Study on the Effect of Radish Sprouts on Short-Chain Fatty Acids and Gut Microbial Diversity in Healthy Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Radish Sprout Cultivation
2.2. Acquisition of Additives for Radish Sprout Cultivation
2.3. Assessment of Health and Dietary Practices in a Study Population
2.4. Collection and Pre-Treatment of Fecal Samples
2.5. Media for In Vitro Cultivation of Intestinal Microbiota
2.6. In Vitro Cultivation of Intestinal Microbiota with Radish Sprout Digesta
2.7. Detection of Short-Chain Fatty Acids
2.8. Detection of Intestinal Microbiota Diversity
2.9. Data Processing
3. Results and Discussion
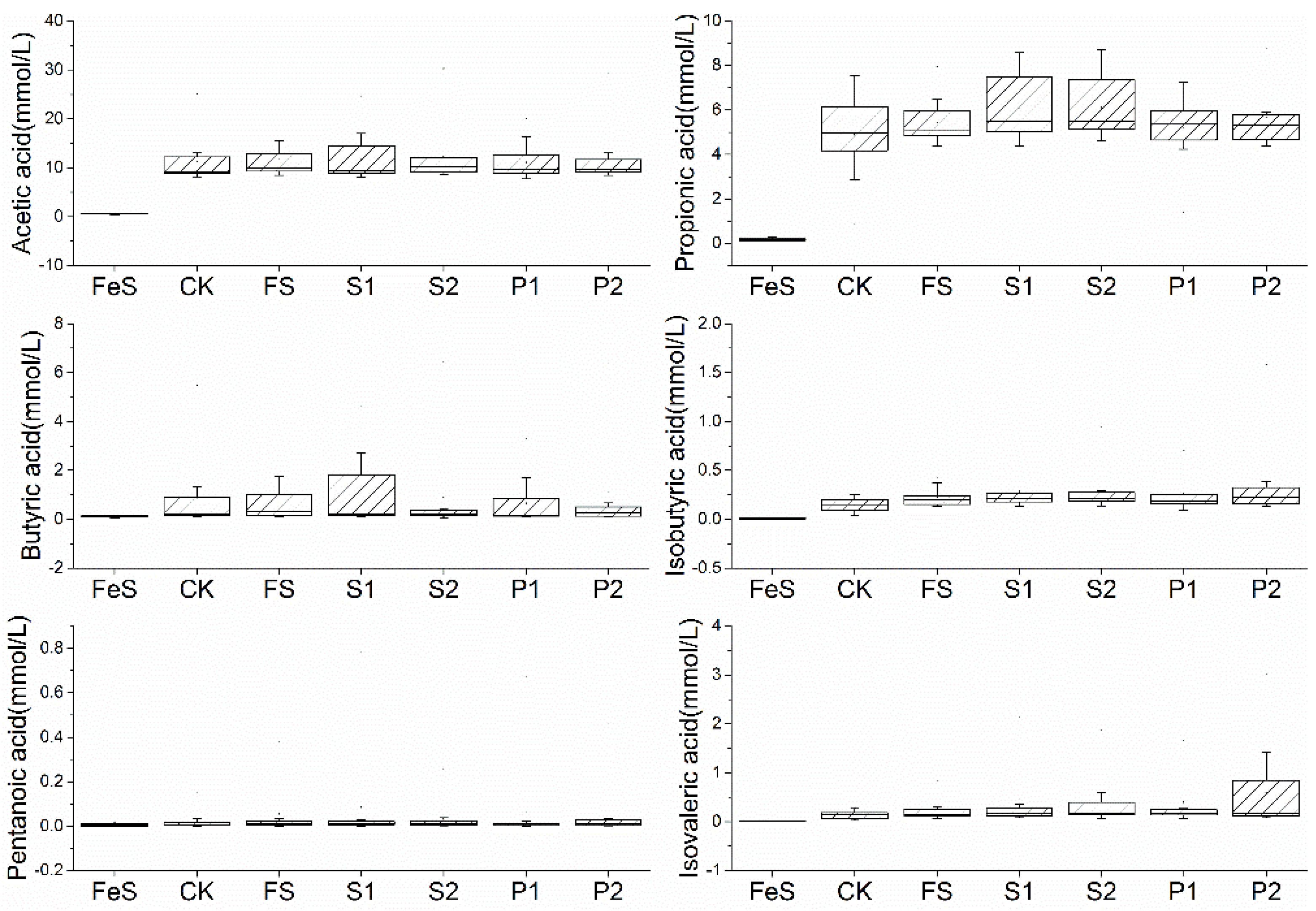
3.1. The Effect of Radish Sprout Digesta Supplementation on Short-Chain Fatty Acids
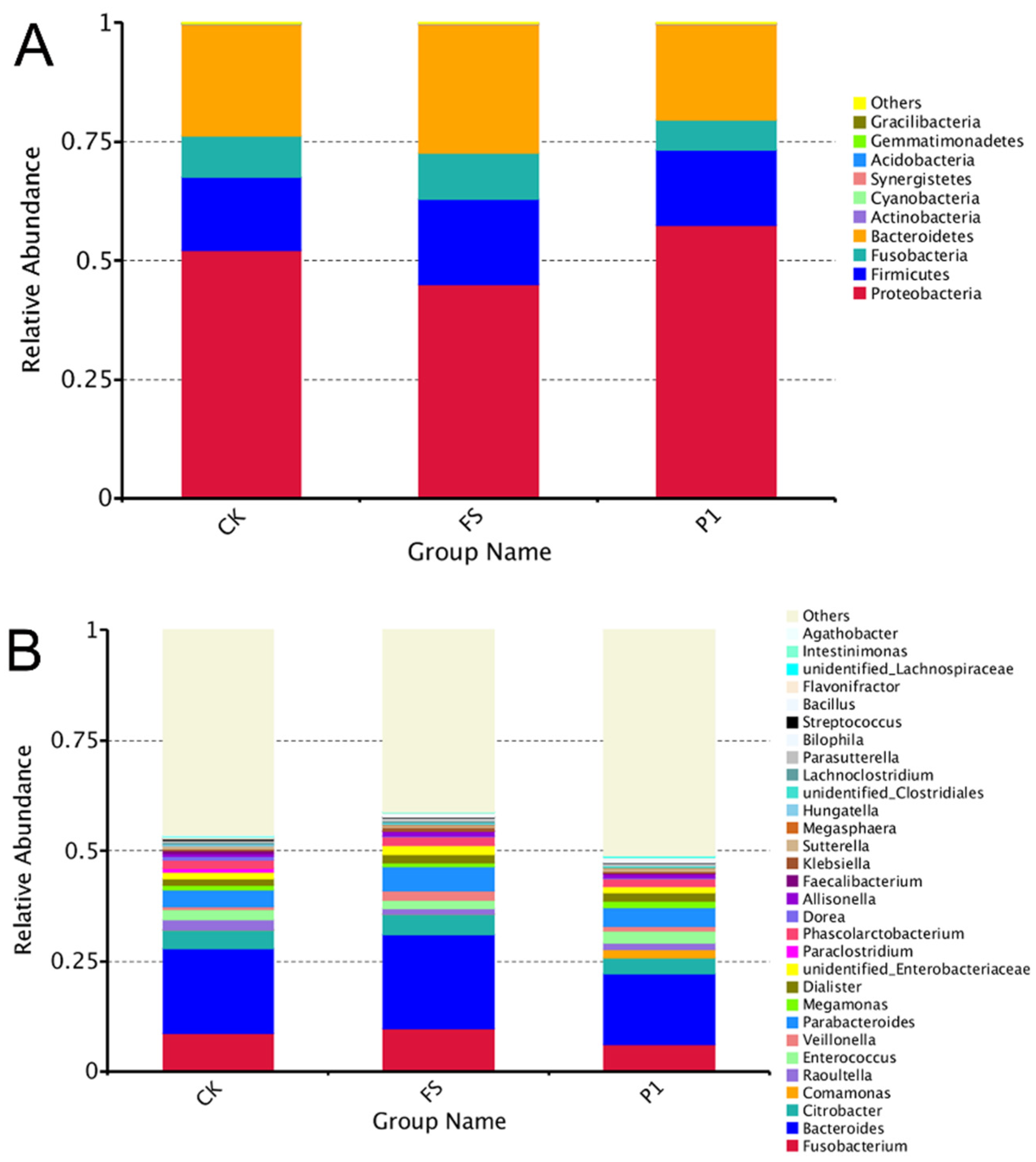
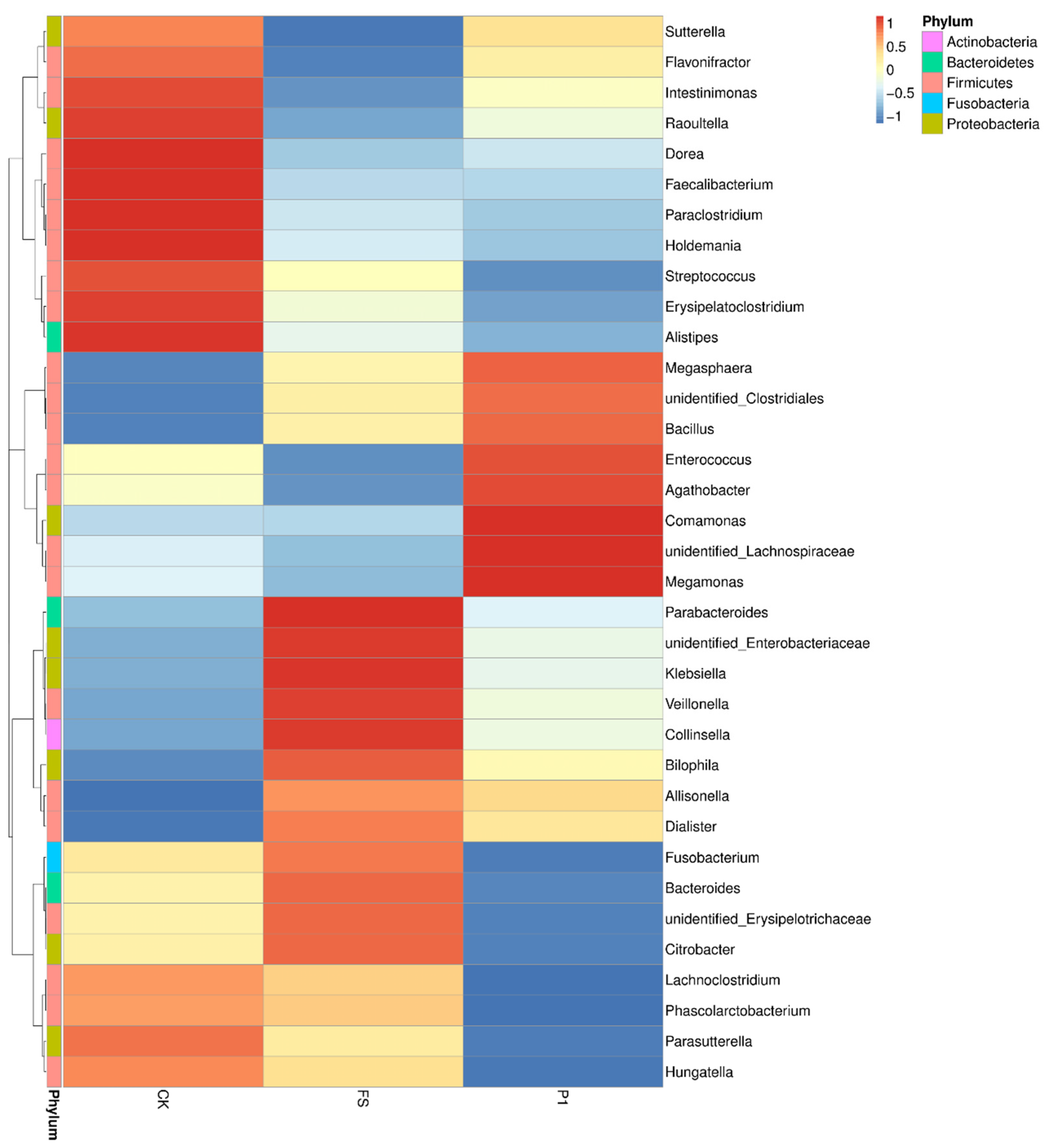
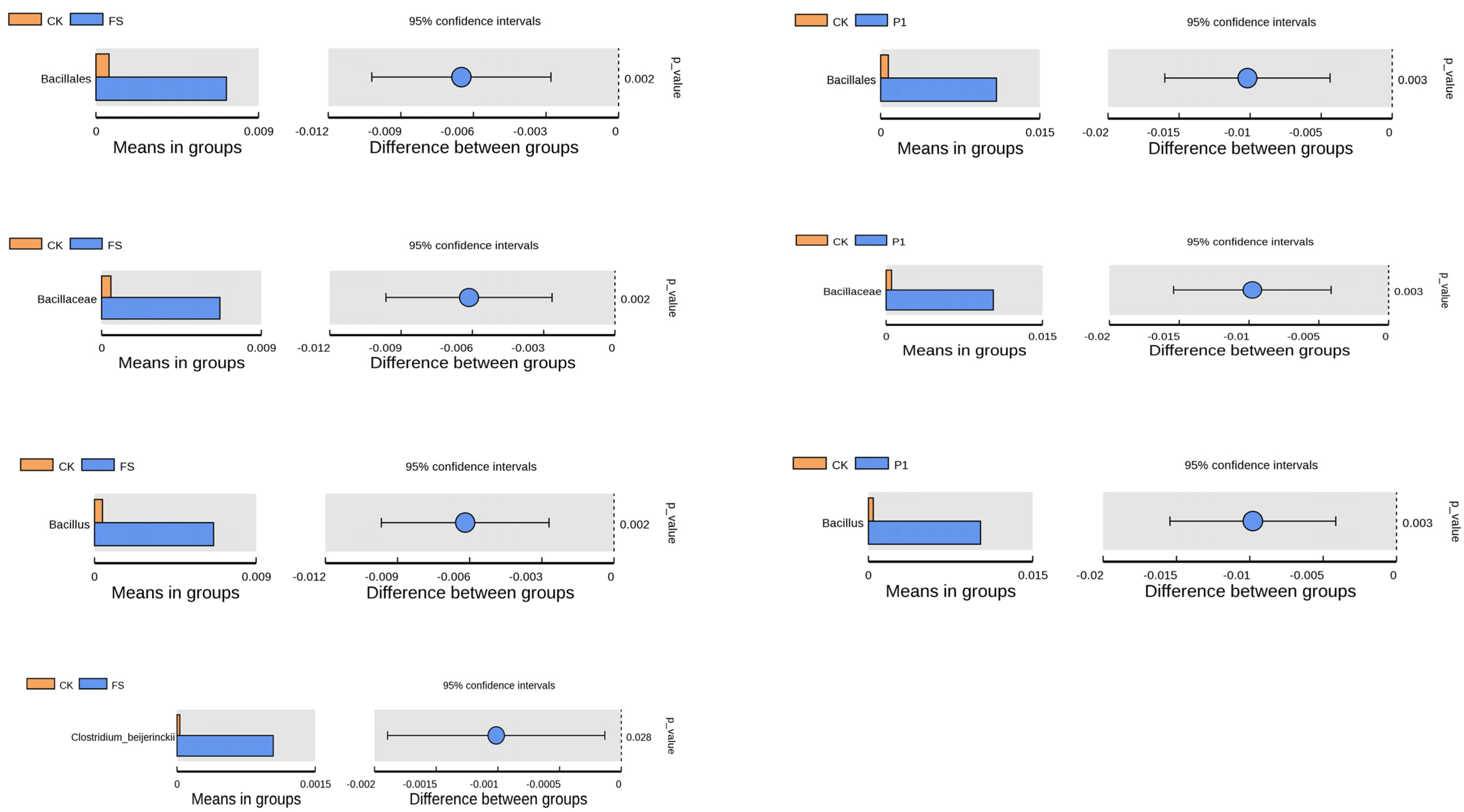
3.2. The Effect of Radish Sprout Digesta Supplementation on Gut Microbiota Diversity—A Comparative Analysis of Microbial Diversity Based on OUT
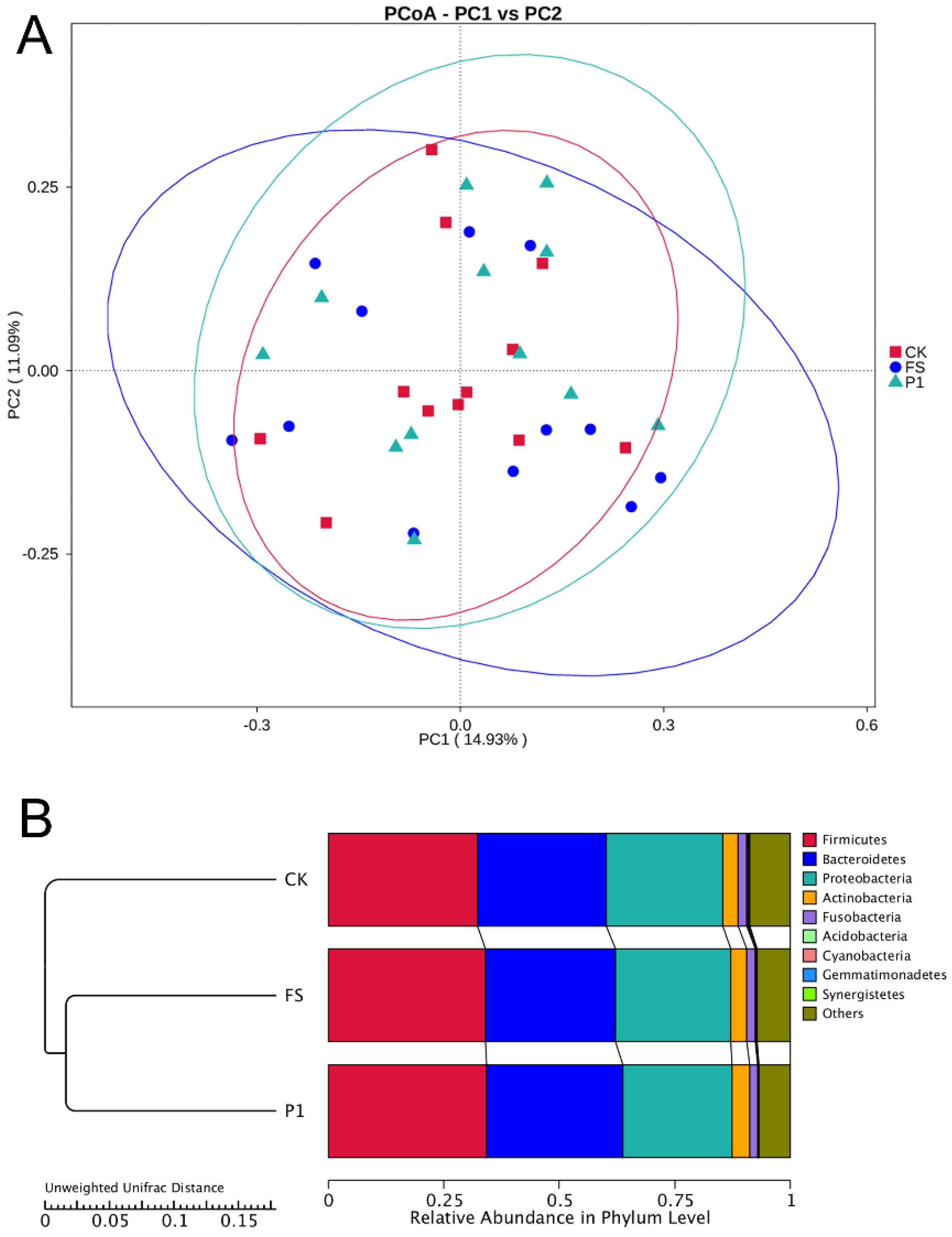
3.3. The Impact of Radish Sprout Digesta Supplementation on Gut Microbiota Diversity—β-Diversity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hitch, T.C.A.; Afrizal, A.; Riedel, T.; Kioukis, A.; Haller, D.; Lagkouvardos, I.; Overmann, J.; Clavel, T. Recent Advances in Culture-Based Gut Microbiome Research. Int. J. Med. Microbiol. 2021, 311, 151485. [Google Scholar] [CrossRef] [PubMed]
- Noecker, C.; Turnbaugh, P.J. Emerging Tools and Best Practices for Studying Gut Microbial Community Metabolism. Nat. Metab. 2024, 6, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-Brain Axis: A Cutting-Edge Approach to Target Neurological Disorders and Potential Synbiotic Application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef] [PubMed]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols On Gut Microbiota, their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The Interplay Between Diet and the Gut Microbiome: Implications for Health and Disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Kumar, S.A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar, R.H.; Gupta, A.; Rosaria, L.M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols On Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition On the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M.; Mildaziene, V. Impact of Seed Color and Storage Time On the Radish Seed Germination and Sprout Growth in Plasma Agriculture. Sci. Rep. 2021, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Kim, J.H.; Kim, D.S.; Lee, E.S.; Lee, H.E. Deciphering the Nutraceutical Potential of Raphanus sativus—A Comprehensive Overview. Nutrients 2019, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.D.; Beres, C.; Brito, F.O.; Zago, L.; Miyahira, R.F. Unlocking the Functional Potential of Sprouts: A Scientific Exploration On Simulated Gastrointestinal Digestion and Colonic Fermentation. J. Funct. Foods 2024, 117, 106235. [Google Scholar] [CrossRef]
- Li, R.; Hao, R.; Zhu, Y. Steam Radish Sprout (Raphanus sativus L.): Active Substances, Antioxidant Activities and Non-Targeted Metabolomics Analysis. Int. J. Food Sci. Technol. 2019, 54, 3138–3148. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Valdes, A.M.; Louca, P.; Visconti, A.; Asnicar, F.; Bermingham, K.; Nogal, A.; Wong, K.; Michelotti, G.A.; Wolf, J.; Segata, N.; et al. Vitamin a Carotenoids, but Not Retinoids, Mediate the Impact of a Healthy Diet On Gut Microbial Diversity. BMC Med. 2024, 22, 321. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Shen, L.; Ji, H. Regulation of Gut Microbiota by Vitamin C, Vitamin E and Β-Carotene. Food Res. Int. 2023, 169, 112749. [Google Scholar] [CrossRef]
- Bernabeu, M.; Gharibzahedi, S.; Ganaie, A.A.; Macha, M.A.; Dar, B.N.; Castagnini, J.M.; Garcia-Bonillo, C.; Meléndez-Martínez, A.J.; Altintas, Z.; Barba, F.J. The Potential Modulation of Gut Microbiota and Oxidative Stress by Dietary Carotenoid Pigments. Crit. Rev. Food. Sci. Nutr. 2023, 64, 12555–12573. [Google Scholar] [CrossRef]
- Li, Z.; Dai, Z.; Shi, E.; Wan, P.; Chen, G.; Zhang, Z.; Xu, Y.; Gao, R.; Zeng, X.; Li, D. Study On the Interaction Between Β-Carotene and Gut Microflora Using an in Vitro Fermentation Model. Food Sci. Human Wellness 2023, 12, 1369–1378. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in Vitro Batch Fermentation Protocol for Studying the Contribution of Food to Gut Microbiota Composition and Functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Y. The Primary Active Components, Antioxidant Properties, and Differential Metabolite Profiles of Radish Sprouts (Raphanus sativus L.) Upon Domestic Storage: Analysis of Nutritional Quality. J. Sci. Food. Agric. 2018, 98, 5853–5860. [Google Scholar] [CrossRef]
- Świeca, M.; Sęczyk, A.; Gawlik-Dziki, U.; Dziki, D. Bread Enriched with Quinoa Leaves–the Influence of Protein–Phenolics Interactions On the Nutritional and Antioxidant Quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Hosseinkhani, F.; Dubbelman, A.; Karu, N.; Harms, A.C.; Hankemeier, T. Towards Standards for Human Fecal Sample Preparation in Targeted and Untargeted LC-HRMS Studies. Metabolites 2021, 11, 364. [Google Scholar] [CrossRef]
- Wang, Z.; Zolnik, C.P.; Qiu, Y.; Usyk, M.; Wang, T.; Strickler, H.D.; Isasi, C.R.; Kaplan, R.C.; Kurland, I.J.; Qi, Q.; et al. Comparison of Fecal Collection Methods for Microbiome and Metabolomics Studies. Front. Cell. Infect. Microbiol. 2018, 8, 301. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The Role of Ph in Determining the Species Composition of the Human Colonic Microbiota. Environ. Microbiol. 2010, 11, 2112–2122. [Google Scholar] [CrossRef]
- Mannaa, M.; Han, G.; Seo, Y.S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- O’ Donnell, M.; Rea, M.; Shanahan, F.; Ross, R. The Use of a Mini-Bioreactor Fermentation System as a Reproducible, High-Throughput Ex Vivo Batch Model of the Distal Colon. Front. Microbiol. 2018, 9, 1844. [Google Scholar] [CrossRef]
- Wu, Q.; Pi, X.; Liu, W.; Chen, H.; Yin, Y.; Yu, H.D.; Wang, X.; Zhu, L. Fermentation Properties of Isomaltooligosaccharides are Affected by Human Fecal Enterotypes. Anaerobe 2017, 48, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; Connor, M.I.O.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and Functional Redundancy in Microbial Systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tian, C.; Dong, L.; Kang, Z.; Wang, J.; Zhao, S.; Li, M.; Tong, X. Mechanisms of Regulation of Glycolipid Metabolism by Natural Compounds in Plants: Effects On Short-Chain Fatty Acids. Nutr. Metab. 2024, 21, 49. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. The Role of Short-Chain Fatty Acids in Cancer Prevention and Cancer Treatment. Arch. Biochem. Biophys. 2024, 761, 110172. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of Microbiota-Derived Short-Chain Fatty Acids in Nervous System Disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction Between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food. Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Sharma, V.; Vashishtha, A.; Jos, A.L.M.; Khosla, A.; Basu, N.; Yadav, R.; Bhatt, A.; Gulani, A.; Singh, P.; Lakhera, S.; et al. Phylogenomics of the Phylum Proteobacteria: Resolving the Complex Relationships. Curr. Microbiol. 2022, 79, 224. [Google Scholar] [CrossRef]
- Pan, X.; Raaijmakers, J.M.; Carrión, V.J. Importance of Bacteroidetes in Host–Microbe Interactions and Ecosystem Functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate Producers, “the Sentinel of Gut”: Their Intestinal Significance with and Beyond Butyrate, and Prospective Use as Microbial Therapeutics. Front. Microbiol. 2022, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- Meile, L.; Ludwig, W.; Rueger, U.; Gut, C.; Kaufmann, P.; Dasen, G.; Wenger, S.; Teuber, M. Bifidobacterium Lactis Sp. Nov., A Moderately Oxygen Tolerant Species Isolated From Fermented Milk. Syst. Appl. Microbiol. 1997, 20, 57–64. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Wang, C.; Wang, L.; He, T.; Hu, H.; Song, J.; Cui, C.; Qiao, J.; Qing, L.; et al. Enterotype Bacteroides is Associated with a High Risk in Patients with Diabetes: A Pilot Study. J. Diabetes Res. 2020, 2020, 6047145. [Google Scholar] [CrossRef]
- Ou, S.; Wang, H.; Tao, Y.; Luo, K.; Ye, J.; Ran, S.; Guan, Z.; Wang, Y.; Hu, H.; Huang, R. Fusobacterium Nucleatum and Colorectal Cancer: From Phenomenon to Mechanism. Front. Cell. Infect. Microbiol. 2022, 12, 1020583. [Google Scholar] [CrossRef]
- Pilar, A.V.C.; Petronella, N.; Dussault, F.M.; Verster, A.J.; Bekal, S.; Levesque, R.C.; Goodridge, L.; Tamber, S. Similar Yet Different: Phylogenomic Analysis to Delineate Salmonella and Citrobacter Species Boundaries. BMC Genom. 2020, 21, 377. [Google Scholar] [CrossRef]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.; Good, C.E.; et al. Parabacteroides Distasonis: Intriguing Aerotolerant Gut Anaerobe with Emerging Antimicrobial Resistance and Pathogenic and Probiotic Roles in Human Health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef]
- Muñiz, P.D.; Jensen, M.D.; Van Dyke, C.T.; Murray, J.A.; Woods, J.A.; Chen, J.; Kashyap, P.C.; Nehra, V. Gut Microbial Carbohydrate Metabolism Hinders Weight Loss in Overweight Adults Undergoing Lifestyle Intervention with a Volumetric Diet. Mayo. Clin. Proc. 2018, 93, 1104–1110. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased Abundance of Sutterella Spp. and Ruminococcus torques in Feces of Children with Autism Spectrum Disorder. Mol. Autism. 2013, 4, 42. [Google Scholar] [CrossRef]
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.L.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in Anti-Inflammatory Gut Bacteria are Associated with Depression in a Sample of Young Adults. Brain Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of Bile Acids and Gut Microbiota in Obesity Induced by High Fat Diet in Rat Model. J. Agric. Food. Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Si, X.; Yang, L.; Wang, H.; Sun, Y.; Liu, N. Association Between Intestinal Microbiota and Inflammatory Bowel Disease. Anim. Model. Exp. Med. 2022, 5, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, P.; Liu, T.; Lin, L.; Li, L.; Kou, G.; Zhou, R.; Li, P.; Li, Y. Involvement of Mucosal Flora and Enterochromaffin Cells of the Caecum and Descending Colon in Diarrhoea-Predominant Irritable Bowel Syndrome. BMC Microbiol. 2021, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium Prausnitzii and Human Intestinal Health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741. [Google Scholar] [CrossRef]
- Yuan, T.; Xia, Y.; Li, B.; Yu, W.; Rao, T.; Ye, Z.; Yan, X.; Song, B.; Li, L.; Lin, F.; et al. Gut Microbiota in Patients with Kidney Stones: A Systematic Review and Meta-Analysis. BMC Microbiol. 2023, 23, 143. [Google Scholar] [CrossRef]
- Le, S.H.; Nguyen Ngoc Minh, C.; de Sessions, P.F.; Jie, S.; Tran Thi Hong, C.; Thwaites, G.E.; Baker, S.; Pham, D.T.; Chung The, H. The Impact of Antibiotics On the Gut Microbiota of Children Recovering From Watery Diarrhoea. Npj Antimicrob. Resist. 2024, 2, 12. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.H.; Chang, Y.; Yang, D.; Joo, K.J.; Cho, Y.; Park, H.J.; Kim, H.; Ryu, S. Gut Microbiota and the Prevalence and Incidence of Renal Stones. Sci. Rep. 2022, 12, 3732. [Google Scholar] [CrossRef]
- Caso, J.R.; MacDowell, K.S.; González-Pinto, A.; García, S.; de Diego-Adeliño, J.; Carceller-Sindreu, M.; Sarramea, F.; Caballero-Villarraso, J.; Gracia-García, P.; De la Cámara, C.; et al. Gut Microbiota, Innate Immune Pathways, and Inflammatory Control Mechanisms in Patients with Major Depressive Disorder. Transl. Psychiatry 2021, 11, 645. [Google Scholar] [CrossRef]
- Cao, C.; Li, F.; Ding, Q.; Jin, X.; Tu, W.; Zhu, H.; Sun, M.; Zhu, J.; Yang, D.; Fan, B. Potassium Sodium Hydrogen Citrate Intervention on Gut Microbiota and Clinical Features in Uric Acid Stone Patients. Appl. Microbiol. Biotechnol. 2024, 108, 51. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, J.; Zhao, D.; Chen, B.; Wang, Q.; Li, Y.; Chen, J.; Bai, C.; Guo, X.; Hu, N.; et al. Microbial Biomarker Discovery in Parkinson’S Disease through a Network-Based Approach. NPJ Park. Dis. 2024, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, J.; Zhang, W.; Doherty, M.; Zhang, Y.; Xie, H.; Li, W.; Wang, N.; Lei, G.; Zeng, C. Gut Dysbiosis in Rheumatic Diseases: A Systematic Review and Meta-Analysis of 92 Observational Studies. EBioMedicine 2022, 80, 104055. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus Spp.-Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Berbert, L.; Santos, A.; Magro, D.O.; Guadagnini, D.; Assalin, H.B.; Lourenço, L.H.; Martinez, C.; Saad, M.; Coy, C. Metagenomics Analysis Reveals Universal Signatures of the Intestinal Microbiota in Colorectal Cancer, Regardless of Regional Differences. Braz. J. Med. Biol. Res. 2022, 55, e11832. [Google Scholar] [CrossRef]
- Blaut, M. Gut Microbiota and Energy Balance: Role in Obesity. Proc. Nutr. Soc. 2015, 74, 227–234. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Lee, N.K.; Kim, W.S.; Paik, H.D. Bacillus Strains as Human Probiotics: Characterization, Safety, Microbiome, and Probiotic Carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Golnari, M.; Bahrami, N.; Milanian, Z.; Rabbani Khorasgani, M.; Asadollahi, M.A.; Shafiei, R.; Fatemi, S.S. Isolation and Characterization of Novel Bacillus Strains with Superior Probiotic Potential: Comparative Analysis and Safety Evaluation. Sci. Rep. 2024, 14, 1457. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Imre, K.; Arslan-Acaroz, D.; Istanbullugil, F.R.; Fang, Y.; Ros, G.; Zhu, K.; Acaroz, U. Mechanisms of Probiotic Bacillus Against Enteric Bacterial Infections. One Health Adv. 2023, 1, 21. [Google Scholar] [CrossRef]
- Amoah, K.; Cai, J.; Huang, Y.; Wang, B.; Shija, V.M.; Wang, Z.; Jin, X.; Cai, S.; Lu, Y.; Jian, J. Identification and Characterization of Four Bacillus Species From the Intestine of Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂), their Antagonistic Role On Common Pathogenic Bacteria, and Effects On Intestinal Health. Fish Shellfish Immunol. 2024, 152, 109795. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Ye, G.; Liu, C.; Zhang, K.; Li, H.; Yang, J. Comparative Genome Analysis of Clostridium Beijerinckii Strains Isolated From Pit Mud of Chinese Strong Flavor Baijiu Ecosystem. G3-Genes Genomes Genet. 2021, 11, jkab317. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, Q.; Fan, Y.; Xu, X.; Liu, Z. Butanol–Isopropanol Fermentation with Oxygen-Tolerant Clostridium Beijerinckii XH29. AMB Express 2022, 12, 57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Chen, X.; Shi, C.; Zhu, Y. Study on the Effect of Radish Sprouts on Short-Chain Fatty Acids and Gut Microbial Diversity in Healthy Individuals. Foods 2025, 14, 170. https://doi.org/10.3390/foods14020170
Li R, Chen X, Shi C, Zhu Y. Study on the Effect of Radish Sprouts on Short-Chain Fatty Acids and Gut Microbial Diversity in Healthy Individuals. Foods. 2025; 14(2):170. https://doi.org/10.3390/foods14020170
Chicago/Turabian StyleLi, Ru, Xuehong Chen, Cong Shi, and Yi Zhu. 2025. "Study on the Effect of Radish Sprouts on Short-Chain Fatty Acids and Gut Microbial Diversity in Healthy Individuals" Foods 14, no. 2: 170. https://doi.org/10.3390/foods14020170
APA StyleLi, R., Chen, X., Shi, C., & Zhu, Y. (2025). Study on the Effect of Radish Sprouts on Short-Chain Fatty Acids and Gut Microbial Diversity in Healthy Individuals. Foods, 14(2), 170. https://doi.org/10.3390/foods14020170






