Assessment of Heavy Metal Contamination, Bioaccumulation, and Nutritional Quality in Fish from the Babina–Cernovca Romanian Sector of the Danube River
Abstract
1. Introduction
2. Materials and Methods
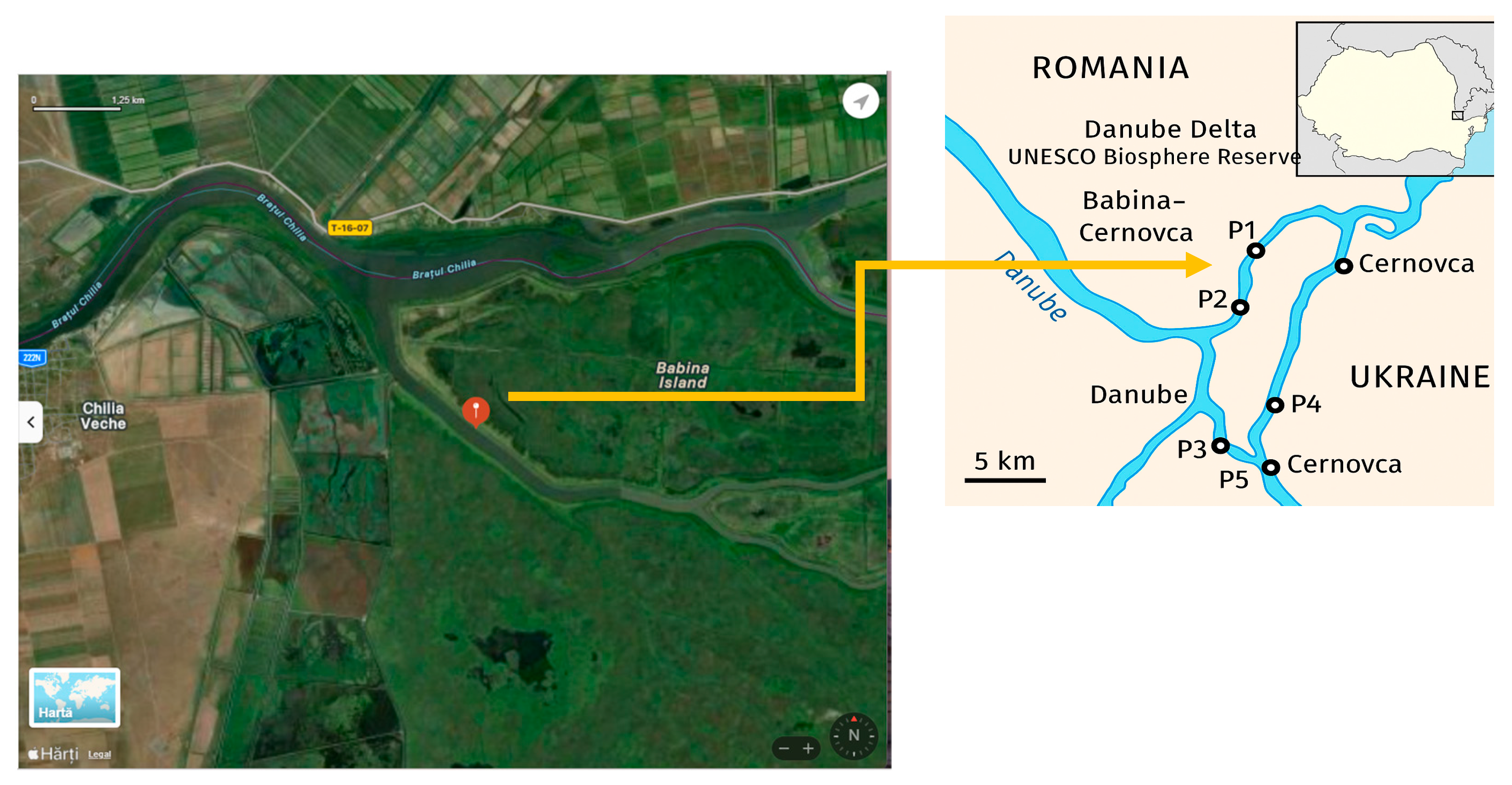
2.1. Location
2.2. Sampling
2.3. Laboratory Analysis
2.4. Bioconcentration Factor (BCF) and Biota-Sediment Accumulation Factor (BSAF)
2.5. Statistics
2.6. Ethical Considerations
3. Results
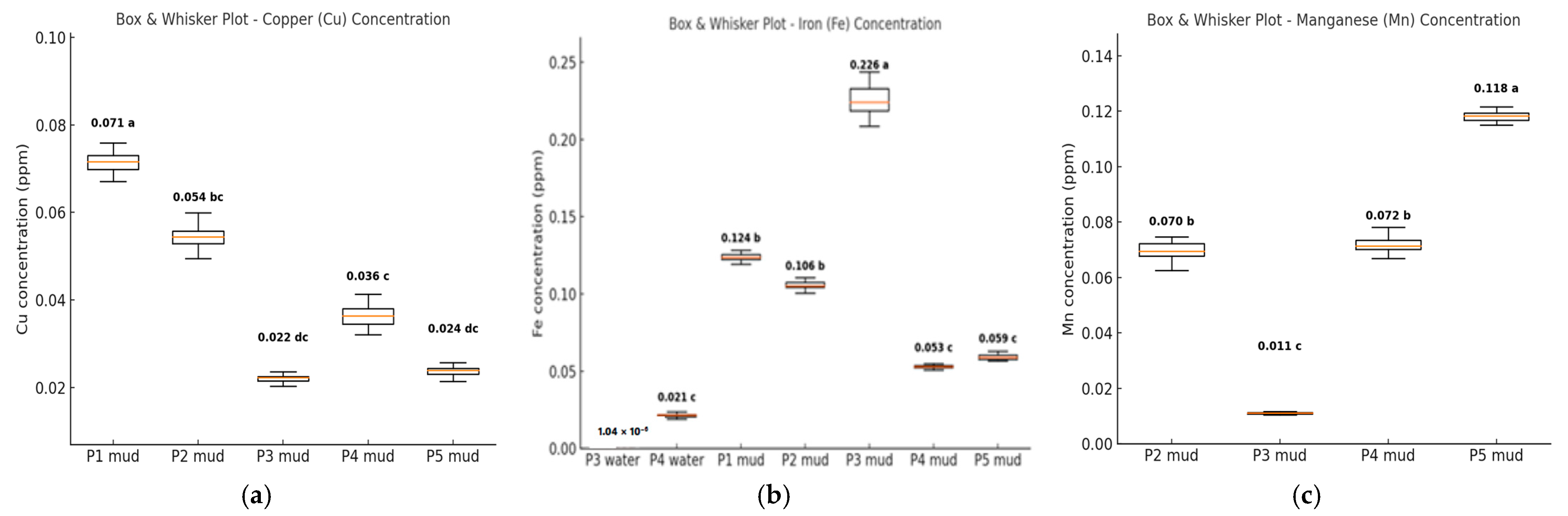
3.1. The Heavy Metals Concetration in Water and Mud in the Samplig Area
3.2. The Proximate Composition and Heavy Metals in the Muscle Tissue of the Fish Species
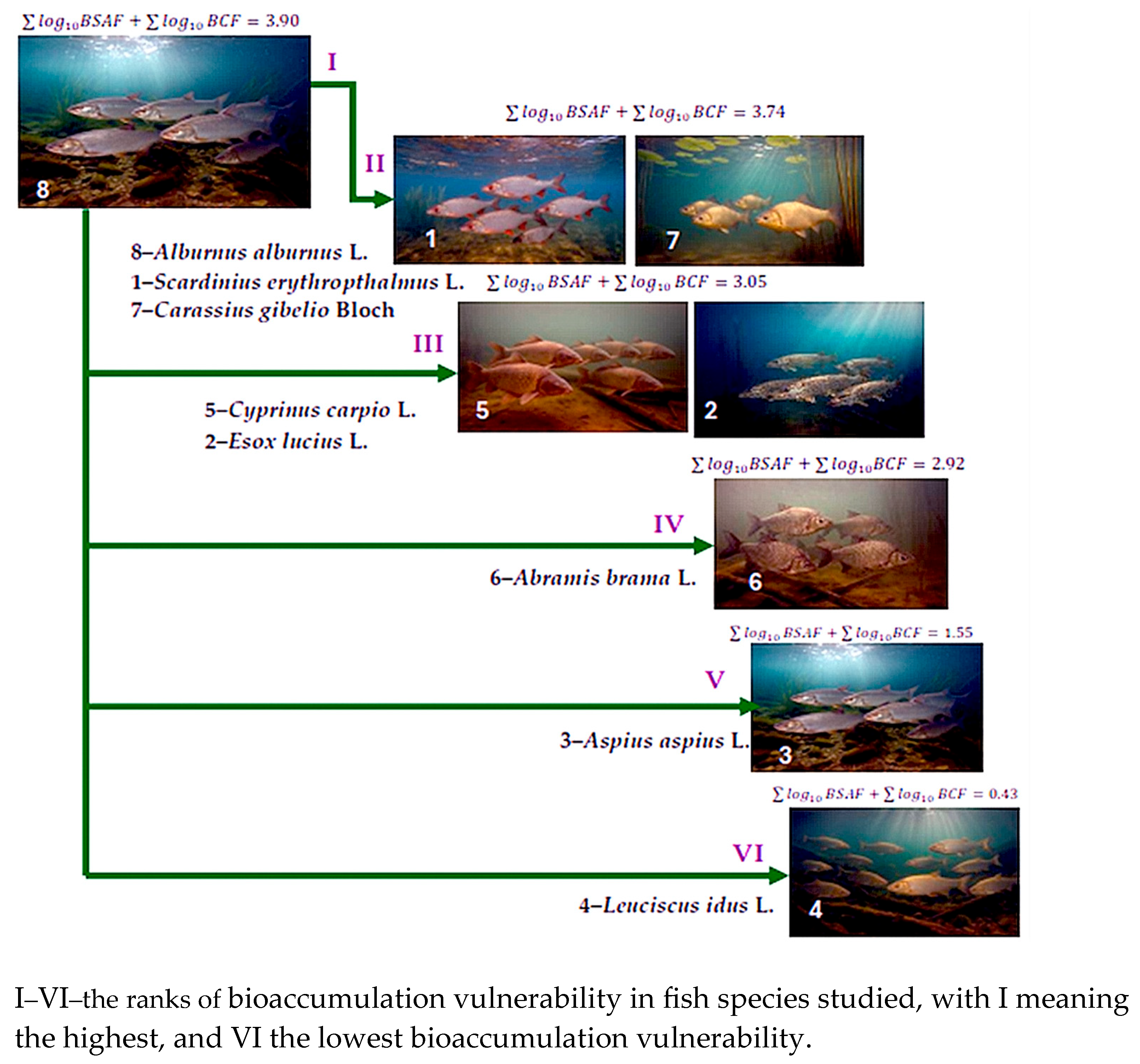
3.3. Bioconcentration Factor (BCF) and Biota-Sediment Accumulation Factor (BSAF) Identified in the Fish Muscle Tissue
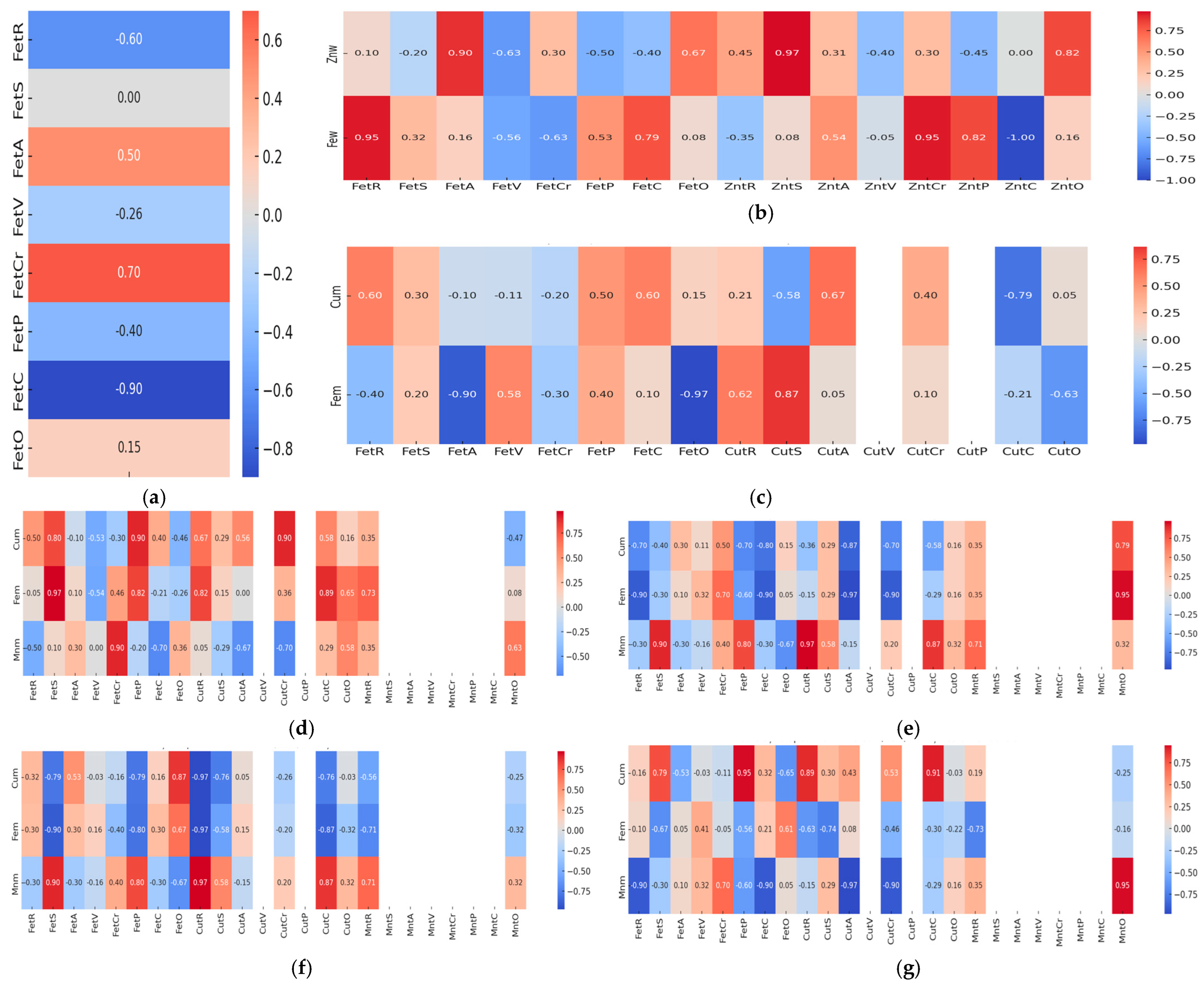
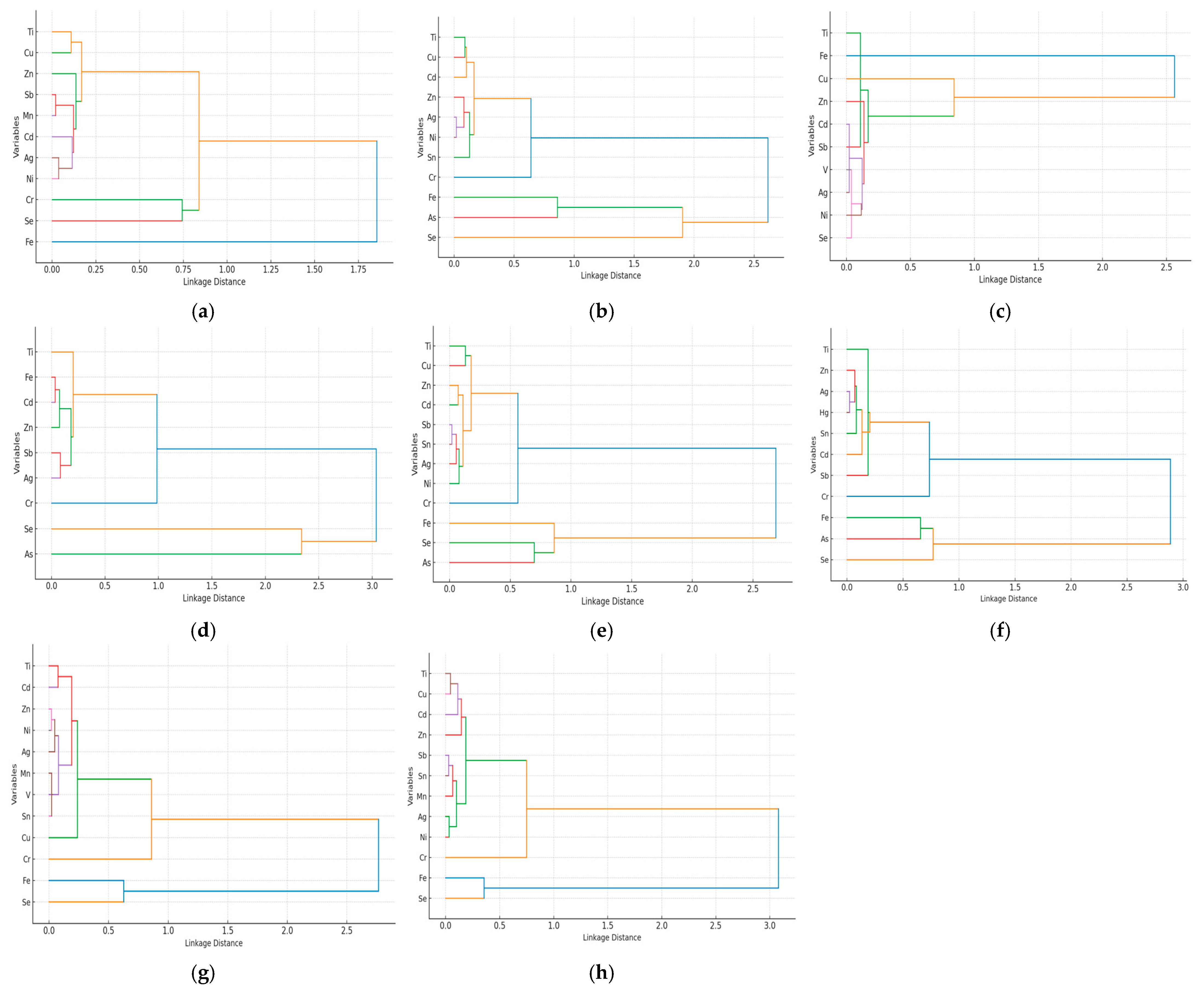
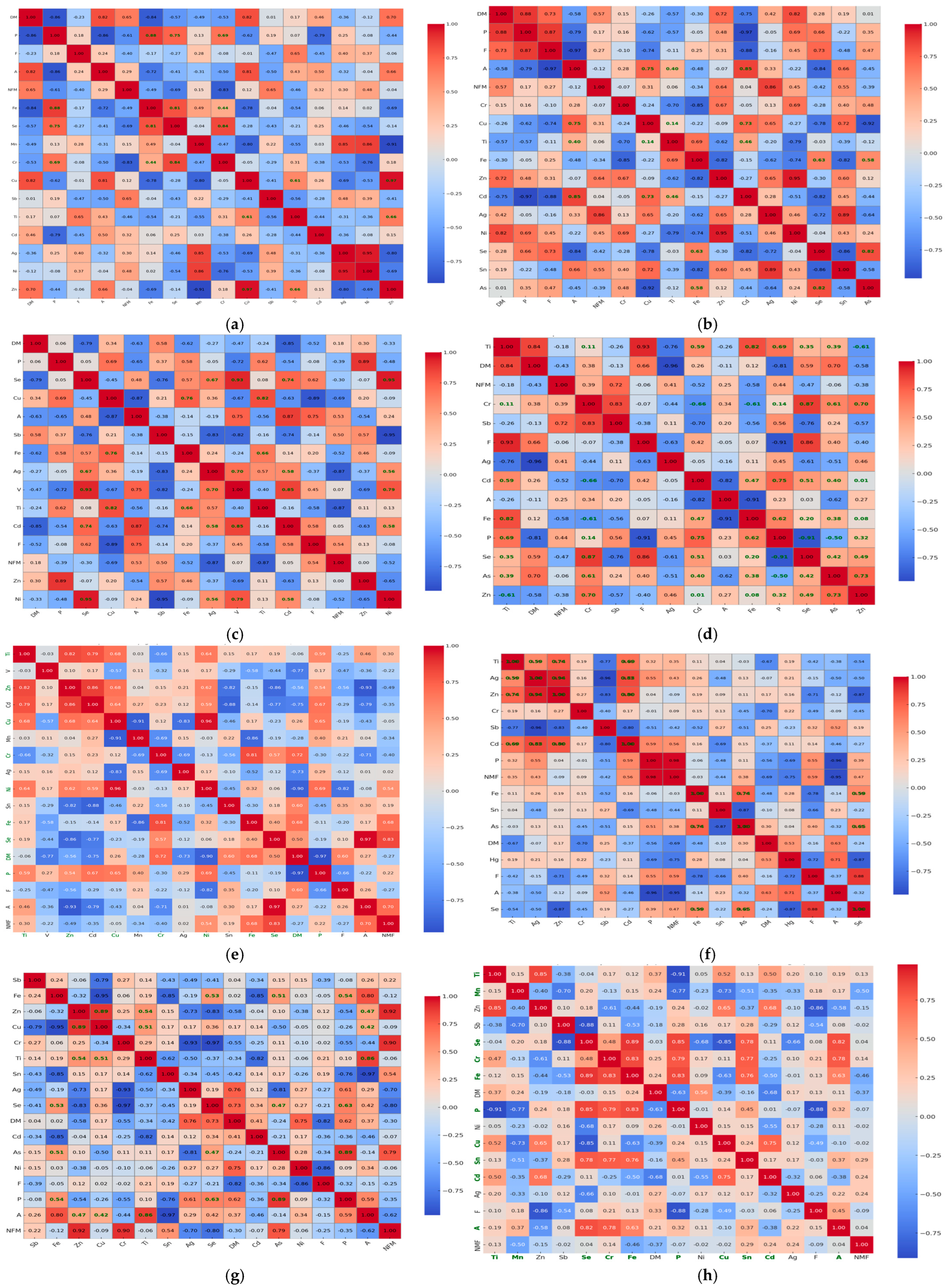
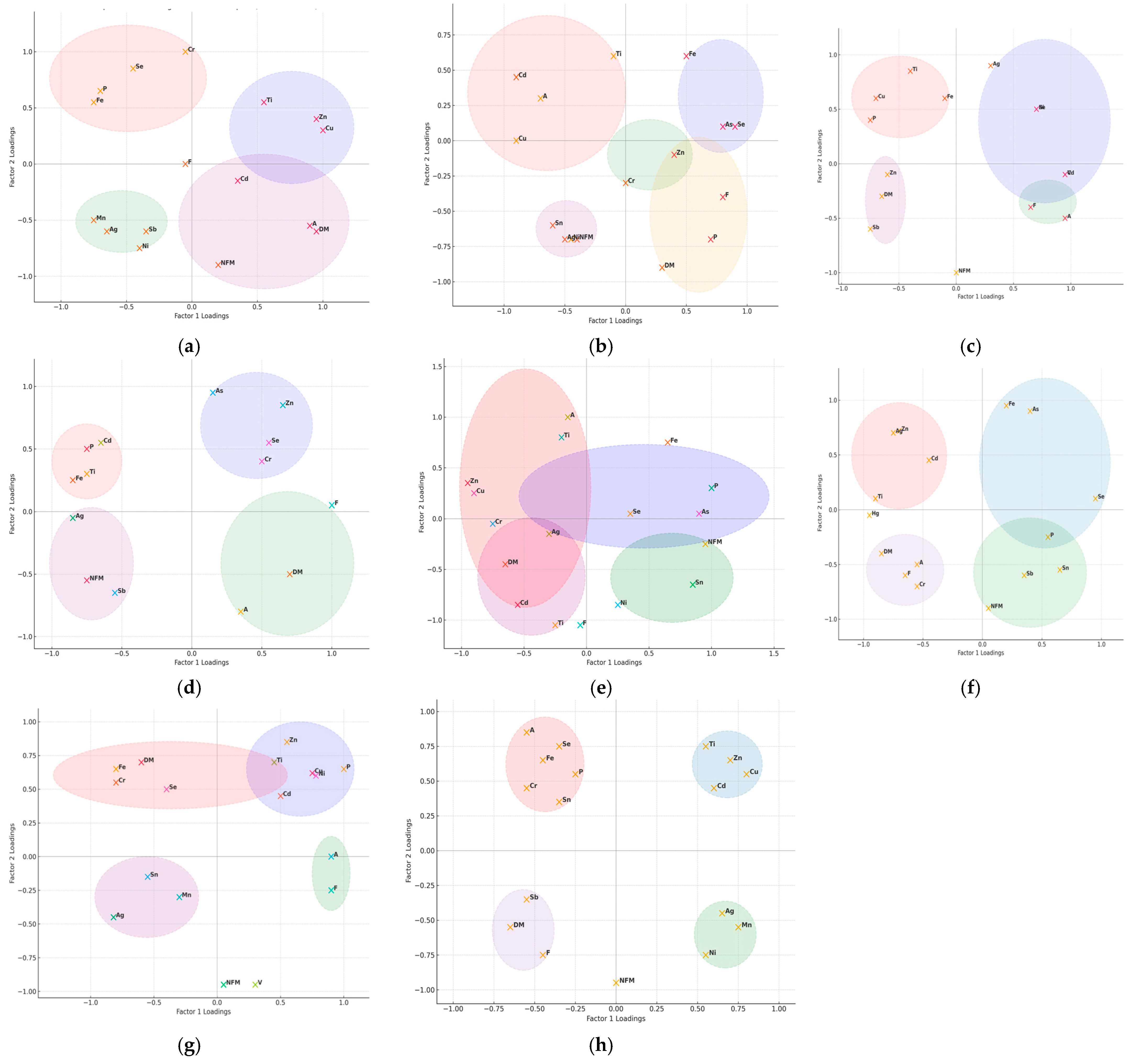
3.4. Correlation and Multivariate Analysis of Heavy Metals Bioaccumulation and Proximate Composition in Fish Muscle Tissue
4. Discussion
4.1. The Heavy Metals Concetration in Water and Mud from the Samplig Area
| Environment | Metal | Current Data (mg/L) | Literature Values (mg/L) | References |
|---|---|---|---|---|
| Water | ||||
| River | Fe | 1.04 × 10−6; 0.021 | 0.330; 0.080; 1244.700; 1.41–0.33 | [42,44,45,49] |
| Delta | - | 1.330; 0.428 | [48] | |
| River | Zn | 0.126 | 0.025–0.118; 0.032; 0.009, 333.78; 0.094–1.46 | [41,42,44,46,49] |
| Delta | - | 0.020; 0.002 | [48] | |
| Lakes | - | 0.020–0.120 | - | |
| Sediment | ||||
| Fe | 0.053–0.226 | 16,104; 15.36; 17,530; 32,720–33,480 | [42,47,49,50] | |
| Mn | 0.011–0.118 | 1147–1359 | - | |
| Cu | 0.024–0.071 | 35.92; 2; 56; 21–26 | [42,49,51,52] |
4.2. The Proximate Composition and Heavy Metals from the Muscle Tissue
| Species | Metal | Current Data (mg/kg) | Literature Values (mg/kg) | References |
|---|---|---|---|---|
| C. gibelio | Fe | 1.71 | 7.25–8.05 | [59] |
| Ni | 0.10 | 1.994–2.246 | [54] | |
| Zn | 0.11 | 10.26; 11.72 | [54,59] | |
| Cd | 0.20 | 0.017; 0.057–0.061; 0.057; 0.13 | [43,54,57,58] | |
| Cr | 0.69 | 1.208; 1.345 | [38,43,60] | |
| Cu | 0.31 | 0.715; 1.500 | [38,43,60] | |
| Hg | - | 0.025; 0.30; 0.094; 0.139 | [38,43,45,59] | |
| As | - | 0.031–0.604; 0.139–0.172 | [38,57] | |
| A. brama | Fe | 1.79 | 1.31; 27.64 | [61,62] |
| Ni | - | 0.02–1.05 | [44] | |
| Zn | 0.10 | 3.15–23.84 | [44,62] | |
| Cd | 0.15 | 0.004; 0.027 | [44,60] | |
| Cr | 0.61 | 0.20; 0.33 | [44,61] | |
| Cu | - | 0.14–1.49 | [44,60] | |
| Hg | 0.07 | 0.08; 0.121; 0.237 | [44,57,63] | |
| As | 1.99 | 0.035; 1.73 | [57,61] | |
| C. carpio | Fe | 1.71 | 7.42–19.62; 1.31; 27.64; 0.008 | [42,60,61,62] |
| Ni | 0.09 | 1.685; 0.02–1.05; 6.16; 59.01 | [44,63,64,67] | |
| Zn | 0.15 | 3.15; 23.84 | [44,62] | |
| Cd | 0.14 | 0.140; 0.005; 0.084 | [42,43,44,63,64,66] | |
| Cr | 0.51 | 0.010; 1.143; 0.20; 0.33 | [44,61,63,65] | |
| Cu | 0.26 | 0.14;1.49; 0.688; 5.100 | [44,60] | |
| Hg | - | 0.207; 0.393–0.466; 0.890 | [42,63,67] | |
| As | 2.07 | 0.01; 0.08; 0.66; 0.035; 1.73 | [42,53,57,61,65,66] | |
| E. lucius | Fe | 1.71 | 9.97–10.10 | [65] |
| Ni | 0.09 | 0.02–0.05 | [44] | |
| Zn | 0.13 | 5.10; 23.90 | [63,64] | |
| Cd | 0.20 | 0.015–0.044 | [57,63] | |
| Cr | 0.55 | 0.781–2.071 | [64] | |
| Cu | 0.28 | 0.548; 2.90 | [64,65] | |
| Hg | - | 0.021–0.025; 0.30; 0.094; 0.139; 0.236 | [43,45,51,54,63] | |
| As | 2.04 | 0.030–0.604; 0.139–0.172; 1.129 | [57,59,63] | |
| A. alburnus | Cd | 0.25 | 0.046; 0.13 | [58,61] |
4.3. Bioconcentration Factor (BCF) and Biota-Sediment Accumulation Factor (BSAF) Identified in the Fish Muscle Tissue
4.4. Correlation and Multivariate Analysis of Heavy Metals Bioaccumulation and Proximate Composition in Fish Muscle Tissue
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| PCA | Principal Components Analysis |
| UNESCO | United Nations Educational, Scientific and Cultural Organization |
| RAMSAR | Convention on Wetlands of International Importance especially as Waterfowl Habitat |
| DM | Dry matter |
| P | Protein |
| F | Fat |
| A | Ash |
| NFM | Nitrogen-free matter |
| BCF | Bioconcentration Factor |
| BSAF | Biota-Sediment Accumulation Factor |
| PC | Principal Component |
| KMO | Keiser–Meyer–Olkin |
| ARBDD | Delta Biosphere Reserve Authority |
| N | Number of replicates |
| X | Average |
| sX | Standard error |
| RS | Spearman coefficient of correlation |
References
- Zahran, E.; Mamdouh, A.-Z.; Elbahnaswy, S.; El-Son, M.M.A.; Risha, E.; ElSayed, A.; El Barbary, M.I.; El Sebaei, M.G. The Impact of Heavy Metal Pollution: Bioaccumulation, Oxidative Stress, and Histopathological Alterations in Fish across Diverse Habitats. Aquac. Int. 2025, 33, 371. [Google Scholar] [CrossRef]
- Peycheva, K.; Panayotova, V.; Stancheva, R.; Makedonski, L.; Merdzhanova, A.; Parrino, V.; Nava, V.; Cicero, N.; Fazio, F. Risk Assessment of Essential and Toxic Elements in Freshwater Fish Species from Lakes near Black Sea, Bulgaria. Toxics 2022, 10, 675. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, G. Toxic Metals. In Metal Pollution in the Aquatic Environment, 2nd ed.; Förstner, U., Wittmann, G.T.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 3–70. [Google Scholar]
- El-Sappah, A.H.; Abdel-Kader, H.H.; Abbas, M.; Zafar, S.; Li, J. Editorial: Impact of Heavy Metal on Aquatic Life and Human Health. Front. Genet. 2025, 16, 1608524. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Förstner, U. Metal Concentrations in River, Lake, and Ocean Waters. In Metal Pollution in the Aquatic Environment, 2nd ed.; Förstner, U., Wittmann, G.T.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 71–109. [Google Scholar]
- Prosi, F. Heavy Metals in Aquatic Organisms. In Metal Pollution in the Aquatic Environment, 2nd ed.; Förstner, U., Wittmann, G.T.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 271–323. [Google Scholar]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.; et al. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Abubakar, I.; Dasuki, A.; Babatunde, T.A.; Ghali-Mohammed, I.; Abdurrasheed, N.; Dauda, A.B. Seasonal Variation of Organochlorine Pesticides Residue in Water and Silver Catfish (Bagrus bajad Fabricius, 1775) from Ajiwa Reservoir, Katsina State. J. Res. For. Wildl. Environ. 2024, 16, 9–16. [Google Scholar]
- Wu, B.; Wang, G.; Wu, J.; Fu, Q.; Liu, C. Sources of heavy metals in surface sediments and an ecological risk assessment from two adjacent Plateau reservoirs. PLoS ONE 2014, 9, e102101. [Google Scholar] [CrossRef]
- Oros, A. Bioaccumulation and Trophic Transfer of Heavy Metals in Marine Fish: Ecological and Ecosystem-Level Impacts. J. Xenobiot. 2025, 15, 59. [Google Scholar] [CrossRef]
- Okwuosa, O.B.; Eyo, J.E.; Omovwohwovie, E.E. Role of Fish as Bioindicators: A Review. IRE J. Iconic Res. Eng. J. 2019, 2, 354–368. [Google Scholar]
- Sharma, A.K.; Sharma, M.; Sharma, S.; Malik, D.S.; Sharma, M.; Sharma, A.K. A Systematic Review on Assessment of Heavy Metals Toxicity in Freshwater Fish Species: Current Scenario and Remedial Approaches. J. Geochem. Explor. 2024, 262, 107472. [Google Scholar] [CrossRef]
- He, M.; Liu, G.; Shi, X.; Wu, L.; Chen, Q. Distribution, Bioaccumulation, Trophic Transfer and Risk Assessment of Trace Elements in Fish from a Typical Shallow Outflow Lake Basin, China. Front. Environ. Sci. Eng. 2024, 18, 89. [Google Scholar] [CrossRef]
- Imsilp, K.; Sirinupong, P.; Yeesin, P.; Thong-asa, W.; Tanhan, P. Potential Risks and Spatial Variation of Heavy Metals in Water and Surface Sediment of Pattani Bay, Thailand. Toxics 2025, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, C.; Jiang, J.; Jiang, T.; Xue, J.; Bah, I.; Gu, M.; Wang, M.; Meng, S. Cadmium Accumulation and Regulation in the Freshwater Mussel Anodonta woodiana. Toxics 2025, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Song, Z.; Song, G.; Li, S.; Sun, H.; Zhang, C.; Li, G. Oxidative Stress and Antioxidant Biomarker Responses in Fish Exposed to Heavy Metals: A Review. Environ. Monit. Assess. 2025, 197, 892. [Google Scholar] [CrossRef]
- Hristov, S.; Kirin, D. Accumulation of Lead (Pb) in Scardinius erythrophthalmus and Ceratophyllum demersum from Freshwater Ecosystem Biosphere Reserve Srebarna, Bulgaria. Sci. Pap. Ser. D Anim. Sci. 2014, LVII, 290–297. [Google Scholar]
- Mîndrescu, M.; Haliuc, A.; Zhang, W.; Carozza, L.; Carozza, J.-M.; Groparu, T.; Valette, P.; Sun, Q.; Nian, X.; Grădinaru, I. A 600 Years Sediment Record of Heavy Metal Pollution History in the Danube Delta. Sci. Total Environ. 2022, 823, 153702. [Google Scholar] [CrossRef]
- Rus, M.-I.; Munteanu, I.; Vaidianu, N.; Aivaz, K.-A. Research Trends Concerning the Danube Delta: A Specific Social-Ecological System Facing Climate Uncertainty. Earth 2025, 6, 7. [Google Scholar] [CrossRef]
- Radu, V.-M.; Ionescu, P.; Deak, G.; Diacu, E.; Ivanov, A.A.; Zamfir, S.; Marcus, M.-I. Overall Assessment of Surface Water Quality in the Lower Danube River. Environ. Monit. Assess. 2020, 192, 135. [Google Scholar] [CrossRef]
- Berceanu, B.; Sandu, C. The Danube Delta Biosphere Reserve—Regional Clusters in Protecting and Promoting the Local Area and Local Fishery Products. Ekon. Vjesn./Econviews Rev. Contemp. Bus. Entrep. Econ. Issues 2015, 28, 495–509. [Google Scholar]
- Emergency Ordinance No. 23 of 5 March 2008 on Fishing and Aquaculture. Official Gazette of Romania, No. 180, 10 March 2008; Romania, 2008 [In Romanian]. Available online: https://legislatie.just.ro/Public/DetaliiDocument/90207 (accessed on 15 July 2025).
- Law No. 317 of 13 November 2009 for the Approval of Government Emergency Ordinance No. 23/2008 on Fishing and Aquaculture, with Amendments and Completions. Official Gazette of Romania, No. 859, 10 December 2009; Romania, 2009 [In Romanian]. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/112447 (accessed on 12 July 2025).
- Danube Delta Biosphere Reserve Administration (ARBDD). Special Permit for Scientific Fishing, Issued by ARBDD and Accompanied by Project Documentation (Vessel and Gear Descriptions, Research Personnel Qualifications). Available online: https://permise.ddbra.ro/ (accessed on 16 July 2025).
- Freyhof, J.; Kottelat, M. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland, 2007; IUCN-2007-011. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- OECD. Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2012. [Google Scholar] [CrossRef]
- Burkhard, L.P. Estimation of Biota-Sediment Accumulation Factor (BSAF) from Paired Observations of Chemical Concentrations in Biota and Sediment. Environ. Toxicol. Chem. 2009, 28, 2267–2274. [Google Scholar] [CrossRef]
- Gobas, F.A.; Morrison, H.A. Bioconcentration and Biomagnification in the Aquatic Environment. In Handbook of Ecotoxicology, 2nd ed.; Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., Cairns, J., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 189–231. [Google Scholar]
- United States Environmental Protection Agency. Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health (2000, Updated 2003): Bioaccumulation; Report No.: EPA-822-R-03-03; US EPA: Washington, DC, USA, 2003.
- Binu, J.A.; Das, P. A Multi-Objective Approach for Inter-Cluster and Intra-Cluster Distance Analysis for Numeric Data. In Soft Computing: Theories and Applications; Kumar, R., Ahn, C.W., Sharma, T.K., Verma, O.P., Agarwal, A., Eds.; Lecture Notes in Networks and Systems; Springer: Singapore, 2022; Volume 425, pp. 381–390. [Google Scholar] [CrossRef]
- Merce, E.; Merce, C. Statistics-Established and Fulfilling Paradigms; Academic Press, Publishing House: Cluj-Napoca, Romania, 2009; pp. 178–190. (In Romanian) [Google Scholar]
- Ministry of Environment and Water Management. Order No. 161 of 16 February 2006 on the Classification of Surface Water Quality for Establishing the Ecological Status of Water Bodies. In Official Gazette of Romania; Part I, No. 511 bis; European Union: Brussels, Belgium, 2006. (In Romanian) [Google Scholar]
- Catianis, I.; Tiron Duțu, L.; Grosu, D. Heavy Metals Occurrence in Lakes of the Danube Delta, Romania. Geo-Eco-Mar. 2022, 28, 49–63. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. National Recommended Water Quality Criteria—Aquatic Life Criteria Table (EPA 822-F-23-002); U.S. EPA, Office of Water: Washington, DC, USA, 2023. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 2 July 2025).
- The Commission Of The European Communities. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 5–24. Available online: https://eur-lex.europa.eu/eli/reg/2006/1881/oj/eng (accessed on 2 July 2025).
- Hermansson, A.L. Zinc in Sediment (Referring to EQS Variability for Zinc in Surface Waters across EU Member States); Chalmers University of Technology: Gothenburg, Sweden, 2022; Available online: https://research.chalmers.se/publication/530951/file/530951_Fulltext.pdf (accessed on 2 July 2025).
- Vorkamp, K.; Sanderson, H. Study on the Variability of Environmental Quality Standards (EQS) for Priority Substances under the Water Framework Directive among EU Member States. 2016. Available online: https://dce2.au.dk/pub/SR198.pdf (accessed on 20 July 2025).
- Tudor, I.M.; Teodorof, L.; Burada, A.; Tudor, M.; Ibram, O.; Despina, C. Long-Term Nutrients and Heavy Metals Concentration Dynamics in Aquatic Ecosystems of Danube Delta. Sci. Ann. Danube Delta Inst. 2016, 22, 149–156. [Google Scholar]
- Milanov, Đ.R.; Krstić, M.P.; Jovanović, D.A.; Marković, R.V.; Baltić, B.M.; Ivanović, J.S.; Jovetić, M.; Baltić, M.Ž. Analysis of Heavy Metals Concentration in Tissues of Three Different Fish Species Included in Human Diet from Danube River, in the Belgrade Region, Serbia. Acta Vet. 2016, 66, 89–102. [Google Scholar] [CrossRef]
- Cordeli (Savescu), A.N.; Oprea, L.; Crețu, M.; Dediu, L.; Coada, M.T.; Mînzala, D.-N. Bioaccumulation of Metals in Some Fish Species from the Romanian Danube River: A Review. Fishes 2023, 8, 387. [Google Scholar] [CrossRef]
- Milošković, A.; Dojčinović, B.; Kovačević, S.; Radojković, N.; Radenković, M.; Milošević, D.; Simić, V. Spatial Monitoring of Heavy Metals in the Inland Waters of Serbia: A Multispecies Approach Based on Commercial Fish. Environ. Sci. Pollut. Res. 2016, 23, 9918–9933. [Google Scholar] [CrossRef]
- Ira-Adeline, S.; Victor, C.; Stefan-Mihai, P.; Alina, M.; Aurelia, N.; Stefan-Adrian, S.; Antoaneta, E.; Ancuta, S.D. Heavy Metal Evaluation in the Lower Sector of Danube River. Available online: https://landreclamationjournal.usamv.ro/pdf/2020/Art1.pdf (accessed on 8 July 2025).
- Burada, A.; Odor, D.S.; Teodorof, L.; Nastasea, C.; Nastase, A.; Navodaru, I.; Georgescu, L.P. Mercury levels in fish tissues with and without commercial value from Danube Delta biosphere reserve. J. Environ. Prot. Ecol. 2014, 15, 842–850. [Google Scholar]
- Popescu, F.; Trumić, M.; Cioabla, A.E.; Vujić, B.; Stoica, V.; Trumić, M.; Opriș, C.; Bogdanović, G.; Trif-Tordai, G. Analysis of Surface Water Quality and Sediments Content on Danube Basin in Djerdap-Iron Gate Protected Areas. Water 2022, 14, 2991. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Cristea, D.S.; Petrea, S.-M.; Mogodan, A.; Jijie, R.; Ciornea, E.; Nicoară, M.; Turek Rahoveanu, M.M.; Cristea, V. Predictive Innovative Methods for Aquatic Heavy Metals Pollution Based on Bioindicators in Support of Blue Economy in the Danube River Basin. Sustainability 2021, 13, 8936. [Google Scholar] [CrossRef]
- Poleksić, V.; Lenhardt, M.; Jarić, I.; Djordjević, D.; Gačić, Z.; Cvijanović, G.; Rašković, B. Liver, Gills, and Skin Histopathology and Heavy Metal Content of the Danube Sterlet (Acipenser ruthenus Linnaeus, 1758). Environ. Toxicol. Chem. 2010, 29, 515–521. [Google Scholar] [CrossRef]
- Ivanović, J.; Janjić, J.; Baltić, M.; Milanov, R.; Bošković, M.; Marković, R.V.; Glamočlija, N. Metal Concentrations in Water, Sediment and Three Fish Species from the Danube River, Serbia: A Cause for Environmental Concern. Environ. Sci. Pollut. Res. 2016, 23, 17105–17112. [Google Scholar] [CrossRef]
- Winkels, H.J.; Kroonenberg, S.B.; Lychagin, M.Y.; Marin, G.; Rusakov, G.V.; Kasimov, N.S. Geochronology of priority pollutants in sedimentation zones of the Volga and Danube delta in comparison with the Rhine delta. Appl. Geochem. 1998, 13, 581–591. [Google Scholar] [CrossRef]
- Culicov, O.A.; Trtíc-Petrovíc, T.; Nekhoroshkov, P.S.; Zinicovscaia, I.; Duliu, O.G. On the Geochemistry of the Danube River Sediments (Serbian Sector). Int. J. Environ. Res. Public Health 2022, 19, 12879. [Google Scholar] [CrossRef]
- Ljubojević, D.; Trbović, D.; Lujic, J.; Bjelić-Čabrilo, O.; Kostić, D.; Novakov, N.; Ćirković, M. Fatty Acid Composition of Fishes from Inland Waters. Bulg. J. Agric. Sci. 2013, 19, 62–74. [Google Scholar]
- Subotić, S.; Jeftić, V.; Spasić, S.; Hegediš, A.; Krpo-Cetković, J.; Lenhardt, M. Distribution and Accumulation of Elements (As, Cu, Fe, Hg, Mn, and Zn) in Tissues of Fish Species from Different Trophic Levels in the Danube River at the Confluence with the Sava River (Serbia). Environ. Sci. Pollut. Res. 2013, 20, 5309–5317. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Heavy Metal Regulations—FAOLEX: Legal Notice No. 66/2003; FAO: Rome, Italy, 2003; Available online: http://faolex.fao.org/docs/pdf/eri42405.pdf (accessed on 11 July 2025).
- Codex Alimentarius Commission. General Standard for Contaminants and Toxins in Food and Feed (CXS 193-1995). 1995. [Revised 1997, 2006, 2008, 2009; Amended 2010–2024]. Available online: https://www.fao.org/fao-who-codexalimentarius/ (accessed on 14 July 2025).
- Starčević, M.; Tasić, A.; Katanić, N.; Laudanović, M.; Baltić, B.; Jovanović, D.; Glamočlija, N. Metal Bioaccumulation in Fish Species from the Danube River in Serbia and Evaluation of Possible Health Risks. Meat Technol. 2025, 66, 53–63. [Google Scholar] [CrossRef]
- Gati, G.; Pop, C.; Brudasca, F.; Gurzau, A.E.; Spinu, M. Assessment of the Heavy Metal Contamination in the Danube Delta from the Bioaccumulation Perspective. Glob. J. Hum. Soc. Sci. 2013, 13, 11–16. [Google Scholar]
- Jovanović, D.A.; Marković, R.V.; Teodorović, V.B.; Šefer, D.S.; Krstić, M.P.; Radulović, S.B.; Ivanović Ćirić, J.S.; Janjić, J.M.; Baltić, M.Ž. Determination of Heavy Metals in Muscle Tissue of Six Fish Species with Different Feeding Habits from the Danube River, Belgrade—Public Health and Environmental Risk Assessment. Environ. Sci. Pollut. Res. 2017, 24, 11383–11391. [Google Scholar] [CrossRef]
- Kostić-Vuković, J.; Kolarević, S.; Kračun-Kolarević, M.; Višnjić-Jeftić, Ž.; Rašković, B.; Poleksić, V.; Gačić, Z.; Lenhardt, M.; Vuković-Gačić, B. Temporal Variation of Biomarkers in Common Bream Abramis brama (L. 1758) Exposed to Untreated Municipal Wastewater in the Danube River in Belgrade, Serbia. Environ. Monit. Assess. 2021, 193, 465. [Google Scholar] [CrossRef]
- Shukerova, S.; Chunchukova, M.; Kuzmanova, D. Cadmium Content in Two Cyprinid Fish Species from the Danube River. Agric. Sci. 2017, 9, 37–42. [Google Scholar]
- Subotić, S.; Spasić, S.; Višnjić-Jeftić, Ž.; Hegediš, A.; Krpo-Cetković, J.; Mićković, B.; Skorić, S.; Lenhardt, M. Heavy Metal and Trace Element Bioaccumulation in Target Tissues of Four Edible Fish Species from the Danube River (Serbia). Ecotoxicol. Environ. Saf. 2013, 98, 196–202. [Google Scholar] [CrossRef]
- Mititelu, M.; Nicolescu, F.; Ioniță, C.-A.; Nicolescu, T.-O. Study of Heavy Metals and Organic Pollutants in Some Fishes of the Danube River. J. Environ. Prot. Ecol. 2012, 13, 869–874. [Google Scholar]
- Lenhardt, M.; Jarić, I.; Višnjić-Jeftić, Ž.; Skorić, S.; Gačić, Z.; Pucar, M.; Hegediš, A. Concentrations of 17 Elements in Muscle, Gills, Liver and Gonads of Five Economically Important Fish Species from the Danube River. Knowl. Manag. Aquat. Ecosyst. 2012, 407, 2. [Google Scholar] [CrossRef]
- Burada, A.; Teodorof, L.; Despina, C.; Seceleanu-Odor, D.; Tudor, M.; Ibram, O.; Navodaru, I.; Murariu, G.; Țopa, C.M.; Tudor, M. Trace Elements in Fish Tissue with Commercial Value of the Danube Delta Biosphere Reserve. Environ. Eng. Manag. J. 2017, 16, 731–738. [Google Scholar] [CrossRef]
- Diaconescu, C.; Urdes, L.; Marius, H.; Ianitchi, D.; Popa, D. The Influence of Heavy Metal Content on Superoxide Dismutase and Glutathione Peroxidase Activity in the Fish Meat Originated from Different Areas of Danube River. Roum. Biotechnol. Lett. 2008, 13, 3859–3862. [Google Scholar]
- Zrnčić, S.; Oraić, D.; Ćaleta, M.; Mihaljević, Z.; Zanella, D.; Bilandžić, N. Biomonitoring of Heavy Metals in Fish from the Danube River. Environ. Monit. Assess. 2013, 185, 1189–1198. [Google Scholar] [CrossRef]
- Aleksić, J.; Glamočlija, N.; Laudanović, M.; Ivanović, S.; Milijašević, M.; Baltić, B.; Starčević, M. The Content and Associated Health Risk Assessment of Toxic Elements, Micro-, and Macrominerals in Common Carp, Wels Catfish, and Silver Carp from the Danube River in Serbia. Environ. Geochem. Health 2025, 47, 60. [Google Scholar] [CrossRef] [PubMed]
- Štrbac, S.; Šajnović, A.; Budakov, L.; Vasić, N.; Kašanin-Grubin, M.; Simonović, P.; Jovančićević, B. Metals in the Sediment and Liver of Four Fish Species from Different Trophic Levels in Tisza River, Serbia. Chem. Ecol. 2013, 29, 1–15. [Google Scholar] [CrossRef]
- Perçin Olgunoğlu, M.; Artar, E.; Olgunoğlu, İ.A. Comparison of Heavy Metal Levels in Muscle and Gills of Four Benthic Fish Species from the Northeastern Mediterranean Sea. Pol. J. Environ. Stud. 2015, 24, 1743–1748. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–161. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj/eng (accessed on 10 July 2025).
| No. | Scientific Name | Common Name | Feeding Behavior | |
|---|---|---|---|---|
| 1 | Scardinius erythropthalmus L. | Common rudd | Omnivorous, primarily benthivorous |  |
| 2 | Esox lucius L. | Northern pike | Piscivorous predator |  |
| 3 | Aspius aspius L. | Asp | Piscivorous predator |  |
| 4 | Leuciscus idus L. | Ide | Omnivorous primarily insectivorous |  |
| 5 | Cyprinus carpio L. | Common carp | Omnivorous and primarily benthivorous |  |
| 6 | Abramis brama L. | Common bream | Omnivorous and primarily benthivorous |  |
| 7 | Carassius gibelio Bloch | Crucian carp | Omnivorous and primarily benthivorous |  |
| 8 | Alburnus alburnus L. | Common bleak | Planktivorous and insectivorous |  |
| In Sampling Point P4 | N | Cu | Zn | Fe | Mn |
|---|---|---|---|---|---|
| X ± sX | X ± sX | X ± sX | X ± sX | ||
| P1 water | - | - | - | - | - |
| P2 water | - | - | - | - | - |
| P3 water | 5 | - | - | 1.04 × 10−6 ± 2.63 × 10−8 | - |
| P4 water | 5 | - | 0.126 ± 0.002 | 0.021 ± 0.001 | - |
| P5 water | - | - | - | - | - |
| P1 mud | 5 | 0.071 ± 0.002 | - | 0.124 ± 0.003 | - |
| P2 mud | 5 | 0.054 ± 0.003 | - | 0.106 ± 0.002 | 0.070 ± 0.003 |
| P3 mud | 5 | 0.022 ± 0.001 | - | 0.226 ± 0.009 | 0.011 ± 0.35 × 10−3 |
| P4 mud | 5 | 0.036 ± 0.002 | - | 0.053 ± 0.001 | 0.072 ± 0.003 |
| P5 mud | 5 | 0.024 ± 0.001 | - | 0.059 ± 0.002 | 0.118 ± 0.002 |
| Species | Dry Matter | Proximate Nutritional Components | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Fat | Ash | Nitrogen-Free Matter | ||||||
| %FT | %DM | %FT | %DM | %FT | %DM | %FT | %DM | ||
| Scardinius erythrophthalmus L. | 30.20 a ± 1.75 | 17.66 a ± 0.36 | 58.48 c ± 0.95 | 2.35 b ± 0.04 | 7.78 b ± 0.011 | 1.17 a ± 0.02 | 3.87 a ± 0.06 | 9.02 a ± 0.40 | 29.87 a ± 1.11 |
| Esox lucius L. | 24.52 bc ± 0.95 | 18.94 a ± 0.62 | 77.24 a ± 1.32 | 1.55 bc ± 0.07 | 6.32 b ± 0.12 | 1.35 a ± 0.08 | 5.51 a ± 0.15 | 2.68 c ± 0.09 | 10.93 c ± 0.25 |
| Aspius aspius L. | 24.14 bc ± 1.28 | 18.18 a ± 0.89 | 75.31 a ± 1.78 | 2.22 b ± 0.07 | 9.20 b ± 0.11 | 0.99 a ± 0.04 | 4.10 a ± 0.07 | 2.75 c ± 0.13 | 11.39 c ± 0.27 |
| Leuciscus idus L. | 24.39 bc ± 0.86 | 16.95 b ± 0.74 | 69.50 b ± 1.52 | 0.78 c ± 0.02 | 3.20 c ± 0.05 | 1.17 a ± 0.05 | 4.80 a ± 0.11 | 5.49 b ± 0.08 | 22.51 b ± 0.18 |
| Cyprinus carpio L. | 29.94 a ± 1.60 | 16.71 a ± 0.56 | 55.81 c ± 1.62 | 6.28 a ± 0.12 | 11.36 a ± 0.31 | 1.52 a ± 0.03 | 5.08 a ± 0.12 | 8.31 a ± 0.68 | 27.76 a ± 1.84 |
| Abramis brama L. | 26.87 b ± 0.79 | 17.64 a ± 0.41 | 65.65 b ± 0.94 | 1.47 bc ± 0.01 | 5.47 bc ± 0.03 | 1.64 a ± 0.05 | 6.10 a ± 0.14 | 6.12 a ± 0.32 | 22.78 b ± 1.02 |
| Carassius gibelio Bloch | 25.73 b ± 1.17 | 20.39 a ± 0.58 | 79.25 a ± 1.26 | 1.05 bc ± 0.06 | 4.08 bc ± 0.13 | 1.15 a ± 0.02 | 4.47 a ± 0.05 | 3.14 c ± 0.06 | 12.20 c ± 0.14 |
| Alburnus alburnus L. | 22.47 c ± 0.60 | 17.51 a ± 0.80 | 77.93 a ± 1.4 | 0.42 c ± 0.02 | 1.87 c ± 0.03 | 1.27 a ± 0.07 | 5.65 a ± 0.02 | 3.27 c ± 0.16 | 14.55 c ± 0.28 |
| Scardinius erythrophthalmus L. | Esox lucius L. | Aspius aspius L. | Leuciscus idus L. | Cyprinus carpio L. | Abramis brama L. | Carassius gibelio Bloch | Alburnus alburnus L. | |
|---|---|---|---|---|---|---|---|---|
| Ti | 0.29 a ± 0.012 | 0.24 a ± 0.007 | 0.28 a ± 0.006 | 0.27 a ± 0.013 | 0.22 a ± 0.010 | 0.23 a ± 0.006 | 0.19 a ± 0.009 | 0.30 a ± 0.011 |
| Fe | 1.80 a ± 0.076 | 1.71 a ± 0.060 | 0.25 b ± 0.015 | 0.18 b ± 0.006 | 1.71 a ± 0.052 | 1.79 a ± 0.040 | 1.98 a ± 0.117 | 2.00 a ± 0.048 |
| Zn | 0.20 a ± 0.005 | 0.13 a ± 0.006 | 0.18 a ± 0.005 | 0.16 ab ± 0.008 | 0.15 ab ± 0.011 | 0.10 b ± 0.003 | 0.11 b ± 0.003 | 0.19 a ± 0.008 |
| Cr | 0.66 a ± 0.028 | 0.55 ab ± 0.031 | - | 0.70 a ± 0.039 | 0.51 a ± 0.016 | 0.61 a ± 0.024 | 0.69 a ± 0.024 | 0.63 a ± 0.037 |
| Cu | 0.27 a ± 0.010 | 0.28 a ± 0.009 | 0.31 a ± 0.017 | - | 0.26 a ± 0.016 | - | 0.31 a ± 0.004 | 0.30 a ± 0.003 |
| Sb | 0.02 b ± 0.003 | - | 0.04 b ± 0.005 | 0.04 b ± 0.004 | 0.03 b ± 0.002 | 0.31 a ± 0.007 | - | 0.06 b ± 0.001 |
| Mn | 0.02 a ± 0.002 | - | - | - | - | - | 0.03 a ± 0.002 | 0.02 a ± 0.004 |
| Cd | 0.14 ab ± 0.006 | 0.20 a ± 0.007 | 0.14 ab ± 0.007 | 0.18 a ± 0.010 | 0.14 ab ± 0.005 | 0.15 ab ± 0.008 | 0.20 a ± 0.014 | 0.25 a ± 0.007 |
| Ag | 0.07 a ± 0.004 | 0.09 a ± 0.003 | 0.07 a ± 0.005 | 0.08 a ± 0.005 | 0.05 a ± 0.004 | 0.07 a ± 0.004 | 0.08 a ± 0.004 | 0.10 a ± 0.005 |
| Ni | 0.09 a ± 0.001 | 0.09 a ± 0.001 | 0.10 a ± 0.007 | - | 0.09 a ± 0.003 | - | 0.10 a ± 0.004 | 0.11 a ± 0.004 |
| Se | 0.99 b ± 0.012 | 2.88 a ± 0.060 | 3.16 a ± 0.094 | 3.03 a ± 0.123 | 2.24 a ± 0.084 | 1.87 a ± 0.127 | 1.92 a ± 0.048 | 1.99 a ± 0.099 |
| V | - | - | 0.05 a ± 0.002 | - | - | - | 0.05 a ± 0.002 | - |
| Sn | - | 0.04 a ± 0.004 | - | - | 0.03 a ± 0.002 | 0.04 a ± 0.001 | 0.04 a ± 0.002 | 0.05 a ± 0.002 |
| Hg | - | - | - | - | - | 0.07 a ± 0.004 | - | - |
| As | - | 2.04 a ± 0.045 | - | 2.05 a ± 0.062 | 2.07 a ± 0.076 | 1.99 a ± 0.097 | - | - |
| Species | BACFe | BACZn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | log10 | P5 | P1 | P2 | P3 | P4 | log10 | P5 | |
| a | - | - | - | 1.59 | 0.2014 | - | - | - | - | 8.57 | 0.9330 | - |
| b | - | - | - | 1.03 | 0.0128 | - | - | - | - | 8.14 | 0.9106 | - |
| c | - | - | - | 1.43 | 0.1553 | - | - | - | - | 1.19 | 0.0755 | - |
| d | - | - | - | 1.27 | 0.1038 | - | - | - | - | 0.86 | 0.0655 | - |
| e | - | - | - | 1.19 | 0.0755 | - | - | - | - | 8.14 | 0.9106 | - |
| f | - | - | - | 0.79 | 0.1024 | - | - | - | - | 8.52 | 0.9304 | - |
| g | - | - | - | 0.87 | 0.0605 | - | - | - | - | 9.43 | 0.9745 | - |
| h | - | - | - | 1.51 | 0.1790 | - | - | - | - | 9.52 | 0.9786 | - |
| Species | BSAF | log10 BSAF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | X ± SE | P1 | P2 | P3 | P4 | P5 | X ± SE | |
| a | 3.80 | 5.00 | 12.27 | 7.50 | 11.25 | 7.96 ± 1.93 | 0.5798 | 0.6990 | 1.0888 | 0.8751 | 1.0512 | 0.8588 ± 0.4690 |
| b | 3.94 | 5.19 | 12.73 | 7.78 | 11.67 | 8.26 ± 1.96 | 0.5955 | 0.7152 | 1.1048 | 0.8910 | 1.0671 | 0.8747 ± 0.4691 |
| c | 4.37 | 5.74 | 14.09 | 8.61 | 12.92 | 9.14 ± 2.07 | 0.6405 | 0.7589 | 1.1489 | 0.9350 | 1.1113 | 0.9189 ± 0.4688 |
| d | - | - | - | - | - | - | - | - | - | - | - | - |
| e | 3.66 | 4.81 | 11.82 | 7.22 | 10.83 | 7.66 ± 1.89 | 0.5635 | 0.6821 | 1.0726 | 0.8585 | 1.0346 | 0.8423 ± 0.4688 |
| f | - | - | - | - | - | - | - | - | - | - | - | - |
| g | 4.37 | 5.74 | 14.09 | 8.61 | 12.92 | 9.14 ± 2.07 | 0.6405 | 0.7589 | 1.1489 | 0.9350 | 1.1113 | 0.9189 ± 0.4688 |
| h | 4.23 | 5.56 | 13.64 | 8.33 | 12.50 | 8.85 ± 2.03 | 0.6263 | 0.7451 | 1.1348 | 0.9206 | 1.0969 | 0.9048 ± 0.4687 |
| Species | BSAF | log10 BSAF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | X ± SE | P1 | P2 | P3 | P4 | P5 | X ± SE | |
| a | 14.52 | 16.98 | 7.96 | 33.96 | 30.51 | 20.78 ± 3.32 | 1.1620 | 1.2299 | 0.9009 | 1.5310 | 1.4844 | 1.2616 ± 0.5065 |
| b | 13.79 | 16.13 | 7.57 | 32.26 | 28.98 | 19.74 ± 3.23 | 1.1396 | 1.2076 | 0.8791 | 1.5087 | 1.4621 | 1.2394 ± 0.5063 |
| c | 2.02 | 2.36 | 1.11 | 4.72 | 4.24 | 2.89 ± 1.23 | 0.3054 | 0.3729 | 0.0453 | 0.6739 | 0.6274 | 0.4050 ± 0.5059 |
| d | 1.45 | 1.70 | 0.80 | 3.40 | 3.05 | 2.08 ± 1.05 | 0.1614 | 0.2304 | −0.0969 | 0.5315 | 0.4843 | 0.2621 ± 0.5059 |
| e | 13.79 | 16.13 | 7.57 | 32.26 | 28.98 | 19.74 ± 3.23 | 1.1396 | 1.2076 | 0.8791 | 1.5087 | 1.4621 | 1.2394 ± 0.5063 |
| f | 14.44 | 16.89 | 7.92 | 33.77 | 30.34 | 20.67 ± 3.31 | 1.1596 | 1.2276 | 0.8987 | 1.5285 | 1.4820 | 1.2593 ± 0.5064 |
| g | 15.97 | 18.68 | 8.76 | 37.36 | 33.56 | 22.86 ± 3.48 | 1.2033 | 1.2714 | 0.9425 | 1.5724 | 1.5258 | 1.3031 ± 0.5064 |
| h | 16.13 | 18.87 | 8.85 | 37.74 | 33.90 | 23.09 ± 3.50 | 1.2076 | 1.2758 | 0.9469 | 1.5768 | 1.5302 | 1.3075 ± 0.5064 |
| Species | BSAF | log10 BSAF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | X ± SE | P1 | P2 | P3 | P4 | P5 | X ± SE | |
| a | - | 0.29 | 1.82 | 0.28 | 0.17 | 0.64 ± 0.88 | - | 0.538 | 0.260 | 0.553 | 0.770 | 0.5303 ± 0.4572 |
| b | - | - | - | - | - | - | - | - | - | - | - | - |
| c | - | - | - | - | - | - | - | - | - | - | - | - |
| d | - | - | - | - | - | - | - | - | - | - | - | - |
| e | - | - | - | - | - | - | - | - | - | - | - | - |
| f | - | - | - | - | - | - | - | - | - | - | - | - |
| g | - | 0.43 | 2.73 | 0.42 | 0.25 | 0.95 ± 1.08 | - | 0.367 | 0.436 | 0.377 | 0.602 | 0.4455 ± 0.3297 |
| h | - | 0.29 | 1.82 | 0.28 | 0.17 | 0.64 ± 0.88 | - | 0.538 | 0.260 | 0.553 | 0.770 | 0.5303 ± 0.4572 |
| Species | PC | Eigenvalue | %Total | Species | PC | Eigenvalue | %Total |
|---|---|---|---|---|---|---|---|
| a—S. erythrophthalmus | PC1 | 6.429895 | 40.18685 | b—E. lucius | PC1 | 6.676482 | 41.72801 |
| PC2 | 4.906004 | 30.66252 | PC2 | 5.884697 | 36.77936 | ||
| Total | 11.3359 | 70.84937 | Total | 12.56118 | 78.50737 | ||
| c—A. aspius | PC1 | 6.602411 | 44.01607 | d—L. idus | PC1 | 6.389428 | 45.63877 |
| PC2 | 4.642895 | 30.95264 | PC2 | 4.093006 | 29.23575 | ||
| Total | 11.24531 | 74.96871 | Total | 10.48243 | 74.87452 | ||
| e—C. carpio | PC1 | 6.946362 | 40.86095 | f—A. brama | PC1 | 6.123379 | 38.27112 |
| PC2 | 5.845441 | 34.38495 | PC2 | 5.561175 | 34.75734 | ||
| Total | 12.7918 | 75.2459 | Total | 11.68455 | 73.02846 | ||
| g—C. gibelio | PC1 | 6.897949 | 40.57617 | h—A. alburnus | PC1 | 6.370362 | 37.47272 |
| PC2 | 5.440019 | 32.00011 | PC2 | 5.652739 | 33.25140 | ||
| Total | 12.33797 | 72.57628 | Total | 12.0231 | 70.72412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oroian, I.; Bulete, B.I.; Matei, E.; Odagiu, A.C.M.; Burduhos, P.; Oroian, C.; Ștefan, O.D.; Bordea, D. Assessment of Heavy Metal Contamination, Bioaccumulation, and Nutritional Quality in Fish from the Babina–Cernovca Romanian Sector of the Danube River. Foods 2025, 14, 3419. https://doi.org/10.3390/foods14193419
Oroian I, Bulete BI, Matei E, Odagiu ACM, Burduhos P, Oroian C, Ștefan OD, Bordea D. Assessment of Heavy Metal Contamination, Bioaccumulation, and Nutritional Quality in Fish from the Babina–Cernovca Romanian Sector of the Danube River. Foods. 2025; 14(19):3419. https://doi.org/10.3390/foods14193419
Chicago/Turabian StyleOroian, Ioan, Bogdan Ioachim Bulete, Ecaterina Matei, Antonia Cristina Maria Odagiu, Petru Burduhos, Camelia Oroian, Ovidiu Daniel Ștefan, and Daniela Bordea. 2025. "Assessment of Heavy Metal Contamination, Bioaccumulation, and Nutritional Quality in Fish from the Babina–Cernovca Romanian Sector of the Danube River" Foods 14, no. 19: 3419. https://doi.org/10.3390/foods14193419
APA StyleOroian, I., Bulete, B. I., Matei, E., Odagiu, A. C. M., Burduhos, P., Oroian, C., Ștefan, O. D., & Bordea, D. (2025). Assessment of Heavy Metal Contamination, Bioaccumulation, and Nutritional Quality in Fish from the Babina–Cernovca Romanian Sector of the Danube River. Foods, 14(19), 3419. https://doi.org/10.3390/foods14193419








