Advancements in Microbial Applications for Sustainable Food Production
Abstract
1. Introduction
2. Literature Search Strategy
2.1. Eligibility Criteria
2.2. Screening and Selection Process
2.3. Data Extraction and Synthesis
3. Fermentation
3.1. Controlled Fermentation Process
3.1.1. SmF
3.1.2. SSF
3.1.3. PF
3.2. Natural Fermentation
3.2.1. Wild or Spontaneous Fermentation (WF)
3.2.2. Traditional Fermentation (TF)
3.2.3. Symbiotic Fermentation (SF)
3.3. Fermentation Pathways and Industrial Applications in Food Biotechnology
- Kombucha
- Miso
- Natto
| Fermented Food | Fermentation Pathway or Process | Fermentation Types Used | Main Bioactive Compounds | Health Benefits | Reference |
|---|---|---|---|---|---|
| Yogurt | Lactic fermentation | TF | Probiotics, peptides | Gut health, immune support | [80] |
| Sauerkraut | Lactic and homolacticfermentation | WF, NF (sometimes TF with starter) | Lactic acid, vitamins | Digestive aid, antioxidant properties | [81,82] |
| Kefir | Lactic and alcoholic fermentation | SF, TF or NF | Probiotics, peptides, and organic acids | Enhances gut microbiota, boosts immune function | [83] |
| Kimchi | Lactic fermentation | WF, NF, TF | Probiotics, vitamins, polyphenols | Supports gut health, anti-inflammatory properties | [74] |
| Tempeh | Fungal enzymes Lactic fermentation | SSF, TF | Isoflavones, peptides, prebiotics Vitamin B12 | Improves digestion, supports bone health | [84] |
4. Food Additives and Ingredients
4.1. Chemical Additives
4.2. Microbial Bioproducts as Food Additives
4.2.1. Microbial Enzymes
4.2.2. Microbial Organic Acid
4.2.3. Other Microbial Bioproducts
- Texturizers and stabilizers
- Food colorants
- Sweeteners
- Flavoring and aroma
- Functional and nutritional bioproducts
4.3. Probiotics, Prebiotics, and Postbiotics
4.3.1. Probiotics
4.3.2. Prebiotics and Dietary Carbohydrates
4.3.3. Postbiotics
5. Food Preservation
5.1. Plant-Derived Preservatives
5.2. Microbial Preservatives
5.2.1. LAB Microorganisms
5.2.2. Antimicrobial Peptides and Other Metabolites
5.2.3. Microbial Biopolymers
5.2.4. Phages and Other Microbial Technologies
5.3. Nanotechnology
6. Microbial Fermenters in Postharvest Disease Management
| Postharvest Disease | Etiologic Microorganism | Microbial Agent | Crop/Host | Reference |
|---|---|---|---|---|
| Green and blue molds | Penicillium digitatum and P. Italicum | Cell-free supernatants lactobacilli strains | Lemmon | [281] |
| Anthracnose | Colletotrichum plurivorum | kernel cake and pineapple peel fermented with lactobacillus plantarum | Mango | [314] |
| Gray mold | Botrytis cinerea | Bacillus fermentates/culture filtrates | Tomato, strawberry, grapes | [315] |
| Pepper rot | Phytophthora capsici | Fermentation supernatant of Lactobacillus plantarum | Pepper | [316] |
| Green mould | Penicillium digitatum | fermentates from Candida peltata | Citrus fruits | [317] |
| White rot | Coniella diplodiella Coniella vitis | metabolites of the PaeniBacillus pisiformis ZBSF and/or the Paenibacillus pisiformis ZB | Grapes | [318] |
7. Bacterial Contaminants in Food
7.1. Conventional Bacteriological-Based Methods
7.2. Rapid Detection Techniques for Foodborne Pathogens
7.3. Immunological Methods
7.4. Molecular-Based Methods
7.5. Novel and Advanced Detection Methods
8. Challenges, Perspectives, and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.M.; Khan, I.; Field, D.L.; Techato, K.; Alameh, K. Powering Agriculture: Present Status, Future Potential, and Challenges of Renewable Energy Applications. Renew. Energy 2022, 188, 731–749. [Google Scholar] [CrossRef]
- Singh, R.; Singh, H.; Raghubanshi, A.S. Challenges and Opportunities for Agricultural Sustainability in Changing Climate Scenarios: A Perspective on Indian Agriculture. Trop. Ecol. 2019, 60, 167–185. [Google Scholar] [CrossRef]
- Azevedo, I.; Barbosa, J.; Albano, H.; Nogueira, T.; Teixeira, P. Lactic Acid Bacteria Isolated from Traditional and Innovative Alheiras as Potential Biocontrol Agents. Food Microbiol. 2024, 119, 104450. [Google Scholar] [CrossRef] [PubMed]
- Shakila, P.J.; Vijaya, T. Biofertilizers: A Review on Advancing Sustainable Agriculture and Enhancing Soil Health. In The Unity of Life: Interdisciplinary Connections across the Sciences; Deep Science Publishing: London, UK, 2025; pp. 46–53. ISBN 978-93-49307-18-6. [Google Scholar]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the Development of Biofertilizers and Biostimulants from Microalgae. Biology 2024, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Benzvi, C.; Vojdani, A. The Frequently Used Industrial Food Process Additive, Microbial Transglutaminase: Boon or Bane. Nutr. Rev. 2025, 83, e1286–e1294. [Google Scholar] [CrossRef] [PubMed]
- Jothyswarupha, K.A.; Venkataraman, S.; Rajendran, D.S.; Shri, S.S.S.; Sivaprakasam, S.; Yamini, T.; Karthik, P.; Kumar, V.V. Immobilized Enzymes: Exploring Its Potential in Food Industry Applications. Food Sci. Biotechnol. 2025, 34, 1533–1555. [Google Scholar] [CrossRef]
- Bajić, B.; Vučurović, D.; Vasić, Đ.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2022, 12, 107. [Google Scholar] [CrossRef]
- Thomsen, P.T.; Nielsen, S.R.; Borodina, I. Recent Advances in Engineering Microorganisms for the Production of Natural Food Colorants. Curr. Opin. Chem. Biol. 2024, 81, 102477. [Google Scholar] [CrossRef]
- Shehzadi, A.; Chaudhary, A.; Aihetasham, A.; Hussain, N.; Naz, S.; Aziz, T.; Alasmari, A.F. Determination of Hydrolyzing and Ethanolic Potential of Cellulolytic Bacteria Isolated from Fruit Waste. Ital. J. Food Sci. 2024, 36, 127–141. [Google Scholar] [CrossRef]
- Ullah, N.; Mujaddad-ur-Rehman, M.; Sarwar, A.; Nadeem, M.; Nelofer, R.; Irfan, M.; Idrees, M.; Ali, U.; Naz, S.; Aziz, T. Effect of Bioprocess Parameters on Alkaline Protease Production by Locally Isolated Bacillus cereus AUST-7 Using Tannery Waste in Submerged Fermentation. Biomass Convers. Biorefinery 2024, 14, 22977–22987. [Google Scholar] [CrossRef]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial Proteases and Their Applications. Front. Microbiol. 2023, 14, 1236368. [Google Scholar] [CrossRef]
- Arifeen, S.; Jamil, J.; Sarwar, A.; Ullah, N.; Nelofer, R.; Aziz, T.; Alharbi, M.; Alasmari, A.F.; Alshammari, A.; Albekairi, T.H. Biosynthesis and optimization of amylase from Bacillus sp. Isolated from soil samples using agro-industrial waste as substrate. Appl. Ecol. Environ. Res. 2024, 22, 2927–2940. [Google Scholar] [CrossRef]
- Kango, N.; Nath, S. Prebiotics, Probiotics and Postbiotics: The Changing Paradigm of Functional Foods. J. Diet. Suppl. 2024, 21, 709–735. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Jan, K.; Sahu, J.K.; Habib, M.; Jan, S.; Bashir, K. A Comprehensive Review on Recent Trends and Utilization of Algal β-Glucan for the Development of Nutraceuticals and Functional Foods. Food Rev. Int. 2025, 41, 469–490. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Wan, Y.; Gao, B.; Rajput, V.D. Alginate-Based Composites as Novel Soil Conditioners for Sustainable Applications in Agriculture: A Critical Review. J. Environ. Manag. 2023, 348, 119133. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, A.; Fouillaud, M.; Dufossé, L.; Haq, I.U.; Mukhtar, H. Microbial Cell Factories: Biodiversity, Pathway Construction, Robustness, and Industrial Applicability. Microbiol. Res. 2024, 15, 247–272. [Google Scholar] [CrossRef]
- Dhiman, S.; Kumar, A.; Sharma, K.; Dhewa, T. Microbial Bioprocessing of Food and Agro-Industrial Residues for a Sustainable Circular Economy. Indian J. Microbiol. 2025, 1–32. [Google Scholar] [CrossRef]
- Mukherjee, G.; Dhiman, S. (Eds.) Value Addition and Utilization of Lignocellulosic Biomass: Through Novel Technological Interventions, 1st ed.; Springer Nature: Singapore, 2025; ISBN 978-981-96-2785-1. [Google Scholar]
- Hernández-Velázquez, R.; Flörl, L.; Lavrinienko, A.; Sebechlebská, Z.; Merk, L.; Greppi, A.; Bokulich, N.A. The Future Is Fermented: Microbial Biodiversity of Fermented Foods Is a Critical Resource for Food Innovation and Human Health. Trends Food Sci. Technol. 2024, 150, 104569. [Google Scholar] [CrossRef]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented Foods, Their Microbiome and Its Potential in Boosting Human Health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef]
- Hassan, M.; Zia, A.; Nauman Ahmad, M.; Baseer Us Salam, M.; Siraj, M.; Sabir, S.; Arif, M.; Naveed Farooq, T.; Aziz, T.; Alshammari, A. Valorization of Banana Waste by Optimizing Nitrocellulose Production, Yield, and Solubility via Nitrating Acid Mixtures and Reaction Time. Ital. J. Food Sci. 2024, 36, 224–230. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial Protein: Future Sustainable Food Supply Route with Low Environmental Footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef]
- Graham, A.E.; Ledesma-Amaro, R. The Microbial Food Revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef] [PubMed]
- Linder, T. Making the Case for Edible Microorganisms as an Integral Part of a More Sustainable and Resilient Food Production System. Food Secur. 2019, 11, 265–278. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Chai, K.F.; Ng, K.R.; Samarasiri, M.; Chen, W.N. Precision Fermentation to Advance Fungal Food Fermentations. Curr. Opin. Food Sci. 2022, 47, 100881. [Google Scholar] [CrossRef]
- Shi, S.; Wang, Z.; Shen, L.; Xiao, H. Synthetic Biology: A New Frontier in Food Production. Trends Biotechnol. 2022, 40, 781–803. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for Future Food Systems: Precision Fermentation Can Complement the Scope and Applications of Traditional Fermentation. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Wang, N.; Liu, X.; Wang, L.; Ning, K. Synergy of Traditional Practices and Modern Technology: Advancing the Understanding and Applications of Microbial Resources and Processes in Fermented Foods. Trends Food Sci. Technol. 2025, 157, 104891. [Google Scholar] [CrossRef]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Bioengineered Enzymes and Precision Fermentation in the Food Industry. Int. J. Mol. Sci. 2023, 24, 10156. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; et al. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 8: Suitability of Taxonomic Units Notified to EFSA until March 2018. EFSA J. 2018, 16, e05315. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J. Cell Factory Engineering for Improved Production of Natural Products. Nat. Prod. Rep. 2019, 36, 1233–1236. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-Enabled Wellness Foods: A Fresh Perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Mummaleti, G.; Sarma, C.; Yarrakula, S.; Urla, R.; Gazula, H. Production, Properties and Applications of Levan Polysaccharide. Food Humanit. 2024, 3, 100369. [Google Scholar] [CrossRef]
- González-Torres, M.; Hernández-Rosas, F.; Pacheco, N.; Salinas-Ruiz, J.; Herrera-Corredor, J.A.; Hernández-Martínez, R. Levan Production by Suhomyces kilbournensis Using Sugarcane Molasses as a Carbon Source in Submerged Fermentation. Molecules 2024, 29, 1105. [Google Scholar] [CrossRef]
- Singh, B.P. Harnessing Probiotic Fermentation for the Generation of Food-Derived Bioactive Peptides: Current Status and Prospects. Eur. Food Res. Technol. 2025, 251, 2061–2076. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhao, W.J.; Wu, R.N.; Sun, Z.H.; Zhang, W.Y.; Wang, J.C.; Bilige, M.; Zhang, H.P. Proteome Analysis of Lactobacillus Helveticus H9 during Growth in Skim Milk. J. Dairy Sci. 2014, 97, 7413–7425. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.E.C.; Ribeiro, B.D.; Coelho, M.A.Z. Characterization and Application of Yarrowia lipolytica Lipase Obtained by Solid-State Fermentation in the Synthesis of Different Esters Used in the Food Industry. Appl. Biochem. Biotechnol. 2019, 189, 933–959. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wai, A.; Guha, T.K.; Hausner, G.; Yuan, Q. Production of Endoglucanase and Xylanase Using Food Waste by Solid-State Fermentation. Waste Biomass Valorization 2018, 9, 2391–2398. [Google Scholar] [CrossRef]
- Benabda, O.; M’hir, S.; Kasmi, M.; Mnif, W.; Hamdi, M. Optimization of Protease and Amylase Production by Rhizopus oryzae Cultivated on Bread Waste Using Solid-State Fermentation. J. Chem. 2019, 2019, 3738181. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Manan, M.A.; Webb, C. Solid-State Fermentation of Food Industry Wastes. In Food Industry Wastes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–161. ISBN 978-0-12-817121-9. [Google Scholar]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-State Fermentation Technology and Innovation for the Production of Agricultural and Animal Feed Bioproducts. Syst. Microbiol. Biomanuf. 2021, 1, 142–165. [Google Scholar] [CrossRef]
- Bamidele, M.O.; Bamikale, M.B.; Cárdenas-Hernández, E.; Bamidele, M.A.; Castillo-Olvera, G.; Sandoval-Cortes, J.; Aguilar, C.N. Bioengineering in Solid-State Fermentation for next Sustainable Food Bioprocessing. Next Sustain. 2025, 6, 100105. [Google Scholar] [CrossRef]
- Vauris, A.; Valcauda, S.; Husson, F.; Coninck, J.D. A Novel Method to Assess Heat Transfer and Impact of Relevant Physicochemical Parameters for the Scaling up of Solid State Fermentation Systems. Biotechnol. Rep. 2022, 36, e00764. [Google Scholar] [CrossRef]
- Milcarz, A.; Harasym, J. Solid State Fermentation—A Promising Approach to Produce Meat Analogues. Foods 2025, 14, 1820. [Google Scholar] [CrossRef]
- Jin, G.; Zhao, Y.; Xin, S.; Li, T.; Xu, Y. Solid-State Fermentation Engineering of Traditional Chinese Fermented Food. Foods 2024, 13, 3003. [Google Scholar] [CrossRef]
- Londoño-Hernández, L.; Ramírez-Toro, C.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar-Gonzalez, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhizopus oryzae—Ancient Microbial Resource with Importance in Modern Food Industry. Int. J. Food Microbiol. 2017, 257, 110–127. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, R.; Li, X.; Zhao, Y.; Huang, J. A Comprehensive Review of Recombinant Technology in the Food Industry: Exploring Expression Systems, Application, and Future Challenges. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70078. [Google Scholar] [CrossRef] [PubMed]
- De Moura Campos, S.; Dos Santos Costa, G.; Karp, S.G.; Thomaz-Soccol, V.; Soccol, C.R. Innovations and Challenges in Collagen and Gelatin Production through Precision Fermentation. World J. Microbiol. Biotechnol. 2025, 41, 63. [Google Scholar] [CrossRef]
- Eastham, J.L.; Leman, A.R. Precision Fermentation for Food Proteins: Ingredient Innovations, Bioprocess Considerations, and Outlook—A Mini-Review. Curr. Opin. Food Sci. 2024, 58, 101194. [Google Scholar] [CrossRef]
- Navarrete, R.C.; Seheult, J.M.; Coffey, M.D. New Bio-Polymers for Drilling, Drill-in, Completions, Spacer Fluids and Coiled Tubing Applications. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference, Kuala Lumpur, Malaysia, 11–13 September 2000; pp. 395–411. [Google Scholar] [CrossRef]
- Mudoor Sooresh, M.; Willing, B.P.; Bourrie, B.C.T. Opportunities and Challenges of Understanding Community Assembly in Spontaneous Food Fermentation. Foods 2023, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Lee, S.H.; Whon, T.W.; Roh, S.W.; Jeon, C.O. Unraveling Microbial Fermentation Features in Kimchi: From Classical to Meta-Omics Approaches. Appl. Microbiol. Biotechnol. 2020, 104, 7731–7744. [Google Scholar] [CrossRef]
- Cuvas-Limon, R.B.; Nobre, C.; Cruz, M.; Rodriguez-Jasso, R.M.; Ruíz, H.A.; Loredo-Treviño, A.; Texeira, J.A.; Belmares, R. Spontaneously Fermented Traditional Beverages as a Source of Bioactive Compounds: An Overview. Crit. Rev. Food Sci. Nutr. 2021, 61, 2984–3006. [Google Scholar] [CrossRef]
- Joseph, B.; M, B.; S, N.; M, L.; R, J.M.; Baskaran, N.; Vignesh, S. Synbiotic Fermented Barnyard Millet Drink: Exploring Its Nutritional Profile, Sensory Attributes, and Bioactive Health Potentials. Food Chem. Adv. 2025, 6, 100872. [Google Scholar] [CrossRef]
- Voidarou, C.; Antoniadou, Μ.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2020, 10, 69. [Google Scholar] [CrossRef]
- Globe Newshire. Fermented Ingredients Business Report 2025: Global Market to Reach $79.3 Billion by 2030 from $47.7 Billion in 2024—Innovations in Microbial Fermentation Techniques Strengthen Prospects; Globe Newswire: El Segundo, CA, USA, 2025. [Google Scholar]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive Compound Encapsulation: Characteristics, Applications in Food Systems, and Implications for Human Health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.-X.; Mutukumira, A.N. Kombucha: Production and Microbiological Research. Foods 2022, 11, 3456. [Google Scholar] [CrossRef]
- Ben Saad, E.; Friedrich, A.; Fischer, F.; Courot, O.; Schacherer, J.; Bleykasten, C. Comprehensive Survey of Kombucha Microbial Communities of Diverse Origins and Fermentation Practices. FEMS Yeast Res. 2025, 25, foaf005. [Google Scholar] [CrossRef]
- Global Market Insights. Fermented Processed Food Market; Global Market Insights: Selbyville, DE, USA, 2024. [Google Scholar]
- Terefe, N.S. Recent Developments in Fermentation Technology: Toward the next Revolution in Food Production. In Food Engineering Innovations Across the Food Supply Chain; Elsevier: Amsterdam, The Netherlands, 2022; pp. 89–106. [Google Scholar]
- Mondal, P.P.; Galodha, A.; Verma, V.K.; Singh, V.; Show, P.L.; Awasthi, M.K.; Lall, B.; Anees, S.; Pollmann, K.; Jain, R. Review on Machine Learning-Based Bioprocess Optimization, Monitoring, and Control Systems. Bioresour. Technol. 2023, 370, 128523. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Ding, N.; Liu, Y.; Zhang, H.; Fang, Y.; Yin, L. Metabolic Engineering of Microorganisms to Produce Pyruvate and Derived Compounds. Molecules 2023, 28, 1418. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-H.; Zhou, X.; Zhang, M.-M.; Wang, Y.-J.; Zhou, B.; Ding, N.; Wu, Q.-F.; Lei, C.-R.; Dong, Z.-Y.; Ren, J.-L.; et al. Integration of Food Raw Materials, Food Microbiology, and Food Additives: Systematic Research and Comprehensive Insights into Sweet Sorghum Juice, Clostridium tyrobutyricum TGL-A236 and Bio-Butyric Acid. Front. Microbiol. 2024, 15, 1410968. [Google Scholar] [CrossRef]
- Smid, E.J.; Kleerebezem, M. Production of Aroma Compounds in Lactic Fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Kim, S.-H. Recent Biotechnological Trends in Lactic Acid Bacterial Fermentation for Food Processing Industries. Syst. Microbiol. Biomanuf. 2022, 2, 14–40. [Google Scholar] [CrossRef]
- Martínez-Leal, J.; Ponce-García, N.; Escalante-Aburto, A. Recent Evidence of the Beneficial Effects Associated with Glucuronic Acid Contained in Kombucha Beverages. Curr. Nutr. Rep. 2020, 9, 163–170. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea—Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Falentin, H.; Deutsch, S.-M.; Jan, G.; Loux, V.; Thierry, A.; Parayre, S.; Maillard, M.-B.; Dherbécourt, J.; Cousin, F.J.; Jardin, J.; et al. The Complete Genome of Propionibacterium freudenreichii CIRM-BIA1T, a Hardy Actinobacterium with Food and Probiotic Applications. PLoS ONE 2010, 5, e11748. [Google Scholar] [CrossRef]
- Kumar, R.; Kaur, G.; Brar, S.K. Tailored Production of Butyric Acid from Mixed Culture Fermentation of Food Waste. Food Bioprod. Process. 2025, 150, 1–11. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Shah, Y.A.; Khan, M.H.; Hussain, M.; Ikram, A.; Ateeq, H.; Noman, M.; Saewan, S.A.; Khashroum, A.O. Miso: A Traditional Nutritious & Health–Endorsing Fermented Product. Food Sci. Nutr. 2022, 10, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, K.-I.; Yamagata, Y.; Tazawa, R.; Kitagawa, M.; Kato, T.; Isobe, K.; Kashiwagi, Y. Japanese Traditional Miso and Koji Making. J. Fungi 2021, 7, 579. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the Microbial Community of Japanese Koji and Miso: A Review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef]
- Ahnan-Winarno, A.D.; Cordeiro, L.; Winarno, F.G.; Gibbons, J.; Xiao, H. Tempeh: A Semicentennial Review on Its Health Benefits, Fermentation, Safety, Processing, Sustainability, and Affordability. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1717–1767. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ge, C.; Yuan, W.; Zhu, R.; Zhang, W.; Du, L.; Xue, J. Characterization of Fermented Black Soybean Natto Inoculated with Bacillus Natto during Fermentation: Characterization of Fermented Black Soybean Natto. J. Sci. Food Agric. 2010, 90, 1194–1202. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Islam, F.; Ateeq, H.; Asghar, A.; Shah, Y.A.; Ofoedu, C.E.; Chacha, J.S. Nutritional Health Perspective of Natto: A Critical Review. Biochem. Res. Int. 2022, 2022, 5863887. [Google Scholar] [CrossRef]
- Zou, H.; Wang, H.; Zhang, Z.; Lin, H.; Li, Z. Immune Regulation by Fermented Milk Products: The Role of the Proteolytic System of Lactic Acid Bacteria in the Release of Immunomodulatory Peptides. Crit. Rev. Food Sci. Nutr. 2024, 64, 10498–10516. [Google Scholar] [CrossRef]
- Giuffrè, D.; Giuffrè, A.M. Fermentation Technology and Functional Foods. Front. Biosci. 2024, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, S.; Liu, W.; Chang, L.; Zhu, X.; Mu, G.; Qian, F. Probiotic Properties of Lactobacillus paraplantarum LS-5 and Its Effect on Antioxidant Activity of Fermented Sauerkraut. Food Biosci. 2023, 52, 102489. [Google Scholar] [CrossRef]
- Apalowo, O.E.; Adegoye, G.A.; Mbogori, T.; Kandiah, J.; Obuotor, T.M. Nutritional Characteristics, Health Impact, and Applications of Kefir. Foods 2024, 13, 1026. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J.; Karaca, A.C.; Capanoglu, E.; Esatbeyoglu, T. Fermented Soy Products: A Review of Bioactives for Health from Fermentation to Functionality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70080. [Google Scholar] [CrossRef]
- Garde, A.; Messenger, G. The Principle of Consistency: The Role of Standards in International Trade Law. In Public Health and International Economic Law; Bristol University Press: Bristol, UK, 2025; pp. 96–118. [Google Scholar] [CrossRef]
- Morshdy, A.E.M.A.; Hafez, A.E.S.; Fouda, O.O.; Dawish, W.S. Food Additives from Classification to Their Use in the Food Industry: A Review. J. Adv. Vet. Res. 2024, 14, 542–546. [Google Scholar]
- Jarmakiewicz-Czaja, S.; Sokal-Dembowska, A.; Filip, R. Effects of Selected Food Additives on the Gut Microbiome and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Medicina 2025, 61, 192. [Google Scholar] [CrossRef]
- Liu, C.; Zhan, S.; Tian, Z.; Li, N.; Li, T.; Wu, D.; Zeng, Z.; Zhuang, X. Food Additives Associated with Gut Microbiota Alterations in Inflammatory Bowel Disease: Friends or Enemies? Nutrients 2022, 14, 3049. [Google Scholar] [CrossRef]
- Kayode, O.T.; Bello, J.A.; Oguntola, J.A.; Kayode, A.A.A.; Olukoya, D.K. The Interplay between Monosodium Glutamate (MSG) Consumption and Metabolic Disorders. Heliyon 2023, 9, e19675. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Frías-Celayeta, J.; Bolton, D.; Botinestean, C. A Comprehensive Review of Cured Meat Products in the Irish Market: Opportunities for Reformulation and Processing. Foods 2024, 13, 746. [Google Scholar] [CrossRef]
- Kobets, T.; Smith, B.P.C.; Williams, G.M. Food-Borne Chemical Carcinogens and the Evidence for Human Cancer Risk. Foods 2022, 11, 2828. [Google Scholar] [CrossRef]
- Shanmugavel, V.; Komala Santhi, K.; Kurup, A.H.; Kalakandan, S.; Anandharaj, A.; Rawson, A. Potassium Bromate: Effects on Bread Components, Health, Environment and Method of Analysis: A Review. Food Chem. 2020, 311, 125964. [Google Scholar] [CrossRef]
- Ambroziewicz, Z.M.; Siemiątkowski, R.; Łata, M.; Dowgiert, S.; Sikorska, M.; Kamiński, J.; Więcław, K.; Grabowska, H.; Chruściel, J.; Mąsior, G. Long-Term Health Effects of Artificially Colored Foods in Adults and Children: A Review of Scientific Literature on Attention Deficits, Carcinogenicity, and Allergy Risks. J. Educ. Health Sport 2024, 76, 56522. [Google Scholar] [CrossRef]
- Kumar, P.; Anita; Joshi, N. Are the Food Additives, Safe or Harmful?—A Review. J. Ayurveda Integr. Med. Sci. 2023, 8, 140–144. [Google Scholar] [CrossRef]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’Auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef]
- Shaher, S.A.A.; Mihailescu, D.F.; Amuzescu, B. Aspartame Safety as a Food Sweetener and Related Health Hazards. Nutrients 2023, 15, 3627. [Google Scholar] [CrossRef]
- Boutillier, S.; Fourmentin, S.; Laperche, B. History of Titanium Dioxide Regulation as a Food Additive: A Review. Environ. Chem. Lett. 2022, 20, 1017–1033. [Google Scholar] [CrossRef]
- De Siena, M.; Raoul, P.; Costantini, L.; Scarpellini, E.; Cintoni, M.; Gasbarrini, A.; Rinninella, E.; Mele, M.C. Food Emulsifiers and Metabolic Syndrome: The Role of the Gut Microbiota. Foods 2022, 11, 2205. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Sweetener Food Additives: A Synoptical Overview on Their Chemical Properties, Applications in Food Products and Side Effects. Emir. J. Food Agric. 2023, 35, 18. [Google Scholar] [CrossRef]
- Huang, L.; Huhulea, E.; Aifuwa, E.; Frishman, W.H.; Aronow, W.S. Sugar-Free but Not Risk-Free? Exploring Artificial Sweeteners and Cardiovascular Disease. Cardiol. Rev. 2025, 10–1097. [Google Scholar] [CrossRef]
- Kossiva, L.; Kakleas, K.; Christodouli, F.; Soldatou, A.; Karanasios, S.; Karavanaki, K. Chronic Use of Artificial Sweeteners: Pros and Cons. Nutrients 2024, 16, 3162. [Google Scholar] [CrossRef]
- Shibata, R.; Nakanishi, Y.; Suda, W.; Nakano, T.; Sato, N.; Inaba, Y.; Kawasaki, Y.; Hattori, M.; Shimojo, N.; Ohno, H. Neonatal Gut Microbiota and Risk of Developing Food Sensitization and Allergy. J. Allergy Clin. Immunol. 2025, 155, 932–946. [Google Scholar] [CrossRef]
- Campbell, D.E.; Mehr, S.; Moscatelli, O.G.; Anderson, R.P.; Tye-Din, J.A. Immune Therapies in Coeliac Disease and Food Allergies: Advances, Challenges, and Opportunities. Semin. Immunol. 2025, 78, 101960. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, Y.; Lin, X.; Zhang, S. Recent Applications and Prospects of Enzymes in Quality and Safety Control of Fermented Foods. Foods 2024, 13, 3804. [Google Scholar] [CrossRef]
- Ojo Omoniyi, O.A.; Dighitoghi Moro, D.; Bridget Afolabi, O. Microbial Proteases: Sources, Significance and Industrial Applications. Int. J. Curr. Microbiol. Appl. Sci. 2024, 13, 1–23. [Google Scholar] [CrossRef]
- Öztekin, S.; Anaya, K.; Yurdunuseven-Yıldız, A. Regulation of Natural Food Additives. In Natural Additives in Foods; Springer International Publishing: Cham, Switzerland, 2023; pp. 343–372. [Google Scholar] [CrossRef]
- Pariza, M.W.; Johnson, E.A. Evaluating the Safety of Microbial Enzyme Preparations Used in Food Processing: Update for a New Century. Regul. Toxicol. Pharmacol. 2001, 33, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, C.M.; Van De Ligt, J.L.G.; Eapen, A.K.; Pavel, A.T.; Hanlon, P.R.; Almond-Abbate, K.; Haugabrooks, E.; Hlywka, J.; Lu, V.; De Mooij, F.; et al. A Tool to Support Food Substance Safety Evaluations in the United States. Regul. Toxicol. Pharmacol. 2025, 161, 105838. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Leroi, F.; Passerini, D.; Siemiatkowska, M.; Sopacua, T.; Andersson, K.; Teixeira, P.; Poças, F.; Heir, E.; Langsrud, S.; et al. Evaluating Biosolutions for Sustainable Food Systems: A Review of Safety, Quality, Regulatory, and Sustainability Considerations Within the European Union. J. Food Prot. 2025, 88, 100606. [Google Scholar] [CrossRef] [PubMed]
- ANVISA—Brazilian Health Regulatory Agency. Resolution of the Collegiate Board–RDC No. 243, of July 26, 2018. Provides for the Sanitary Requirements of Food Supplements. Official Gazette of the Union, 27 July 2018, 1, p. 100. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2018/rdc0243_26_07_2018.pdf (accessed on 25 September 2025).
- ANVISA—Brazilian Health Regulatory Agency. Resolution of the Collegiate Board–RDC No. 241, of July 26, 2018. Provides for the Requirements to Demonstrate the Safety and Health Benefits of Probiotics for Use in Foods. Official Gazette of the Union*, 27 July 2018, 1, p.97. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2018/rdc0241_26_07_2018.pdf (accessed on 25 September 2025).
- Montera, V.D.S.P.; Martins, A.P.B.; Mais, L.A.; Canella, D.S. Information on food additives on food labels in Brazil: A critical analysis. Rev. Saúde Pública 2023, 57, 2. [Google Scholar] [CrossRef]
- CHINA GB 29921-2021; National Food Safety Standard for Food Cultures—National Food Safety Standard Limit of Pathogen in in Prepackaged Foods. National Standard of the People’s Republic of China: Beijing, China, 2021. Available online: https://www.codeofchina.com/standard/GB29921-2021.html (accessed on 25 September 2025).
- CHINA GB 4789.45-2023; National Food Safety Standard—General Rules for Verification of Microbial Test Methods. National Standard of the People’s Republic of China: Beijing, China, 2024. Available online: https://www.codeofchina.com/standard/GB4789.45-2023.html (accessed on 25 September 2025).
- Liu, C.; Wang, N.; Liu, L.X.; Zhang, Y.Y.; Liu, Y.G. An Analytical Overview of the Composition and Characteristics of China’s Food Safety Standards. J. Sci. Food Agric. 2024, 104, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Liu, X.; Beachy, R. New Food Safety Law of China and the Special Issue on Food Safety in China. J. Integr. Agric. 2015, 14, 2136–2141. [Google Scholar] [CrossRef]
- Singh, S.; Kumar Sharma, P.; Chaturvedi, S.; Kumar, P.; Deepak Nannaware, A.; Kalra, A.; Kumar Rout, P. Biocatalyst for the Synthesis of Natural Flavouring Compounds as Food Additives: Bridging the Gap for a More Sustainable Industrial Future. Food Chem. 2024, 435, 137217. [Google Scholar] [CrossRef]
- Patel, A.K.; Dong, C.-D.; Chen, C.-W.; Pandey, A.; Singhania, R.R. Production, Purification, and Application of Microbial Enzymes. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–57. ISBN 978-0-443-19059-9. [Google Scholar]
- Park, G.; Lee, K.M.; Lee, Y.S.; Kim, Y.; Jeon, C.M.; Lee, O.-M.; Kim, Y.-J.; Son, H.-J. Biodegradation and Valorization of Feather Waste Using the Keratinase-Producing Bacteria and Their Application in Environmentally Hazardous Industrial Processes. J. Environ. Manag. 2023, 346, 118986. [Google Scholar] [CrossRef]
- Basketter, D.A.; Kimber, I. Enzymes and Sensitization via Skin Exposure: A Critical Analysis. Regul. Toxicol. Pharmacol. 2022, 129, 105112. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Rosiek, P.; Bauer, T. Microbial Transglutaminase—The Food Additive, a Potential Inducing Factor in Primary Biliary Cholangitis. Molecules 2025, 30, 762. [Google Scholar] [CrossRef]
- EFSA Panel on Food Enzymes (FEZ); Zorn, H.; Barat Baviera, J.M.; Bolognesi, C.; Catania, F.; Gadermaier, G.; Greiner, R.; Mayo, B.; Mortensen, A.; Roos, Y.H.; et al. Safety Evaluation of the Food Enzyme α-amylase from the Genetically Modified Bacillus licheniformis Strain DP-Dzb105. EFSA J. 2025, 23, e9531. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Kaur, J. Protein Engineering Strategies for Tailoring the Physical and Catalytic Properties of Enzymes for Defined Industrial Applications. Curr. Protein Pept. Sci. 2023, 24, 113–129. [Google Scholar] [CrossRef]
- ANVISA—Brazilian Health Regulatory Agency. Resolution of the Collegiate Board—RDC No. 728, of July 1, 2022. Provides for Enzymes and Enzyme Preparations for Use as Processing Aids in the Production of Foods for Human Consumption. *Official Gazette of the Union*, 6 July 2022, Sec. 1, No. 126. Available online: https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-728-de-1-de-julho-de-2022-413366697 (accessed on 25 September 2025).
- Li, L.; Pei, Y.; Cheng, K.; Deng, Y.; Dong, X.; Fang, R.; Chu, B.; Wei, P.; Chen, Q.; Xiao, G. Production and Evaluation of Enzyme-Modified Cheese Adding Protease or Lipase to Improve Quality Properties. J. Biosci. Bioeng. 2023, 135, 389–394. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for Health, Food, and Cosmetics: A Review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E. Hydrolytic Enzymes and Their Directly and Indirectly Effects on Gluten and Dough Properties: An Extensive Review. Food Sci. Nutr. 2021, 9, 3988–4006. [Google Scholar] [CrossRef] [PubMed]
- Pouris, J.; Kolyva, F.; Bratakou, S.; Vogiatzi, C.A.; Chaniotis, D.; Beloukas, A. The Role of Fungi in Food Production and Processing. Appl. Sci. 2024, 14, 5046. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Ben-Mahmoud, A.; Al-Barazie, R. Bacillus amyloliquefaciens: Harnessing Its Potential for Industrial, Medical, and Agricultural Applications—A Comprehensive Review. Microorganisms 2023, 11, 2215. [Google Scholar] [CrossRef]
- Arya, P.S.; Yagnik, S.M.; Rajput, K.N.; Panchal, R.R.; Raval, V.H. Understanding the Basis of Occurrence, Biosynthesis, and Implications of Thermostable Alkaline Proteases. Appl. Biochem. Biotechnol. 2021, 193, 4113–4150. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.M.; Chellappan, S.; Sabu, A. Enzymes in Fruit and Vegetable Processing. In Value-Addition in Food Products and Processing Through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–110. ISBN 978-0-323-89929-1. [Google Scholar]
- De Souza, T.S.P.; Kawaguti, H.Y. Cellulases, Hemicellulases, and Pectinases: Applications in the Food and Beverage Industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Cosme, F.; Inês, A.; Vilela, A. Microbial and Commercial Enzymes Applied in the Beverage Production Process. Fermentation 2023, 9, 385. [Google Scholar] [CrossRef]
- Pandey, P.; Kuila, A.; Tuli, D.K. Cellulase: An Overview. In Current Status and Future Scope of Microbial Cellulases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 95–113. ISBN 978-0-12-821882-2. [Google Scholar]
- Sutaoney, P.; Rai, S.N.; Sinha, S.; Choudhary, R.; Gupta, A.K.; Singh, S.K.; Banerjee, P. Current Perspective in Research and Industrial Applications of Microbial Cellulases. Int. J. Biol. Macromol. 2024, 264, 130639. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current Perspective on Production and Applications of Microbial Cellulases: A Review. Bioresour. Bioprocess. 2021, 8, 95. [Google Scholar] [CrossRef]
- Keshavarz, B.; Khalesi, M. Trichoderma reesei, a Superior Cellulase Source for Industrial Applications. Biofuels 2016, 7, 713–721. [Google Scholar] [CrossRef]
- Suhaimi, H.; Dailin, D.J.; Malek, R.A.; Hanapi, S.Z.; Ambehabati, K.K.; Keat, H.C.; Prakasham, S.; Elsayed, E.A.; Misson, M.; El Enshasy, H. Fungal Pectinases: Production and Applications in Food Industries. In Fungi in Sustainable Food Production; Dai, X., Sharma, M., Chen, J., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 85–115. ISBN 978-3-030-64405-5. [Google Scholar]
- Dhevagi, P.; Ramya, A.; Priyatharshini, S.; Geetha Thanuja, K.; Ambreetha, S.; Nivetha, A. Industrially Important Fungal Enzymes: Productions and Applications. In Recent Trends in Mycological Research; Yadav, A.N., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 263–309. ISBN 978-3-030-68259-0. [Google Scholar]
- Mendonça, M.; Barroca, M.; Collins, T. Endo-1,4-β-Xylanase-Containing Glycoside Hydrolase Families: Characteristics, Singularities and Similarities. Biotechnol. Adv. 2023, 65, 108148. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Zoghi, A.; Azizi, M.H. Effect of Xylanase and Pentosanase Enzymes on Dough Rheological Properties and Quality of Baguette Bread. J. Food Qual. 2022, 2022, 2910821. [Google Scholar] [CrossRef]
- Mu, D.; Li, P.; Ma, T.; Wei, D.; Montalbán-López, M.; Ai, Y.; Wu, X.; Wang, Y.; Li, X.; Li, X. Advances in the Understanding of the Production, Modification and Applications of Xylanases in the Food Industry. Enzym. Microb. Technol. 2024, 179, 110473. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, J.; Khatri, M.; Singh, G.; Arya, S.K. A Multifaceted Enzyme Conspicuous in Fruit Juice Clarification: An Elaborate Review on Xylanase. Int. J. Biol. Macromol. 2021, 193, 1350–1361. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, Y.; Long, S.; Feng, S.; Jia, X.; Hu, Y.; Ma, M.; Liu, J.; Zeng, B. Aspergillus oryzae as a Cell Factory: Research and Applications in Industrial Production. J. Fungi 2024, 10, 248. [Google Scholar] [CrossRef]
- Debnath, S. Trichoderma Enzymes in the Wine and Beer Industry. In Advances in Trichoderma Biology for Agricultural Applications; Amaresan, N., Sankaranarayanan, A., Dwivedi, M.K., Druzhinina, I.S., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2022; pp. 545–555. ISBN 978-3-030-91649-7. [Google Scholar]
- Qeshmi, F.I.; Homaei, A.; Fernandes, P.; Hemmati, R.; Dijkstra, B.W.; Khajeh, K. Xylanases from Marine Microorganisms: A Brief Overview on Scope, Sources, Features and Potential Applications. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2020, 1868, 140312. [Google Scholar] [CrossRef]
- Das, R.; Kayastha, A.M. An Overview on Starch Processing and Key Enzymes. In Industrial Starch Debranching Enzymes; Wu, J., Xia, W., Eds.; Springer Nature: Singapore, 2023; pp. 1–20. ISBN 978-981-19-7025-2. [Google Scholar]
- Li, Z.; Zheng, M.; Zheng, J.; Gänzle, M.G. Bacillus Species in Food Fermentations: An Underappreciated Group of Organisms for Safe Use in Food Fermentations. Curr. Opin. Food Sci. 2023, 50, 101007. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Jamzivar, F.; Razzaghi-Abyaneh, M. Prospective Application of Aspergillus Species: Focus on Enzyme Production Strategies, Advances and Challenges. In Natural Food Additives; Prieto, M.A., Otero, P., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-83968-959-8. [Google Scholar]
- Martín-Miguélez, J.M.; Bross, J.; Prado, D.; Merino, E.; Perisé Moré, R.; Otero, J.; Aduriz, A.L.; Delgado, J. Review: Rhizopus sp. beyond Tempeh. An Occidental Approach to Mold-Based Fermentations. Int. J. Gastron. Food Sci. 2025, 39, 101090. [Google Scholar] [CrossRef]
- Dutta, D.; Bhattacharya, S.; Nandi, S. Microbes in the Baking Industry: Harnessing the Power of Microbes in Baking Products. In Microbial Products for Health and Nutrition; Kothari, V., Ray, S., Kumar, P., Eds.; Springer Nature: Singapore, 2024; pp. 261–284. ISBN 978-981-97-4234-9. [Google Scholar]
- Zheng, L.; Jiang, B.; Wu, Y. Maltodextrin Production: Challenges and Advances in Enzymatic and Metabolic Synthesis for Controlled Polymerization of Degree. Food Rev. Int. 2025, 41, 2094–2112. [Google Scholar] [CrossRef]
- Schulz, P.; Rizvi, S.S.H. Hydrolysis of Lactose in Milk: Current Status and Future Products. Food Rev. Int. 2023, 39, 2875–2894. [Google Scholar] [CrossRef]
- Ambrogi, V.; Bottacini, F.; Cao, L.; Kuipers, B.; Schoterman, M.; Van Sinderen, D. Galacto-Oligosaccharides as Infant Prebiotics: Production, Application, Bioactive Activities and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 753–766. [Google Scholar] [CrossRef]
- Goff, H.D.; Hynes, E.H.; Perotti, M.C.; Kelly, P.M.; Hogan, S.A. Significance of Lactose in Dairy Products. In Advanced Dairy Chemistry; McSweeney, P.L.H., O’Mahony, J.A., Kelly, A.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 39–104. ISBN 978-3-030-92584-0. [Google Scholar]
- Otter, D.E.; Wu, S.; Jayasinghe, D.N.D.S. Galacto-Oligosaccharides and Other Products Derived from Lactose. In Advanced Dairy Chemistry; McSweeney, P.L.H., O’Mahony, J.A., Kelly, A.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 125–228. ISBN 978-3-030-92584-0. [Google Scholar]
- Uwineza, C.; Parchami, M.; Bouzarjomehr, M.; Taherzadeh, M.J.; Mahboubi, A. Recent Developments in the Application of Filamentous Fungus Aspergillus oryzae in Ruminant Feed. Animals 2024, 14, 2427. [Google Scholar] [CrossRef]
- Danilova, I.; Sharipova, M. The Practical Potential of Bacilli and Their Enzymes for Industrial Production. Front. Microbiol. 2020, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Okpara, M.O. Microbial Enzymes and Their Applications in Food Industry: A Mini-Review. Adv. Enzym. Res. 2022, 10, 23–47. [Google Scholar] [CrossRef]
- Cerminati, S.; Paoletti, L.; Aguirre, A.; Peirú, S.; Menzella, H.G.; Castelli, M.E. Industrial Uses of Phospholipases: Current State and Future Applications. Appl. Microbiol. Biotechnol. 2019, 103, 2571–2582. [Google Scholar] [CrossRef]
- Borrelli, G.; Trono, D. Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef]
- Riaz, S.; Amin, U.; Maan, A.A. Natural Emulsifiers as Clean Label Ingredients. In The Age of Clean Label Foods; Galanakis, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 37–72. ISBN 978-3-030-96697-3. [Google Scholar]
- Reyes-Reyes, A.L.; Valero Barranco, F.; Sandoval, G. Recent Advances in Lipases and Their Applications in the Food and Nutraceutical Industry. Catalysts 2022, 12, 960. [Google Scholar] [CrossRef]
- Golgeri, M.D.B.; Mulla, S.I.; Bagewadi, Z.K.; Faniband, B.; Mishra, P.; Bankole, P.O.; Sharma, S.; Américo-Pinheiro, J.H.P.; Bharagava, R.N.; Romanholo Ferreira, L.F. Microbial Naringinase: From Microbial Source to Its Current Applications in Various Fields. Biologia 2025, 80, 977–991. [Google Scholar] [CrossRef]
- Tang, Z.; Shi, L.; Liang, S.; Yin, J.; Dong, W.; Zou, C.; Xu, Y. Recent Advances of Tannase: Production, Characterization, Purification, and Application in the Tea Industry. Foods 2024, 14, 79. [Google Scholar] [CrossRef]
- Kumar, V.V.; Venkataraman, S.; Kumar, P.S.; George, J.; Rajendran, D.S.; Shaji, A.; Lawrence, N.; Saikia, K.; Rathankumar, A.K. Laccase Production by Pleurotus ostreatus Using Cassava Waste and Its Application in Remediation of Phenolic and Polycyclic Aromatic Hydrocarbon-Contaminated Lignocellulosic Biorefinery Wastewater. Environ. Pollut. 2022, 309, 119729. [Google Scholar] [CrossRef]
- Ozdemir, M.B.; Kılıçarslan, E.; Demir, H.; Koca, E.; Salum, P.; Berktaş, S.; Çam, M.; Erbay, Z.; Aydemir, L.Y. Upgrading the Bioactive Potential of Hazelnut Oil Cake by Aspergillus oryzae under Solid-State Fermentation. Molecules 2024, 29, 4237. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, M.; Jiang, H.; Wang, W.; Huang, J.; Ye, S.; Chen, Y.; Liu, S.; Liu, J. Theaflavins Are Improved by the Oxidation of Catechins in Tannase Treatment During Black Tea Fermentation. Molecules 2025, 30, 452. [Google Scholar] [CrossRef]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and Antimicrobial Preservatives: Properties, Mechanism of Action and Applications in Food—A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef]
- Joudaki, H.; Aria, N.; Moravej, R.; Rezaei Yazdi, M.; Emami-Karvani, Z.; Hamblin, M.R. Microbial Phytases: Properties and Applications in the Food Industry. Curr. Microbiol. 2023, 80, 374. [Google Scholar] [CrossRef]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical Characteristics, Applications and Research Trends of Edible Pickering Emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Harirchi, S.; Sar, T.; Ramezani, M.; Aliyu, H.; Etemadifar, Z.; Nojoumi, S.A.; Yazdian, F.; Awasthi, M.K.; Taherzadeh, M.J. Bacillales: From Taxonomy to Biotechnological and Industrial Perspectives. Microorganisms 2022, 10, 2355. [Google Scholar] [CrossRef] [PubMed]
- Todea, A.; Benea, I.C.; Bîtcan, I.; Péter, F.; Klébert, S.; Feczkó, T.; Károly, Z.; Biró, E. One-Pot Biocatalytic Conversion of Lactose to Gluconic Acid and Galacto-Oligosaccharides Using Immobilized β-Galactosidase and Glucose Oxidase. Catal. Today 2021, 366, 202–211. [Google Scholar] [CrossRef]
- Sarfaraz, M.; Sukmawati, D.; Shakir, H.A.; Khan, M.; Franco, M.; Irfan, M. Microbial Production and Applications of Glucose Oxidase. Syst. Microbiol. Biomanuf. 2025, 5, 890–914. [Google Scholar] [CrossRef]
- Naik, B.; Kumar, V.; Goyal, S.K.; Dutt Tripathi, A.; Mishra, S.; Joakim Saris, P.E.; Kumar, A.; Rizwanuddin, S.; Kumar, V.; Rustagi, S. Pullulanase: Unleashing the Power of Enzyme with a Promising Future in the Food Industry. Front. Bioeng. Biotechnol. 2023, 11, 1139611. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The Industrially Important Enzymes from Bacillus Species. In Bacilli in Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Eds.; Bacilli in Climate Resilient Agriculture and Bioprospecting; Springer International Publishing: Cham, 2022; pp. 89–99. ISBN 978-3-030-85464-5. [Google Scholar]
- Coban, H.B. Organic Acids as Antimicrobial Food Agents: Applications and Microbial Productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef]
- Zhang, E.; Breselge, S.; Carlino, N.; Segata, N.; Claesson, M.J.; Cotter, P.D. A Genomics-Based Investigation of Acetic Acid Bacteria across a Global Fermented Food Metagenomics Dataset. iScience 2025, 28, 112139. [Google Scholar] [CrossRef] [PubMed]
- Neffe-Skocińska, K.; Karbowiak, M.; Kruk, M.; Kołożyn-Krajewska, D.; Zielińska, D. Polyphenol and Antioxidant Properties of Food Obtained by the Activity of Acetic Acid Bacteria (AAB)—A Systematic Review. J. Funct. Foods 2023, 107, 105691. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Assadpour, E.; Cong, X.; Jafari, S.M. Cross-Linked Biopolymeric Films by Citric Acid for Food Packaging and Preservation. Adv. Colloid Interface Sci. 2023, 314, 102886. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, A.O.; Lateef, A. Biotechnological Valorization of Cashew Apple Juice for the Production of Citric Acid by a Local Strain of Aspergillus niger LCFS 5. J. Genet. Eng. Biotechnol. 2021, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Ribeiro, H.C. Citric Acid Production by the Solid-State Cultivation Consortium of Aspergillus niger and Trichoderma reesei from Sugarcane Bagasse. Open Biotechnol. J. 2020, 14, 32–41. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, F.; Li, X.; Mao, G.; Xie, H.; Song, A.; Santos, J.C.D.; Zhang, Z. Tailored Production of Citric Acid and Mannitol by Yarrowia lipolytica from Corn Stover Pretreated by Glycerol-Assisted Instant Catapult Steam Explosion. Ind. Crops Prod. 2022, 189, 115820. [Google Scholar] [CrossRef]
- Campos, A.L.B.M.A.; Nascimento, F.V.D.; Secchi, A.R.; Coelho, M.A.Z. Phenomenological Modeling of Polyols, Citric Acid and Bio-Oil Concurrent Production by Yarrowia lipolytica from Glycerol. Clean. Chem. Eng. 2023, 5, 100100. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, C.; Liu, R.; Liu, Q.; Xu, J.; Yao, J. Structure and Properties of Citric Acid Cross-Linked Chitosan/Poly(Vinyl Alcohol) Composite Films for Food Packaging Applications. Carbohydr. Polym. 2023, 312, 120842. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Zheng, X.; Pei, Y.; Tang, K. Citric Acid Crosslinked Soluble Soybean Polysaccharide Films for Active Food Packaging Applications. Food Chem. 2024, 438, 138009. [Google Scholar] [CrossRef]
- Wu, R.; Yang, J.; Jiang, Y.; Xin, F. Advances and Prospects for Lactic Acid Production from Lignocellulose. Enzym. Microb. Technol. 2025, 182, 110542. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, T.; Hu, M.; Chen, J.; Liu, C.; Gu, L.; He, Q.; Li, L. Effect of Capsaicin on Anaerobic Lactic Acid Production from Food Waste. Chem. Eng. J. 2025, 507, 160638. [Google Scholar] [CrossRef]
- Rana, A.K.; Thakur, V.K. Advances and New Horizons in Metabolic Engineering of Heterotrophic Bacteria and Cyanobacteria for Enhanced Lactic Acid Production. Bioresour. Technol. 2025, 419, 131951. [Google Scholar] [CrossRef]
- Yan, Y.; Shan, W.; Wu, Y.; Zhang, C.; Zhang, G.; Liu, G.; Chen, J.; Hu, W. Engineering Bacillus Coagulans with High Osmotic Tolerance for Enhancing L-Lactic Acid Production Using Sweet Sorghum Juice Coupled with Acid-Pretreated Soybean Meal under Unsterile Conditions. Ind. Crops Prod. 2025, 224, 120323. [Google Scholar] [CrossRef]
- Ali, R.; Saravia, F.; Hille-Reichel, A.; Gescher, J.; Horn, H. Propionic Acid Production from Food Waste in Batch Reactors: Effect of pH, Types of Inoculum, and Thermal Pre-Treatment. Bioresour. Technol. 2021, 319, 124166. [Google Scholar] [CrossRef]
- Cavero-Olguin, V.H.; Rahimpour, F.; Dishisha, T.; Alvarez-Aliaga, M.T.; Hatti-Kaul, R. Propionic Acid Production from Glycerol in Immobilized Cell Bioreactor Using an Acid-Tolerant Strain of Propionibacterium acidipropionici Obtained by Adaptive Evolution. Process Biochem. 2021, 110, 223–230. [Google Scholar] [CrossRef]
- Derman, Ü.C.; Erdem, A.; Alemdar, F.; Türker, M. Kinetics of Fermentative Production of Propionic Acid on a Range of Carbon and Nitrogen Sources Using Acidipropionibacterium acidipropionici. Food Biosci. 2024, 57, 103507. [Google Scholar] [CrossRef]
- Ashagrie, H.; Baye, K.; Guibert, B.; Rochette, I.; Tisseyre, P.; Humblot, C. The Use of Propionic and Lactic Acid Bacteria to Produce Cobalamin and Folate in Injera, an Ethiopian Cereal-Based Fermented Food. Int. J. Food Microbiol. 2025, 426, 110909. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Zhou, Y.; Xu, S.; Ren, X.; Wei, Q. Progress on Production of Succinic Acid by Actinobacillus succinogenes—New Opportunities for Cheap Biomass and Waste Gas Utilization. J. Clean. Prod. 2024, 434, 140005. [Google Scholar] [CrossRef]
- Kleps, C.; Malchow, R.; Ettinger, J.; Dalichow, J.; Schneider, R.; Venus, J.; Pleissner, D. Utilization of Acid Whey and Oat Pomace in Succinic Acid Fermentation. New Biotechnol. 2025, 86, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, W.; Zhu, J. Progress on Production of Malic Acid and Succinic Acid by Industrially-Important Engineered Microorganisms. J. Biotechnol. 2025, 400, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Teleky, B.-E.; Nemes, S.-A.; Plamada, D.; Varvara, R.-A.; Pascuta, M.-S.; Ciont, C.; Cocean, A.-M.; Medeleanu, M.; Nistor, A.; et al. Succinic Acid—A Run-through of the Latest Perspectives of Production from Renewable Biomass. Heliyon 2024, 10, e25551. [Google Scholar] [CrossRef]
- Louasté, B.; Eloutassi, N. Succinic Acid Production from Whey and Lactose by Actinobacillus succinogenes 130Z in Batch Fermentation. Biotechnol. Rep. 2020, 27, e00481. [Google Scholar] [CrossRef]
- Olszewska-Widdrat, A.; Da Costa Pereira, L.P.R.; Schneider, R.; Unger, P.; Xiros, C.; Venus, J. Pilot Scale Succinic Acid Production from Fibre Sludge Followed by the Downstream Processing. Food Bioprod. Process. 2025, 151, 118–126. [Google Scholar] [CrossRef]
- Gonçalves, F.; Fernandes, T.; Tulha, J.; Bessa, D.; Pereira, J.; Schuller, D.; Sousa, M.J.; Sampaio, P.; Pais, C.; Franco-Duarte, R. Yeast Mycobiome of Fruit and Vegetable Biowastes Revealed by Culture-Dependent and Metabarcoding Approaches: Screening for the Production of Succinic Acid and Single Cell Oils. Food Biosci. 2024, 62, 105340. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Ahangari, H.; Mousazadeh, S.; Hosseini, S.M.; Dufossé, L. Microbial Pigments as an Alternative to Synthetic Dyes and Food Additives: A Brief Review of Recent Studies. Bioprocess Biosyst. Eng. 2022, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Liang, X.; Li, Y.; Li, M.; Yu, T.; Qin, Y.; Li, B.; An, T.; Wang, G. Biosynthesis and Metabolic Engineering of Natural Sweeteners. AMB Express 2025, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, T.-T.; Guo, R.-R.; Ye, Q.; Zhao, H.-L.; Huang, X.-H. The Regulation of Key Flavor of Traditional Fermented Food by Microbial Metabolism: A Review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef]
- Da Silva, L.I.; De Abreu, D.J.M.; Da Conceição Jesus, E.; Carvalho, E.E.N.; Pereira, M.C.; Teixeira, A.F.D.S.; Pasqual, M.; Dória, J. Optimizing Strawberry Flavour: The Role of Bacterial Inoculants in Enhancing Organoleptic Characteristics. J. Plant Growth Regul. 2025, 44, 4612–4627. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V. Role of Microbial Fermentation in the Bio-Production of Food Aroma Compounds from Vegetable Waste. Fermentation 2024, 10, 132. [Google Scholar] [CrossRef]
- Dhandwal, A.; Bashir, O.; Malik, T.; Salve, R.V.; Dash, K.K.; Amin, T.; Shams, R.; Wani, A.W.; Shah, Y.A. Sustainable Microalgal Biomass as a Potential Functional Food and Its Applications in Food Industry: A Comprehensive Review. Environ. Sci. Pollut. Res. 2024, 32, 19110–19128. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials. Polysaccharides 2024, 5, 241–287. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Cheng, Y.; Yao, W.; Qian, H. Neuroprotective Effects of Polysaccharide from Sparassis crispa on Alzheimer’s Disease-like Mice: Involvement of Microbiota-Gut-Brain Axis. Int. J. Biol. Macromol. 2023, 225, 974–986. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Flavourings (FAF); Castle, L.; Andreassen, M.; Aquilina, G.; Bastos, M.L.; Boon, P.; Fallico, B.; Fitzgerald, R.; Frutos Fernandez, M.J.; Grasl-Kraupp, B.; et al. Re-Evaluation of Pullulan (E 1204) as a Food Additive and New Application for Its Extension of Use. EFSA J. 2025, 23, e9267. [Google Scholar] [CrossRef] [PubMed]
- Aali, R.A.K.A.; Al-Sahlany, S.T.G.A. Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends. Gels 2024, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Martínez-Espinosa, R.M. Production of Microbial Food Colorants. In Application of Bio-Additives for the Food Industry; Sattar Jatoi, A., Mubarak, N.M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 49–65. ISBN 978-3-031-71130-5. [Google Scholar]
- Liu, J.; Luo, Y.; Guo, T.; Tang, C.; Chai, X.; Zhao, W.; Bai, J.; Lin, Q. Cost-Effective Pigment Production by Monascus purpureus Using Rice Straw Hydrolysate as Substrate in Submerged Fermentation. J. Biosci. Bioeng. 2020, 129, 229–236. [Google Scholar] [CrossRef]
- Barros, K.O.; Souza, R.M.; Palladino, F.; Cadete, R.M.; Santos, A.R.O.; Goes-Neto, A.; Berkov, A.; Zilli, J.E.; Vital, M.J.S.; Lachance, M.-A.; et al. Cyberlindnera dasilvae sp. Nov., a Xylitol-Producing Yeast Species Isolated from Rotting Wood and Frass of Cerambycid Larva. Int. J. Syst. Evol. Microbiol. 2021, 71, 004986. [Google Scholar] [CrossRef]
- Nazem-Bokaee, H.; Hom, E.F.Y.; Mathews, S.; Gueidan, C. Analyzing Sorbitol Biosynthesis Using a Metabolic Network Flux Model of a Lichenized Strain of the Green Microalga Diplosphaera chodatii. Microbiol. Spectr. 2025, 13, e03660-23. [Google Scholar] [CrossRef]
- Hosseini, S.V.; Dastgerdi, H.E.; Tahergorabi, R. Marine Mannitol: Extraction, Structures, Properties, and Applications. Processes 2024, 12, 1613. [Google Scholar] [CrossRef]
- Noble, A.J.; Swain, A.; Clarke, C.J.; Zhang, C.; Sabouri, S.; Li, S.; Long, A.W.; Sasidharan Pillai, P.K. Compositions, Preparation and Uses of Paramylon. Patent WO2020178718A1, 10 September 2021. [Google Scholar]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food Thickening Agents: Sources, Chemistry, Properties and Applications—A Review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Deehan, E.C.; Al Antwan, S.; Witwer, R.S.; Guerra, P.; John, T.; Monheit, L. Revisiting the Concepts of Prebiotic and Prebiotic Effect in Light of Scientific and Regulatory Progress—A Consensus Paper from the Global Prebiotic Association. Adv. Nutr. 2024, 15, 100329. [Google Scholar] [CrossRef]
- ANVISA—Brazilian Health Regulatory Agency. Resolution of the Collegiate Board–RDC No. 728, of July 1, 2022. Provides for Enzymes and Enzyme Preparations for Use as Processing Aids in the Production of Foods Intended for Human Consumption. Official Gazette of the Union, 6 July 2022; 1, 217. [Google Scholar]
- Hutkins, R.; Walter, J.; Gibson, G.R.; Bedu-Ferrari, C.; Scott, K.; Tancredi, D.J.; Wijeyesekera, A.; Sanders, M.E. Classifying Compounds as Prebiotics—Scientific Perspectives and Recommendations. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 54–70. [Google Scholar] [CrossRef]
- Hossain, M.I.; Sadekuzzaman, M.; Ha, S.-D. Probiotics as Potential Alternative Biocontrol Agents in the Agriculture and Food Industries: A Review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- Akamine, I.T.; Mansoldo, F.R.P.; Vermelho, A.B. Probiotics in the Sourdough Bread Fermentation: Current Status. Fermentation 2023, 9, 90. [Google Scholar] [CrossRef]
- Bk, J.; Chalannavar, R.K.; Nandini, N.; Malabadi, R.B.; Kolkar, K.P. Divakar Yeast Probiotics Fermented Food Products: Gut Microbiome and Women Health. World J. Adv. Res. Rev. 2025, 27, 1209–1230. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Pham, T.-T.; Nguyen, P.-T.; Le-Buanec, H.; Rabetafika, H.N.; Razafindralambo, H.L. Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules 2024, 14, 1162. [Google Scholar] [CrossRef]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Edo, G.I.; Mafe, A.N.; Akpoghelie, P.O.; Gaaz, T.S.; Yousif, E.; Yusuf, O.S.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; Ayinla, J.L.; et al. The Utilization of Biopolymer Hydrogels to Encapsulate and Protect Probiotics in Foods. Process Biochem. 2025, 153, 66–91. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Duarte, L.G.R.; Bonfim, D.O.; Salgaço, M.K.; Mattoso, L.H.C.; Egea, M.B. Shaping the Future of Functional Foods: Using 3D Printing for the Encapsulation and Development of New Probiotic Foods. Probiotics Antimicrob. Proteins 2025, 17, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Katkowska, M.; Garbacz, K.; Kusiak, A. Probiotics: Should All Patients Take Them? Microorganisms 2021, 9, 2620. [Google Scholar] [CrossRef]
- Ren, W.; Wei, Y.; Lian, G.; Yang, C.; Liu, Y. Lactobacillus plantarum Liver Abscess Following ERCP: A Case Report and Review. BMC Infect. Dis. 2025, 25, 704. [Google Scholar] [CrossRef] [PubMed]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Jarvinen, A. Lactobacillus Bacteremia, Clinical Significance, and Patient Outcome, with Special Focus on Probiotic L. rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef]
- Muñoz, M.; Castaño, G.E.; Esquivel Suman, R.; Alvarado, M. Septicemia Por Bacillus clausii Posterior al Uso de Probióticos. Una Complicación Para Tener Presente. Andes Pediatr. 2023, 94, 379–385. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-Producing Human Gut Symbiont, Clostridium butyricum, and Its Role in Health and Disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef] [PubMed]
- Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.C.; Nouwen, J.L.; Thio, H.B. Butyrate Properties in Immune-Related Diseases: Friend or Foe? Fermentation 2023, 9, 205. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Sadeghi, M.; Haghshenas, B.; Nami, Y. Bifidobacterium Exopolysaccharides: New Insights into Engineering Strategies, Physicochemical Functions, and Immunomodulatory Effects on Host Health. Front. Microbiol. 2024, 15, 1396308. [Google Scholar] [CrossRef]
- Rafiq, S.; Bhat, M.I.; Sofi, S.A.; Muzzafar, K.; Majid, D.; Dar, B.; Makroo, H.A. Bioconversion of Agri-Food Waste and by-Products into Microbial Lipids: Mechanism, Cultivation Strategies and Potential in Food Applications. Trends Food Sci. Technol. 2023, 139, 104118. [Google Scholar] [CrossRef]
- Zhou, Z.; Sarwar, A.; Xue, R.; Hu, G.; Wu, J.; Aziz, T.; Alasmari, A.F.; Yang, Z.; Yang, Z. Metabolomics Analysis of Potential Functional Metabolites in Synbiotic Ice Cream Made with Probiotic Saccharomyces cerevisiae Var. Boulardii CNCM I-745 and Prebiotic Inulin. Food Chem. 2024, 454, 139839. [Google Scholar] [CrossRef]
- Rachwał, K.; Gustaw, K. Lactic Acid Bacteria in Sustainable Food Production. Sustainability 2024, 16, 3362. [Google Scholar] [CrossRef]
- Cramer, J.F.; Kjaer, K.H.; Yde, C.C.; Wichmann, J.; Jensen, H.M.; Kortman, G.A.M.; Dellomonaco, C.; Ewert, J. β-Galactosidase from Bifidobacterium bifidum for Improved in Situ Synthesis of GOS-Oligomers with Prebiotic Effects. Int. Dairy J. 2025, 163, 106164. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; Van Sinderen, D. Carbohydrate Metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef]
- Schell, M.A.; Karmirantzou, M.; Snel, B.; Vilanova, D.; Berger, B.; Pessi, G.; Zwahlen, M.-C.; Desiere, F.; Bork, P.; Delley, M.; et al. The Genome Sequence of Bifidobacterium longum Reflects Its Adaptation to the Human Gastrointestinal Tract. Proc. Natl. Acad. Sci. USA 2002, 99, 14422–14427. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Guo, S.; Wang, T.; Cao, H.; Cao, Y.; Dong, B. Galacto-Oligosaccharides Alleviate Experimental Lactose Intolerance Associated with Gut Microbiota in Mice. Front. Microbiol. 2025, 16, 1530156. [Google Scholar] [CrossRef]
- Tsigoriyna, L.; Stefanov, S.; Armenova, N.; Petrova, P.; Petrov, K. Microbial Conversion of Inulin to Valuable Products: The Biorefinery Concept. Fermentation 2024, 10, 640. [Google Scholar] [CrossRef]
- Chacher, M.F.A.; Kamran, Z.; Ahsan, U.; Ahmad, S.; Koutoulis, K.C.; Qutab Ud Din, H.G.; Cengiz, Ö. Use of Mannan Oligosaccharide in Broiler Diets: An Overview of Underlying Mechanisms. World’s Poult. Sci. J. 2017, 73, 831–844. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.-L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.-D. Emerging Prospects of Macro- and Microalgae as Prebiotic. Microb. Cell Factories 2021, 20, 112. [Google Scholar] [CrossRef]
- Devaprakash, M.; Thirumalaivasan, R.; Sivakumar, N.; Shyam Kumar, R.; Ponmurugan, K. Nutraceuticals and Functional Foods from Algae: Formulation and Health Benefits. In Value Added Products from Bioalgae Based Biorefineries: Opportunities and Challenges; Arya, S.K., Khatri, M., Singh, G., Eds.; Springer Nature: Singapore, 2024; pp. 289–341. ISBN 978-981-97-1661-6. [Google Scholar]
- Olvera-Aguirre, G.; Piñeiro-Vázquez, Á.T.; Sanginés-García, J.R.; Sánchez Zárate, A.; Ochoa-Flores, A.A.; Segura-Campos, M.R.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Using Plant-Based Compounds as Preservatives for Meat Products: A Review. Heliyon 2023, 9, e17071. [Google Scholar] [CrossRef]
- Akpoghelie, P.O.; Edo, G.I.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Owheruo, J.O.; Isoje, E.F.; Igbuku, U.A.; Essaghah, A.E.A.; Makia, R.S.; et al. Lactic Acid Bacteria: Nature, Characterization, Mode of Action, Products and Applications. Process Biochem. 2025, 152, 1–28. [Google Scholar] [CrossRef]
- Naveed, M.; Ishfaq, H.; Rehman, S.U.; Javed, A.; Waseem, M.; Makhdoom, S.I.; Aziz, T.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. GC–MS Profiling of Bacillus spp. Metabolites with an in Vitro Biological Activity Assessment and Computational Analysis of Their Impact on Epithelial Glioblastoma Cancer Genes. Front. Chem. 2023, 11, 1287599. [Google Scholar] [CrossRef]
- Yang, Y.; Kumrungsee, T.; Okazaki, Y.; Watanabe, T.; Inoue, J.; Iguchi, T.; Fukuda, S.; Kuroda, M.; Nishio, K.; Yamaguchi, S.; et al. Potential Roles of Exogenous Proteases and Lipases as Prebiotics. Nutrients 2025, 17, 924. [Google Scholar] [CrossRef] [PubMed]
- Shanuke, D.S.; Ranasinghage, N.B.D.P.; Illippangama, A.U.; Kulathunga, J.; Bandara, M.D. Co-Encapsulation of Probiotics and Prebiotics: Techniques and Applications in Food Fortification. Food Sci. Nutr. 2025, 13, e70426. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, Z.; Šarić, L.; Tomičić, R. Potential Future Applications of Postbiotics in the Context of Ensuring Food Safety and Human Health Improvement. Antibiotics 2025, 14, 674. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Cunningham, M.; Hill, C. Frequently Asked Questions about the ISAPP Postbiotic Definition. Front. Microbiol. 2024, 14, 1324565. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Cruz, A.G.; Pereira, E.; Almeida Da Costa, W.K.; Da Silva Rocha, R.; Targino De Souza Pedrosa, G.; Rocha, C.D.S.; Alves, J.M.; Alvarenga, V.O.; Sant’Ana, A.S.; et al. Postbiotics: An Overview of Concepts, Inactivation Technologies, Health Effects, and Driver Trends. Trends Food Sci. Technol. 2023, 138, 199–214. [Google Scholar] [CrossRef]
- Park, S.-J.; Sharma, A.; Lee, H.-J. Postbiotics against Obesity: Perception and Overview Based on Pre-Clinical and Clinical Studies. Int. J. Mol. Sci. 2023, 24, 6414. [Google Scholar] [CrossRef]
- Mi, X.-J.; Tran, T.H.M.; Park, H.-R.; Xu, X.Y.; Subramaniyam, S.; Choi, H.S.; Kim, J.; Koh, S.C.; Kim, Y.J. Immune-Enhancing Effects of Postbiotic Produced by Bacillus velezensis Kh2-2 Isolated from Korea Foods. Food Res. Int. 2022, 152, 110911. [Google Scholar] [CrossRef]
- Khani, N.; Abedi Soleimani, R.; Homayouni Rad, A. Characterization and Antimicrobial Activity of Postbiotic from Lactobacillus acidophilus LA5 on Staphylococcus aureus in Food Model and In Vitro. Curr. Nutr. Food Sci. 2025, 21, 379–387. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.; Habiba, U.; Pandey, V.K.; Kaur, S.; Rustagi, S. Potential Health Benefits of Postbiotics and Its Utilization as Natural Food Preservatives. Food Humanit. 2025, 5, 100726. [Google Scholar] [CrossRef]
- Molina, D.; Marinas, I.C.; Angamarca, E.; Hanganu, A.; Stan, M.; Chifiriuc, M.C.; Tenea, G.N. Postbiotic-Based Extracts from Native Probiotic Strains: A Promising Strategy for Food Preservation and Antimicrobial Defense. Antibiotics 2025, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Zolfanelli, C.; Lauciello, V.; Di Ciancia, A.; Vagliasindi, A.; Smaoui, S.; Varzakas, T. Using Postbiotics from Functional Foods for Managing Colorectal Cancer: Mechanisms, Sources, Therapeutic Potential, and Clinical Perspectives. Microorganisms 2025, 13, 1335. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Moreira, J.V.; Junior, A.N.; Da Silva, C.R.; Cardoso, V.D.S.; Akamine, I.T. Microbial Preservation and Contamination Control in the Baking Industry. Fermentation 2024, 10, 231. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Li, M.; Lin, J. Fruits and Vegetables Preservation Based on AI Technology: Research Progress and Application Prospects. Comput. Electron. Agric. 2024, 226, 109382. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Zhou, Y.; Peng, Z.; Cui, F.; Zhou, Q.; Man, Z.; Guo, J.; Sun, W. Can Cadmium-Contaminated Rice Be Used to Produce Food Additive Sodium Erythorbate? Food Chem. 2025, 462, 140923. [Google Scholar] [CrossRef] [PubMed]
- Khoshnoud, M.J.; Siavashpour, A.; Bakhshizadeh, M.; Rashedinia, M. Effects of Sodium Benzoate, a Commonly Used Food Preservative, on Learning, Memory, and Oxidative Stress in Brain of Mice. J. Biochem. Mol. Toxicol. 2018, 32, e22022. [Google Scholar] [CrossRef]
- Wong, S.X.E.; Kiew, S.F.; Lau, S.Y.; Pottas, P.W. Procedures to Investigate Potential of Plants as Natural Food Preservatives: Extraction Technology, Phytochemical Characterisation, and Antimicrobial Bioassays. Food Chem. Adv. 2023, 3, 100435. [Google Scholar] [CrossRef]
- Shwaiki, L.N.; Lynch, K.M.; Arendt, E.K. Future of Antimicrobial Peptides Derived from Plants in Food Application—A Focus on Synthetic Peptides. Trends Food Sci. Technol. 2021, 112, 312–324. [Google Scholar] [CrossRef]
- Stefanello, R.F.; Vilela, L.F.; Margalho, L.P.; Nabeshima, E.H.; Matiolli, C.C.; Da Silva, D.T.; Schwan, R.F.; Emanuelli, T.; Noronha, M.F.; Cabral, L.; et al. Dynamics of Microbial Ecology and Their Bio-Preservative Compounds Formed during the Panettones Elaboration Using Sourdough-Isolated Strains as Starter Cultures. Food Biosci. 2024, 60, 104279. [Google Scholar] [CrossRef]
- Rathod, N.B.; Phadke, G.G.; Tabanelli, G.; Mane, A.; Ranveer, R.C.; Pagarkar, A.; Ozogul, F. Recent Advances in Bio-Preservatives Impacts of Lactic Acid Bacteria and Their Metabolites on Aquatic Food Products. Food Biosci. 2021, 44, 101440. [Google Scholar] [CrossRef]
- Viana De Souza, J.; Silva Dias, F. Protective, Technological, and Functional Properties of Select Autochthonous Lactic Acid Bacteria from Goat Dairy Products. Curr. Opin. Food Sci. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Omoregbe, O.; Hart, A.; Miri, T.; Eze, U.A.; Onyeaka, H. Applications of Bacteriocins of Lactic Acid Bacteria in Biotechnology and Food Preservation: A Bibliometric Review. Open Microbiol. J. 2022, 16, e187428582206300. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, W.; Peng, S.; Liang, Z.; Liu, Z.; Zhu, C.; Mou, H. Current Status and Potential of Bacteriocin-Producing Lactic Acid Bacteria Applied in the Food Industry. Curr. Res. Food Sci. 2025, 10, 100997. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Mohamed, M.E.B.; Sebaei, A.S.; Mahmoud, N.M.; Mohammed, N.A.; Hassan, H.A.; Abdel-aal, R.R. Electrochemical and Chromatographic Methods for the Determination of Some Natural Food Preservatives—A Review. Food Chem. 2025, 468, 142491. [Google Scholar] [CrossRef]
- Azimirad, M.; Javaheri-Ghezeldizaj, F.; Yekta, R.; Ezzati Nazhad Dolatabadi, J.; Torbati, M. Mechanistic and Kinetic Aspects of Natamycin Interaction with Serum Albumin Using Spectroscopic and Molecular Docking Methods. Arab. J. Chem. 2023, 16, 105043. [Google Scholar] [CrossRef]
- Epparti, P.; Eligar, S.M.; Sattur, A.P.; Gnanesh Kumar, B.S.; Halami, P.M. Characterization of Dual Bacteriocins Producing Bacillus subtilis SC3.7 Isolated from Fermented Food. LWT 2022, 154, 112854. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, L.; Tang, W.; Li, J.; Tang, T.; Sun, X.; Qiao, X.; He, Z. Characterization of a Novel Circular Bacteriocin from Bacillus velezensis 1-3, and Its Mode of Action against Listeria monocytogenes. Heliyon 2024, 10, e29701. [Google Scholar] [CrossRef]
- Hata, T.; Alemu, M.; Kobayashi, M.; Suzuki, C.; Nitisinprasert, S.; Ohmomo, S. Characterization of a Bacteriocin Produced by Enterococcus faecalis N1-33 and Its Application as a Food Preservative. J. Food Prot. 2009, 72, 524–530. [Google Scholar] [CrossRef]
- Rajapakshe, P.; Rathnasinghe, N.; Guruge, K.; Nilmini, R.; Jayasinghe, R.; Karunaratne, V.; Wijesena, R.; Priyadarshana, G. Strategies to Minimize Post-Harvest Waste of Fruits and Vegetables: Current Solutions and Future Perspectives. J. Future Foods 2025, 6, 400–412. [Google Scholar] [CrossRef]
- Riolo, M.; Villena, A.M.; Calpe, J.; Luz, C.; Meca, G.; Tuccitto, N.; Cacciola, S.O. A Circular Economy Approach: A New Formulation Based on a Lemon Peel Medium Activated with Lactobacilli for Sustainable Control of Post-Harvest Fungal Rots in Fresh Citrus Fruit. Biol. Control. 2024, 189, 105443. [Google Scholar] [CrossRef]
- Volentini, S.I.; Olmedo, G.M.; Grillo-Puertas, M.; Rapisarda, V.A.; Hebert, E.M.; Cerioni, L.; Villegas, J.M. Biological Control of Green and Blue Molds on Postharvest Lemon by Lactic Acid Bacteria. Biol. Control 2023, 185, 105303. [Google Scholar] [CrossRef]
- Rovetto, E.I.; La Spada, F.; El Boumlasy, S.; Conti Taguali, S.; Riolo, M.; Pane, A.; Cacciola, S.O. Biological Control of Green Mold in Simulated Post-Harvest Chain of Citrus Fruit: Efficacy of Candida oleophila Strain O and Molecular Insight into Elicitation of Host Immune System. Biol. Control 2024, 193, 105531. [Google Scholar] [CrossRef]
- Yakir, I.; Cohen, E.; Schlesinger, S.; Hayouka, Z. Random Antimicrobial Peptide Mixtures as Non-Antibiotic Antimicrobial Agents for Cultured Meat Industry. Food Chem. Mol. Sci. 2025, 10, 100240. [Google Scholar] [CrossRef]
- Chauhan, K.; Rao, A. Clean-Label Alternatives for Food Preservation: An Emerging Trend. Heliyon 2024, 10, e35815. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and Pullulan Derivatives as Promising Biomolecules for Drug and Gene Targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef]
- De Souza, C.K.; Ghosh, T.; Lukhmana, N.; Tahiliani, S.; Priyadarshi, R.; Hoffmann, T.G.; Purohit, S.D.; Han, S.S. Pullulan as a Sustainable Biopolymer for Versatile Applications: A Review. Mater. Today Commun. 2023, 36, 106477. [Google Scholar] [CrossRef]
- Wani, S.M.; Rizwan, D.; Khanday, F.A.; Mir, S.A.; Masoodi, F.A. Effect of Pullulan and Pullulan-Chitosan Composite Coating on the Antioxidant Activity, Texture, Color and Shelf-Life of a Local Cultivar of Sweet Cherry. Food Humanit. 2024, 3, 100432. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Tong, Q.; Zhang, Z.; Jin, Z. Effect of Pullulan on the Water Distribution, Microstructure and Textural Properties of Rice Starch Gels during Cold Storage. Food Chem. 2017, 214, 702–709. [Google Scholar] [CrossRef]
- Khalid, M.U.; Phyo, H.M.; Hassan, F.; Mushtaq, A.; Hussain, A.; Hussain, M.; Alsulami, T.; Yao, W. Preparation, Characterization, and Antifungal Capacity of Co-Encapsulated Anisaldehyde and Cinnamaldehyde within Pullulan-Stabilized Nanoemulsions for Bread Preservation. Food Biosci. 2024, 62, 105506. [Google Scholar] [CrossRef]
- Hong, S.J.; Riahi, Z.; Shin, G.H.; Kim, J.T. Development of Innovative Active Packaging Films Using Gelatin/Pullulan-Based Composites Incorporated with Cinnamon Essential Oil-Loaded Metal-Organic Frameworks for Meat Preservation. Int. J. Biol. Macromol. 2024, 267, 131606. [Google Scholar] [CrossRef] [PubMed]
- Adame, M.Y.; Shi, C.; Li, C.; Aziz, T.; Alharbi, M.; Cui, H.; Lin, L. Fabrication and Characterization of Pullulan/Tapioca Starch-Based Antibacterial Films Incorporated with Litsea Cubeba Essential Oil for Meat Preservation. Int. J. Biol. Macromol. 2024, 268, 131775. [Google Scholar] [CrossRef]
- Aayush, K.; Sharma, K.; Singh, G.P.; Chiu, I.; Chavan, P.; Shandilya, M.; Roy, S.; Ye, H.; Sharma, S.; Yang, T. Development and Characterization of Edible and Active Coating Based on Xanthan Gum Nanoemulsion Incorporating Betel Leaf Extract for Fresh Produce Preservation. Int. J. Biol. Macromol. 2024, 270, 132220. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, F.; Xiang, P.; Jiang, F.; Xiao, M. High-Absorbent Antibacterial Konjac Glucomannan/Xanthan Gum/Bamboo Fiber Aerogel with Carvacrol for Chilled Pork Preservation. Int. J. Biol. Macromol. 2025, 306, 141624. [Google Scholar] [CrossRef]
- Li, H.; Xing, R.; Wang, Z.; Li, G. Advancements in Xanthan Gum-Based Film and Coating for Food Packaging. Carbohydr. Polym. 2025, 356, 123409. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Saif Alrasbi, A.N.; Jawad, M.; Koca, E.; Aydemir, L.Y.; Alamoudi, J.A.; Almoshari, Y.; Mohan, S. Structural, Mechanical, Barrier and Antioxidant Properties of Pectin and Xanthan Gum Edible Films Loaded with Grapefruit Essential Oil. Heliyon 2024, 10, e25501. [Google Scholar] [CrossRef]
- Mohammadi, E.; Rahimian, M.; Panahi, B. Bridging the Gap: Phage Manufacturing Processes from Laboratory to Agri-Food Industry. Virus Res. 2025, 353, 199537. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hussain, W.; Chen, Y.; Xu, P.; Yang, X.; Wang, H.; Zhang, X.; Fu, Q.; Wang, S. A New Type of Pseudomonas aeruginosa Phage with Potential as a Natural Food Additive for Eradicating Biofilms and Combating Multidrug-Resistant Strains. Food Control 2025, 168, 110888. [Google Scholar] [CrossRef]
- Qin, X.; Liu, T.; Xue, J.; Zhang, W.; Li, H.; Liu, Y.; Wang, X.; Li, Z.; Ma, Y.; Xia, X.; et al. Characterization of a Novel Salmonella enterica serovar Manhattan phage and Its Inhibitory Effects in Vitro and in Food Matrices. LWT 2025, 222, 117617. [Google Scholar] [CrossRef]
- Bateman, K.R.; Hutchinson, E.S.; Widmalm, G.; Miller, M.J.; Stenmark, P. Structural and Functional Insights into Listeria monocytogenes Phage Endolysin PlyP100—A Promising Food Safety Tool. J. Biol. Chem. 2025, 301, 110295. [Google Scholar] [CrossRef]
- Zanzan, M.; Ezzaky, Y.; Achemchem, F.; Hamadi, F. Leveraging Lactic Acid Bacteria Biofilms and Bioactive Substances to Counteract Pathogenic Biofilms in the Dairy Processing. Food Humanit. 2024, 3, 100448. [Google Scholar] [CrossRef]
- Biswas, R.; Alam, M.; Sarkar, A.; Haque, M.I.; Hasan, M.M.; Hoque, M. Application of Nanotechnology in Food: Processing, Preservation, Packaging and Safety Assessment. Heliyon 2022, 8, e11795. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of Using Nanotechnology for Food Preservation, Safety, and Security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef]
- Qu, B.; Xiao, Z.; Luo, Y. Sustainable Nanotechnology for Food Preservation: Synthesis, Mechanisms, and Applications of Zinc Oxide Nanoparticles. J. Agric. Food Res. 2025, 19, 101743. [Google Scholar] [CrossRef]
- Correia, L.F.; Da Silva Pinho, G.; Da Cruz Neves, T.J.; De Oliveira Vieira, K.C.; Maddela, N.R.; Prasad, R.; Winkelstroter, L.K. Nanotechnology Innovation Combined with Bacteriocins as Emerging Strategy for the Development of Active and Intelligent Food Packaging. Sustain. Chem. Pharm. 2024, 39, 101551. [Google Scholar] [CrossRef]
- Pyne, S.; Paria, K.; Mandal, S.M.; Srivastav, P.P.; Bhattacharjee, P.; Barik, T.K. Green Microalgae Derived Organic Nanodots Used as Food Preservative. Curr. Res. Green Sustain. Chem. 2022, 5, 100276. [Google Scholar] [CrossRef]
- Manna, A.; Mondal, R. Bacteriocin-Mediated Food Preservation in Conjugation with Silver Nanoparticles: A Green Approach. Food Chem. Adv. 2023, 3, 100464. [Google Scholar] [CrossRef]
- Dejene, B.K. Leveraging Synergistic Effects of Metallic Nanoparticles and Essential Oils in Biopolymers: Emerging Nanocomposites for Food Packaging Applications—A Review. J. Agric. Food Res. 2025, 21, 101885. [Google Scholar] [CrossRef]
- Guan, J.; Zeng, K.; Chen, Z. Editorial: Postharvest Disease Management in Fruits and Vegetables: Recent Advances and Mechanisms. Front. Microbiol. 2023, 14, 1203010. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Kamat, S.; Dixit, R.; Pandey, S.; Giri, V.P.; Mishra, A. Microbial Formulation Approaches in Postharvest Disease Management. In Food Security and Plant Disease Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 279–305. ISBN 978-0-12-821843-3. [Google Scholar]
- Lawal, H.; Gaddafi, M.S.; Jamiu, A.M.; Edo, G.S.; Fremah, O.G.; El-yakub, A.U.; Mahunu, G.K.; Wang, K.; Zhang, H.; Yang, Q. Biocontrol and Nanotechnology Strategies for Postharvest Disease Management in Fruits and Vegetables: A Comprehensive Review. Foods 2025, 14, 2782. [Google Scholar] [CrossRef]
- Cuadrado-Osorio, P.D.; Ramírez-Mejía, J.M.; Mejía-Avellaneda, L.F.; Mesa, L.; Bautista, E.J. Agro-Industrial Residues for Microbial Bioproducts: A Key Booster for Bioeconomy. Bioresour. Technol. Rep. 2022, 20, 101232. [Google Scholar] [CrossRef]
- Remolif, G.; Schiavon, G.; Garello, M.; Spadaro, D. Efficacy of Postharvest Application of Aureobasidium pullulans to Control White Haze on Apples and Effect on the Fruit Mycobiome. Horticulturae 2024, 10, 927. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic Mechanisms and Control Strategies of Botrytis cinerea Causing Post-Harvest Decay in Fruits and Vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Ranjith, F.H.; Muhialdin, B.J.; Yusof, N.L.; Mohammed, N.K.; Miskandar, M.H.; Hussin, A.S.M. Effects of Lacto-Fermented Agricultural By-Products as a Natural Disinfectant against Post-Harvest Diseases of Mango (Mangifera indica L.). Plants 2021, 10, 285. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Gao, Y.; Gao, T.; Zhang, D. Inhibitory Abilities of Bacillus Isolates and Their Culture Filtrates against the Gray Mold Caused by Botrytis Cinerea on Postharvest Fruit. Plant Pathol. J. 2019, 35, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, R.; Cao, X.; Hu, N.; Xia, B.; Yi, Y.; Zhou, S.; Zhou, H. Preservation Effect of Lactobacillus Plantarum O2 Fermentation Supernatant on Postharvest Pepper and Its Induced Resistance to Phytophthora capsici. Plant Physiol. Biochem. 2023, 204, 108098. [Google Scholar] [CrossRef]
- Leal Rodrigues, P.; Gleyce Julião Bomfim, A.; Leal Dos Santos, W.; Tiago Correia Oliveira, J.; Aparecida Moreira, K.; Galvão, J.R.; Pacheco, M.J.B. Yeast Biocontrol against Green Mold in Pear Orange Postharvest. Comun. Sci. 2023, 14, e3963. [Google Scholar] [CrossRef]
- Yuan, L.; Jiang, H.; Yin, X.; Wei, Y.; Li, T.; Jiang, X. Paenibacillus pumilus and Application Thereof in Preventing and Treating White Rot of Grape. Patent CN202210960434.0A, 29 October 2024. [Google Scholar]
- AL-Mohaithef, M. Awareness of Foodborne Pathogens among Students: A Cross-Sectional Study in the Kingdom of Saudi Arabia. Int. J. Food Sci. 2021, 2021, 9971748. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef]
- Bintsis, T. Department of International Trade, TEI of West Macedonia, Kastoria, Greece Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Vieira, K.C.D.O.; Silva, H.R.A.D.; Rocha, I.P.M.; Barboza, E.; Eller, L.K.W. Foodborne Pathogens in the Omics Era. Crit. Rev. Food Sci. Nutr. 2022, 62, 6726–6741. [Google Scholar] [CrossRef]
- Disson, O.; Moura, A.; Lecuit, M. Making Sense of the Biodiversity and Virulence of Listeria monocytogenes. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef]
- Söderqvist, K.; Lambertz, S.T.; Vågsholm, I.; Fernström, L.-L.; Alsanius, B.; Mogren, L.; Boqvist, S. Fate of Listeria monocytogenes, Pathogenic Yersinia enterocolitica, and Escherichia coli O157:H7 Gfp+ in Ready-to-Eat Salad during Cold Storage: What Is the Risk to Consumers? J. Food Prot. 2017, 80, 204–212. [Google Scholar] [CrossRef]
- Han, J.; Aljahdali, N.; Zhao, S.; Tang, H.; Harbottle, H.; Hoffmann, M.; Frye, J.G.; Foley, S.L. Infection Biology of Salmonella enterica. EcoSal Plus 2024, 12, eesp-0001-2023. [Google Scholar] [CrossRef] [PubMed]
- Hariri, S. Detection of Escherichia coli in Food Samples Using Culture and Polymerase Chain Reaction Methods. Cureus 2022, 14, e32808. [Google Scholar] [CrossRef] [PubMed]
- WHO. E. Coli; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Paramithiotis, S. Molecular Targets for Foodborne Pathogenic Bacteria Detection. Pathogens 2023, 12, 104. [Google Scholar] [CrossRef]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.M.C.; Bhowmik, S.; Ali, A. Conventional and Advanced Detection Techniques of Foodborne Pathogens: A Comprehensive Review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent Progress and Growth in Biosensors Technology: A Critical Review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G.M. Microbial Detection and Identification Methods: Bench Top Assays to Omics Approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106–3129. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.V.; Jeevanantham, S.; Kamalesh, R.; Sneha, S.; Yaashikaa, P.R. Methods of Detection of Food-Borne Pathogens: A Review. Environ. Chem. Lett. 2021, 19, 189–207. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, C.-W.; Wang, J.; Oh, D.H. Advances in Rapid Detection Methods for Foodborne Pathogens. J. Microbiol. Biotechnol. 2014, 24, 297–312. [Google Scholar] [CrossRef]
- Panwar, S.; Duggirala, K.S.; Yadav, P.; Debnath, N.; Yadav, A.K.; Kumar, A. Advanced Diagnostic Methods for Identification of Bacterial Foodborne Pathogens: Contemporary and Upcoming Challenges. Crit. Rev. Biotechnol. 2023, 43, 982–1000. [Google Scholar] [CrossRef]
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor Nanoengineering: Design, Operation, and Implementation for Biomolecular Analysis. Sens. Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Dong, X.; Huang, A.; He, L.; Cai, C.; You, T. Recent Advances in Foodborne Pathogen Detection Using Photoelectrochemical Biosensors: From Photoactive Material to Sensing Strategy. Front. Sustain. Food Syst. 2024, 8, 1432555. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, K.; Huang, M.; Zeng, M.; Deng, Y.; Li, S.; Chen, H.; Li, W.; Chen, Z. Research Progress on Detection Techniques for Point-of-Care Testing of Foodborne Pathogens. Front. Bioeng. Biotechnol. 2022, 10, 958134. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Tzialla, K.; Voulgari, A.; Dikeoulia, M.; Raptis, I.; Kakabakos, S.E.; Petrou, P. Rapid Detection of Salmonella typhimurium in Drinking Water by a White Light Reflectance Spectroscopy Immunosensor. Sensors 2021, 21, 2683. [Google Scholar] [CrossRef]
- Quintela, I.A.; De Los Reyes, B.G.; Lin, C.-S.; Wu, V.C.H. Simultaneous Colorimetric Detection of a Variety of Salmonella spp. in Food and Environmental Samples by Optical Biosensing Using Oligonucleotide-Gold Nanoparticles. Front. Microbiol. 2019, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Singh, L.; Kaushik, V.; Rajput, S.; Jain, S.; Pal, M.K.; Kumar, M. Electrically Controlled Nanophotonic Slot Structure Based on Photocatalytic Nanocomposite for Optical Detection of Foodborne Pathogens. J. Light. Technol. 2021, 39, 6670–6677. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, Y.; Huang, Q.; Huang, J.; Tao, Y.; Chen, J.; Li, H.-Y.; Liu, H. Electrochemical Protein Biosensors for Disease Marker Detection: Progress and Opportunities. Microsyst. Nanoeng. 2024, 10, 65. [Google Scholar] [CrossRef]
- Feng, K.; Li, T.; Ye, C.; Gao, X.; Yue, X.; Ding, S.; Dong, Q.; Yang, M.; Huang, G.; Zhang, J. A Novel Electrochemical Immunosensor Based on Fe3O4@graphene Nanocomposite Modified Glassy Carbon Electrode for Rapid Detection of Salmonella in Milk. J. Dairy Sci. 2022, 105, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Kabir, A.; Khoubafarin Doust, S.; Kreais, Z.J.; Ray, A. Detection of Bacterial and Viral Pathogens Using Photonic Point-of-Care Devices. Diagnostics 2020, 10, 841. [Google Scholar] [CrossRef]
- Quintela, I.A.; Vasse, T.; Lin, C.-S.; Wu, V.C.H. Advances, Applications, and Limitations of Portable and Rapid Detection Technologies for Routinely Encountered Foodborne Pathogens. Front. Microbiol. 2022, 13, 1054782. [Google Scholar] [CrossRef]
- Prakitchaiwattana, C.; Det-udom, R. Contaminant Sensors: Nanosensors, an Efficient Alarm for Food Pathogen Detection. In Nanobiosensors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 511–572. ISBN 978-0-12-804301-1. [Google Scholar]
- Yeni, F.; Acar, S.; Polat, Ö.G.; Soyer, Y.; Alpas, H. Rapid and Standardized Methods for Detection of Foodborne Pathogens and Mycotoxins on Fresh Produce. Food Control 2014, 40, 359–367. [Google Scholar] [CrossRef]
- Wieneke, A.A.; Gilbert, R.J. The Use of a Sandwich ELISA for the Detection of Staphylococcal Enterotoxin A in Foods from Outbreaks of Food Poisoning. Epidemiol. Infect. 1985, 95, 131–138. [Google Scholar] [CrossRef]
- Priyanka, B.; Patil, R.K.; Dwarakanath, S. A Review on Detection Methods Used for Foodborne Pathogens. Indian J. Med. Res. 2016, 144, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; He, J.; Cao, X.; Huang, K.; Luo, Y.; Xu, W. Development of a Double-Antibody Sandwich ELISA for Rapid Detection of Bacillus Cereus in Food. Sci. Rep. 2016, 6, 16092. [Google Scholar] [CrossRef] [PubMed]
- Ndraha, N.; Lin, H.-Y.; Wang, C.-Y.; Hsiao, H.-I.; Lin, H.-J. Rapid Detection Methods for Foodborne Pathogens Based on Nucleic Acid Amplification: Recent Advances, Remaining Challenges, and Possible Opportunities. Food Chem. Mol. Sci. 2023, 7, 100183. [Google Scholar] [CrossRef]
- Cvrtila, Ž.; Kozačinski, L.; Mikuš, T. Contemporary Methods and Novel Approaches in the Identification and Quantification of Foodborne Pathogens: A Review. Vet. Stanica 2024, 56, 389–404. [Google Scholar] [CrossRef]
- El-Sayed, A.S.; Ibrahim, H.; Farag, M.A. Detection of Potential Microbial Contaminants and Their Toxins in Fermented Dairy Products: A Comprehensive Review. Food Anal. Methods 2022, 15, 1880–1898. [Google Scholar] [CrossRef]
- Xue, Y.; He, S.; Li, M.; Qiu, Y. Development and Application of Four Foodborne Pathogens by TaqMan Multiplex Real-Time PCR. Foodborne Pathog. Dis. 2025, 22, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Treglia, I.; Ciccaglioni, G.; Ortoffi, M.F.; Gattuso, A. Application of a Loop-Mediated Isothermal Amplification (LAMP) Assay for the Detection of Listeria Monocytogenes in Cooked Ham. Foods 2023, 12, 193. [Google Scholar] [CrossRef]
- Aladhadh, M. A Review of Modern Methods for the Detection of Foodborne Pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Ab Mutalib, N.-S.; Chan, K.-G.; Lee, L.-H. Rapid Methods for the Detection of Foodborne Bacterial Pathogens: Principles, Applications, Advantages and Limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef]
- Bilgin, E.; Omeroglu, M.A.; Baltaci, M.O.; Adiguzel, G.; Adiguzel, A. Rapid Detection of Listeria monocytogenes in Chicken Meat By Real-Time PCR without Culture Enrichment. J. Pure Appl. Microbiol. 2024, 18, 2645–2650. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Z.; Li, W.; Xiao, F.; Huang, J.; Xu, Q.; Xu, H. Rapid and Accurate Detection for Listeria monocytogenes in Milk Using Ampicillin-Mediated Magnetic Separation Coupled with Quantitative Real-Time PCR. Microchem. J. 2022, 183, 108063. [Google Scholar] [CrossRef]
- Lungu, B.; Waltman, W.D.; Hofacre, C.L. Comparison of a Real-Time PCR Method with a Culture Method for the Detection of Salmonella enterica Serotype Enteritidis in Naturally Contaminated Environmental Samples from Integrated Poultry Houses. J. Food Prot. 2012, 75, 743–747. [Google Scholar] [CrossRef]
- Foddai, A.C.G.; Grant, I.R. Methods for Detection of Viable Foodborne Pathogens: Current State-of-Art and Future Prospects. Appl. Microbiol. Biotechnol. 2020, 104, 4281–4288. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Zhang, T.; Xu, M.; Wang, H.; Zhang, S.; Zhang, T.; Zhou, W.; Shi, G. Establishing a MALDI-TOF-TOF-MS Method for Rapid Identification of Three Common Gram-Positive Bacteria (Bacillus cereus, Listeria monocytogenes, and Micrococcus luteus) Associated with Foodborne Diseases. Food Sci. Technol. 2022, 42, e117021. [Google Scholar] [CrossRef]
- Xu, X.; Liu, G.; Huang, X.; Li, L.; Lin, H.; Xu, D. MALDI-TOF MS-Based Identification of Bacteria and a Survey of Fresh Vegetables with Pathogenic Bacteria in Beijing, China. Food Biosci. 2021, 41, 100746. [Google Scholar] [CrossRef]
- Fang, S.; Liu, S.; Song, J.; Huang, Q.; Xiang, Z. Recognition of Pathogens in Food Matrixes Based on the Untargeted in Vivo Microbial Metabolite Profiling via a Novel SPME/GC × GC-QTOFMS Approach. Food Res. Int. 2021, 142, 110213. [Google Scholar] [CrossRef]
- Lasch, P.; Beyer, W.; Bosch, A.; Borriss, R.; Drevinek, M.; Dupke, S.; Ehling-Schulz, M.; Gao, X.; Grunow, R.; Jacob, D.; et al. A MALDI-ToF Mass Spectrometry Database for Identification and Classification of Highly Pathogenic Bacteria. Sci. Data 2025, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Monedeiro, F.; Monedeiro-Milanowski, M.; Pomastowski, P.; Buszewski, B. Influence of Culture Medium on Bacterial Molecular Profiles in Different Ionization Modes with the Use of Computational Methods. Int. J. Mass Spectrom. 2021, 466, 116614. [Google Scholar] [CrossRef]
- Liberty, J.T.; Bromage, S.; Peter, E.; Ihedioha, O.C.; Alsalman, F.B.; Odogwu, T.S. CRISPR Revolution: Unleashing Precision Pathogen Detection to Safeguard Public Health and Food Safety. Methods 2025, 240, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Hajikhani, M.; Zhang, Y.; Gao, X.; Lin, M. Advances in CRISPR-Based SERS Detection of Food Contaminants: A Review. Trends Food Sci. Technol. 2023, 138, 615–627. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, J.; Yin, L.; Man, S.; Ma, L. Integration of Logic Gates to CRISPR/Cas12a System for Rapid and Sensitive Detection of Pathogenic Bacterial Genes. Anal. Chim. Acta 2020, 1125, 162–168. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and Portable Nucleic Acid Detection Platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef]
- Wei, N.; Xiong, J.; Ma, J.; Ye, J.; Si, Y.; Cao, S. Development of Efficient, Sensitive, and Specific Detection Method for Encephalomyocarditis Virus Based on CRISPR/Cas13a. J. Virol. Methods 2022, 309, 114592. [Google Scholar] [CrossRef] [PubMed]
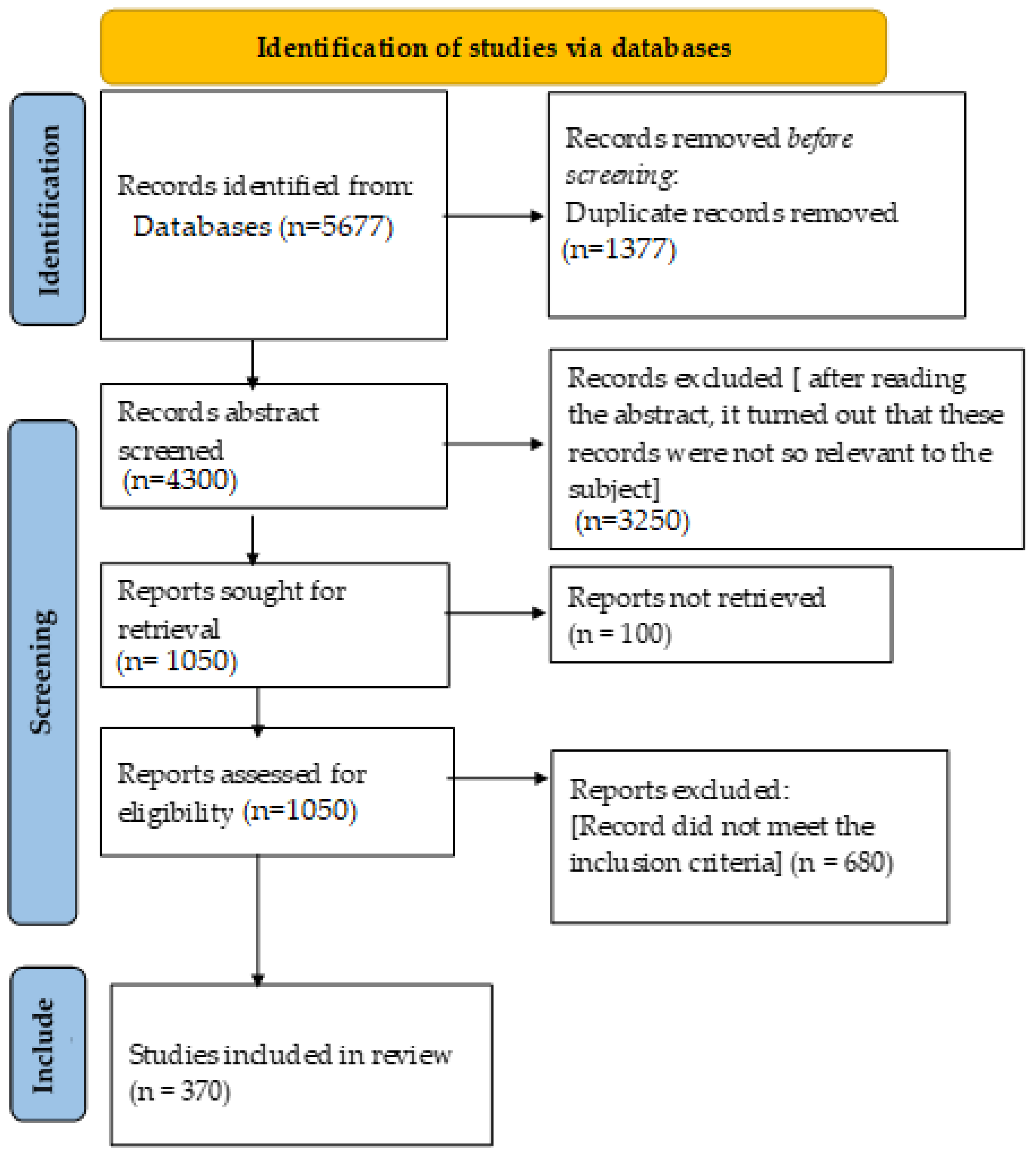
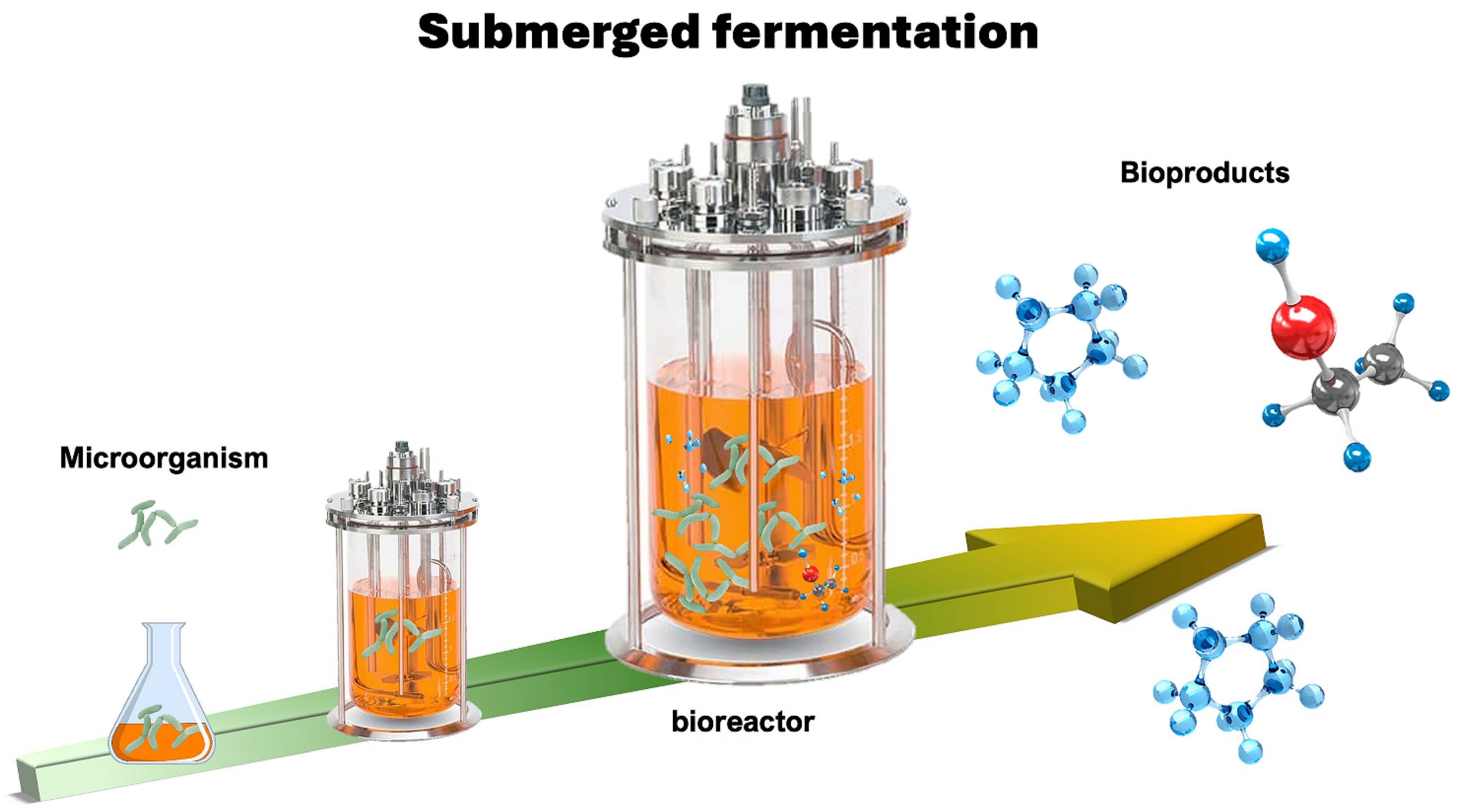
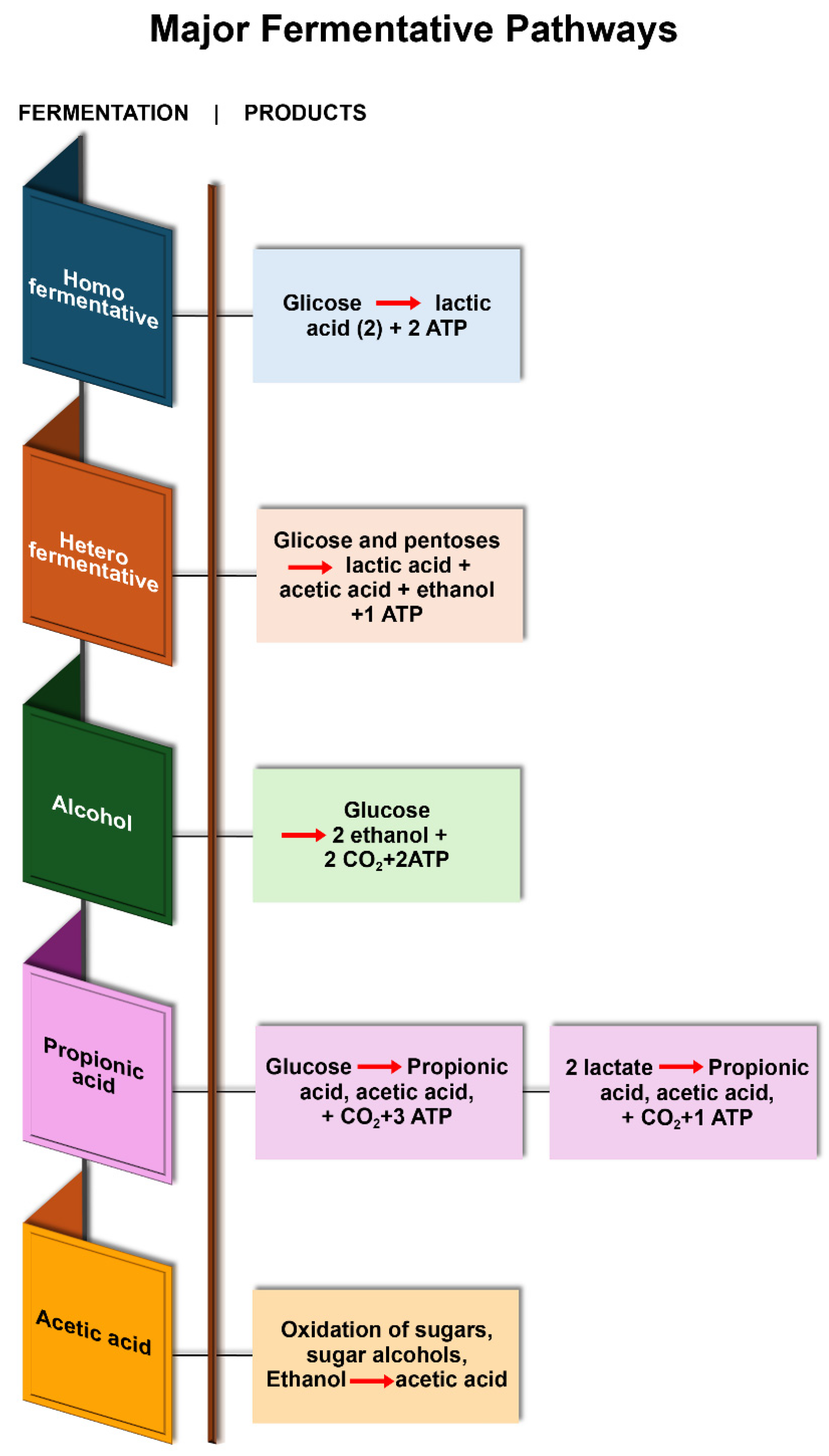
| Parameter | SmF | SSF |
|---|---|---|
| Substrate type | Soluble substrates in liquid media | Solid substrates with minimal or no free water, often using agro-industrial residues |
| Microbial growth preferences | Ideal for bacteria and yeast that thrive in liquid environments | Suited for filamentous fungi and certain bacteria adapted to low-moisture conditions |
| Water activity (aw) | High water activity | Low water activity |
| Aeration and oxygen transfer | Oxygen transfer due to agitation and aeration systems | Relies on diffusion |
| Energy consumption | Higher energy requirements for agitation, aeration, and temperature control | Lower energy demands often passive processes. |
| Contamination risk | Elevated risk due to high moisture and nutrient availability | Reduced risk owing to low moisture |
| Product yield and concentration | Often lower due to dilution in the aqueous phase | Higher concentration of products; easier downstream processing |
| Fermentation time | Typically shorter due to rapid growth in liquid | Longer due to slower metabolism in solid substrates |
| Process monitoring and control | Real-time control of pH, temperature, and others | Difficult to monitor and control accurately |
| Scale-up and industrial application | Well-established with standardized equipment | Challenging due to complexity and heterogeneity |
| Environmental impact | High wastewater and energy use | Minimal wastewater and resource use; sustainable |
| Economic considerations | Higher operational costs | Cost-effective with low-input substrates |
| Technological maturity | Mature and widely adopted | Emerging; increasing industrial interest |
| Additive | Function | Common Foods | Potential Health Impact | References |
|---|---|---|---|---|
| Monosodium glutamate (MSG) | Flavor enhancer | Instant noodles, processed meats | May cause headaches, nausea (in sensitive individuals) | [89] |
| Sodium nitrite | Preservative, color fixative | Cured meats (bacon, ham) | May form carcinogenic nitrosamines | [90] |
| BHA (Butylated hydroxyanisole) | Antioxidant, preservative | Chips, cereals, butter | Possible endocrine disruptor linked to cancer in high doses | [91] |
| Potassium bromate | Dough conditioner | Bread, baked goods | Possible carcinogen, banned in some countries | [92] |
| Tartrazine (E102) | Coloring agent | Soft drinks, candies | Hyperactivity, allergic reactions | [93] |
| Sodium benzoate | Preservative | Sodas, sauces | Trigger allergies, potential carcinogens | [94] |
| Carrageenan | Thickener | Dairy products | Linked to gastrointestinal inflammation | [95] |
| Aspartame | Sweetener | Diet sodas, gums | Headaches, metabolic disturbances | [96] |
| Titanium dioxide (E171) | Coloring agent | Candies, chewing gum | Potential genotoxic effects, banned in some regions | [97] |
| Polysorbate 80 | Emulsifier | Ice cream, salad dressings | Disrupt gut bacteria linked to inflammation | [98] |
| Sorbitol | Sweetener, humectant | Sugar-free gum, candies | Causes bloating, laxative effects | [99] |
| Sucralose | Artificial sweetener | Diet sodas, sugar-free products | Alter gut microbiota, potential metabolic effects, and cardiovascular disease | [100,101] |
| Agency | Region | Key Lists/Frameworks | Safety Requirements | References |
|---|---|---|---|---|
| Food and Drug Administration (FDA) | United States | GRAS—Generally Recognized as Safe | Toxicological safety, history of safe use, scientific consensus on safety for intended use | [106,107,108] |
| European Food Safety Authority (EFSA) | European Union | QPS—Qualified Presumption of Safety | Non-pathogenicity, absence of toxigenicity, genetic stability, absence of antimicrobial resistance genes, allergenicity assessment | [32,109] |
| Agência Nacional de Vigilância Sanitária (ANVISA) | Brazil | RDC Resolutions (e.g., RDC 728/2022)—Official list of approved microbial strains | Non-pathogenic, non-toxigenic, genetically stable strains, and industrial performance consistency | [110,111,112] |
| China Food and Drug Administration (CFDA, currently reorganized under the State Administration for Market Regulation (SAMR) and the National Health Commission (NHC). | China | National Food Safety Standards (GB standards, NFSSs) | Toxicological evaluation, allergenicity testing, and safety under intended conditions of use | [113,114,115,116] |
| Enzyme | Microbial Sources | Main Function | Industrial Applications | References |
|---|---|---|---|---|
| Peptidases | A. oryzae, A. niger, Rhizopus spp., B. subtilis, B. licheniformis | Hydrolysis of peptide bonds; milk coagulation | Cheese production (chymosin/rennet), whey hydrolysis, dough extensibility in baking | [105,125,126,127,128,129,130] |
| Cellulases and Pectinases | T. reesei, A. niger | Degradation of cellulose and pectin; macerating action | Juice extraction and clarification, viscosity reduction, pulp liquefaction | [131,132,133,134,135,136,137,138,139,140] |
| Xylanases | A. niger, A. oryzae, T. reesei, B. subtilis | Hydrolysis of xylan; improvement of rheological properties | Increased bread loaf volume and texture, clarification of juices and beer | [140,141,142,143,144,145,146] |
| α-Amylase and Glucoamylase | A. niger, R. oryzae B. subtilis, B. amyloliquefaciens | Conversion of starch into fermentable sugars | Bread making, syrup production, alcoholic beverages | [147,148,149,150,151,152] |
| Lactase (β-galactosidase) | K. lactis, A. oryzae, B. circulans | Hydrolysis of lactose into glucose and galactose | Low-lactose dairy products, prevention of crystallization in ice creams, production of prebiotic GOS | [153,154,155,156,157,158] |
| Lipases and Phospholipases | T. lanuginosus, R. miehei, C. rugosa, A. oryzae | Hydrolysis and modification of lipids | Cheese flavor development, bakery emulsification, oil refining, structured lipids | [159,160,161,162,163,164,165] |
| Laccase | Trametes versicolor, Pleurotus ostreatus, A. oryzae | Oxidizes phenolic compounds; contributes to beverage stabilization | Juice, wine, and beer clarification improve dough structure in baking | [159,166,167] |
| Naringinase | A. niger, Penicillium decumbens, R. nigricans | Hydrolyzes naringin (a bitter flavonoid) | Reduction of bitterness in citrus juices (e.g., grapefruit) | [164] |
| Tannase | A. niger, A. oryzae | Removes polyphenolic compounds; reduces astringency | Brewing industry and instant tea production | [165,168] |
| Catalase | Micrococcus luteus, A. niger, B. subtilis | Decomposes hydrogen peroxide into water and oxygen | Preserves dairy and packaged foods; extends shelf life and improves microbiological safety | [169] |
| Phytase | A. niger, E. coli, B. amyloliquefaciens | Hydrolyzes phytic acid; releases bioavailable minerals | Animal feed supplementation; enhances mineral absorption, reduces environmental phosphorus output | [170] |
| Transglutaminase | Streptoverticillium mobaraense, Streptomyces mobaraense, B. subtilis, B. sphaericus | Catalyzes covalent cross-links between glutamine and lysine residues in proteins | Improves the texture and elasticity of processed meats, dairy products, and baked goods | [171,172] |
| Glucose oxidase | A. niger, A. oryzae, P. amagasakiense | Oxidizes glucose to gluconic acid and hydrogen peroxide | Improves dough structure and bread volume; reduces glucose in functional beverages | [173,174] |
| Pullulanase | B. acidopullulyticus, B. subtilis, B. deramificans, Klebsiella spp. | Hydrolyzes α-1,6-glycosidic bonds in pullulan and branched starches | Used in starch processing to produce glucose and maltose; enhances saccharification in brewing and syrup production | [175,176] |
| Organic Acid | Main Microbial Producers | Functions in Food Products | Industrial Application | References |
|---|---|---|---|---|
| Acetic acid | Acetobacter spp., Gluconacetobacter spp., Gluconobacter spp. | Flavoring, preservative, functional bioactivity in fermented foods | Vinegar, kombucha, cocoa, sour beer | [177,178,179] |
| Citric acid | Aspergillus niger, Trichoderma reesei, Yarrowia lipolytica | Flavoring, acidity regulator, preservative; component in bioactive food packaging | Soft drinks, effervescent salts, medicinal citrates, biofilm-based packaging | [180,181,182,183,184,185,186] |
| Lactic acid | Lactic acid bacteria (Lactobacillus spp., Lactococcus spp.), Rhizopus oryzae, Mucor spp. | Flavoring, acidity regulator, preservative; antimicrobial activity enhancer | Bakery, jams, candies, beverages, bioplastics/packaging | [187,188,189,190] |
| Propionic acid | Acidipropionibacterium spp., Propionibacterium spp. | Flavoring, preservative in bakery and dairy; nutritional co-products (vitamins) | Bread, cheese, jams, fermented foods | [191,192,193,194] |
| Succinic acid | Actinobacillus succinogenes, Mannheimia succiniciproducens, Basfia succiniciproducens | Flavoring, preservative, bread softener; precursor for biochemicals | Bread, beverages, biochemical platform for plastics and solvents (engineered yeasts) | [195,196,197,198,199,200,201] |
| Additive Type | Compound/Example | Functions in Food Products | Industrial Applications | Main Microbial Producers | References |
|---|---|---|---|---|---|
| Texturizers and Stabilizers | Xanthan gum | Thickener, stabilizer, emulsifier | Low-fat and gluten-free formulations, sauces, dressings | Xanthomonas campestris | [208,209] |
| Pullulan | Thickener, film-former | Edible films, coatings, candies | Aureobasidium pullulans | [210] | |
| Dextran | Stabilizer, prevents crystallization | Confectionery, ice cream, bakery | Leuconostoc spp., Lactobacillus spp. | [208] | |
| Gellan gum | Gelling and stabilizing agent | Beverages, jams, dairy, fruit coatings | Pseudomonas elodea | [211] | |
| Bacterial cellulose | Texture, water retention, fat replacement | Ice cream, tofu gels, meat products | Gluconacetobacter spp. | [208] | |
| Colorants | Carotenoids (β-carotene, astaxanthin) | Natural pigments, antioxidant | Dairy, beverages, nutraceuticals | Blakeslea trispora, Dunaliella salina, Haematococcus pluvialis | [202,212] |
| Monascus pigments | Natural red pigments | Fermented foods, sauces, beverages | Monascus spp. | [213] | |
| Phycocyanin, chlorophylls | Natural pigments, antioxidant | Confectionery, beverages, jellies | Arthrospira sp. (Spirulina), microalgae | [207] | |
| Sweeteners | Xylitol | Natural sweetener, low-calorie | Sugar-free confectionery, chewing gum | Cyberlindnera dasilvae sp. nov., engineered S. cerevisiae | [214] |
| Sorbitol, mannitol | Sweeteners, humectants | Bakery, sugar-free foods, pharmaceuticals | Zymomonas mobilis, Lactobacillus plantarum | [215,216] | |
| 2′-Fucosyllactose (2′-FL) | Functional sweetener, prebiotic | Infant formula, functional foods | Engineered E. coli, B. subtilis | [203] | |
| Flavorings and Aroma | Esters, aldehydes, ketones (e.g., ethyl acetate, benzaldehyde|) | Flavor enhancers | Fermented foods, beverages | Lactobacillus spp., Acetobacter spp., Penicillium spp. | [204] |
| Vanillin (biovanillin) | Aroma compound | Beverages, dairy, bakery, chocolate | Aspergillus niger, Penicillium cinnabarinum | [206] | |
| Fruity aromas (e.g., ethyl lactate) | Flavor enhancers | Fruit juices, fermented products | Ceratocystis fimbriata, yeasts, LAB | [205,206] | |
| Functional and Nutritional Bioproducts | β-glucans (paramylon, selacan) | Gelling, thickening, prebiotic | Functional foods, supplements | Euglena spp., Agrobacterium spp. | [217,218] |
| Polyunsaturated Fatty Acids (PUFA) and vitamins | Nutritional enrichment | Beverages, pasta, baked goods | Arthrospira spp., Chlorella spp., Nannochloropsis spp. | [207] |
| Category | Key Microbial Products | Applications in Food Systems | Emerging Research Trends |
|---|---|---|---|
| Fermentation & Biotransformation | Lactic acid, ethanol, organic acids, alternative proteins | Protein-rich ingredients, improved digestibility, waste valorization | Precision fermentation, strain engineering, CRISPR-enabled production |
| Microbial Food Additives | Bacteriocins, microbial enzymes, bio-pigments, Exopolysaccharide—(pullulan, xanthan) | Clean-label food enhancement, texture and color agents, health-promoting properties | Use of GRAS strains for multifunctional roles, metabolite optimization |
| Biopreservation & Safety | Antimicrobial peptides, reuterin, propionic acid, biosurfactants | Shelf-life extension, pathogen inhibition, reduced need for chemical preservatives | Natural antimicrobial cocktails, synergistic preservation systems |
| Sustainable Packaging | Edible films and coatings using microbial polymers (e.g., pullulan, gellan gum) | Biodegradable alternatives to plastic, smart coatings for perishables | Integration with nanotechnology, biodegradable multi-layered systems |
| Contamination Control & Detection | Biosensors, microbial indicators, diagnostic enzyme systems | Rapid detection of foodborne pathogens, smart packaging, contamination tracing | Point-of-need detection, AI-linked diagnostics, probiotic biosensors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermelho, A.B.; Cardoso, V.d.S.; Domingos, L.T.S.; Akamine, I.T.; Amenu, B.; Osei, B.K.; Neves Junior, A. Advancements in Microbial Applications for Sustainable Food Production. Foods 2025, 14, 3427. https://doi.org/10.3390/foods14193427
Vermelho AB, Cardoso VdS, Domingos LTS, Akamine IT, Amenu B, Osei BK, Neves Junior A. Advancements in Microbial Applications for Sustainable Food Production. Foods. 2025; 14(19):3427. https://doi.org/10.3390/foods14193427
Chicago/Turabian StyleVermelho, Alane Beatriz, Verônica da Silva Cardoso, Levy Tenório Sousa Domingos, Ingrid Teixeira Akamine, Bright Amenu, Bernard Kwaku Osei, and Athayde Neves Junior. 2025. "Advancements in Microbial Applications for Sustainable Food Production" Foods 14, no. 19: 3427. https://doi.org/10.3390/foods14193427
APA StyleVermelho, A. B., Cardoso, V. d. S., Domingos, L. T. S., Akamine, I. T., Amenu, B., Osei, B. K., & Neves Junior, A. (2025). Advancements in Microbial Applications for Sustainable Food Production. Foods, 14(19), 3427. https://doi.org/10.3390/foods14193427







