Multi-Technique Flavoromics for Identifying Key Differential Volatile Compounds Underlying Sensory Profiles in Lager Beers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Materials
2.3. LLE-SAFE and HS-SPME
2.4. Analytical Instrumentation and Conditions
2.4.1. GC-MS Conditions
2.4.2. GC-O-MS Conditions
2.4.3. GC×GC-TOF MS Conditions
2.5. Qualitative and Quantitative Analysis
2.6. Odor Activity Value (OAV) and Taste Activity Value (TAV) Calculation
2.7. Flavor Perception Evaluation of Lager Beers
2.8. Adding Validation Experiment
2.9. Statistical Analysis and Statistical Methods
3. Results and Discussion
3.1. Analysis of Distribution Characteristics of Volatile Compounds
3.2. Flavor Expression Evaluation
3.3. Flavor Perception Evaluation
3.4. Screening of Potential Differential Compounds by PLS-DA
3.5. Validation Experiment Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LLE | Liquid–liquid extraction |
| HS-SPME | Headspace solid-phase microextraction |
| SAFE | Solvent-assisted flavor evaporation |
| GC-MS | Gas chromatography–mass spectrometry |
| GC×GC-TOF MS | Comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry |
| GC-O-MS | Gas chromatography–olfactometry–mass spectrometry |
| OAV | Odor activity value |
| TAV | Taste activity value |
| PLS-DA | Partial least squares discriminant analysis |
| VIP | Variable importance in projection |
| YJ | U8, Beijing Yanjing Brewery Co., Ltd. |
| QD | Classic, Tsingtao Brewery Group Co., Ltd. |
| HR | Brave the World, China Resources Beer (Holdings) Co., Ltd. |
| BW | Ice Beer, Budweiser Asia Pacific Holdings Ltd. |
Appendix A
| Code | Product Name | Manufacturer | Original Wort Concentration | Ingredients |
|---|---|---|---|---|
| YJ | U8 | Beijing Yanjing Brewery Co., Ltd. | 8 °P | Water, malt, rice, hops |
| QD | Classic | Tsingtao Brewery Group Co., Ltd. | 8 °P | Water, malt, rice, hops |
| HR | Brave the World | China Resources Beer (Holdings) Co., Ltd. | 8 °P | Water, malt, brewing syrup, hops |
| BW | Ice Beer | Budweiser Asia Pacific Holdings Ltd. | 8 °P | Water, malt, rice, hop extract, yeast |
| – | Qing Shuang Beer | Beijing Yanjing Brewery Co., Ltd. | 10 °P | Water, malt, rice, hops |
| No. | Compound Name | CAS | Quantifier Ion | Calibration Curve | ||||
|---|---|---|---|---|---|---|---|---|
| n | Slope | Intercept | R2 | Range (mg/L) | ||||
| 1 | Isoamyl acetate | 123-92-2 | 43 | 9 | 0.00005 | −15.2830 | 0.9972 | 2.34–1200.00 |
| 2 | Ethyl hexanoate | 123-66-0 | 88 | 8 | 0.000003 | −0.9841 | 0.9993 | 6.25–800.00 |
| 3 | Ethyl propanoate | 105-37-3 | 29 | 7 | 0.0000005 | 1.2961 | 0.9998 | 0.31–160.00 |
| 4 | Hexyl butanoate | 2639-63-6 | 43 | 9 | 0.000003 | 3.6897 | 0.9974 | 1.25–320.00 |
| 5 | Methyl decanoate | 110-42-9 | 74 | 10 | 0.000003 | 0.8804 | 0.9990 | 0.78–400.00 |
| 6 | Ethyl octanoate | 106-32-1 | 88 | 7 | 0.0000002 | −0.4058 | 0.9976 | 0.43–27.50 |
| 7 | Ethyl decanoate | 110-38-3 | 88 | 8 | 0.0000004 | −0.1858 | 0.9997 | 0.16–40.00 |
| 8 | Isoamyl butyrate | 106-27-4 | 71 | 7 | 0.000004 | −3.6818 | 0.9855 | 3.13–200.00 |
| 9 | Ethyl trans-2-decenoate | 7367-88-6 | 55 | 11 | 0.0000005 | 0.4049 | 0.9996 | 0.16–160.00 |
| 10 | Octyl acetate | 112-14-1 | 43 | 9 | 0.0000007 | −0.3902 | 0.9998 | 0.39–100.00 |
| 11 | Ethyl stearate | 111-61-5 | 88 | 9 | 0.0000004 | −1.2460 | 0.9994 | 0.39–100.00 |
| 12 | Ethyl isovalerate | 108-64-5 | 88 | 6 | 0.000001 | −0.6628 | 0.9988 | 0.39–50.00 |
| 13 | Ethyl myristate | 124-06-1 | 88 | 7 | 0.0000004 | −0.1408 | 0.9999 | 0.39–25.00 |
| 14 | Ethyl lactate | 97-64-3 | 45 | 7 | 0.000002 | −0.6235 | 0.9977 | 0.78–50.00 |
| 15 | Ethyl acetate | 141-78-6 | 43 | 7 | 0.000003 | −1.2344 | 0.9993 | 12.5–800.00 |
| 16 | Ethyl heptanoate | 106-30-9 | 88 | 8 | 0.0000008 | −0.1803 | 0.9992 | 0.16–20.00 |
| 17 | Hexyl acetate | 142-92-7 | 43 | 8 | 0.000003 | 1.0401 | 0.9955 | 1.25–160.00 |
| 18 | Diethyl succinate | 123-25-1 | 101 | 10 | 0.0000009 | 0.1174 | 0.9995 | 0.31–160.00 |
| 19 | Butyl acetate | 123-86-4 | 43 | 9 | 0.000002 | 0.6073 | 0.9928 | 0.08–20.00 |
| 20 | Ethyl 3-hydrobutyrate | 5405-41-4 | 43 | 7 | 0.000002 | 0.0074 | 0.9997 | 0.02–1.25 |
| 21 | Ethyl nonanoate | 123-29-5 | 88 | 8 | 0.0000007 | 0.2045 | 0.9993 | 0.31–40.00 |
| 22 | Ethyl hydrogen succinate | 1070-34-4 | 101 | 6 | 0.000008 | −1.0704 | 0.9992 | 10.00–320.00 |
| 23 | Ethyl valerate | 539-82-2 | 29 | 8 | 0.000002 | 0.3861 | 0.9909 | 0.31–40.00 |
| 24 | Methyl 2-octynate | 111-12-6 | 95 | 9 | 0.000003 | −0.5746 | 0.9981 | 0.78–200.00 |
| 25 | (−)-Ethyl L-lactate | 687-47-8 | 45 | 8 | 0.000003 | −1.9181 | 0.9973 | 0.78–100.00 |
| 26 | 2-Hydroxyethyl acetate | 542-59-6 | 43 | 8 | 0.000002 | 0.1359 | 0.9926 | 0.16–20.00 |
| 27 | Propyl acetate | 109-60-4 | 43 | 8 | 0.000003 | −1.9656 | 0.9971 | 0.16–20.00 |
| 28 | 2,3-Dihydroxypropyl acetate | 106-61-6 | 43 | 9 | 0.000003 | −1.7321 | 0.9967 | 0.16–40.00 |
| 29 | Methyl propionate | 554-12-1 | 29 | 9 | 0.000002 | 0.2601 | 0.9901 | 0.16–40.00 |
| 30 | Isopropyl formate | 625-55-8 | 45 | 8 | 0.000002 | 0.3989 | 0.9995 | 1.25–160.00 |
| 31 | Ethyl dodecanoate | 106-33-2 | 88 | 8 | 0.000002 | 0.6648 | 0.9987 | 0.16–20.00 |
| 32 | Ethyl 3-hexenoate | 2396-83-0 | 29 | 8 | 0.000002 | 1.7706 | 0.9918 | 0.31–40.00 |
| 33 | Ethyl butyrylacetate | 3249-68-1 | 71 | 8 | 0.000003 | 0.7792 | 0.9963 | 0.16–40.00 |
| 34 | Ethyl 3-methylvalerate | 5870-68-8 | 88 | 8 | 0.000001 | −0.4345 | 0.9988 | 0.16–20.00 |
| 35 | Ethyl undecanoate | 627-90-7 | 88 | 8 | 0.000001 | −1.6635 | 0.9984 | 0.16–20.00 |
| 36 | (+)-Diethyl L-tartrate | 87-91-2 | 104 | 8 | 0.000002 | 1.1348 | 0.9984 | 0.16–20.00 |
| 37 | 2,3-Butaneiol | 513-85-9 | 45 | 9 | 0.00003 | 16.4440 | 0.9951 | 4.69–1200.00 |
| 38 | 3-Methyl-1-butanol | 123-51-3 | 55 | 7 | 0.000006 | −32.0320 | 0.9990 | 25.00–1600.00 |
| 39 | 1,2,3-Propanetriol | 56-81-5 | 61 | 7 | 0.000004 | 124.5400 | 0.9992 | 125.00–8000.00 |
| 40 | 2-Methyl-1-propanol | 78-83-1 | 43 | 9 | 0.00001 | −6.9598 | 0.9996 | 4.69–1200.00 |
| 41 | Diisobutylcarbinol | 108-82-7 | 69 | 8 | 0.0000007 | 0.2155 | 0.9941 | 0.39–50.00 |
| 42 | 1-Butanol | 71-36-3 | 56 | 8 | 0.000005 | −0.6615 | 0.9998 | 1.56–200.00 |
| 43 | 1-Octanol | 111-87-5 | 56 | 7 | 0.0000008 | −0.1317 | 0.9995 | 0.31–20.00 |
| 44 | 1-Pentanol | 71-41-0 | 42 | 10 | 0.000001 | −0.4668 | 0.9991 | 0.31–320.00 |
| 45 | 3-Methyl-3-buten-1-ol | 763-32-6 | 41 | 9 | 0.000006 | 0.0446 | 0.9966 | 0.04–10.00 |
| 46 | α-Terpineol | 98-55-5 | 59 | 6 | 0.0000007 | 0.0775 | 0.9993 | 0.16–5.00 |
| 47 | 2-Ethyl-1-hexanol | 104-76-7 | 57 | 8 | 0.0000009 | −0.6655 | 0.998 | 0.31–40.00 |
| 48 | 4-Methyl-1-pentanol | 626-89-1 | 56 | 8 | 0.000003 | 0.7926 | 0.996 | 0.31–80.00 |
| 49 | 1,2-Propylene glycol | 57-55-6 | 45 | 6 | 0.000005 | 1.6696 | 0.9993 | 1.25–40.00 |
| 50 | 2-Hexyl-1-decanol | 2425-77-6 | 57 | 8 | 0.0000009 | −0.6035 | 0.961 | 0.31–40.00 |
| 51 | 1-Hexanol | 111-27-3 | 56 | 7 | 0.000004 | −0.3672 | 0.9996 | 1.56–100.00 |
| 52 | Linalool | 78-70-6 | 71 | 8 | 0.000005 | −0.5625 | 0.9998 | 0.78–100.00 |
| 53 | 1-Decanol | 112-30-1 | 70 | 8 | 0.000005 | −0.5974 | 0.9992 | 0.78–100.00 |
| 54 | Diethylene glycol monoethyl ether | 111-90-0 | 45 | 8 | 0.000005 | −1.2538 | 0.9999 | 1.56–200.00 |
| 55 | (−)-Isolongifolol | 1139-17-9 | 109 | 7 | 0.000004 | −0.0935 | 0.9997 | 1.56–100.00 |
| 56 | Phytol | 150-86-7 | 71 | 7 | 0.000004 | 0.1171 | 0.9996 | 0.39–25.00 |
| 57 | β-Eudesmol | 473-15-4 | 59 | 8 | 0.000001 | 0.0652 | 0.9982 | 3.13–200.00 |
| 58 | Guaiol | 489-86-1 | 161 | 9 | 0.000002 | −0.3251 | 0.9997 | 0.78–200.00 |
| 59 | 2,6-Dimethyl-5,7-octadien-2-ol | 5986-38-9 | 93 | 7 | 0.000001 | 0.0317 | 0.9964 | 1.56–100.00 |
| 60 | Phenethyl alcohol | 60-12-8 | 91 | 9 | 0.000007 | −97.3390 | 0.9851 | 7.81–4000.00 |
| 61 | 2-Phenylethyl acetate | 103-45-7 | 104 | 10 | 0.000003 | −8.9800 | 0.9958 | 0.78–800.00 |
| 62 | Phenol | 108-95-2 | 94 | 10 | 0.000001 | −4.5197 | 0.9993 | 1.17–600.00 |
| 63 | Benzeneacetaldehyde | 122-78-1 | 91 | 7 | 0.00001 | 0.5234 | 0.9916 | 1.25–80.00 |
| 64 | Benzyl alcohol | 100-51-6 | 79 | 7 | 0.0000009 | 0.0445 | 0.9999 | 0.16–10.00 |
| 65 | Ethyl benzoate | 93-89-0 | 105 | 7 | 0.000002 | −0.0393 | 0.9997 | 0.63–40.00 |
| 66 | Benzaldehyde | 100-52-7 | 77 | 6 | 0.000003 | 0.0024 | 0.9904 | 1.25–40.00 |
| 67 | 2,4-Di-tert-butylphenol | 96-76-4 | 191 | 8 | 0.000001 | 2.3582 | 0.9989 | 1.56–200.00 |
| 68 | 2-Methoxy-4-vinylphenol | 7786-61-0 | 135 | 7 | 0.000002 | 5.609 | 0.9955 | 3.13–200.00 |
| 69 | 2,6-Di-tert-butyl-4-methylphenol | 128-37-0 | 205 | 9 | 0.0000004 | 0.4619 | 0.9998 | 2.50–640.00 |
| 70 | Benzoic acid | 65-85-0 | 105 | 6 | 0.000003 | 6.6738 | 0.9964 | 12.5–400.00 |
| 71 | p-Hydroxyphenylethanol | 501-94-0 | 107 | 9 | 0.000001 | 2.0534 | 0.9989 | 0.78–200.00 |
| 72 | Benzeneacetic acid | 103-82-2 | 91 | 6 | 0.000003 | 4.3926 | 0.9943 | 6.25–200.00 |
| 73 | Resorcinol | 108-46-3 | 110 | 6 | 0.000002 | 1.6271 | 0.9992 | 1.56–50.00 |
| 74 | 2,5-Dimethylphenol | 95-87-4 | 122 | 7 | 0.000001 | 1.6717 | 0.9967 | 0.78–50.00 |
| 75 | α-Methylstyrene | 98-83-9 | 118 | 6 | 0.000003 | 0.3579 | 0.9924 | 1.25–40.00 |
| 76 | 1-(2-Hydroxy-5-methylphenyl)ethanone | 1450-72-2 | 135 | 8 | 0.000002 | 0.1579 | 0.9973 | 0.08–10.00 |
| 77 | (R)-(+)-1-Phenylethanol | 1517-69-7 | 107 | 8 | 0.000005 | 0.2135 | 0.9957 | 0.08–10.00 |
| 78 | Toluene | 108-88-3 | 91 | 6 | 0.000004 | −1.9747 | 0.9986 | 7.81–250.00 |
| 79 | Octanoic acid | 124-07-2 | 60 | 8 | 0.000005 | 45.9690 | 0.9888 | 6.25–1600.00 |
| 80 | Hexanoic acid | 142-62-1 | 60 | 8 | 0.000008 | −2.3383 | 0.9990 | 4.69–600.00 |
| 81 | Acetic acid | 64-19-7 | 43 | 6 | 0.00001 | 1.6743 | 0.9987 | 12.5–800.00 |
| 82 | 3-Methylbutanoic acid | 503-74-2 | 60 | 8 | 0.000007 | −0.1555 | 0.9967 | 3.13–400.00 |
| 83 | Decanoic acid | 334-48-5 | 60 | 6 | 0.000003 | 11.6970 | 0.9989 | 12.5–400.00 |
| 84 | Dodecanoic acid | 143-07-7 | 60 | 9 | 0.000004 | 3.1751 | 0.9996 | 3.13–800.00 |
| 85 | Elaidic acid | 112-79-8 | 55 | 8 | 0.000003 | 20.8040 | 0.9958 | 3.13–800.00 |
| 86 | DL-3-Methylvaleric acid | 105-43-1 | 60 | 8 | 0.000001 | 1.7304 | 0.9997 | 1.56–200.00 |
| 87 | Heptanoic acid | 111-14-8 | 60 | 9 | 0.000002 | 3.7580 | 0.9988 | 1.56–400.00 |
| 88 | Tridecanoic acid | 638-53-9 | 73 | 6 | 0.000001 | 1.8894 | 0.9915 | 0.78–50.00 |
| 89 | 2-Methylvaleric acid | 594-61-6 | 59 | 6 | 0.000001 | 1.1722 | 0.9987 | 1.56–50.00 |
| 90 | Pentanoic acid | 109-52-4 | 60 | 7 | 0.000004 | 5.7311 | 0.9998 | 10.00–640.00 |
| 91 | Nonanoic acid | 112-05-0 | 60 | 8 | 0.0000002 | 0.4131 | 0.9962 | 0.31–40.00 |
| 92 | Palmitic acid | 57-10-3 | 43 | 6 | 0.000001 | 10.45 | 0.9993 | 12.5–400.00 |
| 93 | trans-3-Hexenoic acid | 4219-24-3 | 41 | 6 | 0.00001 | 6.6252 | 0.9978 | 0.78–25.00 |
| 94 | 4-Methyl-2-pentenoic acid | 10321-71-8 | 41 | 8 | 0.000001 | 1.5656 | 0.9991 | 0.39–50.00 |
| 95 | Hendecanoic acid | 112-37-8 | 60 | 7 | 0.000002 | 1.1722 | 0.9987 | 0.39–25.00 |
| 96 | Crotonic acid | 3724-65-0 | 86 | 7 | 0.000002 | −1.0557 | 0.9953 | 0.39–25.00 |
| 97 | 2-Methyl-2-pentenoic acid | 3142-72-1 | 41 | 7 | 0.000006 | −2.8264 | 0.9958 | 4.69–300.00 |
| 98 | Furfuryl alcohol | 98-00-0 | 98 | 6 | 0.000007 | 1.2221 | 0.9956 | 1.56–100.00 |
| 99 | 2-Furancarboxylic acid | 88-14-2 | 112 | 6 | 0.000004 | 1.7403 | 0.9987 | 5.00–160.00 |
| 100 | 2-Butylfuran | 4466-24-4 | 81 | 6 | 0.000004 | 1.6517 | 0.9948 | 1.25–80.00 |
| 101 | Furfural | 98-01-1 | 96 | 6 | 0.000002 | 2.345 | 0.9974 | 3.13–100.00 |
| 102 | 2-Acetyl furan | 1192-62-7 | 95 | 11 | 0.000005 | 0.6997 | 0.9985 | 0.08–80.00 |
| 103 | Methyl 2-furoate | 611-13-2 | 95 | 7 | 0.000003 | 0.9577 | 0.9986 | 1.25–80.00 |
| 104 | 5-Hydroxymethyl-2-furaldehyde | 67-47-0 | 97 | 9 | 0.000005 | −7.9761 | 0.9971 | 4.68–1200.00 |
| 105 | 2,5-Dimethyl-4-hydroxy-2H-furan-3-one | 3658-77-3 | 43 | 7 | 0.000006 | 3.1029 | 0.9981 | 1.56–100.00 |
| 106 | 2(5H)-Furanone | 497-23-4 | 55 | 8 | 0.000008 | −0.334 | 0.9933 | 1.56–200.00 |
| 107 | Furfuryl sulfide | 13678-67-6 | 81 | 7 | 0.000002 | −1.394 | 0.9958 | 3.13–200.00 |
| 108 | 2,2′-Difurfuryl ether | 4437-22-3 | 81 | 8 | 0.000003 | −1.8112 | 0.9959 | 1.56–200.00 |
| 109 | (±)-3-Hydroxy-4-butanolide | 5469-16-9 | 44 | 7 | 0.00001 | 19.2420 | 0.9969 | 1.17–1200.00 |
| 110 | γ-Decanolactone | 706-14-9 | 85 | 7 | 0.0000006 | −0.1815 | 0.9998 | 0.39–25.00 |
| 111 | γ-Butyrolactone | 96-48-0 | 42 | 10 | 0.00001 | −7.8209 | 0.998 | 1.56–800.00 |
| 112 | 4-Nonanolide | 104-61-0 | 85 | 6 | 0.000003 | 0.167 | 0.9991 | 0.78–50.00 |
| 113 | γ-Hexanolactone | 695-06-7 | 85 | 8 | 0.000001 | 0.0428 | 0.9999 | 0.08–10.00 |
| 114 | γ-Octalactone | 104-50-7 | 85 | 6 | 0.000004 | −0.6705 | 0.9959 | 1.25–40.00 |
| 115 | δ-Valerolactone | 542-28-9 | 42 | 8 | 0.000001 | 0.1065 | 0.9992 | 0.08–10.00 |
| 116 | γ-Valerolactone | 108-29-2 | 56 | 10 | 0.000005 | 0.7945 | 0.9985 | 0.16–80.00 |
| 117 | Costunolide | 553-21-9 | 81 | 8 | 0.0000007 | −0.4606 | 0.9992 | 0.39–50.00 |
| 118 | 5-Hydroxy-4-pentanolide | 32780-06-6 | 85 | 6 | 0.000004 | −0.3032 | 0.9977 | 2.50–80.00 |
| 119 | 2-Hydroxypyridine | 142-08-5 | 95 | 7 | 0.000002 | 0.6471 | 0.9958 | 0.31–20.00 |
| 120 | (Z)-9-Octadecenamide | 301-02-0 | 59 | 6 | 0.0000007 | 0.0624 | 0.9963 | 2.50–80.00 |
| 121 | 2-Acetyl pyrrole | 1072-83-9 | 94 | 7 | 0.000002 | 0.5324 | 0.9922 | 0.31–20.00 |
| 122 | N-Butyl-acetamide | 1119-49-9 | 30 | 8 | 0.000004 | 0.6875 | 0.9995 | 0.16–20.00 |
| 123 | 2,6-Di-tert-butylpyridine | 585-48-8 | 176 | 9 | 0.000001 | 0.4103 | 0.994 | 0.16–40.00 |
| 124 | 5-Methyl-1H-pyrrole-2-carbaldehyde | 1192-79-6 | 108 | 8 | 0.0000003 | 0.1641 | 0.9996 | 0.08–10.00 |
| 125 | 6-(Ethoxycarbonyl)nicotinic acid | 17874-78-1 | 123 | 7 | 0.000001 | −1.0354 | 0.9992 | 1.25–160.00 |
| 126 | Benzylamine | 100-46-9 | 106 | 7 | 0.000002 | 0.195 | 0.9981 | 0.63–40.00 |
| 127 | Diethylamine | 109-89-7 | 58 | 8 | 0.000003 | 0.2945 | 0.9963 | 0.08–10.00 |
| 128 | 3-Methylthiopropanol | 505-10-2 | 106 | 10 | 0.000006 | −1.3947 | 0.9995 | 1.56–800.00 |
| 129 | Thiophene | 110-02-1 | 84 | 9 | 0.0000005 | −1.7580 | 0.9902 | 0.31–160.00 |
| 130 | Methyl mercaptan | 74-93-1 | 47 | 9 | 0.000006 | −0.711 | 0.9982 | 1.56–100.00 |
| 131 | Dimethyl Sulfoxide | 67-68-5 | 63 | 8 | 0.00002 | 7.776 | 0.9962 | 3.13–400.00 |
| 132 | Dimethyl sulfone | 67-71-0 | 79 | 8 | 0.000007 | 0.5096 | 0.9975 | 0.31–40.00 |
| 133 | Thioacetic acid | 507-09-5 | 43 | 7 | 0.00002 | 9.9358 | 0.9964 | 3.13–200.00 |
| 134 | Tetrahydrothiophene | 110-01-0 | 60 | 7 | 0.00001 | 6.1179 | 0.995 | 1.56–100.00 |
| 135 | 2-Acetyl-2-thiazoline | 29926-41-8 | 43 | 7 | 0.00002 | −0.398 | 0.9984 | 6.25–400.00 |
| 136 | 4-Methyl-5-acetyl thiazole | 38205-55-9 | 126 | 7 | 0.00003 | 0.7128 | 0.9961 | 1.56–100.00 |
| 137 | (E)-2-Nonenal | 18829-56-6 | 43 | 7 | 0.000001 | 0.4996 | 0.9996 | 1.56–100.00 |
| 138 | Isovaleraldehyde | 590-86-3 | 44 | 9 | 0.000001 | −1.3097 | 0.9990 | 1.56–400.00 |
| 139 | Acetaldehyde | 75-07-0 | 29 | 8 | 0.000002 | 0.1996 | 0.9996 | 1.56–50.00 |
| 140 | Hexadecanal | 629-80-1 | 82 | 7 | 0.000001 | 0.2471 | 0.9991 | 0.78–50.00 |
| 141 | 4-Hydroxy-2-butanone | 590–90-9 | 43 | 6 | 0.000002 | 3.5000 | 0.9997 | 5.00–160.00 |
| 142 | (Z)-β-Ionone | 79-77–6 | 177 | 8 | 0.000002 | 2.9139 | 0.9992 | 1.56–200.00 |
| 143 | 3-Hydroxy-2-butanone | 513-86–0 | 45 | 8 | 0.000002 | 1.3398 | 0.9987 | 0.63–160.00 |
| 144 | Cholestane | 481-21-0 | 217 | 8 | 0.000003 | 1.1282 | 0.9989 | 3.13–400.00 |
| 145 | 1-Heptene | 592-76-7 | 56 | 7 | 0.00003 | −10.3550 | 0.9955 | 0.39–50.00 |
| 146 | 1-Undecene | 821-95-4 | 43 | 9 | 0.0000008 | −0.5146 | 0.9998 | 0.16–80.00 |
| 147 | 2-Methyl-2-butene | 513-35-9 | 55 | 7 | 0.000002 | 1.0146 | 0.9972 | 0.78–50.00 |
| 148 | Myrcene | 123-35-3 | 41 | 7 | 0.000002 | 1.0361 | 0.9986 | 0.78–50.00 |
| 149 | Diisopropyl ether | 108-20-3 | 45 | 7 | 0.000002 | 1.8727 | 0.9972 | 0.78–50.00 |
| 150 | 3-Methyl-1-butene | 563-45-1 | 55 | 7 | 0.000003 | 0.0584 | 0.9993 | 0.78–50.00 |
| No. | Compound Name | CAS | RI | Identification Method | Sample Preparation | Presence in Samples | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DB-WAX | DB-FFAP | YJ | QD | HR | BW | |||||
| Alcohols (98) | ||||||||||
| 1 | 2-Propen-1-ol | 107-18-6 | 1016 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 2 | 1-Methoxy-2-propanol | 107-98-2 | 1028 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 3 | 1-Butanol | 71-36-3 | 1044 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 4 | 3-Penten-2-ol | 1569-50-2 | 1070 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 5 | (±)-2-Methyl-1-butanol | 137-32-6 | 1104 | 1145 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 6 | (S)-(−)-2-Methyl-1-butanol | 1565-80-6 | 1106 | 1141 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | √ |
| 7 | 3-Methyl-1-butanol | 123-51-3 | 1107 | 1131 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 8 | 3-Butyn-2-ol | 2028-63-9 | 1111 | MS, RI | LLE-SAFE | √ | ||||
| 9 | 3-Methyl-3-buten-1-ol | 763-32-6 | 1147 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 10 | 1-Pentanol | 71-41-0 | 1149 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 11 | 2-Cyclopropylethanol | 2566-44-1 | 1200 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 12 | 4-Penten-1-ol | 821-09-0 | 1200 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 13 | 4-Methyl-1-pentanol | 626-89-1 | 1213 | 1205 | MS, RI, S | LLE-SAFE, SPME | √ | √ | ||
| 14 | 2-Methyl-2-buten-1-ol | 4675-87-0 | 1219 | MS, RI | LLE-SAFE | √ | ||||
| 15 | 3-Methyl-2-buten-1-ol | 556-82-1 | 1219 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 16 | 2-Pentyn-1-ol | 6261-22-9 | 1235 | MS, RI | LLE-SAFE | √ | ||||
| 17 | Cyclobut-1-enylmethanol | 89182-08-1 | 1235 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 18 | 1,4-Pentadien-3-ol | 922-65-6 | 1235 | MS, RI | LLE-SAFE | √ | ||||
| 19 | 2-Methoxyethanol | 109-86-4 | 1236 | MS, RI | LLE-SAFE | √ | √ | |||
| 20 | 1-Hexanol | 111-27-3 | 1252 | 1227 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 21 | 4-Methyl-3-penten-1-ol | 763-89-3 | 1285 | MS, RI | LLE-SAFE | √ | ||||
| 22 | Propargyl alcohol | 107-19-7 | 1294 | MS, RI | LLE-SAFE | √ | ||||
| 23 | 1-Heptanol | 111-70-6 | 1352 | MS, RI, S | LLE-SAFE | √ | ||||
| 24 | (2S)-2-Oxiranylmethanol | 60456-23-7 | 1356 | MS, RI | LLE-SAFE | √ | √ | |||
| 25 | 2-Ethyl-1-hexanol | 104-76-7 | 1387 | 1311 | MS, RI, S | LLE-SAFE, SPME | √ | |||
| 26 | 2-Methylbutane-2,3-diol | 5396-58-7 | 1411 | MS, RI | LLE-SAFE | √ | √ | |||
| 27 | (R)-(−)-2-Hexanol | 26549-24-6 | 1418 | MS, RI | LLE-SAFE | √ | ||||
| 28 | (R)-2-Octanol | 5978-70-1 | 1418 | MS, RI | LLE-SAFE | √ | ||||
| 29 | (2R,3R)-(−)-2,3-Butanediol | 513-85-9 | 1434 | 1541 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 30 | (S,S)-(+)-1,3-Butaneiol | 19132-06-0 | 1435 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 31 | Linalool | 78-70-6 | 1443 | 1332 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | |
| 32 | 1-Octanol | 111-87-5 | 1456 | 1348 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 33 | 2-(2-Methoxyethoxy)ethanol | 111-77-3 | 1480 | 1955 | MS, RI | LLE-SAFE, SPME | √ | |||
| 34 | (S)-(+)-1,2-Propanediol | 4254-15-3 | 1484 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 35 | 1,2-Propylene glycol | 57-55-6 | 1484 | 1592 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ |
| 36 | 3-Ethoxy-1-propanol | 111-35-3 | 1507 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 37 | Ethylene glycol | 107-21-1 | 1516 | 1627 | MS, RI | LLE-SAFE | √ | √ | √ | √ |
| 38 | Cyclopropanemethanol | 2516-33-8 | 1545 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 39 | Cyclobutanol | 2919-23-5 | 1545 | MS, RI | LLE-SAFE | √ | √ | |||
| 40 | 1-Methoxy-2-butanol | 53778-73-7 | 1550 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 41 | 1,2-Butanediol | 584-03-2 | 1550 | MS, RI | LLE-SAFE | √ | ||||
| 42 | α-Terpineol | 98-55-5 | 1593 | 1386 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | |
| 43 | 4,5-Octanediol | 22607-10-9 | 1598 | MS, RI | LLE-SAFE | √ | ||||
| 44 | 2-Nonanol | 628-99-9 | 1618 | MS, RI | LLE-SAFE | √ | ||||
| 45 | 1,3-Butaneiol | 107-88-0 | 1634 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 46 | 2-Methyl-3-hexanol | 617-29-8 | 1639 | MS, RI | LLE-SAFE | √ | ||||
| 47 | 1-Decanol | 112-30-1 | 1659 | 1420 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | |
| 48 | 1-Nonanol | 143-08-8 | 1659 | MS, RI, S | LLE-SAFE | √ | √ | √ | ||
| 49 | 5-Methyl-1-hepten-4-ol | 99328-46-8 | 1676 | MS, RI | LLE-SAFE | √ | ||||
| 50 | 2-(2-Butoxyethoxy)ethanol | 112-34-5 | 1688 | MS, RI | LLE-SAFE | √ | ||||
| 51 | 2,2-Dimethyl-1,3-propanediol | 126-30-7 | 1697 | MS, RI | LLE-SAFE | √ | ||||
| 52 | 1,1-Oxydi-2-propanol | 110-98-5 | 1726 | 2389 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | |
| 53 | 2-(2-Hydroxypropoxy)-1-propanol | 106-62-7 | 1777 | MS, RI | LLE-SAFE | √ | ||||
| 54 | Diethylene glycol | 111-46-6 | 1865 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 55 | 1-Nonene-4-ol | 35192-73-5 | 1886 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 56 | 1,2-Heptanediol | 3710-31-4 | 1886 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 57 | 1,6-Heptadien-4-ol | 2883-45-6 | 1978 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 58 | 1-Buten-3-ol | 598-32-3 | 1997 | MS, RI | LLE-SAFE | √ | √ | |||
| 59 | 2-Methyl-1-penten-3-ol | 2088/7/5 | 2012 | MS, RI | LLE-SAFE | √ | ||||
| 60 | 2-Hexen-4-ol | 4798-58-7 | 2012 | MS, RI | LLE-SAFE | √ | ||||
| 61 | 1,2,3-Propanetriol | 56-81-5 | 2197 | 2323 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ |
| 62 | Triethylene glycol | 112-27-6 | 2216 | 1942 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | √ |
| 63 | 2-Methyl-2-propanol | 75-65-0 | 2219 | MS, RI, S | LLE-SAFE | √ | ||||
| 64 | 2E,6E-Farnesol | 106-28-5 | 2249 | MS, RI, S | LLE-SAFE | √ | √ | |||
| 65 | Farnesol | 4602-84-0 | 2249 | MS, RI | LLE-SAFE | √ | ||||
| 66 | 2,2-Dimethyl-5-hexen-3-ol | 19550-89-1 | 2261 | MS, RI | LLE-SAFE | √ | ||||
| 67 | 3-Decyn-2-ol | 69668-93-5 | 2469 | MS, RI | LLE-SAFE | √ | ||||
| 68 | 2,3-Epoxy-1-propanol | 57044-25-4 | 2476 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 69 | 1-Dodecanol | 112-53-8 | 2481 | MS, RI | LLE-SAFE | √ | ||||
| 70 | 3-Methyl-2-hexanol | 2313-65-7 | 2503 | MS, RI | LLE-SAFE | √ | ||||
| 71 | 2-(Vinyloxy)ethanol | 764-48-7 | 2503 | 1685 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | √ |
| 72 | 2-Butene-1,4-diol | 110-64-5 | 2561 | MS, RI | LLE-SAFE | √ | ||||
| 73 | Isopropyl alcohol | 67-63-0 | 2596 | MS, RI, S | LLE-SAFE | √ | ||||
| 74 | Di(ethylene glycol) vinyl ether | 929-37-3 | 2600 | MS, RI | LLE-SAFE | √ | √ | |||
| 75 | (R)-(−)-1,2-Propanediol | 4254-14-2 | 2816 | MS, RI | LLE-SAFE | √ | √ | |||
| 76 | 4-Penten-2-ol | 625-31-0 | 2946 | MS, RI | LLE-SAFE | √ | ||||
| 77 | 2-Eyhoxyethanol | 110-80-5 | 3099 | MS, RI | LLE-SAFE | √ | √ | |||
| 78 | Glycidol | 556-52-5 | 3220 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 79 | 2-Methyl-1-propanol | 78-83-1 | 996 | 682 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 80 | 4-Methyl-2-pentanol | 108-11-2 | 2241 | MS, RI | SPME | √ | √ | |||
| 81 | Diisobutylcarbinol | 108-82-7 | 1215 | MS, RI, S | SPME | √ | √ | √ | ||
| 82 | 2,5-Dimethyl-2,5-hexanediol | 110-03-2 | 1269 | MS, RI | SPME | √ | ||||
| 83 | (+)-β-Citronellol | 1117-61-9 | 1340 | MS, RI | SPME | √ | ||||
| 84 | Diethylene glycol monoethyl ether | 111-90-0 | 2248 | MS, RI | SPME | √ | √ | √ | √ | |
| 85 | (−)-Isolongifolol | 1139-17-9 | 2421 | MS, RI | LLE-SAFE | √ | √ | |||
| 86 | Phytol | 150-86-7 | 1061 | MS, RI | SPME | √ | √ | √ | ||
| 87 | 2-Methyl-3-heptanol | 18720-62-2 | 1217 | MS, RI | SPME | √ | √ | √ | ||
| 88 | 4-Methyl-4-nonanol | 23418-38-4 | 2402 | MS, RI | SPME | √ | √ | |||
| 89 | 2-Hexyl-1-decanol | 2425-77-6 | 2377 | MS, RI, aroma, S | LLE-SAFE | √ | √ | |||
| 90 | β-Eudesmol | 473-15-4 | MS, S | LLE-SAFE | √ | √ | √ | √ | ||
| 91 | Pentaethylene glycol | 4792-15-8 | 2006 | MS, RI | SPME | √ | √ | √ | √ | |
| 92 | Guaiol | 489-86-1 | 2203 | MS, RI, S | SPME | √ | √ | √ | ||
| 93 | Octaethylene glycol | 5117-19-1 | 1986 | MS, RI | SPME | √ | √ | √ | √ | |
| 94 | 2,2,4-Trimethyl-3-pentanol | 5162-48-1 | 1261 | MS, RI | SPME | √ | √ | |||
| 95 | Heptaethylene glycol | 5617-32-3 | 2082 | MS, RI | SPME | √ | √ | √ | √ | |
| 96 | 1-Methylcyclohexanol | 590-67-0 | 1050 | MS, RI | SPME | √ | √ | √ | ||
| 97 | 2,6-Dimethyl-5,7-octadien-2-ol | 5986-38-9 | 1387 | MS, RI | SPME | √ | √ | |||
| 98 | 5-Nonanol | 623-93-8 | 1273 | MS, RI | SPME | √ | ||||
| Nitrogen-containing compounds (91) | ||||||||||
| 1 | Triethylamine | 121-44-8 | 1110 | MS, RI | LLE-SAFE | √ | ||||
| 2 | N,N,O-Triacetylhydroxylamine | 17720-63-7 | 1112 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 3 | 2-Methylpyrazine | 109-08-0 | 1162 | MS, RI, S | LLE-SAFE | √ | √ | √ | ||
| 4 | N,N-Dimethylformamide | 1968-12-2 | 1223 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 5 | 2-Oxopropanamide | 631-66-3 | 1531 | MS, RI | LLE-SAFE | √ | √ | |||
| 6 | N-Methylacetamide | 79-16-3 | 1533 | MS, RI | LLE-SAFE | √ | ||||
| 7 | N-Ethylacetamide | 625-50-3 | 1533 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 8 | N-Acetyglycinamide | 2620-63-5 | 1610 | MS, RI | LLE-SAFE | √ | ||||
| 9 | 2,3-Butanedione monoxime | 57-71-6 | 1627 | MS, RI | LLE-SAFE | √ | √ | |||
| 10 | Acetamide | 60-35-5 | 1656 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 11 | Formamide | 1975/12/7 | 1675 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 12 | 3-(Ethoxycarbonyl)pyridine | 614-18-6 | 1706 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 13 | N-Methylsuccinimide | 1121-07-9 | 1793 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 14 | 2-Pyridinecarbaldehyde oxime | 873-69-8 | 1806 | MS, RI | LLE-SAFE | √ | ||||
| 15 | 3-Methylbutyramide | 541-46-8 | 1811 | MS, RI | LLE-SAFE | √ | √ | |||
| 16 | 2-Acetyl pyrrole | 1072-83-9 | 1862 | 1978 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ |
| 17 | 3-Benzylsydnone | 16844-42-1 | 1862 | MS, RI | LLE-SAFE | √ | ||||
| 18 | 2-Pyrrolecarbaldehyde | 1003-29-8 | 1895 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 19 | 2-Pyrrolidinone | 616-45-5 | 1928 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 20 | 1-Methylpyrrole-2-carboxaldehyde | 1192-58-1 | 1989 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 21 | Butanamide | 541-35-5 | 2000 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 22 | Hexanamide | 628-02-4 | 2000 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 23 | 5-Aminopentanamide | 13023-70-6 | 2021 | MS, RI | LLE-SAFE | √ | ||||
| 24 | 2-Piperidinone | 675-20-7 | 2021 | MS, RI | LLE-SAFE | √ | ||||
| 25 | N-Acetylglycine ethyl ester | 1906-82-7 | 2044 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 26 | ε-Caprolactam | 105-60-2 | 2074 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 27 | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one | 28564-83-2 | 2157 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 28 | Ethosuximide | 77-67-8 | 2252 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 29 | Glutarimide | 1121-89-7 | 2264 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 30 | N,N,3,5-Tetramethylaniline | 4913-13-7 | 2269 | MS, RI | LLE-SAFE | √ | ||||
| 31 | N-Acetylethanolamine | 142-26-7 | 2277 | MS, RI | LLE-SAFE | √ | √ | |||
| 32 | Indole | 120-72-9 | 2329 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 33 | 5H-cyclopenta[b]pyridine | 270-91-7 | 2329 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 34 | Succinimide | 123-56-8 | 2355 | MS, RI | LLE-SAFE | √ | ||||
| 35 | N,S-Diacetylcysteamine | 1420-88-8 | 2411 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 36 | N-(2-Phenylethyl)acetamide | 877-95-2 | 2473 | MS, RI | LLE-SAFE | √ | ||||
| 37 | 1-Methyl-1H-tetrazole | 16681-77-9 | 2475 | MS, RI | LLE-SAFE | √ | ||||
| 38 | 4-Amino-1-butanol | 13325-10-5 | 2479 | MS, RI | LLE-SAFE | √ | ||||
| 39 | DL-Pyroglutamic acid | 149-87-1 | 2497 | MS, RI | LLE-SAFE | √ | ||||
| 40 | Ethyl 5-oxo-L-prolinate | 66183-71-9 | 2497 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 41 | L-Pyroglutamicacid | 98-79-3 | 2500 | MS, RI | LLE-SAFE | √ | ||||
| 42 | Benzamide | 55-21-0 | 2575 | MS, RI | LLE-SAFE | √ | √ | |||
| 43 | Phenylacetamide | 103-81-1 | 2607 | MS, RI | LLE-SAFE | √ | ||||
| 44 | Valeramide | 626-97-1 | 2654 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 45 | Enanthamide | 628-62-6 | 2657 | MS, RI | LLE-SAFE | √ | ||||
| 46 | Octanamide | 629-01-6 | 2657 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 47 | Pent-4-enylamine | 22537-07-1 | 2705 | MS, RI | LLE-SAFE | √ | ||||
| 48 | Cyclobutylamine | 2516-34-9 | 2762 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 49 | 3-Indoleethanol | 526-55-6 | 2767 | MS, RI | LLE-SAFE | √ | ||||
| 50 | (2-Hydroxyethyl)hydrazine | 109-84-2 | 2811 | MS, RI | LLE-SAFE | √ | ||||
| 51 | Dodecanamide | 1120-16-7 | 2883 | MS, RI | LLE-SAFE | √ | √ | |||
| 52 | (Z)-9-Octadecenamide | 301-02-0 | 2887 | 2432 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ |
| 53 | 2-Propoxyethylamine | 42185-03-5 | 2937 | MS, RI | LLE-SAFE | √ | ||||
| 54 | 2-Methoxyethylamine | 109-85-3 | 2963 | MS, RI | LLE-SAFE | √ | √ | |||
| 55 | 2-Ethoxyethylamine | 110-76-9 | 2968 | MS, RI | LLE-SAFE | √ | ||||
| 56 | Nonanamide | 1120-07-6 | 3105 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 57 | 1,2-Propanediamine | 78-90-0 | 3147 | MS, RI | LLE-SAFE | √ | √ | |||
| 58 | N-Methylisobutylamine | 625-43-4 | 3222 | MS, RI | LLE-SAFE | √ | ||||
| 59 | N-Methyltyramine | 370-98-9 | 884 | MS, RI | LLE-SAFE | √ | √ | |||
| 60 | 4-Hydroxypyrazole | 4843-98-5 | 924 | MS, RI | LLE-SAFE | √ | ||||
| 61 | 4H-1,2,4-Triazol-4-amine | 584-13-4 | 925 | MS, RI | LLE-SAFE | √ | ||||
| 62 | Benzylamine | 100-46-9 | 1188 | MS, RI | SPME | √ | √ | |||
| 63 | 2,4,6-Triamino-5-nitrosopyrimidine | 1006-23-1 | MS | LLE-SAFE | √ | |||||
| 64 | 1,3,5-Trimethylpyrazole | 1072-91-9 | 2157 | MS, RI | LLE-SAFE | √ | √ | |||
| 65 | Diethylamine | 109-89-7 | 597 | MS, RI | SPME | √ | √ | |||
| 66 | N-Butyl-acetamide | 1119-49-9 | 1118 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | ||
| 67 | cis-13-Docosenoamide | 112-84-5 | MS | LLE-SAFE | √ | √ | ||||
| 68 | 5-Methyl-1H-pyrrole-2-carbaldehyde | 1192-79-6 | 2120 | MS, RI, aroma, S | LLE-SAFE | √ | √ | |||
| 69 | Octadecanamide | 124-26-5 | MS | LLE-SAFE | √ | √ | ||||
| 70 | 2-Hydroxypyridine | 142-08-5 | 1468 | MS, RI, aroma, S | LLE-SAFE | √ | √ | |||
| 71 | 6-(Ethoxycarbonyl)nicotinic acid | 17874-78-1 | MS, aroma, S | LLE-SAFE | √ | √ | ||||
| 72 | Diethyl iminodiacetate | 19617-44-8 | 2035 | MS, RI | SPME | √ | √ | √ | √ | |
| 73 | N-Ethylisopropylamine | 19961-27-4 | 1270 | MS, RI | SPME | √ | √ | √ | ||
| 74 | 2-Hydroxy-Propanamide | 2043-43-8 | 667 | MS, RI | SPME | √ | √ | |||
| 75 | 5,6,7,8-Tetrahydro-1-naphthylamine | 2217-41-6 | 2039 | MS, RI | SPME | √ | ||||
| 76 | 4-Hydroxyindole | 2380-94-1 | 2284 | MS, RI | SPME | √ | ||||
| 77 | 5-Amino-1-pentanol | 2508-29-4 | 1043 | MS, RI | SPME | √ | ||||
| 78 | Pyrazole | 288-13-1 | 2243 | MS, RI | LLE-SAFE | √ | ||||
| 79 | 4-Hydroxy-6-methylpyrimidine | 3524-87-6 | 2172 | MS, RI | LLE-SAFE | √ | ||||
| 80 | 2-Amino-1,3,4-thiadiazole | 4005-51-0 | 1294 | MS, RI | SPME | √ | ||||
| 81 | N-Ethylmaleamic acid | 4166-67-0 | 2233 | MS, RI | SPME | √ | √ | √ | √ | |
| 82 | N-Ethyl-pentanamide | 54007-33-9 | 1267 | MS, RI | SPME | √ | √ | √ | ||
| 83 | Isobutyramide | 563-83-7 | 590 | MS, RI | SPME | √ | √ | |||
| 84 | 2,6-Di-tert-butylpyridine | 585-48-8 | 2457 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | ||
| 85 | Ethylhydrazine | 624-80-6 | 1710 | MS, RI | SPME | √ | ||||
| 86 | Imidazole-4-acetic acid | 645-65-8 | 1185 | MS, RI | SPME | √ | √ | |||
| 87 | 3-Hydroxypiperidine | 6859-99-0 | 1194 | MS, RI | SPME | √ | ||||
| 88 | N,N-Dimethylthioformamide | 758-16-7 | 1786 | MS, RI | SPME | √ | √ | √ | √ | |
| 89 | N,N-Dimethylpropanamide | 758-96-3 | 664 | MS, RI | SPME | √ | √ | |||
| 90 | Ethyl 3-aminopropanoate | 924-73-2 | 1269 | MS, RI | SPME | √ | ||||
| 91 | 2-Ethyl-4-methylimidazole | 931-36-2 | MS | LLE-SAFE | √ | √ | ||||
| Esters (86) | ||||||||||
| 1 | Isoamyl acetate | 123-92-2 | 1024 | 1014 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 2 | 2-Methylbutyl acetate | 624-41-9 | 1024 | MS, RI | LLE-SAFE | √ | √ | |||
| 3 | Pentyl acetate | 628-63-7 | 1024 | MS, RI, S | LLE-SAFE | √ | √ | |||
| 4 | Ethyl valerate | 539-82-2 | 1038 | 1433 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ |
| 5 | Ethyl hexanoate | 123-66-0 | 1135 | 1157 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 6 | Ethyl Pyruvate | 617-35-6 | 1164 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 7 | Hexyl acetate | 142-92-7 | 1171 | 1183 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 8 | Allyl butanoate | 2051-78-7 | 1175 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 9 | Ethyl heptanoate | 106-30-9 | 1232 | 1214 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 10 | (−)-Ethyl L-lactate | 687-47-8 | 1238 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 11 | Ethyl lactate | 97-64-3 | 1238 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 12 | Hexyl formate | 629-33-4 | 1250 | MS, RI, S | LLE-SAFE | √ | ||||
| 13 | 2-Propenoic acid ethenyl ester | 2177-18-6 | 1294 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 14 | Methyl acrylate | 96-33-3 | 1294 | MS, RI | LLE-SAFE | √ | √ | |||
| 15 | Ethyl octanoate | 106-32-1 | 1332 | 1303 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 16 | Heptyl formate | 112-23-2 | 1352 | MS, RI | LLE-SAFE | √ | ||||
| 17 | 3-Methoxybutyl acetate | 4435-53-4 | 1411 | MS, RI | LLE-SAFE | √ | ||||
| 18 | Ethyl 2-hydroxyisobutyrate | 80-55-7 | 1411 | MS, RI | LLE-SAFE | √ | √ | |||
| 19 | Ethyl 3-hydrobutyrate | 5405-41-4 | 1411 | 1876 | MS, RI, S | LLE-SAFE, SPME | √ | √ | ||
| 20 | Octyl formate | 112-32-3 | 1454 | MS, RI | LLE-SAFE | √ | √ | |||
| 21 | 2-Hydroxypropyl acetate | 627-69-0 | 1467 | MS, RI | LLE-SAFE | √ | ||||
| 22 | Ethyl levulinate | 539-88-8 | 1500 | MS, RI | LLE-SAFE | √ | ||||
| 23 | 2-Hydroxyethyl formate | 628-35-3 | 1514 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 24 | 2-Hydroxyethyl acetate | 542-59-6 | 1528 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 25 | Ethyl decanoate | 110-38-3 | 1535 | 1377 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | |
| 26 | Diethyl succinate | 123-25-1 | 1570 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 27 | Dimethyl glutarate | 1119-40-0 | 1593 | MS, RI | LLE-SAFE | √ | √ | |||
| 28 | Isopropyl isobutyrate | 617-50-5 | 1600 | MS, RI | LLE-SAFE | √ | ||||
| 29 | Propyl acetate | 109-60-4 | 1630 | MS, RI, S | LLE-SAFE | √ | √ | |||
| 30 | 1,3-Diacetoxy-propane | 628-66-0 | 1632 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 31 | Neryl formate | 2142-94-1 | 1744 | MS, RI | LLE-SAFE | √ | ||||
| 32 | 1,1-Ethanediol diacetate | 542-10-9 | 1749 | MS, RI | LLE-SAFE | √ | √ | |||
| 33 | Butyl butyrate | 109-21-7 | 1760 | MS, RI | LLE-SAFE | √ | ||||
| 34 | Butyl 2-methyl propanoate | 97-87-0 | 1760 | MS, RI, S | LLE-SAFE | √ | ||||
| 35 | Pentyl 2-methylprop-2-enoate | 2849-98-1 | 1936 | MS, RI | LLE-SAFE | √ | ||||
| 36 | Allyl propionate | 2408-20-0 | 2012 | MS, RI | LLE-SAFE | √ | ||||
| 37 | Vinyl butyrate | 123-20-6 | 2012 | MS, RI | LLE-SAFE | √ | ||||
| 38 | Allyl isobutyrate | 15727-77-2 | 2015 | MS, RI | LLE-SAFE | √ | √ | |||
| 39 | Ethyl cinnamate | 103-36-6 | 2019 | MS, RI | LLE-SAFE | √ | ||||
| 40 | Vinyl acetate | 108-05-4 | 2038 | MS, RI | LLE-SAFE | √ | ||||
| 41 | Methyl acetate | 79-20-9 | 2102 | MS, RI, S | LLE-SAFE | √ | ||||
| 42 | Methyl tridecanoate | 1731-88-0 | 2112 | MS, RI | LLE-SAFE | √ | ||||
| 43 | Glycidyl acrylate | 106-90-1 | 2139 | MS, RI | LLE-SAFE | √ | √ | |||
| 44 | 2,3-Dihydroxypropyl acetate | 106-61-6 | 2173 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 45 | Ethyl cyclohexanepropionate | 10094-36-7 | 2233 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 46 | Ethyl nonanoate | 123-29-5 | 2233 | 1285 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 47 | Allyl propyl ether | 1471-03-0 | 2589 | MS, RI | LLE-SAFE | √ | ||||
| 48 | 2-Oxooctadecanoic acid methyl ester | 2380-18-9 | 2607 | MS, RI | LLE-SAFE | √ | ||||
| 49 | L-Lactide | 4511-42-6 | 2630 | MS, RI | LLE-SAFE | √ | ||||
| 50 | Ethenyl formate | 692-45-5 | 2716 | MS, RI | LLE-SAFE | √ | √ | |||
| 51 | Methyl propionate | 554-12-1 | 2729 | MS, RI, S | LLE-SAFE, SPME | √ | √ | |||
| 52 | Butyl lactate | 138-22-7 | 2751 | MS, RI, S | LLE-SAFE | √ | √ | |||
| 53 | Lactic acid methyl ester | 547-64-8 | 2811 | MS, RI | LLE-SAFE | √ | ||||
| 54 | Isopropyl formate | 625-55-8 | 2939 | MS, RI | LLE-SAFE | √ | √ | |||
| 55 | DL-Lactide | 95-96-5 | 3127 | MS, RI | LLE-SAFE | √ | √ | |||
| 56 | Ethyl acetate | 141-78-6 | 913 | 606 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 57 | Isobutyl acetate | 110-19-0 | 990 | MS, RI, S | LLE-SAFE | √ | ||||
| 58 | Butyl acetate | 123-86-4 | 990 | MS, RI, S | LLE-SAFE | √ | √ | |||
| 59 | 2,2-Dimethoxypropionic acid methyl ester | 10076-48-9 | 2237 | MS, RI | SPME | √ | √ | |||
| 60 | sec-Butyl Crotonate | 10371-45-6 | 1304 | MS, RI | SPME | √ | √ | |||
| 61 | Ethyl propanoate | 105-37-3 | 2101 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 62 | Isoamyl butyrate | 106-27-4 | 1102 | MS, RI, S | SPME | √ | ||||
| 63 | Ethyl dodecanoate | 106-33-2 | 2256 | MS, RI | LLE-SAFE | √ | √ | |||
| 64 | Ethyl hydrogen succinate | 1070-34-4 | 2388 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 65 | Ethyl isovalerate | 108-64-5 | 1302 | MS, RI, S | SPME | √ | ||||
| 66 | Methyl decanoate | 110-42-9 | 2215 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 67 | Methyl 2-octynate | 111-12-6 | 2415 | MS, RI, aroma, S | LLE-SAFE | √ | √ | |||
| 68 | Ethyl Stearate | 111-61-5 | 1301 | MS, RI, S | SPME | √ | √ | √ | √ | |
| 69 | Octyl Acetate | 112-14-1 | 1298 | MS, RI, S | SPME | √ | √ | |||
| 70 | Ethyl myristate | 124-06-1 | 1382 | MS, RI, S | SPME | √ | √ | √ | √ | |
| 71 | Ccrotonic acid isopropyl ester | 18060-77-0 | 1263 | MS, RI | SPME | √ | √ | √ | ||
| 72 | Ethyl nonadecanoate | 18281-04-4 | 1299 | MS, RI | SPME | √ | √ | |||
| 73 | Methyl (Z)-3,7-dimethylocta-2,6-dienoate | 1862-61-9 | 1394 | MS, RI | SPME | √ | ||||
| 74 | Ethyl 3-hexenoate | 2396-83-0 | 1206 | MS, RI, S | SPME | √ | √ | √ | ||
| 75 | 2-Ethylhexyl Butyrate | 25415-84-3 | 1320 | MS, RI | SPME | √ | ||||
| 76 | Hexyl butanoate | 2639-63-6 | 2144 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 77 | Ethyl butyrylacetate | 3249-68-1 | 2297 | MS, RI | SPME | √ | √ | √ | ||
| 78 | Heptadecanyl margarate | 36617-50-2 | 1422 | MS, RI | SPME | √ | √ | |||
| 79 | Dimethyl dl-malate | 38115-87-6 | 1197 | MS, RI | SPME | √ | √ | |||
| 80 | Methyl 4-methoxyacetoacetate | 41051-15-4 | 2275 | MS, RI | SPME | √ | ||||
| 81 | Ethyl 3-methylvalerate | 5870-68-8 | 1299 | MS, RI | SPME | √ | ||||
| 82 | Ethyl undecanoate | 627-90-7 | 1271 | MS, RI | SPME | √ | √ | |||
| 83 | Dodecyl isobutyrate | 6624-71-1 | 2332 | MS, RI | SPME | √ | √ | |||
| 84 | Ethyl trans-2-decenoate | 7367-88-6 | 1194 | MS, RI, S | SPME | √ | ||||
| 85 | Isobutyric acid 2-ethyl-3-hydroxyhexyl ester | 74367-31-0 | 677 | MS, RI | SPME | √ | ||||
| 86 | (+)-Diethyl L-tartrate | 87-91-2 | 1526 | MS, RI | SPME | √ | ||||
| Alkanes (57) | ||||||||||
| 1 | 1,1-Dimethyl cyclopropane | 1630-94-0 | 886 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 2 | s-Trioxane | 110-88-3 | 1063 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 3 | Nonane | 111-84-2 | 1105 | MS, RI | LLE-SAFE | √ | ||||
| 4 | 2,4-Dimethyldecane | 2801-84-5 | 1105 | MS, RI | LLE-SAFE | √ | ||||
| 5 | n-Hendecane | 1120-21-4 | 1105 | MS, RI | LLE-SAFE | √ | ||||
| 6 | 2,3-Dimethylbutane | 79-29-8 | 1111 | MS, RI | LLE-SAFE | √ | ||||
| 7 | Decane | 124-18-5 | 1201 | MS, RI | LLE-SAFE | √ | √ | |||
| 8 | 3,7-Dimethyldecane | 17312-54-8 | 1201 | MS, RI | LLE-SAFE | √ | ||||
| 9 | 2-Methylnonane | 871-83-0 | 1201 | MS, RI | LLE-SAFE | √ | ||||
| 10 | Tridecane | 629-50-5 | 1203 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 11 | Propyl cyclopropane | 2415-72-7 | 1250 | MS, RI | LLE-SAFE | √ | ||||
| 12 | Cyclododecane | 294-62-2 | 1659 | MS, RI | LLE-SAFE | √ | ||||
| 13 | 2,3-Dimethyloxirane | 3266-23-7 | 1713 | MS, RI | LLE-SAFE | √ | ||||
| 14 | 1,4- Diacetoxybutane | 628-67-1 | 1769 | MS, RI | LLE-SAFE | √ | ||||
| 15 | trans-1,2-Dimethylcyclopropane | 2402/6/4 | 2039 | MS, RI | LLE-SAFE | √ | ||||
| 16 | 1,2-Epoxyhexane | 1436-34-6 | 2155 | MS, RI | LLE-SAFE | √ | √ | |||
| 17 | Methylcyclobutane | 598-61-8 | 2264 | MS, RI | LLE-SAFE | √ | √ | |||
| 18 | 6-Ethyl-2-methyldecane | 62108-21-8 | 2401 | MS, RI | LLE-SAFE | √ | ||||
| 19 | 2,7-Dimethyloctane | 1072-16-8 | 2491 | MS, RI | LLE-SAFE | √ | ||||
| 20 | Dimethylmethane | 74-98-6 | 2514 | MS, RI | LLE-SAFE | √ | √ | |||
| 21 | 2,6,10-Trimethyldodecane | 3891-98-3 | 2709 | MS, RI | LLE-SAFE | √ | ||||
| 22 | Hexadecane | 544-76-3 | 2805 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 23 | 2-Methylpropane | 75-28-5 | 2996 | MS, RI | LLE-SAFE | √ | √ | |||
| 24 | Butane | 106-97-8 | 3044 | MS, RI | LLE-SAFE | √ | √ | |||
| 25 | 1,3,6-Trioxocane | 1779-19-7 | 3054 | MS, RI | LLE-SAFE | √ | ||||
| 26 | Pentane | 109-66-0 | 881 | MS, RI | LLE-SAFE | √ | ||||
| 27 | Bicyclo[2,1,0]pentane | 185-94-4 | 889 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 28 | Ethylene oxide | 75-21-8 | 893 | MS, RI | LLE-SAFE | √ | √ | |||
| 29 | Isobutylene oxide | 558-30-5 | 910 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 30 | Cyclopropane | 75-19-4 | 998 | MS, RI | LLE-SAFE | √ | √ | |||
| 31 | 1,3-Dimethoxybutane | 10143-66-5 | 2290 | MS, RI | SPME | √ | ||||
| 32 | Acetaldehyde dipropyl acetal | 105-82-8 | 2254 | MS, RI | SPME | √ | √ | √ | ||
| 33 | Methylcyclohexane | 108-87-2 | MS | LLE-SAFE | √ | √ | √ | √ | ||
| 34 | Tetratriacontane | 14167-59-0 | MS, aroma, S | LLE-SAFE | √ | √ | ||||
| 35 | Cycloheptane | 291-64-5 | 1237 | MS, RI | SPME | √ | ||||
| 36 | Cyclooctane | 292-64-8 | 1415 | MS, RI | SPME | √ | ||||
| 37 | 1,2,3,4-Tetramethoxybutane | 3011-85-6 | 2305 | MS, RI | SPME | √ | √ | √ | ||
| 38 | 1,1,3-Trimethylcyclohexane | 3073-66-3 | MS | LLE-SAFE | √ | √ | ||||
| 39 | 1,2-Dicyclohexylethane | 3321-50-4 | MS | LLE-SAFE | √ | √ | √ | |||
| 40 | 2,2-Dimethoxybutane | 3453-99-4 | 2017 | MS, RI | SPME | √ | ||||
| 41 | Cholestane | 481-21-0 | MS, aroma, S | LLE-SAFE | √ | √ | √ | √ | ||
| 42 | 11-Decyl-docosane | 55401-55-3 | MS | LLE-SAFE | √ | |||||
| 43 | 1-(1-ethoxyethoxy)-Butane | 57006-87-8 | 671 | MS, RI | SPME | √ | √ | |||
| 44 | 2,4-Dimethylhexane | 589-43-5 | 1303 | MS, RI | LLE-SAFE | √ | ||||
| 45 | Heptacosane | 593-49-7 | 2304 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 46 | 1-Ethyl-2-propylcyclohexane | 62238-33-9 | MS | LLE-SAFE | √ | |||||
| 47 | n-Pentadecane | 629-62-9 | MS | LLE-SAFE | √ | √ | √ | |||
| 48 | n-Heneicosane | 629-94-7 | MS | LLE-SAFE | √ | |||||
| 49 | n-Pentacosane | 629-99-2 | MS | LLE-SAFE | √ | |||||
| 50 | Hexacosane | 630-01-3 | MS, aroma, S | LLE-SAFE | √ | √ | √ | |||
| 51 | n-Octacosane | 630-02-4 | MS | LLE-SAFE | √ | √ | √ | √ | ||
| 52 | n-Nonacosane | 630-03-5 | MS | LLE-SAFE | √ | √ | √ | |||
| 53 | Hentriacontane | 630-04-6 | 2406 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 54 | n-Triacontane | 638-68-6 | MS | LLE-SAFE | √ | √ | √ | |||
| 55 | Tetracosane | 646-31-1 | MS | LLE-SAFE | √ | √ | √ | |||
| 56 | 1,3,3-Trimethoxybutane | 6607-66-5 | 2367 | MS, RI | SPME | √ | √ | |||
| 57 | Tetratetracontane | 7098-22-8 | MS, aroma, S | LLE-SAFE | √ | √ | ||||
| Furans (42) | ||||||||||
| 1 | 2-Methyltetrahydrofuran-3-one | 3188-00-9 | 1160 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 2 | 2,2,5,5-Tetramethyltetrahydrofuran | 15045-43-9 | 1307 | MS, RI | LLE-SAFE | √ | ||||
| 3 | Furfural | 1998/1/1 | 1354 | 1303 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | √ |
| 4 | 2-Acetyl furan | 1192-62-7 | 1396 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 5 | 5-Methyl-2-furaldehyde | 620-02-0 | 1462 | MS, RI, S | LLE-SAFE | √ | ||||
| 6 | 2-Propionylfuran | 3194-15-8 | 1465 | MS, RI | LLE-SAFE | √ | ||||
| 7 | Furfuryl alcohol | 98-00-0 | 1555 | 1375 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 8 | Tetrahydrofuran-2-ylmethylacetat | 637-64-9 | 1566 | MS, RI | LLE-SAFE | √ | ||||
| 9 | 2,2-Di(2-tetrahydrofuryl)propane | 89686-69-1 | 1604 | MS, RI | LLE-SAFE | √ | ||||
| 10 | 3-Methyl-2(5H)-furanone | 22122-36-7 | 1605 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 11 | 2(5H)-Furanone | 497-23-4 | 1639 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 12 | 2,5-Dimethyl-4-hydroxy-2H-furan-3-one | 3658-77-3 | 1749 | 1865 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ |
| 13 | 2-(1-Oxo-2-hydroxyethyl)furan | 17678-19-2 | 1892 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 14 | 5-Acetyltetrahydrofuran-2-one | 29393-32-6 | 1945 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 15 | 4-Hydroxy-5-methyl-3-furanone | 19322-27-1 | 2009 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 16 | 2H-Pyran-2,6(3H)-dione | 5926-95-4 | 2009 | MS, RI | LLE-SAFE | √ | ||||
| 17 | 2-Furaldehyde diethyl acetal | 13529-27-6 | 2012 | MS, RI | LLE-SAFE | √ | ||||
| 18 | 4-Methoxy-5H-furan-2-one | 69556-70-3 | 2056 | MS, RI | LLE-SAFE | √ | ||||
| 19 | 3-Furanmethanol | 4412-91-3 | 2085 | MS, RI | LLE-SAFE | √ | ||||
| 20 | Ethyl 5-oxooxolane-2-carboxylate | 1126-51-8 | 2116 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 21 | Methyl 5-oxotetrahydrofuran-2-carboxylate | 3885-29-8 | 2116 | MS, RI | LLE-SAFE | √ | ||||
| 22 | Tetrahydrofurfruyl alcohol | 97-99-4 | 2155 | MS, RI, S | LLE-SAFE | √ | √ | |||
| 23 | 2-Furanmethanamine | 617-89-0 | 2170 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 24 | 2-Methyl-4H,5H-furo[3,2-c]pyridin-4-one | 26956-44-5 | 2269 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 25 | 5-Hydroxymethyl-2-furaldehyde | 67-47-0 | 2387 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 26 | 5-(Hydroxymethyl)dihydro-2(3H)-furanone | 10374-51-3 | 2390 | MS, RI | LLE-SAFE | √ | ||||
| 27 | 5-(1-Piperidyl)furan-2-carbaldehyde | 22868-60-6 | 2433 | MS, RI | LLE-SAFE | √ | ||||
| 28 | 5-(Hydroxymethyl)furfuryl alcohol | 1883-75-6 | 2469 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 29 | 3-Furaldehyde | 498-60-2 | 2514 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 30 | 2,3-Dihydrofuran | 1191-99-7 | 1116 | MS, RI | SPME | √ | ||||
| 31 | Furfuryl sulfide | 13678-67-6 | MS | LLE-SAFE | √ | |||||
| 32 | 4,6-Dimethoxy-2,3-dihydrobenzofuran-3-one | 4225-35-8 | 2389 | MS, RI | LLE-SAFE | √ | ||||
| 33 | 2,2′-Difurfuryl ether | 4437-22-3 | 1190 | MS, RI | SPME | √ | √ | √ | ||
| 34 | 2-Butylfuran | 4466-24-4 | 1186 | MS, RI, S | SPME | √ | ||||
| 35 | Phthalan | 496-14-0 | 1371 | MS, RI | SPME | √ | ||||
| 36 | 2,3-Dihydrobenzofuran | 496-16-2 | 2402 | MS, RI | LLE-SAFE | √ | ||||
| 37 | Methyl 2-furoate | 611-13-2 | 2021 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 38 | 2,5-Furandicarboxaldehyde | 823-82-5 | 1999 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 39 | 2-Furancarboxylic acid | 88-14-2 | 2249 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | ||
| 40 | 3-Methylfuran | 930-27-8 | 1206 | MS, RI | SPME | √ | √ | |||
| 41 | 5-Methoxy-2,3-dihydrobenzofuran-3-acetic acid | 93198-71-1 | MS | LLE-SAFE | √ | |||||
| 42 | 2-Methyltetrahydrofuran | 96-47-9 | 690 | MS, RI | SPME | √ | ||||
| Aromatic compounds (41) | ||||||||||
| 1 | Styrene | 100-42-5 | 1152 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 2 | Benzocyclobutene | 694-87-1 | 1152 | MS, RI | LLE-SAFE | √ | ||||
| 3 | Benzaldehyde | 100-52-7 | 1411 | MS, RI, S | LLE-SAFE | √ | ||||
| 4 | Benzeneacetaldehyde | 122-78-1 | 1531 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 5 | Ethyl benzoate | 93-89-0 | 1558 | MS, RI, S | LLE-SAFE | √ | ||||
| 6 | 2-Phenylethyl acetate | 103-45-7 | 1706 | 1444 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 7 | Benzyl alcohol | 100-51-6 | 1767 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 8 | Phenethyl alcohol | 1960/12/8 | 1801 | 1471 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 9 | Toluene | 108-88-3 | 1803 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 10 | 2,6-Di-tert-butyl-4-methylphenol | 128-37-0 | 1807 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 11 | DL-β-Ethylphenethyl alcohol | 2035-94-1 | 1876 | MS, RI | LLE-SAFE | √ | ||||
| 12 | Phenol | 108-95-2 | 1894 | 2009 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ |
| 13 | 3-Phenoxypropionic acid | 7170-38-9 | 1964 | MS, RI | LLE-SAFE | √ | ||||
| 14 | 2-Methoxy-4-vinylphenol | 7786-61-0 | 2083 | 1647 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 15 | 3-Hydroxy-4-phenylbutane-2-one | 5355-63-5 | 2146 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 16 | Ethyl 2-hydroxy-3-phenylpropanoate | 15399-05-0 | 2164 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 17 | 1,3-Dioxolane,2-(1-phenylethyl) | 4362-22-5 | 2165 | MS, RI | LLE-SAFE | √ | √ | |||
| 18 | 2,4-Di-tert-butylphenol | 96-76-4 | 2207 | 2309 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ |
| 19 | 1-Phenyl-2-propanone | 103-79-7 | 2219 | MS, RI | LLE-SAFE | √ | √ | |||
| 20 | 4-Vinylphenol | 2628-17-3 | 2281 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 21 | 1,3-Diphenylpropan-2-ol | 5381-92-0 | 2400 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 22 | 3-Hydroxy-3-phenylbutan-2-one | 3155/1/9 | 2410 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 23 | α-Methylstyrene | 98-83-9 | 2679 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 24 | 4-n-Propylphenol | 645-56-7 | 2703 | MS, RI, S | LLE-SAFE | √ | ||||
| 25 | 4-(2-Acetoxy-ethyl)phenol | 58556-55-1 | 2821 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 26 | p-Hydroxybenzaldehyde | 123-08-0 | 2836 | MS, RI, S | LLE-SAFE | √ | ||||
| 27 | 4-Methoxyresorcinol | 6100-60-3 | 2846 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 28 | 2-Methoxyhydroquinone | 824-46-4 | 2846 | MS, RI, S | LLE-SAFE | √ | √ | √ | ||
| 29 | 4-Hydroxyphenylethanol | 501-94-0 | 2893 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 30 | Benzeneacetic acid | 103-82-2 | MS, aroma, S | LLE-SAFE | √ | √ | √ | √ | ||
| 31 | Resorcinol | 108-46-3 | 2084 | MS, RI, aroma, S | LLE-SAFE | √ | ||||
| 32 | 1-(2-Hydroxy-5-methylphenyl)ethanone | 1450-72-2 | 1647 | MS, RI | SPME | √ | √ | |||
| 33 | (R)-(+)-1-Phenylethanol | 1517-69-7 | 1220 | MS, RI | SPME | √ | √ | √ | ||
| 34 | 4-Phenylbutanal | 18328-11-5 | 1428 | MS, RI | SPME | √ | √ | |||
| 35 | 4-Methoxyphenoxyacetic acid | 1877-75-4 | MS | LLE-SAFE | √ | √ | √ | |||
| 36 | 4-Hydroxyphenoxyacetic acid | 1878-84-8 | MS | LLE-SAFE | √ | |||||
| 37 | 3,5-Dimethoxyphenol | 500-99-2 | MS, S | LLE-SAFE | √ | |||||
| 38 | 2,3,5,6-Tetramethyl-pheno | 527-35-5 | 1645 | MS, RI, S | SPME | √ | √ | |||
| 39 | 2,5-Di-tert-butylphenol | 5875-45-6 | 1701 | MS, RI | SPME | √ | √ | √ | √ | |
| 40 | Benzoic acid | 65-85-0 | 2449 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 41 | 2,5-Dimethylphenol | 95-87-4 | 2196 | MS, RI, aroma, S | LLE-SAFE | √ | ||||
| Ketones (37) | ||||||||||
| 1 | 3-Penten-2-one | 3102-33-8 | 1026 | MS, RI | LLE-SAFE | √ | ||||
| 2 | 3-Penten-2-one | 625-33-2 | 1026 | MS, RI | LLE-SAFE | √ | √ | |||
| 3 | Cyclopentanone | 120-92-3 | 1082 | MS, RI | LLE-SAFE | √ | √ | |||
| 4 | 2,2,5-Trimethylhexane-3,4-dione | 20633-03-8 | 1105 | MS, RI | LLE-SAFE | √ | ||||
| 5 | 3-Hydroxy-2-butanone | 513-86-0 | 1179 | 1277 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ |
| 6 | Hydroxyacetone | 116-09-6 | 1191 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 7 | 2-Cyclopenten-1-one | 930-30-3 | 1248 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 8 | 2-Hydroxy-3-pentanone | 5704-20-1 | 1250 | MS, RI | LLE-SAFE | √ | √ | |||
| 9 | 4-Hydroxy-4-methyl-2-pentanone | 123-42-2 | 1254 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 10 | 4-Hydroxy-3-hexanone | 4984-85-4 | 1302 | MS, RI | LLE-SAFE | √ | ||||
| 11 | 4-Hydroxy-2-pentanone | 4161-60-8 | 1350 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 12 | 2-Butanone | 78-93-3 | 1359 | MS, RI | LLE-SAFE | √ | √ | |||
| 13 | 2,5-Hexanedione | 110-13-4 | 1394 | MS, RI | LLE-SAFE | √ | √ | |||
| 14 | 4-Hydroxy-2-butanone | 590-90-9 | 1427 | 1737 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ |
| 15 | 3-Hydroxy-3-methyl-2-butanone | 115-22-0 | 1451 | MS, RI | LLE-SAFE | √ | √ | |||
| 16 | Isophorone | 78-59-1 | 1483 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 17 | 3-Methylcyclopentane-1,2-dione | 765-70-8 | 1726 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 18 | 3,4-Dihydroxy-2-butanone | 57011-15-1 | 1819 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 19 | 1,3-Dioxolan-2-one | 96-49-1 | 1827 | MS, RI | LLE-SAFE | √ | √ | |||
| 20 | 1-Hydroxy-2-pentanone | 64502-89-2 | 1978 | MS, RI | LLE-SAFE | √ | ||||
| 21 | 4-Methyl-2,3-pentanedione | 7493-58-5 | 2003 | MS, RI | LLE-SAFE | √ | ||||
| 22 | 2-Ethyl-cyclopentanone | 4971-18-0 | 2009 | MS, RI | LLE-SAFE | √ | ||||
| 23 | 2,3-Butanedione | 431-03-8 | 2038 | MS, RI, S | LLE-SAFE | √ | ||||
| 24 | 4-Acetoxy-2-butanone | 10150-87-5 | 2424 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 25 | 4-Hydroxy-β-damascone | 102488-09-5 | 2425 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 26 | 4-(Hydroxymethyl)-1,3-dioxolan-2-one | 931-40-8 | 2716 | MS, RI | LLE-SAFE | √ | √ | |||
| 27 | Acetone | 67-64-1 | 917 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 28 | 2,3-Pentanedione | 600-14-6 | 982 | MS, RI | LLE-SAFE | √ | √ | |||
| 29 | 2,2,6-Trimethylcyclohexanone | 2408-37-9 | MS | LLE-SAFE | √ | √ | ||||
| 30 | 2-Ethyl-3-methoxy-2-cyclopentenone | 25112-86-1 | MS | LLE-SAFE | √ | |||||
| 31 | 3-Methyl-4-nonanone | 35778-39-3 | 681 | MS, RI | SPME | √ | √ | |||
| 32 | 3,4,5,6-Tetrahydropseudoionone | 4433-36-7 | MS | LLE-SAFE | √ | |||||
| 33 | 5-Methyl-3-heptanone | 541-85-5 | 2215 | MS, RI | SPME | √ | ||||
| 34 | 4-Octanone | 589-63-9 | 1150 | MS, RI | SPME | √ | √ | √ | √ | |
| 35 | 3-Methyl-cyclohexanone | 591-24-2 | 1188 | MS, RI | SPME | √ | ||||
| 36 | (Z)-β-Ionone | 79-77-6 | MS, aroma, S | LLE-SAFE | √ | √ | ||||
| 37 | Verbenone | 80-57-9 | 1645 | MS, RI | SPME | √ | ||||
| Acids (33) | ||||||||||
| 1 | 2-Methylvaleric acid | 594-61-6 | 1411 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 2 | Acetic acid | 64-19-7 | 1473 | 1296 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 3 | 4-Methyl-3-pentenoic acid | 504-85-8 | 1478 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 4 | 3-Hydroxypropionic acid | 503-66-2 | 1489 | MS, RI | LLE-SAFE | √ | ||||
| 5 | Malonic acid | 141-82-2 | 1715 | MS, RI | LLE-SAFE | √ | ||||
| 6 | Butanoic acid | 107-92-6 | 1718 | MS, RI | LLE-SAFE | √ | ||||
| 7 | Hexanoic acid | 142-62-1 | 1824 | 1441 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 8 | 4-Methylvaleric acid | 646-07-1 | 1832 | MS, RI | LLE-SAFE | √ | ||||
| 9 | Pentanoic acid | 109-52-4 | 1870 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 10 | 4-Methylpent-4-enoic acid | 1001-75-8 | 2006 | MS, RI | LLE-SAFE | √ | ||||
| 11 | Octanoic acid | 124-07-2 | 2006 | 1544 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 12 | 2-Methyl-2-pentenoic acid | 3142-72-1 | 2011 | 1199 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 13 | 3-Methylbutanoic acid | 503-74-2 | 2062 | 1379 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 14 | 2-Propynyl acetate | 627-09-8 | 2112 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 15 | 2-Oxopropionic acid | 127-17-3 | 2229 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 16 | Decanoic acid | 334-48-5 | 2322 | 1684 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 17 | Nonanoic acid | 112-05-0 | 2342 | MS, RI, S | LLE-SAFE | √ | ||||
| 18 | L-Lactic acid | 79-33-4 | 2947 | MS, RI | LLE-SAFE | √ | ||||
| 19 | 4-Methyl-2-pentenoic acid | 10321-71-8 | 1955 | MS, RI | LLE-SAFE | √ | √ | |||
| 20 | DL-3-Methylvaleric acid | 105-43-1 | 1373 | MS, RI, S | SPME | √ | ||||
| 21 | Heptanoic acid | 111-14-8 | 1619 | MS, RI, S | SPME | √ | √ | √ | ||
| 22 | Hendecanoic acid | 112-37-8 | 1492 | MS, RI | SPME | √ | √ | √ | ||
| 23 | Elaidic acid | 112-79-8 | MS, aroma, S | LLE-SAFE | √ | √ | √ | |||
| 24 | 3-Ethylheptanoic acid | 14272-47-0 | 1629 | MS, RI | SPME | √ | √ | √ | ||
| 25 | Dodecanoic acid | 143-07-7 | 2486 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 26 | 2-(2-Methoxyethoxy)acetic acid | 16024-56-9 | 2311 | MS, RI | SPME | √ | ||||
| 27 | 4-Methoxybutyric acid | 29006-02-8 | 2321 | MS, RI | SPME | √ | √ | √ | ||
| 28 | Crotonic acid | 3724-65-0 | 2395 | MS, RI | LLE-SAFE | √ | ||||
| 29 | trans-3-Hexenoic acid | 4219-24-3 | 2128 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 30 | 3-Ethoxypropionic acid | 4324-38-3 | 1859 | MS, RI | SPME | √ | √ | |||
| 31 | Palmitic acid | 1957/10/3 | MS, aroma, S | LLE-SAFE | √ | √ | √ | √ | ||
| 32 | Tridecanoic acid | 638-53-9 | 1620 | MS, RI, S | SPME | √ | √ | √ | √ | |
| 33 | Citric acid | 77-92-9 | 1730 | MS, RI | SPME | √ | ||||
| Sulfur-containing compounds (27) | ||||||||||
| 1 | Thiazole | 288-47-1 | 1145 | MS, RI | LLE-SAFE | √ | ||||
| 2 | Acetyl Sulfide | 3232-39-1 | 1164 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 3 | 2-(Methylthio)ethanol | 5271-38-5 | 1422 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 4 | Dimethyl sulfoxide | 67-68-5 | 1467 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 5 | 3-(Methylthio)-1-propanol | 505-10-2 | 1610 | 1714 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ |
| 6 | Thioacetic acid | 507-09-5 | 1630 | MS, RI, S | LLE-SAFE | √ | √ | |||
| 7 | S-Ethyl ethanethioate | 625-60-5 | 1704 | MS, RI, S | LLE-SAFE | √ | ||||
| 8 | Dimethyl sulfone | 67-71-0 | 1785 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 9 | 4-methyl-5-hydroxyethyl thiazole | 137-00-8 | 2197 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 10 | Methyl p-tolyl sulfone | 3185-99-7 | 2504 | MS, RI | LLE-SAFE | √ | ||||
| 11 | Methyl mercaptan | 74-93-1 | 893 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 12 | Tetrahydrothiophene | 110-01-0 | 1679 | MS, RI | SPME | √ | √ | |||
| 13 | Thiophene | 110-02-1 | 1031 | MS, RI, aroma, S | LLE-SAFE, SPME | √ | √ | √ | √ | |
| 14 | 2,3-Dihydrothiophene | 1120-59-8 | 2395 | MS, RI | LLE-SAFE | √ | ||||
| 15 | 2-Thiopheneacetic acid | 1918-77-0 | 1187 | MS, RI | SPME | √ | √ | √ | ||
| 16 | (Butylthio)acetic acid | 20600-61-7 | 1989 | MS, RI | SPME | √ | ||||
| 17 | [(2-Methylpropyl)thio]acetic acid | 20600-62-8 | 2282 | MS, RI | SPME | √ | ||||
| 18 | 3-(n-Butylsulphanyl)propionic acid | 22002-73-9 | 2383 | MS, RI | SPME | √ | ||||
| 19 | 2-Pyrrolidinethione | 2295-35-4 | 1219 | MS, RI | SPME | √ | √ | |||
| 20 | Isothiazole | 288-16-4 | 652 | MS, RI | LLE-SAFE | √ | √ | |||
| 21 | 2-Acetyl-2-thiazoline | 29926-41-8 | 1631 | MS, RI | SPME | √ | ||||
| 22 | 4-Methyl-5-acetyl thiazole | 38205-55-9 | 1349 | MS, RI | SPME | √ | ||||
| 23 | Thiazolidine-4-carboxylic acid | 444-27-9 | 2219 | MS, RI | SPME | √ | √ | √ | ||
| 24 | Thiazolidine | 504-78-9 | 1893 | MS, RI | SPME | √ | ||||
| 25 | Diethyl sulfite | 623-81-4 | 2166 | MS, RI | SPME | √ | √ | √ | ||
| 26 | 5-Methylthio-1,3,4-thiadiazole-2-thiol | 6264-40-0 | 2158 | MS, RI | SPME | √ | ||||
| 27 | Divinyl sulfide | 627-51-0 | 2399 | MS, RI | LLE-SAFE | √ | ||||
| Lactones (24) | ||||||||||
| 1 | α-Methyl-γ-butyrolactone | 1679-47-6 | 1476 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 2 | 4,4-Dimethylbut-2-en-4-olide | 20019-64-1 | 1496 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 3 | γ-Valerolactone | 108-29-2 | 1496 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 4 | 3-Methyl-4-butanolide | 1679-49-8 | 1500 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 5 | γ-Butyrolactone | 96-48-0 | 1512 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 6 | γ-Hexanolactone | 695-06-7 | 1589 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 7 | δ-Hexanolide | 823-22-3 | 1679 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 8 | δ-Valerolactone | 542-28-9 | 1688 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 9 | 3-Methyl-2-buten-4-olide | 6124-79-4 | 1769 | 1903 | MS, RI | LLE-SAFE | √ | √ | √ | √ |
| 10 | γ-Octalactone | 104-50-7 | 1803 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 11 | 3-Methyl-2-penten-5-olide | 2381-87-5 | 1903 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 12 | 4-Nonanolide | 104-61-0 | 1915 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 13 | L-(+)-Pantolactone | 5405-40-3 | 1917 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 14 | D-(−)-Pantolactone | 599-04-2 | 1917 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 15 | DL-Pantoyl Lacyone | 79-50-5 | 1917 | MS, RI | LLE-SAFE | √ | ||||
| 16 | 2-Hydroxy-γ-butyrolactone | 19444-84-9 | 2053 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 17 | Dihydroactinidiolide | 15356-74-8 | 2224 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 18 | (R)-Dihydro-actinidiolide | 17092-92-1 | 2224 | MS, RI | LLE-SAFE | √ | ||||
| 19 | 5-Hydroxy-4-pentanolide | 32780-06-6 | 2364 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 20 | DL-Mevalonolactone | 674-26-0 | 2428 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 21 | (±)-3-Hydroxy-4-butanolide | 5469-16-9 | 2479 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| 22 | β-Butyrolactone | 3068-88-0 | 2531 | MS, RI | LLE-SAFE | √ | ||||
| 23 | Costunolide | 553-21-9 | MS, aroma, S | LLE-SAFE | √ | √ | ||||
| 24 | γ-Decanolactone | 706-14-9 | 2040 | MS, RI, aroma, S | LLE-SAFE | √ | √ | √ | √ | |
| Aldehydes (19) | ||||||||||
| 1 | 3-Methyl-2-butenal | 107-86-8 | 1096 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 2 | Nonanal | 124-19-6 | 1289 | MS, RI, S | LLE-SAFE | √ | √ | |||
| 3 | 1,3,5,7-Tetroxane | 293-30-1 | 1418 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 4 | Methyl glyoxal | 78-98-8 | 1458 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 5 | Glutaraldehyde | 111-30-8 | 1545 | 2353 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | |
| 6 | (Z)-2-Methyl-2-butenal | 1115-11-3 | 1636 | MS, RI | LLE-SAFE | √ | ||||
| 7 | Methacrolein | 78-85-3 | 1686 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 8 | 2-Ethyl-4-pentenal | 5204-80-8 | 2038 | MS, RI | LLE-SAFE | √ | ||||
| 9 | Methoxy acetaldehyde | 10312-83-1 | 2216 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 10 | cis-6-Nonenal | 2277-19-2 | 2517 | MS, RI | LLE-SAFE | √ | ||||
| 11 | Glyoxylic acid | 298-12-4 | 2643 | MS, RI | LLE-SAFE | √ | ||||
| 12 | 2,2-Dimethylocta-3,4-dienal | 590-71-6 | 2796 | MS, RI | LLE-SAFE | √ | ||||
| 13 | 3-Hydroxybutyraldehyde | 107-89-1 | 2929 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 14 | Acetaldehyde | 75-07-0 | 890 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 15 | Hexanal | 66-25-1 | 993 | MS, RI | LLE-SAFE | √ | √ | |||
| 16 | trans-2-Nonenal | 18829-56-6 | 1414 | MS, RI, S | SPME | √ | √ | |||
| 17 | Isovaleraldehyde | 590-86-3 | 2047 | MS, RI, S | SPME | √ | ||||
| 18 | (E,E)-2,4-Nonadienal | 5910-87-2 | 1123 | MS, RI | SPME | √ | √ | |||
| 19 | Hexadecanal | 629-80-1 | MS | LLE-SAFE | √ | √ | ||||
| Acetals (2) | ||||||||||
| 1 | 2-Benzyl-1,3-dioxolane | 101-49-5 | 2165 | MS, RI | LLE-SAFE | √ | √ | |||
| 2 | Tetraethylene glycol | 112-60-7 | 2597 | 2144 | MS, RI | LLE-SAFE, SPME | √ | √ | √ | √ |
| Others (37) | ||||||||||
| 1 | Myrcene | 123-35-3 | 1066 | 1056 | MS, RI, S | LLE-SAFE, SPME | √ | √ | √ | |
| 2 | Dimethyl ether | 115-10-6 | 1110 | MS, RI | LLE-SAFE | √ | √ | |||
| 3 | Acetic anhydride | 108-24-7 | 1134 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 4 | 1,3,5,7-Cyclooctatetraene | 629-20-9 | 1152 | MS, RI | LLE-SAFE | √ | ||||
| 5 | 3,5-Dimethylhex-1-ene | 7423-69-0 | 1659 | MS, RI | LLE-SAFE | √ | ||||
| 6 | Ethoxyacetylene | 927-80-0 | 1676 | MS, RI | LLE-SAFE | √ | ||||
| 7 | 1,5-Heptadien-3-yne | 3511-27-1 | 1806 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 8 | Isobutyl anhydride | 97-72-3 | 2012 | MS, RI | LLE-SAFE | √ | √ | |||
| 9 | 2-Methyl-2-butene | 513-35-9 | 2039 | MS, RI, S | LLE-SAFE | √ | √ | √ | √ | |
| 10 | 3-Methylene-nonane | 51655-64-2 | 2039 | MS, RI | LLE-SAFE | √ | ||||
| 11 | Valeric anhydride | 2082-59-9 | 2116 | MS, RI | LLE-SAFE | √ | ||||
| 12 | 2-Methylsuccinic anhydride | 4100-80-5 | 2264 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 13 | 1-Docosene | 1599-67-3 | 2481 | MS, RI | LLE-SAFE | √ | ||||
| 14 | 1-Hexadecene | 629-73-2 | 2481 | MS, RI | LLE-SAFE | √ | ||||
| 15 | 1,4-Dioxane | 123-91-1 | 2713 | MS, RI | LLE-SAFE | √ | ||||
| 16 | Cyclopentene | 142-29-0 | 889 | MS, RI | LLE-SAFE | √ | ||||
| 17 | Propene | 115-07-1 | 908 | MS, RI | LLE-SAFE | √ | √ | √ | √ | |
| 18 | 1,3-Butadiyne | 460-12-8 | 918 | MS, RI | LLE-SAFE | √ | ||||
| 19 | 1-Buten-3-yne | 689-97-4 | 921 | MS, RI | LLE-SAFE | √ | ||||
| 20 | 1-Nonyne | 3452/9/3 | 1115 | MS, RI | SPME | √ | ||||
| 21 | cis-2-Octene | 7642/4/8 | 1292 | MS, RI | SPME | √ | √ | √ | ||
| 22 | Diisopropyl ether | 108-20-3 | 2325 | MS, RI | SPME | √ | √ | √ | ||
| 23 | 2-Pentene | 109-68-2 | 606 | MS, RI, aroma, S | LLE-SAFE | √ | √ | |||
| 24 | 3-Ethyl-3-hexene | 16789-51-8 | 1282 | MS, RI | SPME | √ | ||||
| 25 | β-Pinene | 18172-67-3 | 1040 | MS, RI, S | SPME | √ | √ | √ | ||
| 26 | 2-Methyl-2-hexene | 2738-19-4 | 1412 | MS, RI | SPME | √ | ||||
| 27 | (E)-2-Hexene | 4050-45-7 | 1175 | MS, RI | SPME | √ | ||||
| 28 | Dodecyl ether | 4542-57-8 | MS | LLE-SAFE | √ | √ | √ | |||
| 29 | 3-Methyl-1-butene | 563-45-1 | 605 | MS, RI | LLE-SAFE | √ | √ | √ | ||
| 30 | 1,2-Butadiene | 590-19-2 | 613 | MS, RI | LLE-SAFE | √ | ||||
| 31 | 1-Heptene | 592-76-7 | 1228 | MS, RI, S | SPME | √ | √ | √ | √ | |
| 32 | 1-Octadecyne | 629-89-0 | MS | LLE-SAFE | √ | √ | √ | |||
| 33 | 1,1-Diethoxy-3,7-dimethylocta-2,6-diene | 7492-66-2 | 1216 | MS, RI | SPME | √ | √ | |||
| 34 | 2-Methyl-1-pentene | 763-29-1 | 1050 | MS, RI | SPME | √ | √ | √ | ||
| 35 | 2-Methyl-1,3-butadiene | 78-79-5 | 605 | MS, RI | LLE-SAFE | √ | ||||
| 36 | 1-Undecene | 821-95-4 | 1349 | MS, RI, S | SPME | √ | √ | √ | √ | |
| 37 | Vinyl isopropyl ether | 926-65-8 | 1044 | MS, RI | SPME | √ | √ | |||
| No. | Compound Name | CAS | Sniffing Description | YJ | BW | HR | QD |
|---|---|---|---|---|---|---|---|
| 1 | 4-Vinylguaiacol | 7786-61-0 | Coffee bean, nut shell, sour | 4 | 3 | 1.5 | 3 |
| 2 | γ-Decalactone | 706-14-9 | Sweet, floral, gardenia, creamy | 4 | 4.5 | 4 | 4 |
| 3 | Phenylacetic acid | 103-82-2 | Floral, rose, sweet, grassy | 3.5 | 4 | 2.5 | 2 |
| 4 | Isovaleric acid | 503-74-2 | Sour, durian-like | 3.5 | 3 | 3.5 | 2.5 |
| 5 | 2-Phenylethyl acetate | 103-45-7 | Sweet, floral, fruity | 3 | 2.5 | 3.5 | 1 |
| 6 | Acetic acid | 64-19-7 | Sour, muddy | 3 | 2.5 | 4 | 3 |
| 7 | Octanoic acid | 124-07-2 | Grassy, vegetal | 3 | 2.5 | 2.5 | 2 |
| 8 | Hexyl butyrate | 2639-63-6 | Sweet, floral, honey-like | 2.5 | 2 | 2 | 1.5 |
| 9 | 6-Methyl-5-hepten-2-one | 3658-77-3 | Caramel, sweet | 2.5 | 3 | 2.5 | 2 |
| 10 | Phenylethanol | 1960/12/8 | Rose-like, sweet | 2.5 | 3.5 | 3.5 | 2.5 |
| 11 | Pineapple alcohol | 505-10-2 | Sour, muddy | 2.5 | 2.5 | 3 | 3 |
| 12 | 2,3-Butanediol | 513-85-9 | Roasted, dark wheat | 2 | 1 | - | - |
| 13 | Hexanoic acid | 142-62-1 | Lemon-like, sour, bitter | 2 | 2 | 2.5 | 0.5 |
| 14 | Decanoic acid | 334-48-5 | Sour, hoppy, grassy | 2 | 1.5 | 1.5 | 0.5 |
| 15 | 2-Acetylpyrrole | 1072-83-9 | Malty, sweet, caramel | 1.5 | 2.5 | 1.5 | 1 |
| 16 | Furoic acid | 88-14-2 | Rooty, wheaty | 1.5 | 2 | - | 1.5 |
| 17 | 4-Hydroxyphenylethanol | 501-94-0 | Floral, sweet, fruity | 1.5 | 2 | 3 | 1 |
| 18 | Ethyl propionate | 105-37-3 | Floral, fruity, strawberry-like | 1 | 2 | 1.5 | 1 |
| 19 | Hexacosane | 630-01-3 | Floral, honey-like | 1 | 2 | 0.5 | - |
| 20 | Resorcinol | 108-46-3 | Burnt, sweet | - | 2 | - | - |
| 21 | trans-Oleic acid | 112-79-8 | Dark wheat | - | 2 | - | 2 |
| 22 | 5-Methylpyrrole-2-carboxaldehyde | 1192-79-6 | Rooty | 1.5 | 1.5 | - | - |
| 23 | trans-3-Hexenoic acid | 4219-24-3 | Floral, sweet, burnt | 1.5 | 1.5 | 1 | - |
| 24 | Isoamyl alcohol | 123-51-3 | Nut shell, roasted | 1 | 1.5 | 2 | 1 |
| 25 | Furfuryl alcohol | 98-00-0 | Sour, putrid | 1 | 1.5 | 0.5 | - |
| 26 | Palmitic acid | 1957/10/3 | Dark wheat | 1 | 1.5 | - | - |
| 27 | (±)-3-Hydroxy-4-butyrolactone | 5469-16-9 | Floral, woody | 1 | 1.5 | 1 | 1.5 |
| 28 | Monoethyl succinate | 1070-34-4 | Malty | - | 1.5 | - | - |
| 29 | 2,4-Di-tert-butylphenol | 96-76-4 | Roasted, nut shell, yeasty | 1 | 1 | 1 | 1.5 |
| 30 | Ethyl hexanoate | 123-66-0 | Sweet, fruity, floral | 0.5 | 1 | 1.5 | 1.5 |
| 31 | 4-Hydroxy-2-butanone | 590-90-9 | Floral, oily, sweet | 0.5 | 1 | 2 | 0.5 |
| 32 | Methyl octanoate | 111-12-6 | Dusty, moldy, bitter | - | 1 | 1 | - |
| 33 | 6-(Ethoxycarbonyl)nicotinic acid | 17874-78-1 | Smoky | - | 1 | - | - |
| 34 | (Z)-β-Ionone | 79-77-6 | Floral, sweet | - | 1 | - | 0.5 |
| 35 | Cholestane | 481-21-0 | Roasted, dark wheat | 1 | 0.5 | - | - |
| 36 | 1,2-Propanediol | 57-55-6 | Nut shell | - | 0.5 | - | - |
| 37 | Isoamyl acetate | 123-92-2 | Floral | - | 0.5 | - | - |
| 38 | Tetratetracontane | 7098-22-8 | Nut shell | - | 0.5 | - | - |
| 39 | Methyl decanoate | 110-42-9 | Apple-like, sweet, floral | 1.5 | - | - | 1 |
| 40 | (Z)-9-Octadecenamide | 301-02-0 | Sweet, yeasty | 1 | - | - | 1 |
| 41 | 2,6-Di-tert-butylpyridine | 585-48-8 | Ashy | 1 | - | - | - |
| 42 | 5-Hydroxymethylfurfural | 67-47-0 | Yeasty | 1 | - | - | - |
| 43 | Benzoic acid | 65-85-0 | Ashy | 1 | - | - | - |
| 44 | Lauric acid | 143-07-7 | Fruity | 1 | - | - | - |
| 45 | 2-Hexyl-1-decanol | 2425-77-6 | Floral | 0.5 | - | 0.5 | - |
| 46 | Thiophene | 110-02-1 | Garlic, oily | - | - | 2.5 | - |
| 47 | 2-Hydroxypyridine | 142-08-5 | Hoppy, bitter | - | - | 2 | - |
| 48 | 2-Pentene | 109-68-2 | Burnt, plastic-like | - | - | 2 | - |
| 49 | 2,5-Dimethylphenol | 95-87-4 | Nut shell | - | - | 1 | - |
| 50 | Methyl furoate | 611-13-2 | Nut shell | - | - | 0.5 | - |
| 51 | N-Butylacetamide | 1119-49-9 | Sweet | - | - | - | 1 |
| 52 | Tetratriacontane | 14167-59-0 | Sweet | - | - | - | 2 |
| 53 | Costunolide | 553-21-9 | Floral | - | - | - | 0.5 |
| No. | Compound Name | Odor Threshold (μg/L) | OAV | Taste Threshold (μg/L) | TAV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| YJ | QD | HR | BW | YJ | QD | HR | BW | ||||
| 1 | Isoamyl acetate | 0.13 | 2,046,526 | 1,217,009 | 4,338,735 | 2,821,490 | 2.63 | 102,326 | 60,850 | 216,937 | 141,074 |
| 2 | Ethyl hexanoate | 4.35 | 8113 | 6941 | 4690 | 10,366 | 4.35 | 8113 | 6941 | 4690 | 10,366 |
| 3 | Ethyl propanoate | 8.88 | 4468 | 2403 | 6090 | 5094 | 8.88 | 4468 | 2403 | 6090 | 5094 |
| 4 | Hexyl butanoate | 172.75 | 1354 | 718 | 1797 | 1547 | — | — | — | — | — |
| 5 | Methyl decanoate | 3.75 | 19,448 | 16,822 | 29,701 | 45,970 | 871.00 | 84 | 72 | 128 | 198 |
| 6 | Ethyl octanoate | 16.73 | 105 | 30 | 79 | 97 | 0.09 | 20,170 | 5840 | 15,231 | 18,693 |
| 7 | Ethyl decanoate | 4.31 | 249 | — | 320 | 634 | 17.24 | 62 | — | 80 | 158 |
| 8 | Isoamyl butyrate | 12.93 | 3658 | — | — | — | — | — | — | — | — |
| 9 | Ethyl trans-2-decenoate | 10,000.00 | — | — | <1 | — | — | — | — | — | — |
| 10 | Octyl acetate | 0.04 | 99 | 22 | — | — | 182.28 | 22 | 5 | — | — |
| 11 | Ethyl stearate | 0.53 | 26 | 23 | 12 | 27 | — | — | — | — | — |
| 12 | Ethyl isovalerate | 0.10 | — | 31,502 | — | — | 0.17 | — | 17,326 | — | — |
| 13 | Ethyl myristate | 3440.00 | 1 | <1 | <1 | 1 | — | — | — | — | — |
| 14 | Ethyl lactate | 51,550.00 | <1 | <1 | <1 | <1 | 257,750.00 | <1 | <1 | <1 | <1 |
| 15 | Ethyl acetate | 50.00 | 11,099 | 8987 | 10,306 | 7351 | 6765.00 | 82 | 66 | 76 | 54 |
| 16 | Ethyl heptanoate | 1.65 | 350 | 409 | 254 | 623 | 147.90 | 4 | 5 | 3 | 7 |
| 17 | Hexyl acetate | 20.00 | 286 | 392 | 574 | 551 | 34.80 | 164 | 225 | 330 | 317 |
| 18 | Diethyl succinate | 104,700.00 | <1 | <1 | <1 | <1 | 1,094,115.00 | <1 | <1 | <1 | <1 |
| 19 | Butyl acetate | 51.04 | — | — | 13 | 14 | 88.00 | — | — | 8 | 8 |
| 20 | Ethyl 3-hydrobutyrate | 1000.00 | — | — | <1 | <1 | — | — | — | — | — |
| 21 | Ethyl nonanoate | 326.48 | 7 | 2 | 9 | 16 | 1039.20 | 2 | 1 | 3 | 5 |
| 22 | Ethyl hydrogen succinate | 1,141,000.00 | <1 | <1 | <1 | <1 | 1,369,200.00 | <1 | <1 | <1 | <1 |
| 23 | Ethyl valerate | 5.08 | 774 | 679 | 596 | 623 | 787.50 | 5 | 4 | 4 | 4 |
| 24 | Methyl 2-octynate | 23.00 | — | — | 721 | 575 | — | — | — | — | — |
| 25 | (−)-Ethyl L-lactate | — | — | — | — | — | — | — | — | — | — |
| 26 | 2-Hydroxyethyl acetate | — | — | — | — | — | — | — | — | — | — |
| 27 | Propyl acetate | 1776.00 | <1 | <1 | — | — | 710.40 | 1 | <1 | — | — |
| 28 | 2,3-Dihydroxypropyl acetate | — | — | — | — | — | 1,616,040.00 | <1 | <1 | <1 | <1 |
| 29 | Methyl propionate | 4209.00 | — | <1 | — | — | 53.07 | — | 73 | — | — |
| 30 | Isopropyl formate | — | — | — | — | — | — | — | — | — | — |
| 31 | Ethyl dodecanoate | 5091.70 | 1 | — | <1 | — | 284.79 | 24 | — | 17 | — |
| 32 | Ethyl 3-hexenoate | 10,000.00 | 3 | — | <1 | <1 | — | — | — | — | — |
| 33 | Ethyl butyrylacetate | — | — | — | — | — | — | — | — | — | — |
| 34 | Ethyl 3-methylvalerate | — | — | — | — | — | — | — | — | — | — |
| 35 | Ethyl undecanoate | — | — | — | — | — | 859.00 | 56 | — | 3 | — |
| 36 | (+)-Diethyl L-tartrate | — | — | — | — | — | — | — | — | — | — |
| 37 | 2,3-Butaneiol | 100,200.00 | 2 | <1 | 4 | 5 | 50,100.00 | 5 | 1 | 8 | 11 |
| 38 | 3-Methyl-1-butanol | 792.82 | 710 | 531 | 1045 | 913 | 202.25 | 2785 | 2082 | 4098 | 3579 |
| 39 | 1,2,3-Propanetriol | 25,000,000.00 | <1 | <1 | <1 | <1 | 6,562,500.00 | <1 | <1 | <1 | <1 |
| 40 | 2-Methyl-1-propanol | 11.00 | 14,796 | 12,246 | 15,732 | 6183 | 8.83 | 18,426 | 15,251 | 19,592 | 7700 |
| 41 | Diisobutylcarbinol | 1051.70 | — | — | 3 | 2 | — | — | — | — | — |
| 42 | 1-Butanol | 3483.00 | 3 | <1 | <1 | <1 | 162,000.00 | <1 | <1 | <1 | <1 |
| 43 | 1-Octanol | 104.04 | 4 | 5 | 11 | 8 | 44.66 | 10 | 11 | 27 | 18 |
| 44 | 1-Pentanol | 121.81 | 2030 | 1596 | 2460 | 2104 | 64,880.00 | 4 | 3 | 5 | 4 |
| 45 | 3-Methyl-3-buten-1-ol | 466.70 | <1 | <1 | <1 | <1 | — | — | — | — | — |
| 46 | α-Terpineol | 1116.00 | <1 | <1 | <1 | — | 279.00 | 2 | 2 | 1 | — |
| 47 | 2-Ethyl-1-hexanol | 21,227.00 | — | — | — | <1 | — | — | — | — | — |
| 48 | 4-Methyl-1-pentanol | 673.22 | 1 | — | — | 99 | — | — | — | — | — |
| 49 | 1,2-Propylene glycol | 352,240.00 | <1 | <1 | <1 | <1 | 1,450,400.00 | <1 | <1 | <1 | <1 |
| 50 | 2-Hexyl-1-decanol | — | — | — | — | — | — | — | — | — | — |
| 51 | 1-Hexanol | 318.27 | 10 | 24 | 13 | 10 | 162.80 | 20 | 47 | 26 | 20 |
| 52 | Linalool | 941.34 | 71 | 6 | 3 | — | 23.49 | 2854 | 242 | 114 | — |
| 53 | 1-Decanol | 642.48 | — | 16 | 16 | — | 19.07 | — | 548 | 536 | — |
| 54 | Diethylene glycol monoethyl ether | — | — | — | — | — | 47,952.00 | <1 | <1 | <1 | <1 |
| 55 | (−)-Isolongifolol | — | — | — | — | — | — | — | — | — | — |
| 56 | Phytol | — | — | — | — | — | — | — | — | — | — |
| 57 | β-Eudesmol | — | — | — | — | — | — | — | — | — | — |
| 58 | Guaiol | — | — | — | — | — | — | — | — | — | — |
| 59 | 2,6-Dimethyl-5,7-octadien-2-ol | — | — | — | — | — | — | — | — | — | — |
| 60 | Phenethyl alcohol | 397.80 | 5968 | 3462 | 8289 | 4426 | 50,100.00 | 5 | 1 | 8 | 11 |
| 61 | 2-Phenylethyl acetate | 257.58 | 100 | 16 | 231 | 72 | 102,000.00 | 23 | 14 | 32 | 17 |
| 62 | Phenol | 59,000.00 | <1 | <1 | <1 | <1 | 20.64 | 1248 | 205 | 2877 | 894 |
| 63 | Benzeneacetaldehyde | 6.80 | 4206 | 5867 | 990 | 633 | 5890.50 | <1 | <1 | 1 | 1 |
| 64 | Benzyl alcohol | 2660.80 | <1 | <1 | <1 | <1 | 9.71 | 2944 | 4107 | 693 | 443 |
| 65 | Ethyl benzoate | 58.06 | 615 | — | — | — | 5747.50 | <1 | <1 | <1 | <1 |
| 66 | Benzaldehyde | 783.93 | — | — | 2 | — | 313,500.00 | <1 | — | — | — |
| 67 | 2,4-Di-tert-butylphenol | 443.50 | 18 | 157 | 73 | 88 | 313.20 | — | — | 5 | — |
| 68 | 2-Methoxy-4-vinylphenol | 20.69 | 1128 | 1171 | 1740 | 1290 | — | — | — | — | — |
| 69 | 2,6-Di-tert-butyl-4-methylphenol | 1048.00 | 500 | 274 | 572 | 327 | 21.78 | 1072 | 1113 | 1653 | 1226 |
| 70 | Benzoic acid | 1080.00 | 102 | 126 | 133 | 82 | — | — | — | — | — |
| 71 | p-Hydroxyphenylethanol | — | — | — | — | — | 313,200.00 | <1 | <1 | <1 | <1 |
| 72 | Benzeneacetic acid | 12,972.00 | 8 | 6 | 6 | 7 | — | — | — | — | — |
| 73 | Resorcinol | 7620.00 | — | — | — | <1 | 108.10 | 936 | 696 | 684 | 846 |
| 74 | 2,5-Dimethylphenol | 388.40 | — | — | 6 | — | 93,980.00 | — | — | — | <1 |
| 75 | α-Methylstyrene | — | — | — | — | — | 485.50 | — | — | 5 | — |
| 76 | 1-(2-Hydroxy-5-methylphenyl)ethanone | — | — | — | — | — | — | — | — | — | — |
| 77 | (R)-(+)-1-Phenylethanol | — | — | — | — | — | — | — | — | — | — |
| 78 | Toluene | — | — | — | — | — | — | — | — | — | — |
| 79 | Octanoic acid | 2730.00 | 151 | 101 | 210 | 190 | 4550.00 | 91 | 60 | 126 | 114 |
| 80 | Hexanoic acid | 0.60 | 258,687 | 198,095 | 474,974 | 419,423 | 0.56 | 279,058 | 213,694 | 512,378 | 452,452 |
| 81 | Acetic acid | 23,078.00 | <1 | <1 | <1 | <1 | 74,479.00 | <1 | <1 | <1 | <1 |
| 82 | 3-Methylbutanoic acid | 1110.00 | 17 | 27 | 31 | 23 | 462.50 | 40 | 64 | 75 | 54 |
| 83 | Decanoic acid | 8930.00 | 8 | 9 | 14 | 22 | 4554.30 | 16 | 18 | 27 | 42 |
| 84 | Dodecanoic acid | 10,000.00 | 9 | 6 | 15 | 12 | 529.80 | 172 | 121 | 277 | 221 |
| 85 | Elaidic acid | — | — | — | — | — | — | — | — | — | — |
| 86 | DL-3-Methylvaleric acid | 42.78 | — | 183 | — | — | 9.30 | — | 843 | — | — |
| 87 | Heptanoic acid | 587.52 | 11 | — | 11 | 9 | 2111.40 | 3 | — | 3 | 3 |
| 88 | Tridecanoic acid | 9582.00 | <1 | <1 | <1 | <1 | — | — | — | — | — |
| 89 | 2-Methylvaleric acid | — | — | — | — | — | 11,868,480.00 | <1 | <1 | <1 | <1 |
| 90 | Pentanoic acid | 15,963.00 | 13 | 7 | 20 | 20 | 469.50 | 426 | 247 | 682 | 684 |
| 91 | Nonanoic acid | 4167.60 | — | — | — | <1 | 1359.00 | — | — | — | <1 |
| 92 | Palmitic acid | 8520.00 | 5 | 28 | 7 | 11 | — | — | — | — | — |
| 93 | trans-3-Hexenoic acid | — | — | — | — | — | — | — | — | — | — |
| 94 | 4-Methyl-2-pentenoic acid | — | — | — | — | — | — | — | — | — | — |
| 95 | Hendecanoic acid | 8900.00 | <1 | — | <1 | <1 | 890.00 | 5 | — | 3 | 2 |
| 96 | Crotonic acid | — | — | — | — | — | — | — | — | — | — |
| 97 | 2-Methyl-2-pentenoic acid | — | — | — | — | — | — | — | — | — | — |
| 98 | Furfuryl alcohol | 5108.10 | 3 | 2 | 5 | 4 | 1135.00 | 14 | 9 | 24 | 18 |
| 99 | 2-Furancarboxylic acid | — | — | — | — | — | 23,832.00 | <1 | <1 | — | <1 |
| 100 | 2-Butylfuran | 4.43 | — | — | 708 | — | — | — | — | — | — |
| 101 | Furfural | 3480.00 | <1 | 1 | 2 | 2 | 9280.00 | <1 | <1 | <1 | <1 |
| 102 | 2-Acetyl furan | 16,498.00 | <1 | <1 | <1 | <1 | 87,840.00 | <1 | <1 | <1 | <1 |
| 103 | Methyl2-furoate | — | — | — | — | — | — | — | — | — | — |
| 104 | 5-Hydroxymethyl-2-furaldehyde | 1,243,000.00 | <1 | <1 | <1 | <1 | 1,243,000.00 | <1 | <1 | <1 | <1 |
| 105 | 2,5-Dimethyl-4-hydroxy-2H-furan-3-one | 167.84 | 37 | 245 | 37 | 48 | 10.49 | 592 | 3926 | 600 | 772 |
| 106 | 2(5H)-Furanone | — | — | — | — | — | — | — | — | — | — |
| 107 | Furfuryl sulfide | — | — | — | — | — | — | — | — | — | — |
| 108 | 2,2′-Difurfuryl ether | — | — | — | — | — | — | — | — | — | — |
| 109 | (±)-3-Hydroxy-4-butanolide | — | — | — | — | — | — | — | — | — | — |
| 110 | γ-Decanolactone | 2.46 | 1192 | 1474 | 2091 | 3559 | 379.20 | 8 | 10 | 14 | 23 |
| 111 | γ-Butyrolactone | 1120.00 | 5 | 6 | 20 | 22 | 11,200.00 | <1 | <1 | 2 | 2 |
| 112 | 4-Nonanolide | 26.35 | 265 | 164 | 272 | 389 | 976.00 | 7 | 4 | 7 | 11 |
| 113 | γ-Hexanolactone | 12,525.00 | <1 | <1 | <1 | <1 | 13,026.00 | <1 | <1 | <1 | <1 |
| 114 | γ-Octalactone | 17.56 | 480 | 271 | 509 | 659 | 93.20 | 90 | 51 | 96 | 124 |
| 115 | δ-Valerolactone | — | — | — | — | — | 1,105,000.00 | <1 | <1 | <1 | <1 |
| 116 | γ-Valerolactone | 10,000.00 | <1 | <1 | <1 | <1 | 105,000.00 | <1 | <1 | <1 | <1 |
| 117 | Costunolide | — | — | — | — | — | — | — | — | — | — |
| 118 | 5-Hydroxy-4-pentanolide | — | — | — | — | — | — | — | — | — | — |
| 119 | 2-Hydroxypyridine | — | — | — | — | — | — | — | — | — | — |
| 120 | (Z)-9-Octadecenamide | — | — | — | — | — | — | — | — | — | — |
| 121 | 2-Acetyl pyrrole | — | — | — | — | — | 111,430.00 | <1 | <1 | <1 | <1 |
| 122 | N-Butyl-acetamide | — | — | — | — | — | — | — | — | — | — |
| 123 | 2,6-Di-tert-butylpyridine | — | — | — | — | — | — | — | — | — | — |
| 124 | 5-Methyl-1H-pyrrole-2-carbaldehyde | — | — | — | — | — | — | — | — | — | — |
| 125 | 6-(Ethoxycarbonyl)nicotinic acid | — | — | — | — | — | — | — | — | — | — |
| 126 | Benzylamine | 2,943,000.00 | <1 | — | — | <1 | 209,934.00 | <1 | <1 | <1 | <1 |
| 127 | Diethylamine | 14,989.00 | <1 | — | — | <1 | 7070.00 | <1 | <1 | <1 | <1 |
| 128 | 3-Methylthiopropanol | 71.07 | 349 | 345 | 563 | 383 | 2060.00 | 12 | 12 | 19 | 13 |
| 129 | Thiophene | 88,284.00 | 1 | 1 | 1 | 1 | 8.41 | 12,028 | 15,518 | 13,595 | 15,080 |
| 130 | Methyl mercaptan | 0.17 | 6794 | 1432 | 6750 | 9059 | 1.73 | 679 | 143 | 675 | 906 |
| 131 | Dimethyl Sulfoxide | — | — | — | — | — | — | — | — | — | — |
| 132 | Dimethyl sulfone | — | — | — | — | — | — | — | — | — | — |
| 133 | Thioacetic acid | 100.00 | 183 | — | — | 105 | — | — | — | — | — |
| 134 | Tetrahydrothiophene | — | — | — | — | — | — | — | — | — | — |
| 135 | 2-Acetyl-2-thiazoline | — | — | — | — | — | 1.17 | — | 70,957 | — | — |
| 136 | 4-Methyl-5-acetyl thiazole | — | — | — | — | — | — | — | — | — | — |
| 137 | (E)-2-Nonenal | 0.16 | 149,938 | 41,011 | — | — | 0.21 | 113,953 | 31,168 | — | — |
| 138 | Isovaleraldehyde | 0.95 | 8845 | — | — | — | 36.39 | 231 | — | — | — |
| 139 | Acetaldehyde | 49.46 | 53 | 33 | 17 | 15 | 7850.00 | <1 | <1 | <1 | <1 |
| 140 | Hexadecanal | — | — | — | — | — | — | — | — | — | — |
| 141 | 4-Hydroxy-2-butanone | — | — | — | — | — | — | — | — | — | — |
| 142 | (Z)-β-Ionone | 0.01 | — | 14,243,050 | — | 12,784,795 | — | — | — | — | — |
| 143 | 3-Hydroxy-2-butanone | 14.18 | 5325 | 4822 | 2838 | 5525 | 17,221.00 | 4 | 4 | 2 | 5 |
| 144 | Cholestane | — | — | — | — | — | — | — | — | — | — |
| 145 | 1-Heptene | 1045.50 | 478 | 441 | 578 | 489 | — | — | — | — | — |
| 146 | 1-Undecene | — | — | — | — | — | — | — | — | — | — |
| 147 | 2-Methyl-2-butene | — | — | — | — | — | — | — | — | — | — |
| 148 | Myrcene | 3.88 | 1083 | — | 962 | — | 13.13 | 320 | — | 284 | — |
| 149 | Diisopropyl ether | — | — | — | — | — | — | — | — | — | — |
| 150 | 3-Methyl-1-butene | — | — | — | — | — | — | — | — | — | — |
References
- Kaczyński, P.; Iwaniuk, P.; Hrynko, I.; Łuniewski, S.; Łozowicka, B. The effect of the multi-stage process of wheat beer brewing on the behavior of pesticides according to their physicochemical properties. Food Control 2024, 160, 110356. [Google Scholar] [CrossRef]
- Biazon, C.L.; Brambilla, R.; Rigacci, A.; Pizzolato, T.M.; dos Santos, J.H.Z. Combining silica-based adsorbents and SPME fibers in the extraction of the volatiles of beer: An exploratory study. Anal. Bioanal. Chem. 2009, 394, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.G. The Production of Secondary Metabolites with Flavour Potential during Brewing and Distilling Wort Fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Perrault, K.A.; Dubois, L.M.; L’Homme, B.; Allen, C.; Loughnane, C.; Ochiai, N.; Focant, J.-F. Advanced method optimization for volatile aroma profiling of beer using two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2017, 1507, 45–52. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Chua, J.Y.; Liu, S.Q. Impact of simultaneous fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii on volatile and non-volatile constituents in beer. LWT 2018, 91, 26–33. [Google Scholar] [CrossRef]
- Lodolo, E.J.; Kock, J.L.F.; Axcell, B.C.; Brooks, M. The yeast Saccharomyces cerevisiae– the main character in beer brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef]
- Lehnhardt, F.; Becker, T.; Gastl, M. Flavor stability assessment of lager beer: What we can learn by comparing established methods. Eur. Food Res. Technol. 2020, 246, 1105–1118. [Google Scholar] [CrossRef]
- Richter, T.M.; Eyres, G.T.; Silcock, P.; Bremer, P.J. Comparison of four extraction methods for analysis of volatile hop-derived aroma compounds in beer. J. Sep. Sci. 2017, 40, 4366–4376. [Google Scholar] [CrossRef] [PubMed]
- Andres-Iglesias, C.; Montero, O.; Sancho, D.; Blanco, C.A. New trends in beer flavour compound analysis. J. Sci. Food Agric. 2015, 95, 1571–1576. [Google Scholar] [CrossRef]
- Vieira, A.C.; Pereira, A.C.; Marques, J.C.; Reis, M.S. Multi-target optimization of solid phase microextraction to analyse key flavour compounds in wort and beer. Food Chem. 2020, 317, 126466. [Google Scholar] [CrossRef]
- Bettenhausen, H.M.; Benson, A.; Fisk, S.; Herb, D.; Hernandez, J.; Lim, J.; Queisser, S.H.; Shellhammer, T.H.; Vega, V.; Yao, L.; et al. Variation in Sensory Attributes and Volatile Compounds in Beers Brewed from Genetically Distinct Malts: An Integrated Sensory and Non-Targeted Metabolomics Approach. J. Am. Soc. Brew. Chem. 2020, 78, 136–152. [Google Scholar] [CrossRef]
- Bertrand, E.; Meyer, X.-M.; Machado-Maturana, E.; Berdague, J.-L.; Kondjoyan, A. Modelling the Maillard reaction during the cooking of a model cheese. Food Chem. 2015, 184, 229–237. [Google Scholar] [CrossRef]
- Nan, F.; Li, X.; Feng, J.; Lv, J.; Liu, Q.; Liu, X.; Liu, Y.; Zhang, R.; Bai, B.; Xie, S. Production, characterization and antioxidant analysis on the Undaria-based alcoholic beverages using response surface method and HS-SPME-GC × GC-TOF-MS. Food Chem. X 2025, 27, 102428. [Google Scholar] [CrossRef]
- Yu, M.; Yang, P.; Song, H.; Guan, X. Research progress in comprehensive two-dimensional gas chromatography-mass spectrometry and its combination with olfactometry systems in the flavor analysis field. J. Food Compos. Anal. 2022, 114, 104790. [Google Scholar] [CrossRef]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Application of gas chromatography-olfactometry (GC-O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Reglitz, K.; Fechir, M.; Mall, V.; Voigt, J.; Steinhaus, M. The impact of caramel and roasted wheat malts on aroma compounds in top-fermented wheat beer. J. Inst. Brew. 2022, 128, 138–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Meng, Q.; Song, H.; Wang, X. Characterization of Key Odor-Active Compounds in Draft Beers for the Chinese Market Using Molecular Sensory Science Approaches. Molecules 2024, 29, 2537. [Google Scholar] [CrossRef]
- Fechir, M.; Reglitz, K.; Mall, V.; Voigt, J.; Steinhaus, M. Molecular Insights into the Contribution of Specialty Barley Malts to the Aroma of Bottom-Fermented Lager Beers. J. Agric. Food Chem. 2021, 69, 8190–8199. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, N.; Chen, S.; Chen, J.; Chen, M.; Tu, J.; Xiang, Z. Unravelling the key flavor components of beer based on a new online recombination olfactometry of mixed targeted compounds. Food Chem. 2025, 479, 143702. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Yin, R.; Wang, S.; Hong, J.; Wu, Y.; Guo, L.; Song, Y.; Zhao, D.; Sun, J.; et al. Exploration of key flavor quality factors in beer and its distilled spirits based on flavor metabolomics. Lwt-Food Sci. Technol. 2025, 224, 117832. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R. Volatiles organic compounds in craft beers produced in Basilicata (Southern Italy). Nat. Product. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.J.; Mannion, D.T.; Oconnor, C.; Kilcawley, K.N. Comparison of head space solid phase micro extraction with conventional and comprehensive gas chromatography mass spectrometry for volatile profiling of Irish whiskey. Talanta Open 2024, 10, 100346. [Google Scholar] [CrossRef]
- Hong, J.; Huang, H.; Zhao, D.; Sun, J.; Huang, M.; Sun, X.; Sun, B. Investigation on the key factors associated with flavor quality in northern strong aroma type of Baijiu by flavor matrix. Food Chem. 2023, 426, 136576. [Google Scholar] [CrossRef]
- Fang, X.; Chen, Y.; Gao, J.; Run, Z.; Chen, H.; Shi, R.; Li, Y.; Zhang, H.; Liu, Y. Application of GC–TOF/MS and GC×GC–TOF/MS to Discriminate Coffee Products in Three States (Bean, Powder, and Brew). Foods 2023, 12, 3123. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Van Gemert, L. Flavour thresholds. In Compilations of Flavour Threshold Values in Water and Other Media, 2nd ed.; Oliemans Punter & Partner: Utrecht, The Netherlands, 2011. [Google Scholar]
- Van Gemert, L. Odour thresholds. In Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans, Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Nuzzi, M.; Lo Scalzo, R.; Testoni, A.; Rizzolo, A. Evaluation of Fruit Aroma Quality: Comparison Between Gas Chromatography–Olfactometry (GC–O) and Odour Activity Value (OAV) Aroma Patterns of Strawberries. Food Anal. Methods 2008, 1, 270–282. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Tang, F.; Chen, B.; Liu, L.; Hayashi, K. Machine-Learning-Based Olfactometry: An Auxiliary System for Human Assessors in Olfactory Measurement. bioRxiv 2022. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, J.; Yuan, X.; Zhang, C.; Hong, J.; Zhao, Z.; Zhao, D.; Sun, B.; Wang, S.; Sun, J.; et al. Flavoromics-based profiling of flavor compound dynamics across the strong-flavor Baijiu production chain. J. Food Compos. Anal. 2025, 148, 108132. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, H.; Sun, Y.; Huang, H.; Chen, Y.; Hong, J.; Liu, X.; Wei, H.; Tian, W.; Zhao, D.; et al. Integration of Chemometrics and Sensory Metabolomics to Validate Quality Factors of Aged Baijiu (Nianfen Baijiu) with Emphasis on Long-Chain Fatty Acid Ethyl Esters. Foods 2023, 12, 3087. [Google Scholar] [CrossRef]
- Jespersen, L.; Jakobsen, M. Specific spoilage organisms in breweries and laboratory media for their detection. Int. J. Food Microbiol. 1996, 33, 139–155. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of chemical and sensory properties during aging of top-fermented beer. J. Agric. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef]
- Blankenship, M.; Grigorova, M.; Katz, D.; Maier, J. Retronasal Odor Perception Requires Taste Cortex, but Orthonasal Does Not. Curr. Biol. 2019, 29, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Han, P. Neurocognitive mechanisms of odor-induced taste enhancement: A systematic review. Int. J. Gastron. Food Sci. 2022, 28, 100535. [Google Scholar] [CrossRef]
- Clapperton, J.F. FATTY ACIDS CONTRIBUTING TO CAPRYLIC FLAVOUR IN BEER. THE USE OF PROFILE AND THRESHOLD DATA IN FLAVOUR RESEARCH. J. Inst. Brew. 1978, 84, 107–112. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Gamero, A.; Ferreira, V.; Pretorius, I.S.; Querol, A. Wine, Beer and Cider: Unravelling the Aroma Profile. In Molecular Mechanisms in Yeast Carbon Metabolism; Piškur, J., Compagno, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 261–297. [Google Scholar]
- Shao, D.; Cheng, W.; Jiang, C.; Pan, T.; Li, N.; Li, M.; Li, R.; Lan, W.; Du, X. Machine-Learning-Assisted Aroma Profile Prediction in Five Different Quality Grades of Nongxiangxing Baijiu Fermented During Summer Using Sensory Evaluation Combined with GC×GC–TOF-MS. Foods 2025, 14, 1714. [Google Scholar] [CrossRef]
- Akarachantachote, N.; Chadcham, S.; Saithanu, K. CUTOFF THRESHOLD OF VARIABLE IMPORTANCE IN PROJECTION FOR VARIABLE SELECTION. Int. J. Pure Appl. Math. 2014, 94, 307–322. [Google Scholar] [CrossRef]
- Dong, L.; Hou, Y.; Li, F.; Piao, Y.; Zhang, X.; Zhang, X.; Li, C.; Zhao, C. Characterization of volatile aroma compounds in different brewing barley cultivars. J. Sci. Food Agric. 2015, 95, 915–921. [Google Scholar] [CrossRef]
- Takoi, K.; Itoga, Y.; Koie, K.; Kosugi, T.; Shimase, M.; Katayama, Y.; Nakayama, Y.; Watari, J. The Contribution of Geraniol Metabolism to the Citrus Flavour of Beer: Synergy of Geraniol and β-Citronellol Under Coexistence with Excess Linalool. J. Inst. Brew. 2010, 116, 251–260. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, X.; Xing, J.; Li, N.; Li, J.; Su, Q.; Chen, Y.; Zhang, B.; Zhu, B. Accurate Determination of 12 Lactones and 11 Volatile Phenols in Nongrape Wines through Headspace-Solid-Phase Microextraction (HS-SPME) Combined with High-Resolution Gas Chromatography-Orbitrap Mass Spectrometry (GC-Orbitrap-MS). J. Agric. Food Chem. 2022, 70, 1971–1983. [Google Scholar] [CrossRef]
- Wanikawa, A.; Hosoi, K.; Kato, T. Conversion of Unsaturated Fatty Acids to Precursors of γ-Lactones by Lactic Acid Bacteria during the Production of Malt Whisky. J. Am. Soc. Brew. Chem. 2000, 58, 51–56. [Google Scholar] [CrossRef]
- Techakriengkrai, I.; Paterson, A.; Piggott, J. Relationships of Sweetness in Lager to Selected Volatile Congeners. J. Inst. Brew. 2004, 110, 360–366. [Google Scholar] [CrossRef]
- Schieberle, P. Primary odorants of pale lager beer. Z. Lebensm.-Unters. Forsch. 1991, 193, 558–565. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.D.; Godbole, A.; Dunshea, F.R. Chemical characterization of aromas in beer and their effect on consumers liking. Food Chem. 2019, 293, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Meier-Dörnberg, T.; Hutzler, M.; Michel, M.; Methner, F.-J.; Jacob, F. The Importance of a Comparative Characterization of Saccharomyces Cerevisiae and Saccharomyces Pastorianus Strains for Brewing. Fermentation 2017, 3, 41. [Google Scholar] [CrossRef]
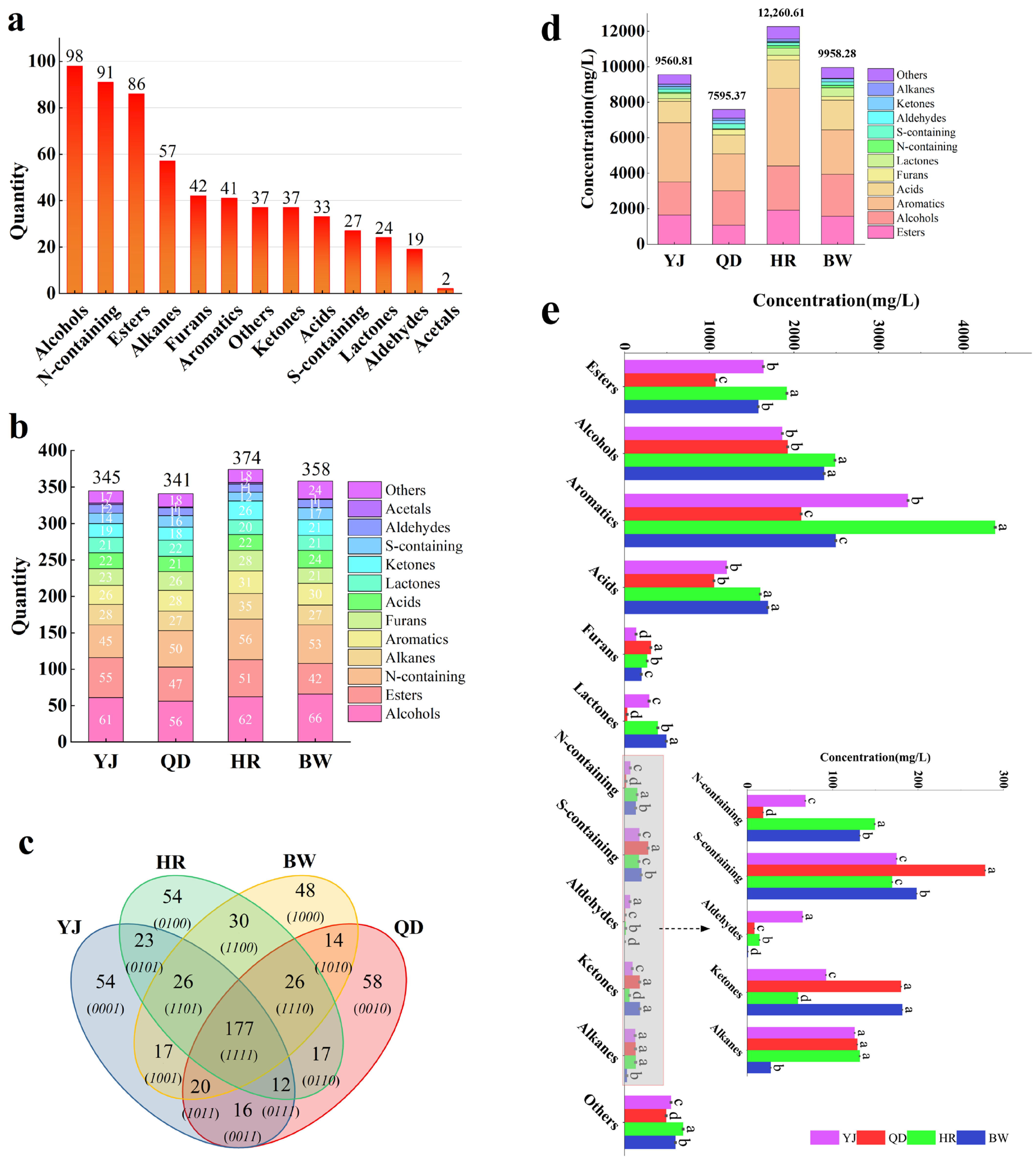




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Huang, H.; Yin, R.; He, X.; Guo, L.; Song, Y.; Zhao, D.; Sun, J.; Li, J.; Huang, M.; et al. Multi-Technique Flavoromics for Identifying Key Differential Volatile Compounds Underlying Sensory Profiles in Lager Beers. Foods 2025, 14, 3428. https://doi.org/10.3390/foods14193428
Chen Y, Huang H, Yin R, He X, Guo L, Song Y, Zhao D, Sun J, Li J, Huang M, et al. Multi-Technique Flavoromics for Identifying Key Differential Volatile Compounds Underlying Sensory Profiles in Lager Beers. Foods. 2025; 14(19):3428. https://doi.org/10.3390/foods14193428
Chicago/Turabian StyleChen, Yiyuan, He Huang, Ruiyang Yin, Xiuli He, Liyun Guo, Yumei Song, Dongrui Zhao, Jinyuan Sun, Jinchen Li, Mingquan Huang, and et al. 2025. "Multi-Technique Flavoromics for Identifying Key Differential Volatile Compounds Underlying Sensory Profiles in Lager Beers" Foods 14, no. 19: 3428. https://doi.org/10.3390/foods14193428
APA StyleChen, Y., Huang, H., Yin, R., He, X., Guo, L., Song, Y., Zhao, D., Sun, J., Li, J., Huang, M., & Sun, B. (2025). Multi-Technique Flavoromics for Identifying Key Differential Volatile Compounds Underlying Sensory Profiles in Lager Beers. Foods, 14(19), 3428. https://doi.org/10.3390/foods14193428







