Screening of Lactic Acid Bacteria Isolated from Fermented Cowpea and Optimization of Biomass Production Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Isolation and Screening of LAB Strains from Fermented Cowpea
2.3. DNA Extraction and PCR Amplification for 16S rRNA Gene Sequence
2.4. Preliminary Selection of Strains
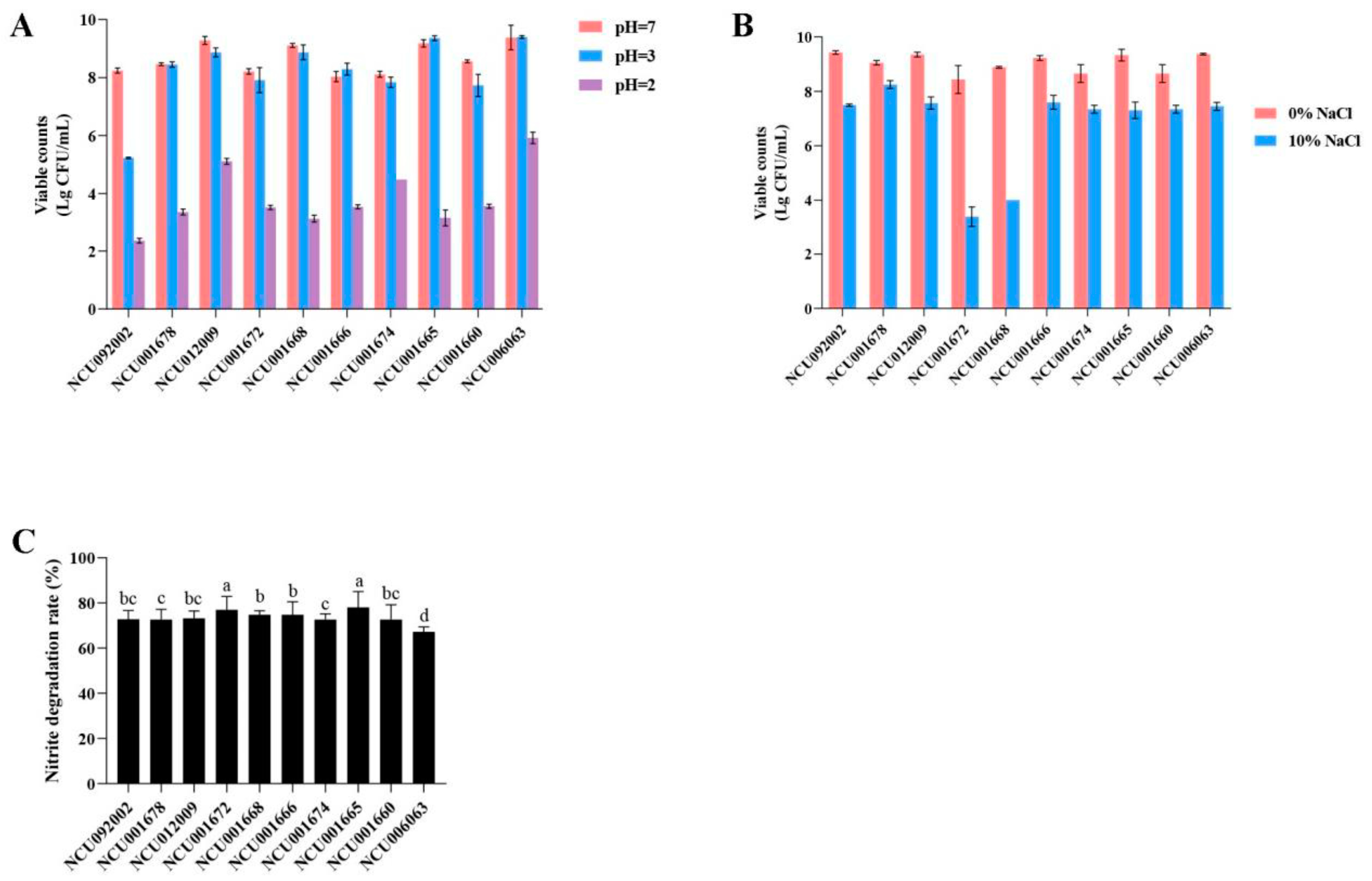
2.4.1. Determination of Acid Production Capacity
2.4.2. Determination of Acid Tolerance Capacity
2.4.3. Determination of Salt Tolerance Capacity
2.4.4. Determination of Degrade Nitrite Capacity
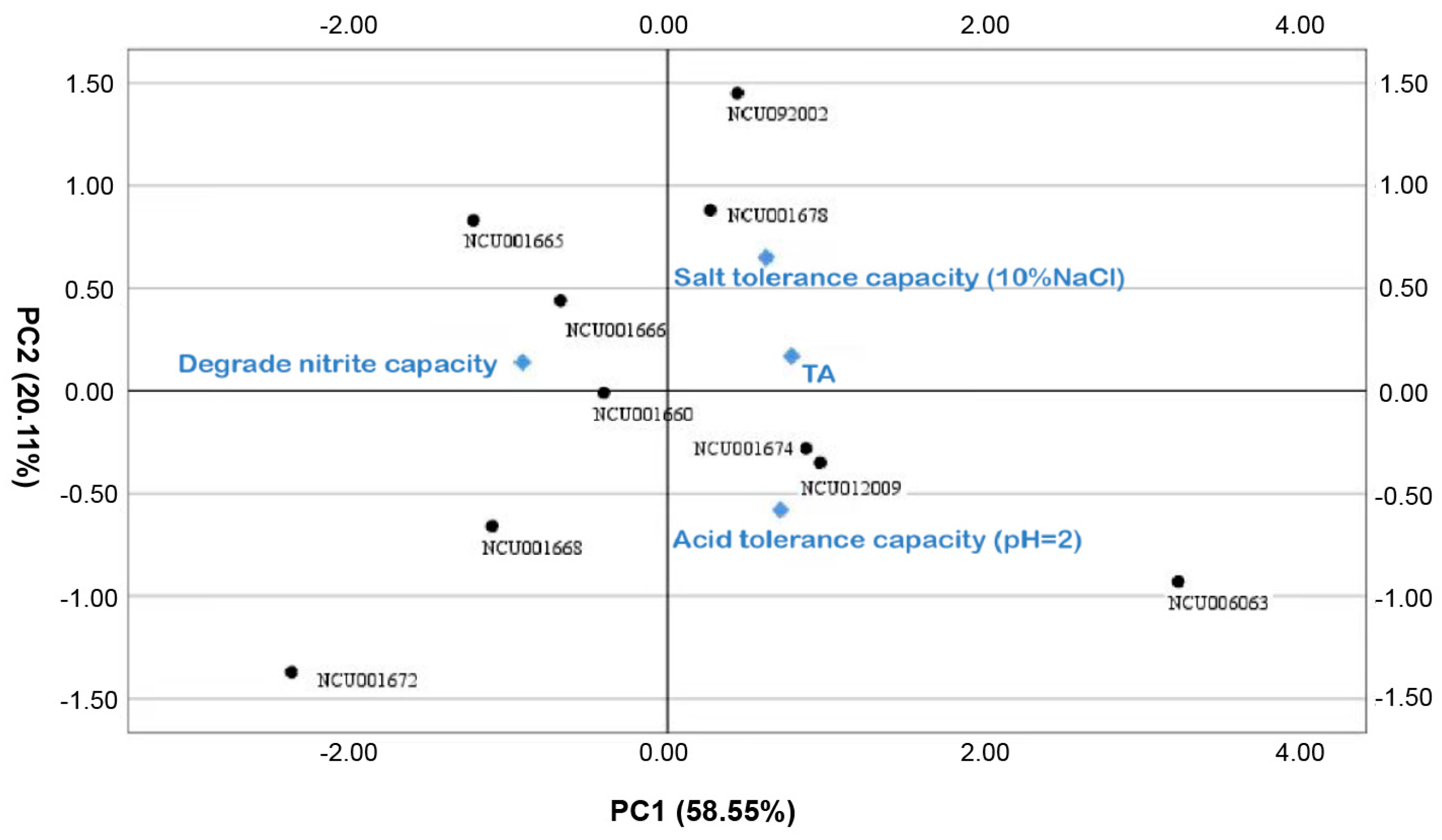
2.4.5. Principal Component Analysis for the Screening of Bacterial Strain
2.5. Effects of the Composition and Culture Conditions of MRS Broth on Dominant Strain Growth
2.5.1. The One Factor per Time (OFT) Approach
2.5.2. Plackett–Burman Design (PBD)
2.5.3. Steepest Ascent Experiment
2.5.4. The Response Surface Analysis (RSM) Approach Utilizing the Central Composite Design (CCD) Technique
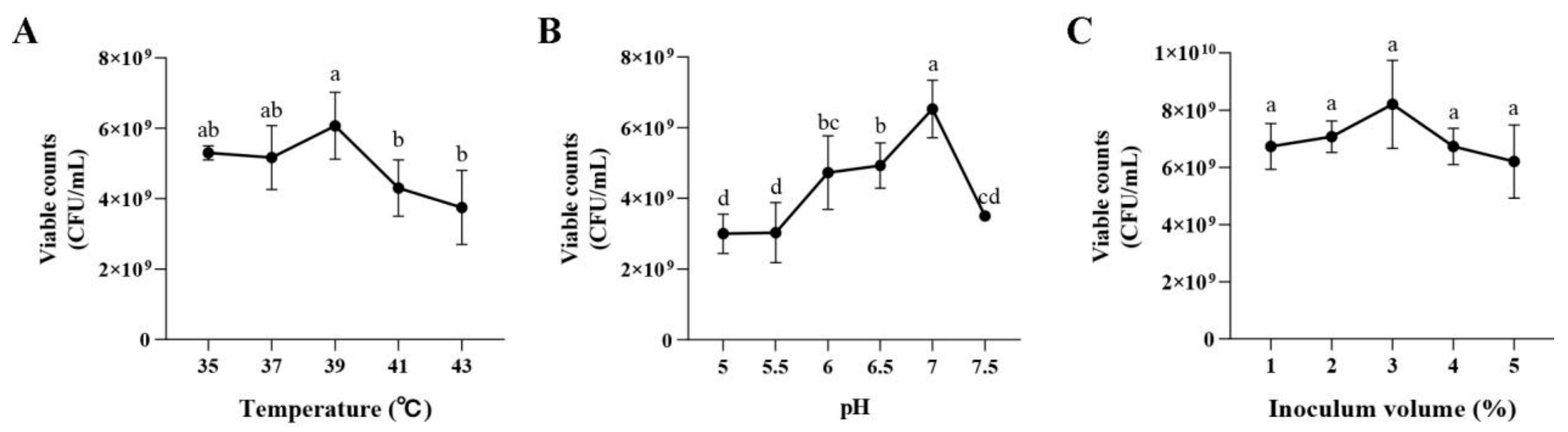
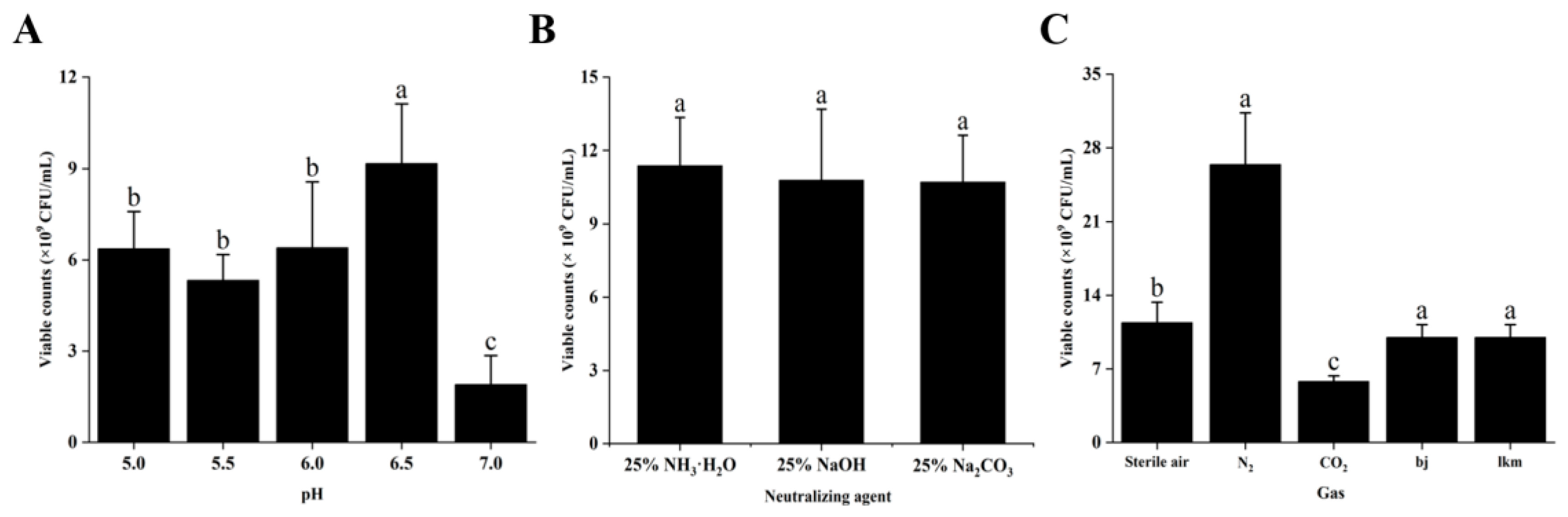
2.5.5. Effects of the MRS Broth Culture Conditions on Dominant Strain Growth
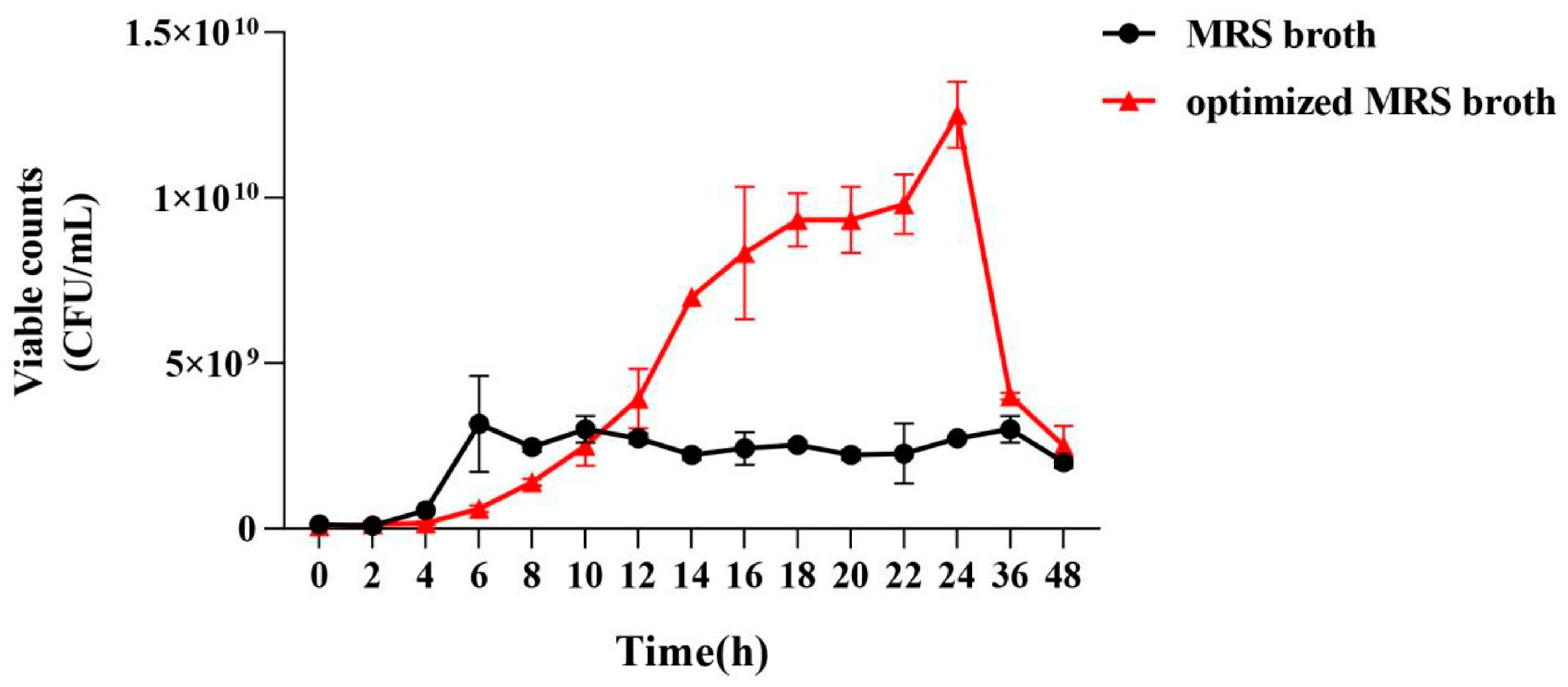
2.5.6. Comparison of Growth Curves
2.6. Optimization of Biomass Production Process Parameters
2.7. Statistical Analysis
3. Results
3.1. Strain Screening
3.1.1. Determination of Acid Production Capacity
3.1.2. Determination of Acid Tolerance Capacity
3.1.3. Determination of Salt Tolerance Capacity
3.1.4. Determination of Degrade Nitrite Capacity
3.1.5. Principal Component Analysis of Bacterial Strain Properties
3.2. Effects of the Compositions and Culture Conditions of MRS Broth on P. pentosaceus NCU006063 Growth
3.2.1. Effects of the Compositions of MRS Broth on P. pentosaceus NCU006063 Growth
3.2.2. Screening of Significant Variables Using Plackett–Burman Design
3.2.3. Steepest Ascent Experiment
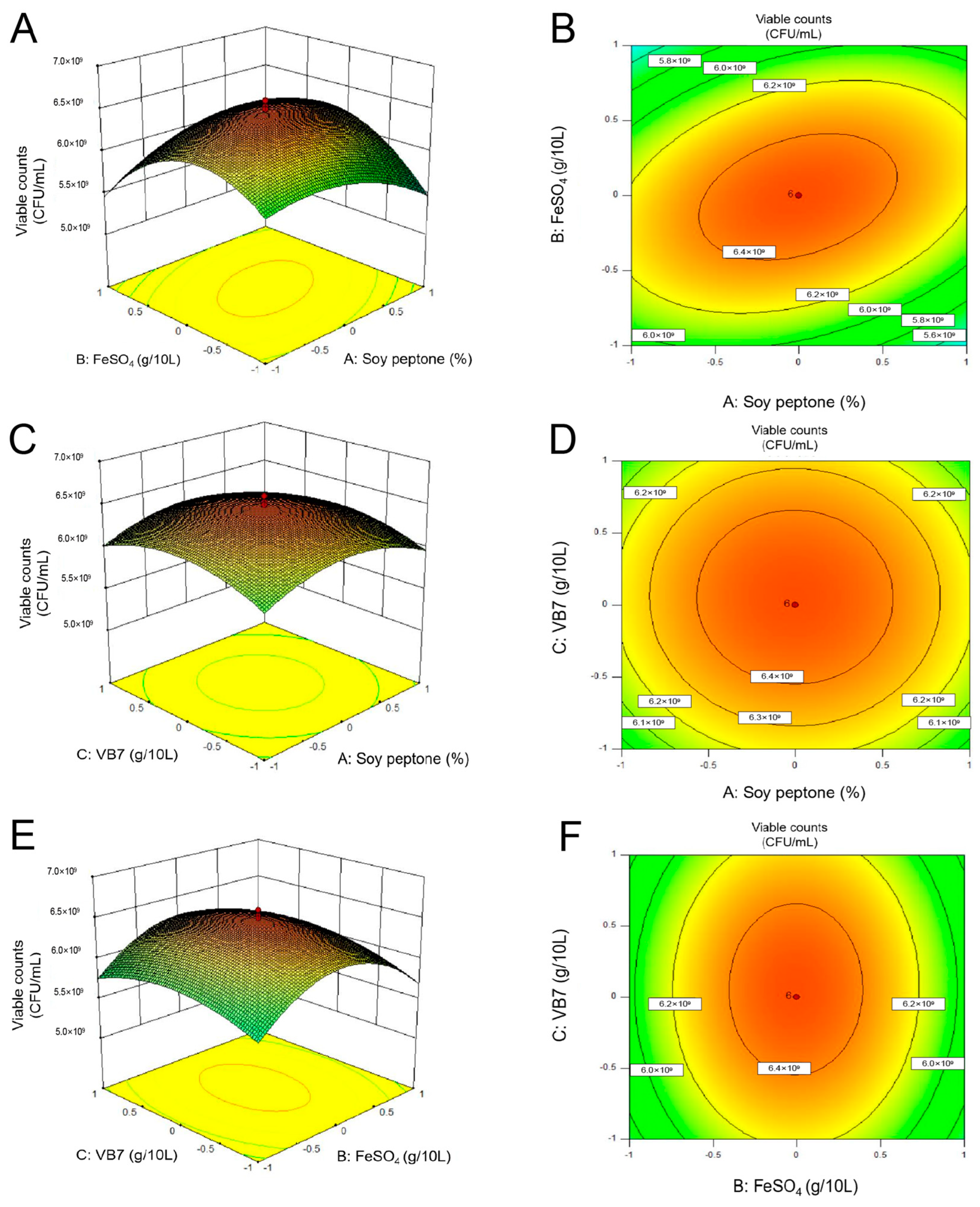
3.2.4. Center Combination Design (CCD)
3.2.5. Effects of the MRS Broth Culture Conditions on P. pentosaceus NCU006063 Growth
3.2.6. Comparison of the Growth Curves of P. pentosaceus NCU006063
3.3. Optimization of Biomass Production Process Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L.): Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Madode, Y.E.; Nout, M.J.R.; Bakker, E.J.; Linnemann, A.R.; Hounhouigan, D.J.; van Boekel, M. Enhancing the digestibility of cowpea (Vigna unguiculata) by traditional processing and fermentation. LWT-Food Sci. Technol. 2013, 54, 186–193. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Wang, Y.; Chen, M.; Sui, Z. Influence of Changes of Fermentation Ways on Texture and Anti-oxidation Performance of Vegetable pickle with Flavor of Garlic. Food Ferment. Ind. 2007, 33, 26–28. [Google Scholar]
- Zheng, X.F.; Yang, Z.Q.; Zhang, H.; Jin, W.X.; Xu, C.W.; Gao, L.; Rao, S.Q.; Jiao, X.A. Isolation of virulent phages infecting dominant mesophilic aerobic bacteria in cucumber pickle fermentation. Food Microbiol. 2020, 86, 103330. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, X.Y.; Yang, S.; Wang, K.L.; Zeng, C.L.; Hou, Z.Z.; Cui, H.L.; Liu, S.K.; Zhu, L. Denitrifying halophilic archaea derived from salt dominate the degradation of nitrite in salted radish during pickling. Food Res. Int. 2022, 152, 110906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Lai, H.M.; He, W.; Wang, Y.L.; Huang, Y.L.; Zhao, M.H.; Li, Y.S.; Zhu, S.Y.; Hu, X.J.; Zhu, Y.Q.; et al. Reduction of biogenic amine and nitrite production in low-salt Paocai by controlled package during storage: A study comparing vacuum and aerobic package with conventional salt solution package. Food Control 2021, 123, 107858. [Google Scholar] [CrossRef]
- Hansen, E.B. Commercial bacterial starter cultures for fermented foods of the future. Int. J. Food Microbiol. 2002, 78, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Ren, H.Y.; Liu, D.Y.; Zhu, W.Y.; Wang, W. Effects of inoculating Lactobacillus sakei starter cultures on the microbiological quality and nitrite depletion of Chinese fermented sausages. Food Control 2013, 32, 591–596. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zou, H.F.; Qu, C.; Zhang, L.Q.; Liu, T.; Wu, H.; Li, Y.H. Dominant Microorganisms during the Spontaneous Fermentation of Suan Cai, a Chinese Fermented Vegetable. Food Sci. Technol. Res. 2014, 20, 915–926. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhao, L.J.; Li, M.Y.; Zhu, Y.D.; Liang, D.; Ma, Y.Y.; Sun, L.X.; Zhao, G.M.; Tu, Q.C. Probiotic Bacillus as fermentation agents: Status, potential insights, and future perspectives. Food Chem. X 2024, 22, 101465. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Sun, M.; Ma, T.; Wang, W.; Lv, B.; Wang, J.; Su, X.; Li, S.; Guo, Y. Revealing the formation mechanisms of key flavor components during the fermentation of bamboo shoots by combining flavoromics and metagenomics. Food Res. Int. 2024, 198, 115361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-P.; Cao, Q.; Meng, T.-T.; Shi, W.; Zhang, X.-J.; Chai, L.-J.; Shi, J.-S.; Chen, F.-W.; Wang, S.-T.; Zhang, S.-Y.; et al. Yeast community in the first-round fermentation of sauce-flavor Baijiu: Source, succession and metabolic function. Food Res. Int. 2025, 200, 115466. [Google Scholar] [CrossRef]
- Zuo, X.; Lu, L.; Li, Y.; Xiong, X. Strategy of high cell-density culture of microorganisms. Shengwu Jiagong Guocheng 2016, 14, 81–86. [Google Scholar] [CrossRef]
- de Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus plantarum—Survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sun, H.R.; Fan, H.R.; Tian, Z.G.; Fan, J.Y.; Zhao, Y.J.; Duan, C.C.; Kang, L.N. The optimization of high cell density culturing of probiotic Lactobacillus plantarum K25. Food Sci. Technol. 2018, 43, 20–25. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.Z.; Xia, X.D.; Zhao, Y.C.; Shao, W.L. Probiotic potential of Lactobacillus paracasei FM-LP-4 isolated from Xinjiang camel milk yoghurt. Int. Dairy J. 2016, 62, 28–34. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.Z.; Lu, J.; Ma, Y.H.; Zhang, Z.C.; Zhang, L.X.; Huang, K.H. High cell density culture and releasing property of lactic acid bacteria in microencapsulation. Jiangsu J. Agric. Sci. 2011, 27, 658–662. [Google Scholar]
- Oh, C.K.; Oh, M.C.; Kim, S.H. The depletion of sodium nitrite by lactic acid bacteria isolated from kimchi. J. Med. Food 2004, 7, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Li, J.Y.; Huang, T.; Xiao, Y.S.; Peng, Z.; Xie, M.Y.; Xiong, T. Comparison of the bacterial communities in home-made Nanfeng yancai with and without salt. Food Res. Int. 2019, 125, 108509. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.O.; Shamila-Syuhada, A.K.; Liong, M.T.; Rosma, A.; Thong, K.L.; Rusul, G. Physio-chemical, microbiological properties of tempoyak and molecular characterisation of lactic acid bacteria isolated from tempoyak. Food Microbiol. 2016, 58, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Vaccalluzzo, A.; Solieri, L.; Romeo, F.V.; Todaro, A.; Caggia, C.; Arroyo-López, F.N.; Bautista-Gallego, J.; Randazzo, C.L. Effect of Sequential Inoculum of Beta-Glucosidase Positive and Probiotic Strains on Brine Fermentation to Obtain Low Salt Sicilian Table Olives. Front. Microbiol. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Maragkoudakis, P.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Dodds, K.L.; Collinsthompson, D.L. Incidence of Nitrite-Depleting Lactic-Acid Bacteria in Cured Meats and in Meat Starter Cultures. J. Food Prot. 1984, 47, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Hwanhlem, N.; Chobert, J.M.; H-Kittikun, A. Bacteriocin-producing lactic acid bacteria isolated from mangrove forests in southern Thailand as potential bio-control agents in food: Isolation, screening and optimization. Food Control 2014, 41, 202–211. [Google Scholar] [CrossRef]
- Song, X.J.; Zhang, X.C.; Kuang, C.H.; Zhu, L.Y.; Guo, N. Optimization of fermentation parameters for the biomass and DHA production of Schizochytrium limacinum OUC88 using response surface methodology. Process Biochem. 2007, 42, 1391–1397. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiment. In Environmental and Engineering Geoscience; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.-J.; Gao, P.-F.; Zhang, H.-P.; Chen, Y.-F. Optimization of Cultural Conditions of Probiotic Bacteria Lactobacillus casei Zhang for High Cell Density Cultivation. Microbiology 2009, 36, 181–186. [Google Scholar]
- Liu, S.; Li, Z.; Zhou, L.; Ge, Y.; Liu, W.; Zhang, C. Optimization of Fermentation Conditions for Cellulase Production by Aspergillus niger Using Response Surface Methodology. Food Sci. 2013, 34, 225–229. [Google Scholar]
- Yan, P.M.; Xue, W.T.; Cheng, Y.Q.; Hu, X.P.; Li, X.Y.; Zhang, Z.L.; You, L.L. Study on the reasons for the formation and disappearance of nitrite peak in cabbage fermentation. In Proceedings of the International Conference on Innovation and Development of Urban Agricultural Engineering, Beijing, China, 14–16 September 2005; pp. 457–466. [Google Scholar]
- Zhang, Q.; Li, M.; Wang, X.; Hu, S.; Yu, S.; Chi, N. Research progress of microbial nitrite reductase. Microbiol. China 2019, 46, 3148–3157. [Google Scholar]
- Li, Y.Y.; Ten, M.M.Z.; Zwe, Y.H.; Li, D. Lactiplantibacillus plantarum 299v as starter culture suppresses Enterobacteriaceae more efficiently than spontaneous fermentation of carrots. Food Microbiol. 2022, 103, 103952. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.S.; He, Z.F.; Luo, P.W.; He, L.; Zhou, K.; Hu, X.J.; Han, X.F.; Yan, Z.C.; Liu, S.L.; Hu, B. Quality analysis of mustard pickles in different fermentation process. Food Ferment. Ind. 2015, 41, 111–116. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.X.; Ibrahim, S.A.; Khaskheli, S.G.; Yang, H.; Wang, Y.F.; Huang, W. Characterization of Lactobacillus pentosus as a starter culture for the fermentation of edible oyster mushrooms (Pleurotus spp.). LWT-Food Sci. Technol. 2016, 68, 21–26. [Google Scholar] [CrossRef]
- Lin, J.; Hui, Z.; Kunjie, L.; Shenglan, H.; Wenfeng, H. Solid-state fermentation of fish meal with mixed culture of microorganisms. Feed Ind. 2017, 38, 35–40. [Google Scholar] [CrossRef]
- Zeng, Z.; Lin, J.; Gong, D. Identification of Lactic Acid Bacterial Strains with High Conjugated Linoleic Acid-Producing Ability from Natural Sauerkraut Fermentations. J. Food Sci. 2009, 74, M154–M158. [Google Scholar] [CrossRef] [PubMed]
- Gezginc, Y.; Topcal, F.; Comertpay, S.; Akyol, I. Quantitative analysis of the lactic acid and acetaldehyde produced by Streptococcus thermophilus and Lactobacillus bulgaricus strains isolated from traditional Turkish yogurts using HPLC. J. Dairy Sci. 2015, 98, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Du, R.P.; Ge, J.P.; Wang, X.Y.; Mu, L.Q.; Ping, W.X. Impact of Lactobacillus paracasei HD1.7 as a Starter Culture on Characteristics of Fermented Chinese Cabbage (Brassica rapa var. pekinensis). Food Sci. Technol. Res. 2016, 22, 325–330. [Google Scholar] [CrossRef]
- Duan, Y.; Jin, Y.; Jin, Z.; Tong, L. Growth and acid-production ability of lactic acid bacteria from traditional sausages in Inner Mongolia. Sci. Technol. Food Ind. 2012, 33, 206. [Google Scholar]
- Di Cagno, R.; Surico, R.F.; Minervini, G.; De Angelis, M.; Rizzello, C.G.; Gobbetti, M. Use of autochthonous starters to ferment red and yellow peppers (Capsicum annum L.) to be stored at room temperature. Int. J. Food Microbiol. 2009, 130, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Verón, H.E.; Di Risio, H.D.; Isla, M.I.; Torres, S. Isolation and selection of potential probiotic lactic acid bacteria from Opuntia ficus-indica fruits that grow in Northwest Argentina. LWT-Food Sci. Technol. 2017, 84, 231–240. [Google Scholar] [CrossRef]
- Dunne, C.; O’Mahony, L.; Murphy, L.; Thornton, G.; Morrissey, D.; O’Halloran, S.; Feeney, M.; Flynn, S.; Fitzgerald, G.; Daly, C.; et al. In vitro selection criteria for probiotic bacteria of human origin: Correlation with in vivo findings. Am. J. Clin. Nutr. 2001, 73, 386S–392S. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.N.; Nielsen, V.R.; Hayford, A.E.; Moller, P.L.; Michaelsen, K.F.; Pærregaard, A.; Sandstrom, B.; Tvede, M.; Jakobsen, M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999, 65, 4949–4956. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, Y.; Pan, S. Study on the Change of the Cowpea Quality Under Different Pickling Conditions. Food Sci. 2006, 27, 122–125. [Google Scholar]
- Panagou, E.Z.; Schillinger, U.; Franz, C.; Nychas, G.J.E. Microbiological and biochemical profile of cv. Conservolea naturally black olives during controlled fermentation with selected strains of lactic acid bacteria. Food Microbiol. 2008, 25, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Jia, X.Z.; Yu, J.J.; Chen, Y.H.; Liu, D.M.; Liang, M.H. Effect of different lactic acid bacteria on nitrite degradation, volatile profiles, and sensory quality in Chinese traditional paocai. LWT-Food Sci. Technol. 2021, 147, 111597. [Google Scholar] [CrossRef]
- Oh, C.-K.; Oh, M.C.; Kim, S.-H. Depletion of Nitrite by Lactic Acid Bacteria Isolated from Commercial Kimchi. J. Korean Soc. Food Sci. Nutr. 2009, 38, 892–901. [Google Scholar]
- Ji, F.D.; Ji, B.P.; Li, B.; Lu, F. Effect of fermentation on nitrate, nitrite and organic acid contents in traditional pickled chinese cabbage. J. Food Process. Preserv. 2009, 33, 175–186. [Google Scholar] [CrossRef]
- Walker, R. Naturally occuring nitrate/nitrite in foods. J. Sci. Food Agric. 1975, 26, 1735–1742. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, W.; Liu, Y.; Wang, R.; Wang, Z.; Li, X. Isolation and Identification of Lactic Acid Bacteria from Traditional Fermented Milk in Western China and Their Nitrite Degradation Capability. Food Sci. 2018, 39, 199–205. [Google Scholar]
- Zhai, Q.X.; Yin, R.J.; Yu, L.L.; Wang, G.; Tian, F.W.; Yu, R.P.; Zhao, J.X.; Liu, X.M.; Chen, Y.Q.; Zhang, H.; et al. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control 2015, 54, 23–30. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.X.; Dang, H.; Liu, X.M.; Tian, F.W.; Zhao, J.X.; Chen, Y.Q.; Zhang, H.; Chen, W. Screening for potential new probiotic based on probiotic properties and α-glucosidase inhibitory activity. Food Control 2014, 35, 65–72. [Google Scholar] [CrossRef]
- Cai, T.; Wu, H.Z.; Qin, J.L.; Qiao, J.N.; Yan, Y.X.; Wu, Y.; Qiao, D.R.; Xu, H.; Cao, Y. In vitro evaluation by PCA and AHP of potential antidiabetic properties of lactic acid bacteria isolated from traditional fermented food. LWT-Food Sci. Technol. 2019, 115, 108455. [Google Scholar] [CrossRef]
- Huaxi, Y.I.; Han, X.; Ming, D.U.; Zhang, L. Effect of MRS medium components on bacteriocin Bac-J23 production from Lactobacillus J23. China Brew. 2012, 31, 56–58. [Google Scholar]
- Liu, C.; Zhang, J.; Fang, Y.; Gao, Z.; Wei, T. Studies on the effects of isomaltooligosaccharides regulating intestinal microbial flora proliferation and relieving constipated function. Sci. Technol. Food Ind. 2018, 39, 298–302. [Google Scholar]
- Xu, Q.S.; Yan, Y.S.; Feng, J.S. Characterization of a Recombinant Raw Starch-digesting Glucoamylase from Penicillium oxalicum. Guangxi Sci. 2017, 24, 83–91. [Google Scholar]
- Li, Y. Quality Changes and Control of Lactobacillus Fermented Fruit and Vegetable Juice During Processing and Storage. Master’s Thesis, South China University of Technology, Guangzhou, China, 2017. [Google Scholar]
- Wang, C.H.; Wang, C.C.; Wei, Y.H. Mitochondrial dysfunction in insulin insensitivity: Implication of mitochondrial role in type 2 diabetes. In Mitochondrial Research in Translational Medicine; Wei, Y.H., Tzeng, C.R., Lee, H.M., Eds.; Wiley-Blackwell: Malden, MA, USA, 2010; Volume 1201, pp. 157–165. [Google Scholar]
- Yang, J.; Duan, Z.; Ding, W.; Xu, J.; Gu, Z.; Liang, X. Effects of VB7 and VB12 on Biofilm Formation and Larval Metamorphosis of the Mussel Mytilus coruscus. Prog. Fish. Sci. 2021, 42, 113–123. [Google Scholar] [CrossRef]
- Li, M.T.; Gao, S.H.; Yang, P.C.; Li, H.J. Improvement of ribonucleic acid production in Cyberlindnera jadinii and optimization of fermentation medium. AMB Express 2024, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Basmak, S.; Turhan, I. Production of β-mannanase, inulinase, and oligosaccharides from coffee wastes and extracts. Int. J. Biol. Macromol. 2024, 261, 129798. [Google Scholar] [CrossRef]
- Chettri, D.; Verma, A.K. Statistical optimization of cellulase production from Bacillus sp. YE16 isolated from yak dung of the Sikkim Himalayas for its application in bioethanol production using pretreated sugarcane bagasse. Microbiol. Res. 2024, 281, 127623. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, H.; de Almeida, J.M.; Matias, A.; Saraiva, C.; Jorge, P.A.; Coelho, L.C. Detection of biogenic amines in several foods with different sample treatments: An overview. Trends Food Sci. Technol. 2021, 113, 86–96. [Google Scholar] [CrossRef]
- Schirone, M.; Esposito, L.; D’Onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic amines in meat and meat products: A review of the science and future perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Alan, Y.; Topalcengiz, Z.; Digrak, M. Biogenic amine and fermentation metabolite production assessments of Lactobacillus plantarum isolates for naturally fermented pickles. LWT-Food Sci. Technol. 2018, 98, 322–328. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]

| Variable Code | Variable | Low Level (−1) | High Level (+1) |
|---|---|---|---|
| A | Maltose (g/L) | 10 | 30 |
| B | Soy peptone (g/L) | 15 | 35 |
| C | Buffer salt (g/L) | 5.16 | 7.16 |
| D | Tween 80 (g/L) | 1 | 2 |
| E | FeSO4 (mg/L) | 100 | 300 |
| F | MnSO4·H2O (mg/L) | 40 | 60 |
| G | VB7 (mg/L) | 300 | 500 |
| Variable Code | Variable | Level | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | ||
| A | Soy peptone (%) | 3.34 | 3.5 | 4.0 | 4.5 | 4.66 |
| B | FeSO4 (g/10 L) | 0.71 | 0.8 | 1.1 | 1.4 | 1.49 |
| C | VB7 (g/10 L) | 1.84 | 2.0 | 2.5 | 3.0 | 3.16 |
| NCU092002 | NCU001678 | NCU012009 | NCU001672 | NCU001668 | NCU001666 | NCU001674 | NCU001665 | NCU001660 | NCU006063 | |
|---|---|---|---|---|---|---|---|---|---|---|
| P. acidilactici | L. plantarum | L. paracasei | L. plantarum | L. plantarum | L. plantarum | L. plantarum | L. plantarum | L. plantarum | P. pentosaceus | |
| pH | ||||||||||
| 0 h | 6.23 | 6.23 | 6.23 | 6.23 | 6.23 | 6.23 | 6.23 | 6.23 | 6.23 | 6.23 |
| 2 h | 5.93 | 5.82 | 5.72 | 5.88 | 5.69 | 5.48 | 5.81 | 5.42 | 5.56 | 4.62 |
| 4 h | 5.49 | 5.47 | 5 | 5.57 | 5.15 | 5.53 | 5.66 | 4.89 | 5.2 | 4.39 |
| 6 h | 5.02 | 4.58 | 4.39 | 4.67 | 4.36 | 4.62 | 4.77 | 4.1 | 4.43 | 4.24 |
| 8 h | 4.7 | 4.1 | 4.16 | 4.33 | 4.02 | 4.21 | 4.39 | 3.98 | 4.13 | 4.05 |
| 10 h | 4.49 | 3.99 | 3.96 | 4.12 | 3.94 | 4.04 | 4.19 | 3.79 | 4.03 | 4.03 |
| 12 h | 3.84 | 3.84 | 3.84 | 3.99 | 3.84 | 3.95 | 3.93 | 3.79 | 3.9 | 3.99 |
| 24 h | 3.67 | 3.71 | 3.71 | 3.83 | 3.67 | 3.76 | 3.69 | 3.7 | 3.73 | 3.84 |
| 36 h | 3.92 | 3.73 | 3.78 | 3.83 | 3.72 | 3.74 | 3.67 | 3.74 | 3.68 | 3.8 |
| 48 h | 3.85 | 3.66 | 3.8 | 3.72 | 3.6 | 3.64 | 3.62 | 3.65 | 3.63 | 3.87 |
| TA (g/kg) | ||||||||||
| 0 h | 0.17 ± 0.82 | 0.17 ± 0.00 | 0.17 ± 0.82 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 |
| 2 h | 2.31 ± 0.66 | 2.31 ± 0.66 | 3.20 ± 0.76 | 2.49 ± 0.48 | 2.49 ± 0.48 | 2.31 ± 0.66 | 2.13 ± 0.84 | 3.02 ± 0.94 | 2.67 ± 0.03 | 2.31 ± 0.66 |
| 4 h | 3.56 ± 0.04 | 3.02 ± 0.94 | 4.98 ± 0.96 | 2.67 ± 0.03 | 3.38 ± 0.58 | 2.67 ± 0.03 | 2.49 ± 0.48 | 4.09 ± 0.86 | 3.20 ± 0.76 | 5.88 ± 0.06 |
| 6 h | 4.81 ± 0.14 | 4.81 ± 0.14 | 9.08 ± 0.82 | 6.05 ± 0.88 | 6.59 ± 0.34 | 6.23 ± 0.07 | 4.81 ± 0.14 | 8.19 ± 0.72 | 6.23 ± 0.07 | 7.48 ± 0.44 |
| 8 h | 6.59 ± 0.34 | 8.37 ± 0.54 | 11.04 ± 0.84 | 6.94 ± 0.98 | 9.80 ± 0.01 | 7.84 ± 0.08 | 6.59 ± 0.34 | 9.97 ± 0.92 | 8.37 ± 0.54 | 8.91 ± 0.00 |
| 10 h | 7.84 ± 0.08 | 9.62 ± 0.28 | 12.65 ± 0.22 | 8.19 ± 0.72 | 10.33 ± 1.35 | 9.08 ± 0.82 | 8.01 ± 0.09 | 11.04 ± 0.84 | 9.08 ± 0.82 | 10.3 ± 0.35 |
| 12 h | 8.350 ± 0.56 | 12.65 ± 0.22 | 13.22 ± 0.17 | 10.87 ± 0.02 | 13.54 ± 0.32 | 11.58 ± 0.03 | 11.40 ± 0.48 | 14.07 ± 0.78 | 12.65 ± 0.22 | 11.76 ± 0.12 |
| 24 h | 13.04 ± 0.77 | 15.89 ± 0.49 | 18.78 ± 0.87 | 12.97 ± 0.17 | 16.62 ± 0.57 | 14.06 ± 0.79 | 16.26 ± 0.03 | 15.89 ± 0.49 | 16.26 ± 0.03 | 15.71 ± 0.22 |
| 36 h | 16.17 ± 0.92 | 15.71 ± 0.22 | 21.39 ± 0.83 | 14.25 ± 0.06 | 17.17 ± 0.38 | 15.34 ± 0.68 | 17.53 ± 0.92 | 16.07 ± 0.76 | 17.35 ± 0.65 | 17.90 ± 0.46 |
| 48 h | 18.78 ± 0.87 | 16.62 ± 0.57 | 17.20 ± 0.37 | 14.98 ± 0.14 | 17.17 ± 0.38 | 15.52 ± 0.95 | 17.17 ± 0.38 | 16.44 ± 0.03 | 15.52 ± 0.95 | 19.00 ± 0.08 |
| Strains | Y1 | Y2 | Y3 | Y |
|---|---|---|---|---|
| NCU006063 | 3.21 | −0.93 | −0.29 | 1.64 |
| NCU012009 | 0.96 | −0.35 | 0.58 | 0.59 |
| NCU001674 | 0.87 | −0.28 | 0.37 | 0.51 |
| NCU001678 | 0.27 | 0.88 | 0.4 | 0.4 |
| NCU092002 | 0.44 | 1.45 | −1.39 | 0.33 |
| NCU001660 | −0.4 | −0.01 | 0.6 | −0.14 |
| NCU001666 | −0.67 | 0.44 | 0.91 | −0.16 |
| NCU001665 | −1.22 | 0.83 | 0.29 | −0.5 |
| NCU001668 | −1.1 | −0.66 | −1.34 | −0.99 |
| NCU001672 | −2.36 | −1.37 | −0.14 | −1.68 |
| Factors | Coefficient Estimates | Standard Error | SS | MS | t-Values | p-Values |
|---|---|---|---|---|---|---|
| Intercept | 9.1308 | 0.0521 | 2.69 | 0.38 | 11.79 | 0.0156 * |
| Maltose | 0.0066 | 0.0521 | 0.0005 | 0.0005 | 0.016 | 0.9047 |
| Soy peptone | 0.1798 | 0.0521 | 0.39 | 0.39 | 11.92 | 0.0260 * |
| Buffer salt | 0.1108 | 0.0521 | 0.15 | 0.15 | 4.53 | 0.1004 |
| Tween 80 | −0.0642 | 0.0521 | 0.05 | 0.05 | 1.52 | 0.285 |
| FeSO4 | −0.3403 | 0.0521 | 1.39 | 1.39 | 42.71 | 0.0028 ** |
| MnSO4·H2O | 0.1415 | 0.0521 | 0.24 | 0.24 | 7.38 | 0.0532 |
| VB7 | −0.1979 | 0.0521 | 0.47 | 0.47 | 14.45 | 0.019 * |
| Experiment | Soy Peptone (%) | FeSO4 (g/10 L) | VB7 (g/10 L) | Viable Counts (×109 CFU/mL) a |
|---|---|---|---|---|
| 1 | 2.5 | 2.0 | 4.0 | 3.50 |
| 2 | 3.0 | 1.7 | 3.5 | 3.33 |
| 3 | 3.5 | 1.4 | 3.0 | 4.60 |
| 4 | 4.0 | 1.1 | 2.5 | 6.15 |
| 5 | 4.5 | 0.8 | 2.0 | 5.73 |
| 6 | 5.0 | 0.5 | 1.5 | 3.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Li, D.; Jiang, X.; Pei, Q.; Li, Z.; Madjirebaye, P.; Xie, M.; Xiong, T.; Liu, Z. Screening of Lactic Acid Bacteria Isolated from Fermented Cowpea and Optimization of Biomass Production Conditions. Foods 2025, 14, 150. https://doi.org/10.3390/foods14020150
Xu H, Li D, Jiang X, Pei Q, Li Z, Madjirebaye P, Xie M, Xiong T, Liu Z. Screening of Lactic Acid Bacteria Isolated from Fermented Cowpea and Optimization of Biomass Production Conditions. Foods. 2025; 14(2):150. https://doi.org/10.3390/foods14020150
Chicago/Turabian StyleXu, Hong, Danyang Li, Xue Jiang, Qi Pei, Zhengqin Li, Philippe Madjirebaye, Mingyong Xie, Tao Xiong, and Zhanggen Liu. 2025. "Screening of Lactic Acid Bacteria Isolated from Fermented Cowpea and Optimization of Biomass Production Conditions" Foods 14, no. 2: 150. https://doi.org/10.3390/foods14020150
APA StyleXu, H., Li, D., Jiang, X., Pei, Q., Li, Z., Madjirebaye, P., Xie, M., Xiong, T., & Liu, Z. (2025). Screening of Lactic Acid Bacteria Isolated from Fermented Cowpea and Optimization of Biomass Production Conditions. Foods, 14(2), 150. https://doi.org/10.3390/foods14020150






