstructure level, to related quality changes. Food Chem. 2023, 403, 134309. [Google Scholar] [CrossRef]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Gornik, S.G.; Albalat, A.; Theethakaew, C.; Neil, D.M. Shelf life extension of whole Norway lobster Nephrops norvegicus using modified atmosphere packaging. Int. J. Food Microbiol. 2013, 167, 369–377. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Ferrocino, I.; Michailidou, S.; Argiriou, A.; Haroutounian, S.A.; Kokokiris, L.; Rantsiou, K.; Boziaris, I.S. Microbiota and volatilome profile of fresh and chill-stored deepwater rose shrimp (Parapenaeus longirostris). Food Res. Int. 2020, 132, 109057. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Z.; Yang, S.-P.; Cheng, Y.; Qian, Y.-F. Study on the spoilage potential of Pseudomonas fluorescens on salmon stored at different temperatures. J. Food Sci. Technol. 2018, 55, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, C.; Wu, H.; Jie, J.; Zeng, M.; Liu, Z.; Wang, C.; Yang, H. Spoilage of refrigerated (4°C) Litopenaeus vannamei: Cooperation between Shewanella species and contribution of cyclo-(L-Pro-L-Leu)-dependent quorum sensing. Int. J. Food Sci. Technol. 2017, 52, 1517–1526. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, A.; Feng, L.; Gao, H. Quorum sensing signals affect spoilage of refrigerated large yellow croaker (Pseudosciaena crocea) by Shewanella baltica. Int. J. Food Microbiol. 2016, 217, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Madigan, T.L.; Bott, N.J.; Torok, V.A.; Percy, N.J.; Carragher, J.F.; de Barros Lopes, M.A.; Kiermeier, A. A microbial spoilage profile of half shell Pacific oysters (Crassostrea gigas) and Sydney rock oysters (Saccostrea glomerata). Food Microbiol. 2014, 38, 219–227. [Google Scholar] [CrossRef]
- Hovda, M.B.; Lunestad, B.T.; Sivertsvik, M.; Rosnes, J.T. Characterisation of the bacterial flora of modified atmosphere packaged farmed Atlantic cod (Gadus morhua) by PCR-DGGE of conserved 16S rRNA gene regions. Int. J. Food Microbiol. 2007, 117, 68–75. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Combined effects of high voltage cold atmospheric plasma and antioxidants on the qualities and shelf-life of Asian sea bass slices. Innov. Food Sci. Emerg. Technol. 2019, 54, 113–122. [Google Scholar] [CrossRef]
- Yu, D.; Jiang, Q.; Xu, Y.; Xia, W. The shelf life extension of refrigerated grass carp (Ctenopharyngodon idellus) fillets by chitosan coating combined with glycerol monolaurate. Int. J. Biol. Macromol. 2017, 101, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.R.; Lee, H.y.; Uhm, K.-N.; Kim, H.K. Production of 4-Ethyl Malate through Position-Specific Hydrolysis of Photobacterium lipolyticum M37 Lipase. J. Microbiol. Biotechnol. 2022, 32, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Broekaert, K.; Noseda, B.; Heyndrickx, M.; Vlaemynck, G.; Devlieghere, F. Volatile compounds associated with Psychrobacter spp. and Pseudoalteromonas spp., the dominant microbiota of brown shrimp (Crangon crangon) during aerobic storage. Int. J. Food Microbiol. 2013, 166, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, T.; Wang, D.; Zhang, L.; Chen, G. Study on the volatile profile characteristics of oyster Crassostrea gigas during storage by a combination sampling method coupled with GC/MS. Food Chem. 2009, 115, 1150–1157. [Google Scholar] [CrossRef]
- Liu, Z.; Kang, B.; Duan, X.; Hu, Y.; Li, W.; Wang, C.; Li, D.; Xu, N. Metabolomic profiles of the liquid state fermentation in co-culture of A. oryzae and Z. Rouxii. Food Microbiol. 2022, 103, 103966. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Xie, J. Characterization of metabolite, genome and volatile organic compound changes provides insights into the spoilage and cold adaptive markers of Acinetobacter johnsonii XY27. LWT 2022, 162, 113453. [Google Scholar] [CrossRef]
- Syropoulou, F.; Parlapani, F.F.; Bosmali, I.; Madesis, P.; Boziaris, I.S. HRM and 16S rRNA gene sequencing reveal the cultivable microbiota of the European sea bass during ice storage. Int. J. Food Microbiol. 2020, 327, 108658. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, H.; Zhu, W.; Chen, S.; Zou, Y.; Xia, X. Studies on Quality Deterioration and Metabolomic Changes in Oysters Induced by Spoilage Bacteria During Chilled Storage. Foods 2025, 14, 193. https://doi.org/10.3390/foods14020193
Dou H, Zhu W, Chen S, Zou Y, Xia X. Studies on Quality Deterioration and Metabolomic Changes in Oysters Induced by Spoilage Bacteria During Chilled Storage. Foods. 2025; 14(2):193. https://doi.org/10.3390/foods14020193
Chicago/Turabian StyleDou, Hanzheng, Wenxiu Zhu, Siyang Chen, Yue Zou, and Xiaodong Xia. 2025. "Studies on Quality Deterioration and Metabolomic Changes in Oysters Induced by Spoilage Bacteria During Chilled Storage" Foods 14, no. 2: 193. https://doi.org/10.3390/foods14020193
APA StyleDou, H., Zhu, W., Chen, S., Zou, Y., & Xia, X. (2025). Studies on Quality Deterioration and Metabolomic Changes in Oysters Induced by Spoilage Bacteria During Chilled Storage. Foods, 14(2), 193. https://doi.org/10.3390/foods14020193




