Influence of Culture Conditions on Bioactive Compounds in Cordyceps militaris: A Comprehensive Review
Abstract
1. Introduction
2. Main Bioactive Compounds in C. militaris and Factors Affecting Their Production
2.1. Cordycepin
2.2. Polysaccharides
2.3. Adenosine
2.4. D-Mannitol
2.5. Carotenoids
2.6. Ergosterol
2.7. Bioactive Proteins
2.8. Cross-Compound Integration and Industrial Implications
3. Solid-State Culture with Grain-Based and Insect-Based Substrates for Enhanced Growth and Bioactive Compound Production
3.1. Solid-State Fermentation and Substrates Roles in C. militaris Cultivation
3.1.1. Grain-Based Culture Media for C. militaris
3.1.2. Insect-Based Culture Media for C. militaris
3.1.3. Mixed Grain- and Insect-Based Culture Media for C. militaris
3.2. Solid-State Fermentation and the Role of Physical Conditions
3.2.1. Solid-State Fermentation and the Role of Temperature in C. militaris Cultivation
3.2.2. Solid-State Fermentation and the Role of Light in C. militaris Cultivation
3.3. Solid-State Fermentation and the Role of Minerals in the Growth of C. militaris Mycelia and Fruiting Bodies
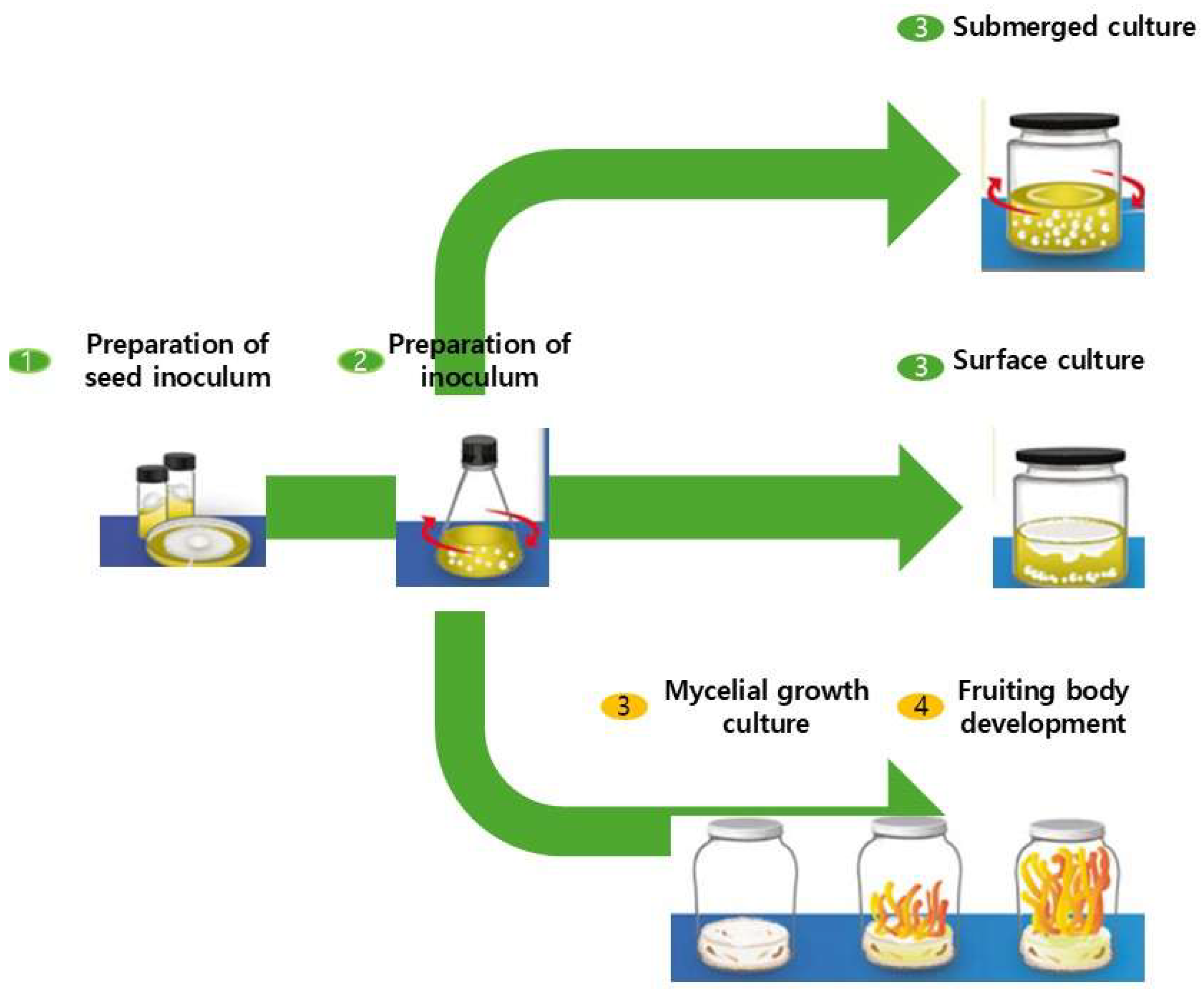
4. Liquid State Fermentation
4.1. Overview of Liquid State Fermentation
4.2. Nutrient Composition of Liquid Fermentation Media
4.3. Liquid Fermentation Conditions: Submerged vs. Surface Culture
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, L.; Zhao, L.; Yang, F.; Yang, W.; Sun, Y.; Hu, Q. Evaluation of anti-fatigue property of the extruded product of cereal grains mixed with Cordyceps militaris on mice. J. Int. Soc. Sports Nutr. 2017, 14, 15. [Google Scholar] [CrossRef]
- Ontawong, A.; Pengnet, S.; Thim-Uam, A.; Munkong, N.; Narkprasom, N.; Narkprasom, K.; Kuntakhut, K.; Kamkeaw, N.; Amornlerdpison, D. A randomized controlled clinical trial examining the effects of Cordyceps militaris beverage on the immune response in healthy adults. Sci. Rep. 2024, 14, 7994. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yu, X.; Li, T.; Zhu, Z. Structure and hypoglycemic activity of a novel exopolysaccharide of Cordyceps militaris. Int. J. Biol. Macromol. 2021, 166, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.Q.; Dat, T.T.H.; Quy, P.T.; Hai, N.T.T.; Thai, N.M.; Phu, N.V.; Tuan, L.V.; Huynh, L.K.; Li, M.S.; Nhung, N.T.A. Identification of potential anti-hyperglycemic compounds in Cordyceps militaris ethyl acetate extract: In vitro and in silico studies. J. Biomol. Struct. Dyn. 2025, 43, 627–643. [Google Scholar] [CrossRef]
- Park, B.J.; Dhong, K.R.; Park, H.J. Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice. Int. J. Mol. Sci. 2024, 25, 10642. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, J.H.; Park, B.J.; Park, H.J. Chitosan Nanoparticle-Encapsulated Cordyceps militaris Grown on Germinated Rhynchosia nulubilis Reduces Type II Alveolar Epithelial Cell Apoptosis in PM(2.5)-Induced Lung Injury. Int. J. Mol. Sci. 2025, 26, 1105. [Google Scholar] [CrossRef]
- Phull, A.R.; Ahmed, M.; Park, H.J. Cordyceps militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms. Microorganisms 2022, 10, 405. [Google Scholar] [CrossRef]
- Park, H.-J. The Genus Cordyceps Sensu Lato: Their Chemical Constituents, Biological Activities, and Therapeutic Effects on Air Pollutants Related to Lung and Vascular Diseases. Life 2025, 15, 935. [Google Scholar] [CrossRef]
- Afzal, M.; Abusalah, M.; Shehzadi, N.; Absar, M.; Ahmed, N.; Khan, S.; Naseem, Y.; Mehmood, N.; Singh, K.K.B. Investigation of biometabolites and novel antimicrobial peptides derived from promising source Cordyceps militaris and effect of non-small cell lung cancer genes computationally. PLoS ONE 2025, 20, e0310103. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Van Pham, T.; Andriana, Y.; Truong, M.N. Cordyceps militaris-Derived Bioactive Gels: Therapeutic and Anti-Aging Applications in Dermatology. Gels 2025, 11, 33. [Google Scholar] [CrossRef]
- Das, S.K.; Masuda, M.; Hatashita, M.; Sakurai, A.; Sakakibara, M. Optimization of culture medium for cordycepin production using Cordyceps militaris mutant obtained by ion beam irradiation. Process Biochem. 2010, 45, 129–132. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Cordyceps—A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef]
- Wu, X.; Wu, T.; Huang, A.; Shen, Y.; Zhang, X.; Song, W.; Wang, S.; Ruan, H. New Insights Into the Biosynthesis of Typical Bioactive Components in the Traditional Chinese Medicinal Fungus Cordyceps militaris. Front. Bioeng. Biotechnol. 2021, 9, 201721. [Google Scholar] [CrossRef]
- Soommat, P.; Raethong, N.; Ruengsang, R.; Thananusak, R.; Laomettachit, T.; Laoteng, K.; Saithong, T.; Vongsangnak, W. Light-Exposed Metabolic Responses of Cordyceps militaris through Transcriptome-Integrated Genome-Scale Modeling. Biology 2024, 13, 139. [Google Scholar] [CrossRef]
- Yang, S.; Jin, L.; Ren, X.; Lu, J.; Meng, Q. Optimization of fermentation process of Cordyceps militaris and antitumor activities of polysaccharides in vitro. J. Food Drug Anal. 2014, 22, 468–476. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.H.; Kim, H.R.; Chun, Y.; Lee, J.H.; Yoo, H.Y.; Park, C.; Kim, S.W. Improved Cordycepin Production by Cordyceps militaris KYL05 Using Casein Hydrolysate in Submerged Conditions. Biomolecules 2019, 9, 461. [Google Scholar] [CrossRef]
- Peng, T.; Guo, J.; Tong, X. Advances in biosynthesis and metabolic engineering strategies of cordycepin. Front. Microbiol. 2024, 15, 1386855. [Google Scholar] [CrossRef]
- Lin, L.-T.; Lai, Y.-J.; Wu, S.-C.; Hsu, W.-H.; Tai, C.-J. Optimal conditions for cordycepin production in surface liquid-cultured Cordyceps militaris treated with porcine liver extracts for suppression of oral cancer. J. Food Drug Anal. 2018, 26, 135–144. [Google Scholar] [CrossRef]
- Kang, C.; Wen, T.-C.; Kang, J.-C.; Meng, Z.-B.; Li, G.-R.; Hyde, K.D. Optimization of large-scale culture conditions for the production of cordycepin with Cordyceps militaris by liquid static culture. Sci. World J. 2014, 2014, 510627. [Google Scholar] [CrossRef]
- Masuda, M.; Urabe, E.; Honda, H.; Sakurai, A.; Sakakibara, M. Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzym. Microb. Technol. 2007, 40, 1199–1205. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, X.; Jiao, Y.; Zheng, X. Improved Cordycepin Production by Cordyceps militaris Using Corn Steep Liquor Hydrolysate as an Alternative Protein Nitrogen Source. Foods 2024, 13, 813. [Google Scholar] [CrossRef]
- Park, S.E.; Yoo, H.S.; Jin, C.Y.; Hong, S.H.; Lee, Y.W.; Kim, B.W.; Lee, S.H.; Kim, W.J.; Cho, C.K.; Choi, Y.H. Induction of apoptosis and inhibition of telomerase activity in human lung carcinoma cells by the water extract of Cordyceps militaris. Food Chem. Toxicol. 2009, 47, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.R.; Chen, W.; Dai, G.S.; Huang, Y.L. Cordycepin suppresses the migration and invasion of human liver cancer cells by downregulating the expression of CXCR4. Int. J. Mol. Med. 2020, 45, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, S.; Chen, C.; Gong, Y.; Ding, K.; Li, G.; Jiang, W.; Zhang, Z.; He, B.; Hu, Z.; et al. Cordycepin combined with antioxidant effects improves fatigue caused by excessive exercise. Sci. Rep. 2025, 15, 8141. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Dhong, K.R.; Kwon, H.K.; Park, H.J. Immunostimulatory Activity of Cordyceps militaris Fermented with Pediococcus pentosaceus SC11 Isolated from a Salted Small Octopus in Cyclophosphamide-Induced Immunocompromised Mice and Its Inhibitory Activity against SARS-CoV 3CL Protease. Microorganisms 2022, 10, 2321. [Google Scholar] [CrossRef]
- Wu, N.; Ge, X.; Yin, X.; Yang, L.; Chen, L.; Shao, R.; Xu, W. A review on polysaccharide biosynthesis in Cordyceps militaris. Int. J. Biol. Macromol. 2024, 260, 129336. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Tomilova, O.G.; Yaroslavtseva, O.N.; Wen, T.-C.; Kryukova, N.A.; Polenogova, O.V.; Tokarev, Y.S.; Glupov, V.V. Temperature adaptations of Cordyceps militaris, impact of host thermal biology and immunity on mycosis development. Fungal Ecol. 2018, 35, 98–107. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, Z.; Wu, S.; Wang, J.; Chen, M.; Liu, W.; Huang, A.; Zhang, J.; Wu, Q.; Ding, Y. Polysaccharides from Cordyceps militaris prevent obesity in association with modulating gut microbiota and metabolites in high-fat diet-fed mice. Food Res. Int. 2022, 157, 111197. [Google Scholar] [CrossRef]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P. A2A receptors in inflammation and injury: Lessons learned from transgenic animals. J. Leukoc. Biol. 2008, 83, 447–455. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Haskó, G. Adenosine and inflammation: It’s time to (re)solve the problem. Trends Pharmacol. Sci. 2022, 43, 43–55. [Google Scholar] [CrossRef]
- Chen, L.X.; Alabdullah, M.; Mahnke, K. Adenosine, bridging chronic inflammation and tumor growth. Front. Immunol. 2023, 14, 1258637. [Google Scholar] [CrossRef]
- Dickenson, J.M.; Reeder, S.; Rees, B.; Alexander, S.; Kendall, D. Functional expression of adenosine A2A and A3 receptors in the mouse dendritic cell line XS-106. Eur. J. Pharmacol. 2003, 474, 43–51. [Google Scholar] [CrossRef]
- Garcia-Garcia, L.; Olle, L.; Martin, M.; Roca-Ferrer, J.; Muñoz-Cano, R. Adenosine Signaling in Mast Cells and Allergic Diseases. Int. J. Mol. Sci. 2021, 22, 5203. [Google Scholar] [CrossRef]
- Hua, X.; Chason, K.D.; Fredholm, B.B.; Deshpande, D.A.; Penn, R.B.; Tilley, S.L. Adenosine induces airway hyperresponsiveness through activation of A3 receptors on mast cells. J. Allergy Clin. Immunol. 2008, 122, 107–113.e107. [Google Scholar] [CrossRef]
- Dong, J.; Lei, C.; Zheng, X.; Ai, X.; Wang, Y.; Wang, Q. Light Wavelengths Regulate Growth and Active Components of Cordyceps militaris Fruit Bodies. J. Food Biochem. 2013, 37, 578–584. [Google Scholar] [CrossRef]
- Dong, J.Z.; Liu, M.R.; Lei, C.; Zheng, X.J.; Wang, Y. Effects of selenium and light wavelengths on liquid culture of Cordyceps militaris Link. Appl. Biochem. Biotechnol. 2012, 166, 2030–2036. [Google Scholar] [CrossRef]
- Shih, I.-L.; Tsai, K.-L.; Hsieh, C. Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem. Eng. J. 2007, 33, 193–201. [Google Scholar] [CrossRef]
- Ng, T.B.; Wang, H.X. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2005, 57, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Park, J.P.; Kim, Y.M.; Kim, S.W.; Hwang, H.J.; Cho, Y.J.; Lee, Y.S.; Song, C.H.; Yun, J.W. Effect of aeration rate on the mycelial morphology and exo-biopolymer production in Cordyceps militaris. Process Biochem. 2002, 37, 1257–1262. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Sung, T.-H.; Hou, S.-J.; Khumsupan, D.; Santoso, S.P.; Cheng, K.-C.; Lin, S.-P. Current Progress Regarding Cordyceps militaris, Its Metabolite Function, and Its Production. Appl. Sci. 2024, 14, 4610. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.T.; Yang, Y.C.; Li, Y.H.; Mau, J.L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ćirić, A.; van Griensven, L.J.L.D.; Soković, M.; Ferreira, I.C.F.R. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef]
- Liang, Z.C.; Liang, C.H.; Wu, C.Y. Various grain substrates for the production of fruiting bodies and bioactive compounds of the medicinal caterpillar mushroom, Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2014, 16, 569–578. [Google Scholar] [CrossRef]
- Schreibman, D.L.; Hong, C.M.; Keledjian, K.; Ivanova, S.; Tsymbalyuk, S.; Gerzanich, V.; Simard, J.M. Mannitol and Hypertonic Saline Reduce Swelling and Modulate Inflammatory Markers in a Rat Model of Intracerebral Hemorrhage. Neurocrit Care 2018, 29, 253–263. [Google Scholar] [CrossRef]
- Pera, M.F., Jr.; Harder, H.C. Effects of mannitol or furosemide diuresis on cis-dichlorodiammineplatinum (II) antitumor activity and toxicity to host-renewing cell populations in rats. Cancer Res. 1979, 39, 1279–1286. [Google Scholar]
- Castro-Torres, I.G.; De la O-Arciniega, M.; Naranjo-Rodríguez, E.B.; Castro-Torres, V.A.; Domínguez-Ortíz, M.Á.; Martínez-Vázquez, M. The hypocholesterolemic effects of Eryngium carlinae F. Delaroche are mediated by the involvement of the intestinal transporters ABCG5 and ABCG8. Evid.-Based Complement. Altern. Med. 2017, 2017, 3176232. [Google Scholar] [CrossRef]
- Coker, A.; Coker, I.; Huseyinov, A.; Sokmen, S.; Karademir, S. Is mannitol effective against platelet-activating factor (PAF)-induced liver damage in obstructive jaundice? Hepato-Gastroenterol. 2001, 48, 1134–1137. [Google Scholar]
- Lan, L.; Wang, S.; Duan, S.; Zhou, X.; Li, Y. Cordyceps militaris Carotenoids Protect Human Retinal Endothelial Cells against the Oxidative Injury and Apoptosis Resulting from H2O2. Evid. Based Complement. Altern. Med. 2022, 2022, 1259093. [Google Scholar] [CrossRef]
- Yang, Y.; Bu, N.; Wang, S.; Zhang, J.; Wang, Y.; Dong, C. Carotenoid Production by Caterpillar Medicinal Mushrooms, Cordyceps militaris (Ascomycetes), under Different Culture Conditions. Int. J. Med. Mushrooms 2020, 22, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Guo, M.; Yang, H.; Guo, S.; Dong, C. The blue-light receptor CmWC-1 mediates fruit body development and secondary metabolism in Cordyceps militaris. Appl. Microbiol. Biotechnol. 2016, 100, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, J.; Lian, T.; Wang, W.; Dong, C.H. Process Optimization for Extraction of Carotenoids from Medicinal Caterpillar Fungus, Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2014, 16, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Nallathamby, N.; Guan-Serm, L.; Vidyadaran, S.; Abd Malek, S.N.; Raman, J.; Sabaratnam, V. Ergosterol of Cordyceps militaris Attenuates LPS Induced Inflammation in BV2 Microglia Cells. Nat. Prod. Commun. 2015, 10, 885–886. [Google Scholar] [CrossRef]
- Umaña, M.; Eim, V.; Garau, M.C.; Rosselló, C.; Simal, S. Ultrasound-assisted extraction of ergosterol and antioxidant components from mushroom by-products and the attainment of a β-glucan rich residue. Food Chem. 2020, 332, 127390. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Cortes, H.; Singh, Y.D.; Panda, M.K.; Mishra, A.P.; Nigam, M.; Saklani, S. Cordyceps spp.: A review on its immune-stimulatory and other biological potentials. Front. Pharmacol. 2021, 11, 602364. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.-H.; Cai, X.; Nam, K.-S.; Lee, S.-J.; An, H.-S.; Jeong, E.-H.; Yun, S.-H.; Sung, S.-K.; Lee, S.-J.; et al. In vitro Antitumor Activity of Ergosterol Peroxide Isolated from Cordyceps militaris on Cancer Cell Lines from Korean Patients. Korean J. Mycol. 2001, 29, 61–66. [Google Scholar]
- Wong, J.H.; Ng, T.B.; Wang, H.; Sze, S.C.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar] [CrossRef]
- Bai, K.C.; Sheu, F. A novel protein from edible fungi Cordyceps militaris that induces apoptosis. J. Food Drug Anal. 2018, 26, 21–30. [Google Scholar] [CrossRef]
- Jung, E.C.; Kim, K.D.; Bae, C.H.; Kim, J.C.; Kim, D.K.; Kim, H.H. A mushroom lectin from ascomycete Cordyceps militaris. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2007, 1770, 833–838. [Google Scholar] [CrossRef]
- Gang, J.; Liu, H.; Liu, Y. Optimization of Liquid Fermentation Conditions and Protein Nutrition Evaluation of Mycelium from the Caterpillar Medicinal Mushroom, Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2016, 18, 745–752. [Google Scholar] [CrossRef]
- Jian, L.; Li, Z. Effect of plant growth regulator on cordycepin and adenosine production of Cordyceps militaris cultured on wheat solid substrate. Acad. J. Agric. Res. 2017, 5, 279–286. [Google Scholar]
- Wen, T.C.; Li, G.R.; Kang, J.C.; Kang, C.; Hyde, K.D. Optimization of Solid-state Fermentation for Fruiting Body Growth and Cordycepin Production by. Chiang Mai J. Sci. 2014, 41, 858–872. [Google Scholar]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. LWT-Food Sci. Technol. 2015, 63, 1317–1324. [Google Scholar] [CrossRef]
- Showkat, M.; Narayanappa, N.; Umashankar, N.; Saraswathy, B.P.; Doddanagappa, S.; Ashraf, S.; Gani, S.; Fatimah, N.; Nabi, A.; Perveen, K.; et al. Optimization of Fermentation Conditions of Cordyceps militaris and In Silico Analysis of Antifungal Property of Cordycepin Against Plant Pathogens. J. Basic. Microbiol. 2024, 64, e2400409. [Google Scholar] [CrossRef]
- Tang, J.; Yiting, L.; Li, Z. Optimization of fermentation conditions and purification of cordycepin from Cordyceps militaris. Prep. Biochem. Biotechnol. 2014, 44, 90–106. [Google Scholar] [CrossRef]
- Borde, M.; Singh, S.K. Enhanced production of cordycepin under solid-state fermentation of Cordyceps militaris by using combinations of grains/substrates. Braz. J. Microbiol. 2023, 54, 2765–2772. [Google Scholar] [CrossRef]
- Turk, A.; Abdelhamid, M.A.A.; Yeon, S.W.; Ryu, S.H.; Lee, S.; Ko, S.M.; Kim, B.S.; Pack, S.P.; Hwang, B.Y.; Lee, M.K. Cordyceps mushroom with increased cordycepin content by the cultivation on edible insects. Front. Microbiol. 2022, 13, 1017576. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Liu, W.; Guan, H.; Li, C.; Wang, J.; Wang, L. Advances in biosynthesis of cordycepin from Cordyceps militaris. Chin. J. Biotechnol. 2020, 36, 1293–1304. [Google Scholar] [CrossRef]
- Lin, Q.; Long, L.; Wu, L.; Zhang, F.; Wu, S.; Zhang, W.; Sun, X. Evaluation of different agricultural wastes for the production of fruiting bodies and bioactive compounds by medicinal mushroom Cordyceps militaris. J. Sci. Food Agric. 2017, 97, 3476–3480. [Google Scholar] [CrossRef]
- Oh, J.; Yoon, D.-H.; Shrestha, B.; Choi, H.-K.; Sung, G.-H. Metabolomic profiling reveals enrichment of cordycepin in senescence process of Cordyceps militaris fruit bodies. J. Microbiol. 2019, 57, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Paje, L.A.; Kwon, B.; Noh, J.; Lee, S. Quantitative analysis of cordycepin in Cordyceps militaris under different extraction methods. J. Appl. Biol. Chem. 2021, 64, 153–158. [Google Scholar] [CrossRef]
- Adnan, M.; Ashraf, S.A.; Khan, S.; Alshammari, E.; Awadelkareem, A.M. Effect of pH, temperature and incubation time on cordycepin production from Cordyceps militaris using solid-state fermentation on various substrates. CyTA-J. Food 2017, 15, 617–621. [Google Scholar] [CrossRef]
- Bamidele, M.O.; Bamikale, M.B.; Cárdenas-Hernández, E.; Bamidele, M.A.; Castillo-Olvera, G.; Sandoval-Cortes, J.; Aguilar, C.N. Bioengineering in Solid-State Fermentation for next sustainable food bioprocessing. Next Sustain. 2025, 6, 100105. [Google Scholar] [CrossRef]
- Kang, N.; Lee, H.-H.; Park, I.; Seo, Y.-S. Development of high cordycepin-producing Cordyceps militaris strains. Mycobiology 2017, 45, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Turk, A.; Kim, B.S.; Ko, S.M.; Yeon, S.W.; Ryu, S.H.; Kim, Y.-G.; Hwang, B.Y.; Lee, M.K. Optimization of cultivation and extraction conditions of pupae-Cordyceps for cordycepin production. Nat. Product. Sci. 2021, 27, 187–192. [Google Scholar]
- Trung, N.Q.; Dat, N.T.; Anh, H.N.; Tung, Q.N.; Nguyen, V.T.H.; Van, H.N.B.; Van, N.M.N.; Minh, T.N. Substrate Influence on Enzymatic Activity in Cordyceps militaris for Health Applications. Chemistry 2024, 6, 517–530. [Google Scholar] [CrossRef]
- Fan, D.-d.; Wang, W.; Zhong, J.-J. Enhancement of cordycepin production in submerged cultures of Cordyceps militaris by addition of ferrous sulfate. Biochem. Eng. J. 2012, 60, 30–35. [Google Scholar] [CrossRef]
- Tang, J.; Qian, Z.; Wu, H. Enhancing cordycepin production in liquid static cultivation of Cordyceps militaris by adding vegetable oils as the secondary carbon source. Bioresour. Technol. 2018, 268, 60–67. [Google Scholar] [CrossRef]
- Stasinopoulos, S.; Seviour, R. Stimulation of exopolysaccharide production in the fungus Acremonium persicinum with fatty acids. Biotechnol. Bioeng. 1990, 36, 778–782. [Google Scholar] [CrossRef]
- Bode, H.B. Insect-associated microorganisms as a source for novel secondary metabolites with therapeutic potential. In Insect Biotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 77–93. [Google Scholar]
- Xie, B.; Zhu, Y.; Chu, X.; Pokharel, S.S.; Qian, L.; Chen, F. Research progress and production status of edible insects as food in China. Foods 2024, 13, 1986. [Google Scholar] [CrossRef] [PubMed]
- Raethong, N.; Laoteng, K.; Vongsangnak, W. Uncovering global metabolic response to cordycepin production in Cordyceps militaris through transcriptome and genome-scale network-driven analysis. Sci. Rep. 2018, 8, 9250. [Google Scholar] [CrossRef]
- Shrestha, B.; Han, S.K.; Sung, J.M.; Sung, G.H. Fruiting Body Formation of Cordyceps militaris from Multi-Ascospore Isolates and Their Single Ascospore Progeny Strains. Mycobiology 2012, 40, 100–106. [Google Scholar] [CrossRef]
- Suraporn, S. Growth of Cordyceps militaris on Plant Substrates Supplemented with Pupal Powder of Thai Variety of the Silkworm, Bombyx mori. Int. J. Wild Silkmoth Silk 2015, 19, 13–17. [Google Scholar] [CrossRef]
- Sibounnavong, P. Study on the Cordyceps militaris Cultivation in Differential Media and Substrates Formulation. Souphanouvong Univ. J. Multidiscip. Res. Dev. 2024, 9, 1–11. [Google Scholar] [CrossRef]
- Jędrejko, K.; Kała, K.; Sułkowska-Ziaja, K.; Krakowska, A.; Szewczyk, A.; Muszyńska, B. Determination of cordycepin, ergothioneine, and other bioactive compounds in Cordyceps militaris fruiting bodies cultivated on different substrates. Int. J. Food Sci. Technol. 2025, 60, vvaf097. [Google Scholar] [CrossRef]
- Hung, L.; Keawsompong, S.; Hanh, V.; Sivichai, S.; Hywel-Jones, N. Effect of temperature on cordycepin production in Cordyceps militaris. Thai J. Agric. Sci. 2009, 42, 219–225. [Google Scholar]
- Zhong SiMin, Z.S.; Du Mei, D.M.; Chen WangBin, C.W.; Zhang Song, Z.S. Liquid culture conditions for promoting cordycepin secreted from Cordyceps militaris mycelia. Mycosystema 2011, 30, 229–234. [Google Scholar]
- Cui, J.D. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit. Rev. Biotechnol. 2015, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. Enrichment of cordycepin for cosmeceutical applications: Culture systems and strategies. Appl. Microbiol. Biotechnol. 2019, 103, 1681–1691. [Google Scholar] [CrossRef]
- Krishna, K.V.; Balasubramanian, B.; Park, S.; Bhattacharya, S.; Kadanthottu Sebastian, J.; Liu, W.-C.; Pappuswamy, M.; Meyyazhagan, A.; Kamyab, H.; Chelliapan, S.; et al. Conservation of Endangered Cordyceps sinensis Through Artificial Cultivation Strategies of C. militaris, an Alternate. Mol. Biotechnol. 2025, 67, 1382–1397. [Google Scholar] [CrossRef]
- Lin, C.-H.; Huang, H.-L.; Chen, Y.-H.; Lee, C.-L. Deep Ocean Water Minerals Promotes the Growth and Cordycepin Production of Cordyceps militaris Fruiting Bodies through Proteomics Regulation. Fermentation 2022, 8, 481. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Wang, J.-J.; Wei, B.-L.; Lee, C.-L. Effect of the salts of deep ocean water on the production of cordycepin and adenosine of Cordyceps militaris-fermented product. AMB Express 2015, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Zhang, H.; Xiao, L.; Lei, Z.; Kaul, S.C.; Wadhwa, R.; Zhang, Z. Low Dose of Fluoride in the Culture Medium of Cordyceps militaris Promotes Its Growth and Enhances Bioactives with Antioxidant and Anticancer Properties. J. Fungi 2021, 7, 342. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, K.S.; Shaw, J.F.; Chen, J.R.; Kuo, W.S.; Shen, G.; Grumezescu, A.M.; Holban, A.M.; Wang, Y.T.; Wang, J.S.; et al. Trends in the Immunomodulatory Effects of Cordyceps militaris: Total Extracts, Polysaccharides and Cordycepin. Front. Pharmacol. 2020, 11, 575704. [Google Scholar] [CrossRef]
- Dong, J.Z.; Lei, C.; Ai, X.R.; Wang, Y. Selenium enrichment on Cordyceps militaris link and analysis on its main active components. Appl. Biochem. Biotechnol. 2012, 166, 1215–1224. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Golo, P.S.; de Souza Ribeiro-Silva, C.; Muniz, E.R.; de Oliveira Franco, A.; Kobori, N.N.; Fernandes, É.K.K. Advances in submerged liquid fermentation and formulation of entomopathogenic fungi. Appl. Microbiol. Biotechnol. 2024, 108, 451. [Google Scholar] [CrossRef]
- Mao, X.-B.; Eksriwong, T.; Chauvatcharin, S.; Zhong, J.-J. Optimization of carbon source/nitrogen ratio for cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Process Biochem. 2005, 40, 1667–1672. [Google Scholar] [CrossRef]
- Park, J.-P.; Kim, S.-W.; Hwang, H.-J.; Cho, Y.-J.; Yun, J.-W. Stimulatory effect of plant oils and fatty acids on the exo-biopolymer production in Cordyceps militaris. Enzym. Microb. Technol. 2002, 31, 250–255. [Google Scholar] [CrossRef]
- Sari, N.; Suparmin, A.; Kato, T.; Park, E. Improved cordycepin production in a liquid surface culture of Cordyceps militaris isolated from wild strain. Biotechnol. Bioprocess. Eng. 2016, 21, 595–600. [Google Scholar] [CrossRef]
- Mao, X.-B.; Zhong, J.-J. Hyperproduction of Cordycepin by Two-Stage Dissolved Oxygen Control in Submerged Cultivation of Medicinal Mushroom Cordyceps militaris in Bioreactors. Biotechnol. Prog. 2004, 20, 1408–1413. [Google Scholar] [CrossRef]
- Raethong, N.; Wang, H.; Nielsen, J.; Vongsangnak, W. Optimizing cultivation of Cordyceps militaris for fast growth and cordycepin overproduction using rational design of synthetic media. Comput. Struct. Biotechnol. J. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Suparmin, A.; Kato, T.; Dohra, H.; Park, E.Y. Insight into cordycepin biosynthesis of Comparison between a liquid surface culture and a submerged culture through transcriptomic analysis. PLoS ONE 2017, 12, e0187052. [Google Scholar] [CrossRef]
- Chiang, S.-S.; Liang, Z.-C.; Wang, Y.-C.; Liang, C.-H. Effect of light-emitting diodes on the production of cordycepin, mannitol and adenosine in solid-state fermented rice by Cordyceps militaris. J. Food Compos. Anal. 2017, 60, 51–56. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Xu, C.P.; Sung, J.M.; Choi, J.W.; Yun, J.W. Optimization of submerged culture process for the production of mycelial biomass and exo-polysaccharides by Cordyceps militaris C738. J. Appl. Microbiol. 2003, 94, 120–126. [Google Scholar] [CrossRef]
| Substrate | Cordycepin (mg/g) | Adenosine (mg/g) | D-Mannitol (mg/g) | Crude Polysaccharides (mg/g) | Cost-Effectiveness/Sustainability | Reference |
|---|---|---|---|---|---|---|
| rice (Control) | 8.92 | 4.57 | 150 | 34.5 | Moderate cost, widely available | [72] |
| corn cob particles (CCP) | 9.45 | 5.86 | 100 | 26.9 | Very cost-effective, agro-industrial by-product | [72] |
| cottonseed shells (CS) | 8.6 | 3.98 | 120 | 23.4 | Low-cost agricultural waste | [72] |
| Italian poplar sawdust (IPS) | 2.7 | 1.22 | 80 | <17.25 (less than half of control) | Low-cost residue | [72] |
| spent substrate (SS) by the mushroom Flammulina velutipes | 2.6 | 1.49 | 75 | <17.25 | Low-cost residue | [72] |
| rice (Oryza sativa) | 1.1 | 2 | - | - | Moderate cost, widely available | [69] |
| rice, wheat (Triticum), and jowar (Sorghum bicolor) (1:1:1) | 1.6 | 2.8 | - | - | Moderate cost, widely available | [69] |
| rice, wheat, and bajra (Pennisetum glaucum) | 1.1 | 2.1 | - | - | Moderate cost, widely available | [69] |
| rice, wheat, jowar, and bajra | 1.7 | 2.9 | - | - | Moderate cost, widely available | [69] |
| rice, wheat, bajra, and ragi (Eleusine coracana) | 1.4 | 3 | - | - | Moderate cost, widely available | [69] |
| rice, wheat, bajra, and ragi (Eleusine coracana) | 1.8 | 2.9 | - | - | Moderate cost, widely available | [69] |
| rice, wheat, jowar, and sugarcane bagasse | 2 | 3.3 | - | - | Moderate cost, widely available | [69] |
| rice, wheat, jowar, ragi, and bajra | 2.1 | 2.8 | - | - | Moderate cost, widely available | [69] |
| rice, wheat, jowar, bajra, and sugarcane bagasse | 2 | 2.5 | - | - | Moderate cost, widely available | [69] |
| rice, wheat, jowar, bajra, sugarcane bagasse, and ragi | 1.9 | 2.8 | - | - | Moderate cost, widely available | [69] |
| brown rice | 8.21 | 0.5 | 117.94 | - | Moderate cost, widely available | [47] |
| brown rice + 1% peptone | 5.83 | 0.54 | 167.97 | - | Moderate cost, widely available | [47] |
| brown rice + 1% yeast extract | 4.74 | 0.66 | 124.98 | - | Moderate cost, widely available | [47] |
| brown rice + 1% ammonium sulfate | 10.05 | 0.48 | 90.25 | - | Moderate cost, widely available | [47] |
| brown rice + 1% monosodium glutamate | 11.93 | 0.36 | 121.13 | - | Moderate cost, widely available | [47] |
| plumule rice | 10.1 | 0.42 | 125.85 | - | Moderate cost, widely available | [47] |
| plumule rice + 1% peptone | 11.45 | 0.42 | 123.23 | - | Moderate cost, widely available | [47] |
| plumule rice + 1% yeast extract | 6.84 | 0.68 | 152.35 | - | Moderate cost, widely available | [47] |
| plumule rice + 1% ammonium sulfate | 8 | 0.64 | 135.05 | - | Moderate cost, widely available | [47] |
| plumule rice + 1% monosodium glutamate | 16.44 | 0.24 | 109.86 | - | Moderate cost, widely available | [47] |
| wheat | 19.12 | 0.48 | 101.76 | - | Moderate cost, widely available | [47] |
| wheat + 1% peptone | 13.54 | 0.54 | 110.82 | - | Moderate cost, widely available | [47] |
| wheat + 1% yeast extract | 13.59 | 0.55 | 125.04 | - | Moderate cost, widely available | [47] |
| wheat + 1% ammonium sulfate | 13.05 | 0.52 | 92.62 | - | Moderate cost, widely available | [47] |
| wheat + 1% monosodium glutamate | 22.14 | 0.61 | 198.35 | - | Moderate cost, widely available | [47] |
| pearl barley | 3.96 | 0.43 | 103.32 | - | Moderate cost, widely available | [47] |
| pearl barley + 1% peptone | 8.96 | 0.48 | 112.25 | - | Moderate cost, widely available | [47] |
| pearl barley + 1% yeast extract | 2.09 | 0.44 | 96.56 | - | Moderate cost, widely available | [47] |
| pearl barley + 1% ammonium sulfate | 10.92 | 0.61 | 141.32 | - | Moderate cost, widely available | [47] |
| pearl barley + 1% monosodium glutamate | 14.5 | 0.4 | 134.71 | - | Moderate cost, widely available | [47] |
| brown rice medium | 6.63 | - | - | - | Moderate cost, | [77] |
| soybean | 8.33 | - | - | - | Moderate cost | [74] |
| chickpea | 11.12 | Moderate cost | [74] | |||
| black bean | 10.43 | Moderate cost | [74] | |||
| silkworm pupae medium | 8.1 | - | - | - | High cost, limited scalability | [77] |
| Bombyx mori | 0.2 | - | - | - | High cost, limited scalability | [78] |
| Protaetia brevitarsis | 4.3 | - | - | - | High cost, limited scalability | [78] |
| Tenebrio molitor | 0.3 | - | - | - | High cost, limited scalability | [78] |
| Allomyrina dichotoma | 8.9 | - | - | - | Expensive, less scalable | [78] |
| Gryllus bimaculatus | 1.5 | - | - | - | Expensive, less scalable | [78] |
| Locusta migratoria | 3.4 | - | - | - | Expensive, less scalable | [78] |
| Brihaspa atrostigmella | 2.932 | 1.062 | - | - | Expensive, less scalable | [79] |
| Allomyrina dichotoma larva | 89.5 | - | - | - | Expensive, less scalable | [70] |
| Mineral | Reported Effect | Notes | Reference |
|---|---|---|---|
| Zn2+ | enhances cordycepin production (up to ~1.55 g/L with supplementation) | cofactor role in nucleic acid metabolism | [96] |
| Fe2+ | adding 1 g/L FeSO4 increased cordycepin by ~70% (to ~596.6 mg/L) | redirects purine metabolism toward adenosine branch | [96] |
| Mg2+ | present in medium (MgSO4·7H2O), supports biomass and metabolite biosynthesis | essential for enzymatic activity | [65] |
| Ca2+, K+, Na+, Se | listed as nutritional minerals present in C. militaris fruiting bodies and mycelia | recognized as essential nutrients | [99] |
| Fermentation Type | C/N Ratio (Carbon: Nitrogen) | Light Wavelength | Observations | Outcome Summary | Reference |
|---|---|---|---|---|---|
| Submerged fermentation | 2.66:1 (42 g/L glucose: 15.8 g/L peptone) | - | achieved maximum cordycepin production (345.4 mg/L; ~19.2 mg/L per day) | Optimal for cordycepin production with glucose as a carbon source; opimal for cell growth with galactose media | [101] |
| Submerged fermentation | 1:1.5 (by mass) | - | adenosine & cordycepin content increased at this ratio; declined at 1:3 | Reduced biomass and productivity | [18] |
| Submerged fermentation | 8:1 | - | 3.5-fold increase in cordycepin production | Optimal for cordycepin production on biomass with glucose as a carbon source | [105] |
| Submerged fermentation | 12:1 | - | Maximal mycelial growth | Optimal for C. militaris growth | [105] |
| surface fermentation | - | red light (620–630 nm) | Stimulated biomass formation | Optimal for mycelial growth and adenosine accumulation | [40] |
| surface fermentation | - | blue light (440–450 nm) | optimal for cordycepin synthesis | Activation of purine metabolism pathways and cordycepin increase | [40] |
| surface fermentation | - | - | Hypoxic growth conditions | Hypoxia induced upregulation of purine metabolism | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J. Influence of Culture Conditions on Bioactive Compounds in Cordyceps militaris: A Comprehensive Review. Foods 2025, 14, 3408. https://doi.org/10.3390/foods14193408
Park H-J. Influence of Culture Conditions on Bioactive Compounds in Cordyceps militaris: A Comprehensive Review. Foods. 2025; 14(19):3408. https://doi.org/10.3390/foods14193408
Chicago/Turabian StylePark, Hye-Jin. 2025. "Influence of Culture Conditions on Bioactive Compounds in Cordyceps militaris: A Comprehensive Review" Foods 14, no. 19: 3408. https://doi.org/10.3390/foods14193408
APA StylePark, H.-J. (2025). Influence of Culture Conditions on Bioactive Compounds in Cordyceps militaris: A Comprehensive Review. Foods, 14(19), 3408. https://doi.org/10.3390/foods14193408





