The Probiotication of a Lychee Beverage with Saccharomyces boulardii: An Alternative to Dairy-Based Probiotic Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Reagents
2.1.2. Lychee Juice
2.1.3. Probiotic Culture
2.2. Methods
2.2.1. Preparation of S. boulardii Inoculum
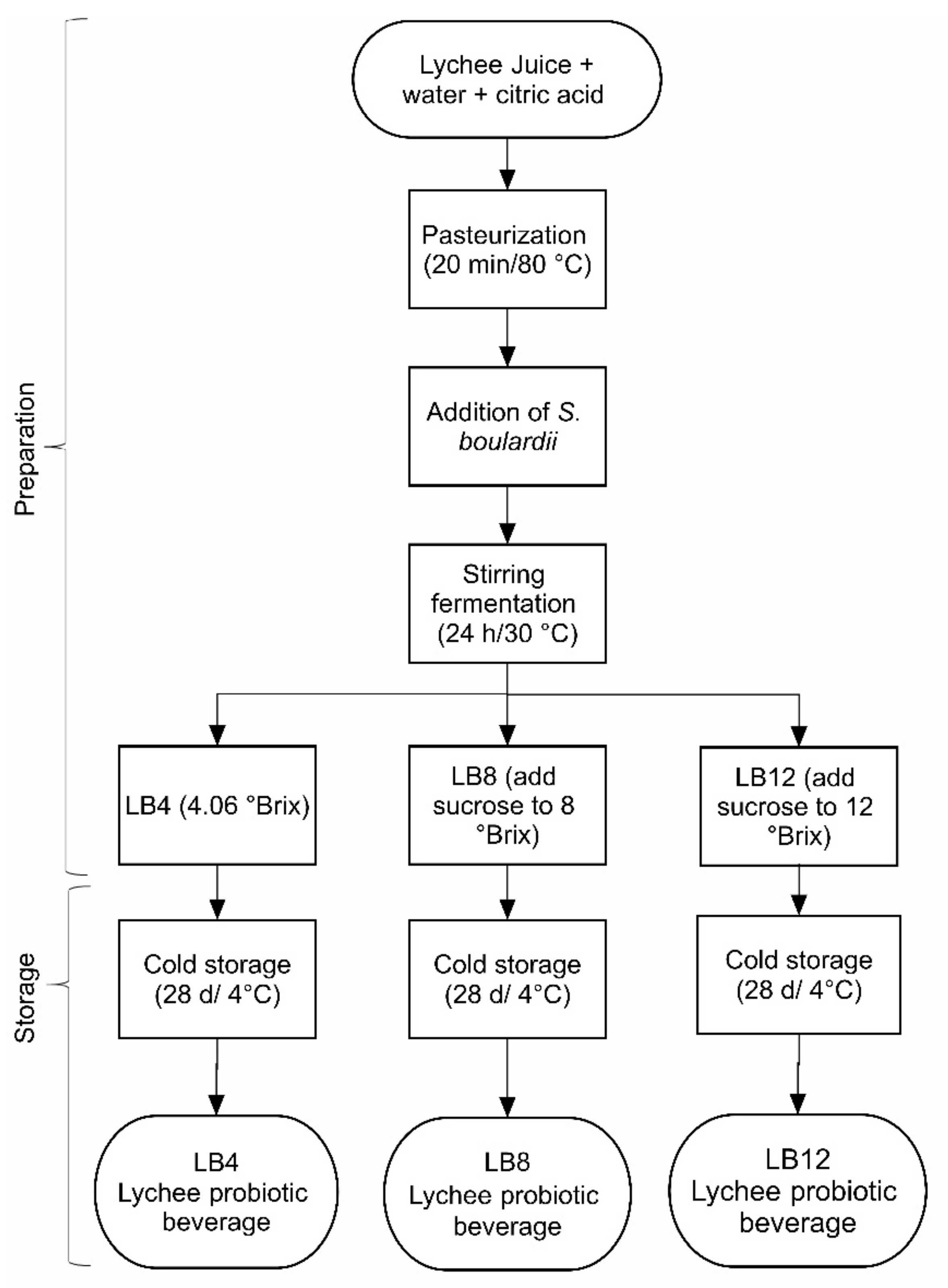
2.2.2. Probiotication of Lychee Juice
2.2.3. Viability of S. boulardii
2.2.4. Physicochemical Analysis
Chromatographic Analyses: HPLC and UHPLC
2.2.5. Sensory Acceptance
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of S. boulardii Probiotic on the Physicochemical Characteristics of Lychee Beverages
3.2. Effect of TSS and Cold Storage on Probiotic Lychee Beverages
3.2.1. S. boulardii Viability
3.2.2. Total Soluble Solids (TSS) and pH
3.2.3. Ethanol Content
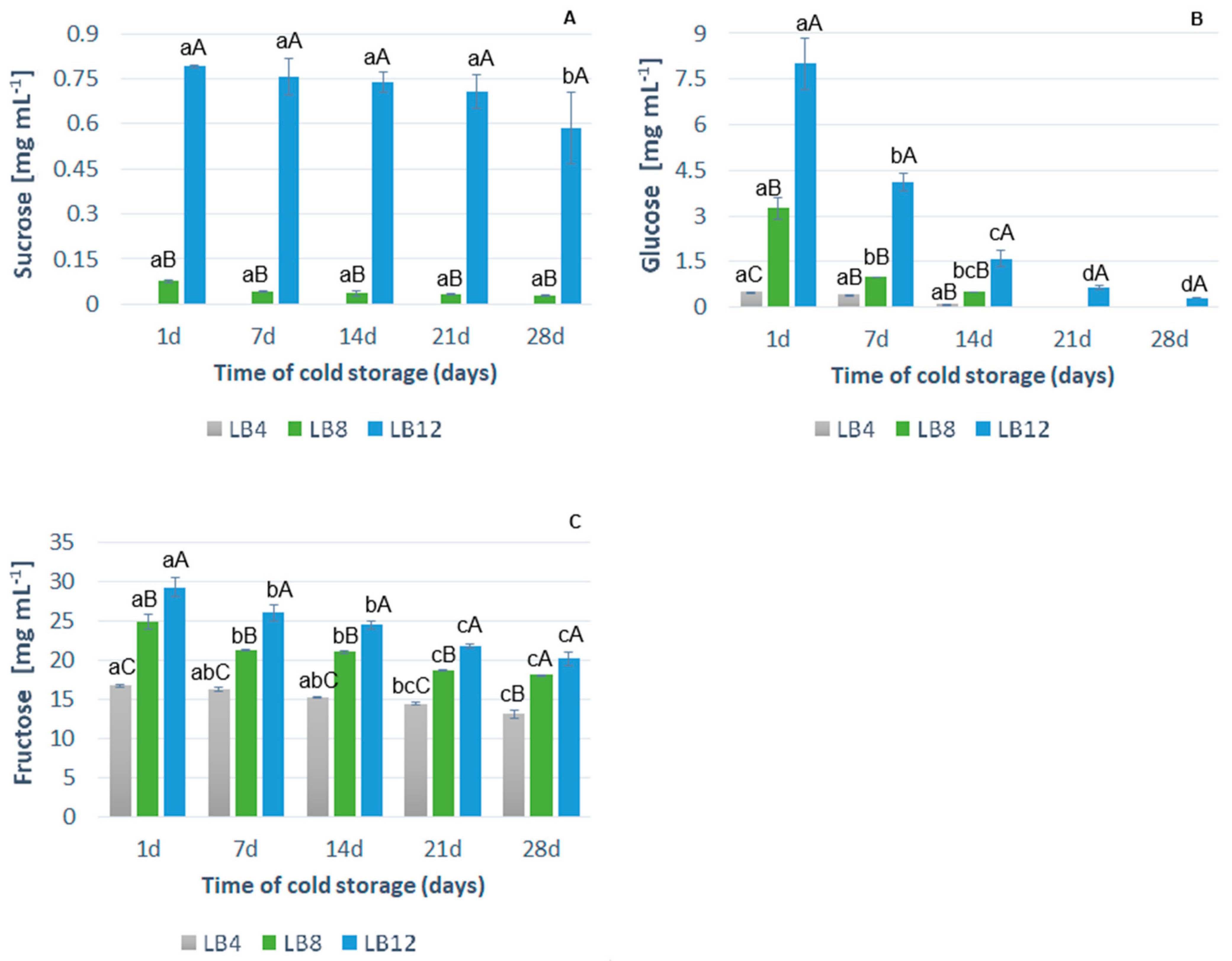
3.2.4. Sugars Content
3.2.5. Organic Acid Content
3.2.6. Total Phenolic Compounds (TPCs) and Antioxidant Activity (AA)
3.2.7. Phenolic Acid, Flavonoid and Methylxanthine Contents
3.3. Sensory Acceptability of Probiotic Lychee Beverages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WGO. World Gastroenterology Organization. Practice Guideline Probiotics and Prebiotics. 2024. Available online: https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2023.pdf (accessed on 25 November 2024).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Common Organisms and Probiotics: Saccharomyces boulardii. In The Microbiota in Gastrointestinal Pathophysiology; Floch, H., Ringel, Y., Walker, W.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 145–164. ISBN 9780128040621. [Google Scholar]
- Czeruca, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Cardinale, F.; Russo, I.; Iuliano, C.; Tremonte, P.; Coppola, R.; Nazzaro, F. Ability of symbiotic encapsulated Saccharomyces cerevisiae boulardii to grow in berry juice and to survive under simulated gastrointestinal conditions. J. Microencapsul. 2014, 31, 299–305. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yilmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Egea, M.B.; Oliveira-Filho, J.G.D.; Lemes, A.C. Investigating the Efficacy of Saccharomyces boulardii in Metabolic Syndrome Treatment: A Narrative Review of What Is Known So Far. Int. J. Mol. Sci. 2023, 24, 12015. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miraglia, N.; Putignano, P.; Castagliuolo, I.; Brun, P.; Dall’acqua, S.; Peroni, G.; Faliva, M.A.; Naso, M.; Nichetti, M.; et al. Effects of 60-day Saccharomyces boulardii and superoxide dismutase supplementation on body composition, hunger sensation, pro/antioxidant ratio, inflammation and hormonal lipo-metabolic biomarkers in obese adults: A double-blind, placebo-controlled trial. Nutrients 2021, 13, 2512. [Google Scholar] [CrossRef]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Gabriella, S.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisae var. boulardii in co-fermentations with S. cerevisae for the production of craft beers with potential healthy value added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Datta, S.; Timson, D.J.; Annapure, U.S. Antioxidant properties and global metabolite screening of the probiotic yeast Saccharomyces cerevisiae var. boulardii. J. Sci. Food Agric. 2017, 97, 3039–3049. [Google Scholar] [CrossRef]
- Czeruca, D.; Rampal, P. Experimental effects of Saccharomices boulardii on diarrheal pathogens. Microbes Infect. 2002, 4, 733–739. [Google Scholar] [CrossRef]
- Mojikon, F.D.; Kasimin, M.E.; Molujin, A.M.; Gansau, J.A.; Jawan, R. Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products. Nutrients 2022, 14, 3457. [Google Scholar] [CrossRef] [PubMed]
- Değirmencioğlu, N.; Gurbuz, O.; Ahan, Y.S. The monitoring, via an in vitro digestion system, of the bioactive content of vegetable juice fermented with Saccharomyces cerevisiae and Saccharomyces boulardii. J. Food Process Preserv. 2016, 40, 798–811. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Serna-Saldívar, S.O.; Rosales-Medina, M.F.; Tinoco-Alvear, M.; Briones-García, M. Application of Saccharomyces cerevisiae var. boulardii in food processing: A review. J. Appl. Microbiol. 2018, 125, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cota, G.Y.; López-Villegas, E.O.; Jiménez-Aparicio, A.R.; Hernández-Sánchez, H. Modeling the Ethanol Tolerance of the Probiotic Yeast Saccharomyces cerevisiae var. boulardii CNCM I-745 for its Possible Use in a Functional Beer. Probiotics Antimicrob. Proteins 2021, 13, 187–194. [Google Scholar] [CrossRef]
- Mulero-Cerezo, J.; Tuñón-Molina, A.; Cano-Vicent, A.; Pérez-Colomer, L.; Martí, M.; Serrano-Aroca, Á. Alcoholic and non-alcoholic rosé wines made with Saccharomyces cerevisiae var. boulardii probiotic yeast. Arch. Microbiol. 2023, 20, 201. [Google Scholar] [CrossRef]
- Gallo, M.; Bevilacqua, A.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. Alginate beds and apple pieces as carriers for Saccharomyces ceverisae var. boulardii, as representative of yeast functional starter cultures. Int. J. Food Sci. 2014, 49, 2092–2100. [Google Scholar] [CrossRef]
- Farinazzo, F.S.; Farinazzo, E.S.; Spinosa, W.A.; Garcia, S. Saccharomyces boulardii: Optimization of simultaneous saccharification and fermentation of cell production in organic and conventional apple substrate pulp. Food Sci. Biotechnol. 2017, 26, 969–977. [Google Scholar] [CrossRef]
- Profir, A.G.; Vizireanu, C. Sensorial Analysis of a Functional Beverage Based on Vegetables Juice. Acta Biol. 2013, 57, 145–148. [Google Scholar]
- Fratianni, F.; Cardinale, F.; Russo, I.; Iuliano, C.; Cucciniello, A.C.; Maione, M.; D’Acierno, A.; Nazzaro, F. Fermentation of tomato juice with the probiotic yeast Saccharomyces boulardii. In Functional Foods: Sources, Biotechnology Applications, and Health Challenges; Robinson, A., Emerson, D., Eds.; Nova Science Publisher: New York, NY, USA, 2013; pp. 143–152. ISBN 9781624174353. [Google Scholar]
- Lourens-Hattingh, A.; Viljoen, B.C. Growth and survival of a probiotic yeast in dairy products. Food Res. Int. 2011, 34, 791–796. [Google Scholar] [CrossRef]
- Karaolis, C.; Botsaris, G.; Pantelides, I.; Tsaltas, D. Potential application of Saccharomyces boulardii as a probiotic in goat’s yoghurt: Survival and organoleptic effects. Int. J. Food Sci. 2013, 48, 1445–1452. [Google Scholar] [CrossRef]
- Heenan, C.N.; Adams, M.C.; Hosken, R.W.; Fleet, G.H. Survival and sensory acceptability of probiotic microorganisms in a nonfermented frozen vegetarian dessert. LWT 2004, 37, 461–466. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Liu, S.-Q. Fortifying foods with synbiotic and postbiotic preparations of the probiotic yeast, Saccharomyces boulardii. Curr. Opin. Food Sci. 2022, 43, 216–224. [Google Scholar] [CrossRef]
- Talebi, M.; Frink, L.A.; Patil, R.A.; Armstrong, D.W. Examination of the varied and changing ethanol content of commercial kombucha products. Food Anal. Methods 2017, 10, 4062–4067. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. Global Regulatory Frameworks for Fermented Foods: A Review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate wine consumption and health: A narrative review. Nutrients 2023, 15, 175. [Google Scholar] [CrossRef]
- Paula, B.P.; Chávez, D.W.H.; Lemos Junior, W.J.F.; Guerra, A.F.; Corrêa, M.F.D.; Pereira, K.S.; Coelho, M.A.Z. Growth Parameters and Survivability of Saccharomyces boulardii for Probiotic Alcoholic Beverages Development. Front. Microbiol. 2019, 10, 2092. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.A.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Diaz, F.C.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zeng, Q.S.; Deng, Y.Y.; Zhang, M.W.; Wei, Z.C.; Zhang, Y.; Tang, X.J. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 2013, 136, 1169–1176. [Google Scholar] [CrossRef]
- Zeng, X.A.; Chen, X.D.; Qin, F.G.F.; Zhang, L. Composition analysis of litchi juice and litchi wine. Int. J. Food Eng. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Su, D.; Ti, H.; Zhang, R.; Zhang, M.; Wei, Z.; Deng, Y.; Guo, J. Structural elucidation and cellular antioxidant activity evaluation of major antioxidant phenolics in lychee pulp. Food Chem. 2014, 158, 385–391. [Google Scholar] [CrossRef]
- Sathya, R.; Arasu, M.V.; Ilavenil, S.; Rejiniemon, T.S.; Vijayaraghavan, P. Cosmeceutical potentials of litchi fruit and its by-products for a sustainable revalorization. Biocatal. Agric. Biotechnol. 2023, 50, 10268. [Google Scholar] [CrossRef]
- Alves, J.A.; Lima, L.C.O.; Dias, D.R.; Nunes, C.A.; Schwan, R.F. Effects of spontaneous and inoculated fermentation on the volatile profile of lychee (Litchi chinensis Sonn) fermented beverages. Int. J. Food Sci. Technol. 2010, 45, 2358–2365. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Sánchez-González, I.; Jiménez-Escrig, A.; Saura-Calixto, F. In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter). Food Chem. 2005, 90, 133–139. [Google Scholar] [CrossRef]
- Pauli, E.D.; Cristiano, V.; Nixdorf, S.L. Method for determining carbohydrates used in screening for adulterations in coffee. Quim. Nova 2011, 34, 689–694. [Google Scholar] [CrossRef]
- Terhaag, M.M.; Constatino, L.V.; Watanabe, L.S.; Madeira, T.B.; Nixdorf, S.L.; Prudencio, S.H. Carbohydrates, organic acids and phenolic compounds in yerba mate leaves and infusion. Rev. Mundi Amb. Agric. 2021, 61–20. Available online: https://periodicos.ifpr.edu.br/index.php/MundiMAA/article/view/1475/886 (accessed on 17 December 2024).
- Bagewadi, Z.; Mulla, S.; Ninnekar, H.Z. Purification and characterization of endo b-1,4-D-glucanase from Trichoderma harzianum strain HZN11 and its application in production of bioethanol from sweet sorghum bagasse. 3 Biotech 2016, 6, 101. [Google Scholar] [CrossRef]
- Villanueva, N.D.M.; Petenate, A.J.; Da Silva, M.A.A.P. Performance of hybrid hedonic scale as compared to the traditional hedonic, self-adjusting and ranking scales. Food Qual. Prefer. 2005, 16, 691–703. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A Guide for its Bootstrap procedures in multiple comparisons. Cienc. Agrotecnol. 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Statsoft. Statistic for Windows: Computer Program Manual, 7.1 version; Software Inc.: Tulsa, OK, USA, 2006.
- Lee, Y.L.; Salminen, S. The coming of age of probiotics. Trends Food Sci. Technol. 1996, 6, 241–245. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; W.H. Freeman: New York, NY, USA, 2017; p. 1308. ISBN 9781464126116. [Google Scholar]
- Reitenbach, A.F.; Iwassa, I.J.; Barros, B.C.B. Production of functional beer with the addition of probiotic: Saccharomyces boulardii. Res. Soc. Dev. 2021, 10, e5010212211. [Google Scholar] [CrossRef]
- Bai, F.W.; Anderson, W.A.; Woo-Young, M. Ethanol fermentation Technologies from sugar and starch feedstocks. Biot. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Gaboardi, G.; Dos Santos, D.G.; Mendes, L.; Centeno, L.; Meireles, T.; Vargas, S.; Griep, E.; Silva, A.C.J.; Moreira, N.A.; Conceição, F.R. Bioremediation and biomass production from the cultivation of probiotic Saccharomyces boulardii in parboiled rice effluent. J. Environ. Manag. 2018, 226, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hedin, K.A. Establishing Saccharomyces boulardii as an Advanced Microbiome Therapeutic Platform. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2022. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/351170515/PhD_Thesis_Karl_Alex_Hedin.pdf (accessed on 10 June 2024).
- Suter, P.M.; Schutz, Y. The Effect of Exercise, Alcohol or Both Combined on Health and Physical Performance. Int. J. Obes. 2008, 32, S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Mena, B.; Aryana, K.J. Influence of ethanol on probiotic and culture bacteria Lactobacillus bulgaricus and Streptococcus thermophilus within a Therapeutic Product. J. Med. Microbiol. 2012, 2, 3. [Google Scholar] [CrossRef]
- Sulieman, A.K.; Putra, M.D.; Abasaeed, A.E.; Gaily, M.H.; Al-Zahrani, S.M.; Zeinelabdeen, M.A. Kinetic modelling of the simultaneous production of ethanol and fructose by Saccharomyces cerevisiae. Electron. J. Biotechnol. 2018, 34, 1–8. [Google Scholar] [CrossRef]
- Parker, K.; Salas, M.; Nwosu, V.C. High Fructose Corn Syrup: Production, Uses and Public Health Concerns. Biotechnol. Mol. Biol. Rev. 2010, 5, 71–78. Available online: https://academicjournals.org/article/article1380113250_Parker%20et%20al.pdf (accessed on 17 December 2024).
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces cerevisiae var. boulardii: Valuable Probiotic Starter for Craft Beer Production. Appl. Sci. 2019, 9, 3250. [Google Scholar] [CrossRef]


| Parameters | Step | Time | Formulations 2 | ||
|---|---|---|---|---|---|
| LB4 | LB8 | LB12 | |||
| Viability (log CFU/mL) | Preparation 3 | 0 h | 5.0 ± 0.0 dA | 5.0 ± 0.0 eA | 5.0 ± 0.0 fA |
| 24 h | 6.8 ± 0.0 cA | 6.8 ± 0.0 cdA | 6.8 ± 0.0 deA | ||
| Cold storage | 1 d | 7.4 ± 3.6 bB | 7.3 ± 3.4 bB | 7.5 ± 2.7 cA | |
| 7 d | 7.6 ± 2.9 aC | 7.7 ± 1.0 aB | 7.8 ± 3.6 aA | ||
| 14 d | 6.3 ± 0.9 cB | 6.9 ± 0.8 cB | 7.8 ± 3.5 bA | ||
| 21 d | 5.9 ± 0.1 cB | 5.8 ± 0.1 cdB | 6.9 ± 0.5 dA | ||
| 28 d | 5.7 ± 0.3 cA | 5.7 ± 0.0 cdA | 5.8 ± 0.0 eA | ||
| TSS (°Brix) | Preparation 3 | 0 h | 12.0 ± 0.0 aA | 12.0 ± 0.0 aA | 12.0 ± 0.0 aA |
| 24 h | 4.1 ± 0.0 bA | 4.1 ± 0.0 fA | 4.1 ± 0.0 bA | ||
| Cold storage | 1 d | 3.7 ± 0.1 cC | 7.0 ± 0.1 bB | 10.1 ± 0.0 bA | |
| 7 d | 3.7 ± 0.0 cC | 5.9 ± 0.0 bB | 9.5 ± 0.0 cA | ||
| 14 d | 3.7 ± 0.0 cC | 5.1 ± 0.0 cB | 8.5 ± 0.1 dA | ||
| 21 d | 3.7 ± 0.0 cC | 4.7 ± 0.0 dB | 7.6 ± 0.1 eA | ||
| 28 d | 3.7 ± 0.0 cC | 4.6 ± 0.1 eB | 6.6 ± 0.4 fA | ||
| pH | Preparation 3 | 0 h | 4.5 ± 0.0 aA | 4.5 ± 0.0 aA | 4.5 ± 0.0 aA |
| 24 h | 3.6 ± 0.0 bcA | 3.8 ± 0.0 dA | 3.8 ± 0.0 cA | ||
| Cold storage | 1 d | 3.7 ± 0.0 cA | 3.7 ± 0.1 eB | 3.6 ± 0.0 eC | |
| 7 d | 3.8 ± 0.0 bB | 3.9 ± 0.0 bA | 3.8 ± 0.0 bB | ||
| 14 d | 3.8 ± 0.0 bcB | 3.8 ± 0.0 cA | 3.7 ± 0.0 dC | ||
| 21 d | 3.8 ± 0.0 bcA | 3.8 ± 0.0 dA | 3.6 ± 0.0 dC | ||
| 28 d | 3.4 ± 0.0 dB | 3.5 ± 0.1 fA | 3.5 ± 0.0 fA | ||
| Ethanol (%) | Preparation 3 | 0 h | 0.0 ± 0.0 cA | 0.0 ± 0.0 cA | 0.0 ± 0.0 fA |
| 24 h | 4.0 ± 0.1 bA | 4.0 ± 0.1 bA | 4.0 ± 0.1 eA | ||
| Cold storage | 1 d | 5.6 ± 0.1 aB | 7.5 ± 0.29 aA | 6.2 ± 0.2 dB | |
| 7 d | 5.6 ± 0.1 aB | 7.5 ± 0.02 aA | 7.1 ± 0.0 cA | ||
| 14 d | 5.8 ± 0.0 aB | 7.8 ± 0.77 aA | 7.8 ± 0.1 cA | ||
| 21 d | 6.1 ± 0.1 aB | 8.1 ± 0.03 aA | 8.7 ± 0.0 bA | ||
| 28 d | 6.2 ± 0.2 aC | 8.1 ± 0.06 aB | 10.1 ± 0.9 aA | ||
| Parameters | Time of Cold Storage (Days) | Formulations 2 | ||
|---|---|---|---|---|
| LB4 | LB8 | LB12 | ||
| TPC 3 | 1 | 151.67 ± 7.32 Ab | 133.70 ± 6.67 bcB | 190.08 ± 4.30 abA |
| 7 | 144.46 ± 3.09 abB | 157.01 ± 2.38 abB | 202.10 ± 34.00 Aa | |
| 14 | 129.34 ± 4.56 abB | 169.49 ± 2.65 aA | 176.68 ± 5.89 bcA | |
| 21 | 121.87 ± 1.17 bB | 123.55 ± 3.28 bC | 153.17 ± 1.46 cdA | |
| 28 | 121.87 ± 1.97 aB | 122.80 ± 2.44 aC | 140.13 ± 0.84 aD | |
| DPPH 4 | 1 | 0.73 ± 0.04 Aa | 0.80 ± 0.11 aA | 0.51 ± 0.15 abB |
| 7 | 0.59 ± 0.09 abA | 0.69 ± 0.20 aA | 0.68 ± 0.13 aA | |
| 14 | 0.43 ± 0.13 bcAB | 0.64 ± 0.04 aA | 0.41 ± 0.11 bB | |
| 21 | 0.33 ± 0.02 aC | 0.27 ± 0.01 aB | 0.33 ± 0.01 aB | |
| 28 | 0.31 ± 0.02 aC | 0.25 ± 0.01 aB | 0.30 ± 0.03 aB | |
| FRAP 4 | 1 | 1.20 ± 0.07 aA | 1.19 ± 0.02 Aa | 1.13 ± 0.02 aB |
| 7 | 0.82 ± 0.06 bcB | 1.24 ± 0.10 Aa | 1.23 ± 0.03 aA | |
| 14 | 0.90 ± 0.02 cB | 1.00 ± 0.02 Bb | 1.18 ± 0.02 abA | |
| 21 | 0.79 ± 0.05 abC | 0.73 ± 0.01 Cb | 0.85 ± 0.01 aC | |
| 28 | 0.78 ± 0.01 aC | 0.78 ± 0.02 Ca | 0.71 ± 0.04 aD | |
| ABTS 4 | 1 | 6.11 ± 0.33 aA | 5.42 ± 0.71 bB | 5.61 ± 0.13 abB |
| 7 | 4.23 ± 0.02 bB | 6.29 ± 0.16 aA | 6.29 ± 0.16 aA | |
| 14 | 2.44 ± 0.15 bC | 3.32 ± 0.05 aC | 3.30 ± 0.10 aC | |
| 21 | 1.71 ± 0.15 aD | 0.29 ± 0.06 bD | 0.28 ± 0.08 bD | |
| 28 | 0.87 ± 0.05 aB | 0.11 ± 0.05 bD | 0.03 ± 0.00 bD | |
| Parameters 3 | |||||
|---|---|---|---|---|---|
| Formulations 2 | Color | Aroma | Flavor | Texture | Global Acceptability |
| LB12/1 | 7.5 ± 1.5 ab | 7.8 ± 1.6 a | 7.7 ± 1.9 a | 7.9 ± 1.6 a | 7.7 ± 1.7 a |
| LB12/7 | 6.6 ± 2.2 c | 7.6 ± 1.8 a | 7.4 ± 1.9 a | 7.8 ± 1.6 a | 7.4 ± 1.7 a |
| LB12/14 | 7.8 ± 1.4 a | 7.9 ± 1.4 a | 7.5 ± 1.8 a | 8.0 ± 1.5 a | 7.7 ± 1.5 a |
| LB12/21 | 6.9 ± 2.1 bc | 7.7 ± 1.4 a | 7.6 ± 1.8 a | 7.9 ± 1.2 a | 7.5 ± 1.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira Terhaag, M.; Sakai, O.A.; Ruiz, F.; Garcia, S.; Bertusso, F.R.; Prudêncio, S.H. The Probiotication of a Lychee Beverage with Saccharomyces boulardii: An Alternative to Dairy-Based Probiotic Products. Foods 2025, 14, 156. https://doi.org/10.3390/foods14020156
Moreira Terhaag M, Sakai OA, Ruiz F, Garcia S, Bertusso FR, Prudêncio SH. The Probiotication of a Lychee Beverage with Saccharomyces boulardii: An Alternative to Dairy-Based Probiotic Products. Foods. 2025; 14(2):156. https://doi.org/10.3390/foods14020156
Chicago/Turabian StyleMoreira Terhaag, Marcela, Otávio Akira Sakai, Fabiana Ruiz, Sandra Garcia, Fernando Rodrigo Bertusso, and Sandra Helena Prudêncio. 2025. "The Probiotication of a Lychee Beverage with Saccharomyces boulardii: An Alternative to Dairy-Based Probiotic Products" Foods 14, no. 2: 156. https://doi.org/10.3390/foods14020156
APA StyleMoreira Terhaag, M., Sakai, O. A., Ruiz, F., Garcia, S., Bertusso, F. R., & Prudêncio, S. H. (2025). The Probiotication of a Lychee Beverage with Saccharomyces boulardii: An Alternative to Dairy-Based Probiotic Products. Foods, 14(2), 156. https://doi.org/10.3390/foods14020156






