Emulsification Properties of Plant and Milk Protein Concentrate Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Analysis and pH Determination
2.3. Blending of Plant Protein Samples with MPC
2.4. Technofunctional Property Analysis
2.4.1. Emulsification
2.4.2. Apparent Viscosity (ηapp)
2.4.3. Water Holding Capacity (WHC) and Oil Binding Capacity (OBC)
2.4.4. Solubility
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis and Sample Properties
3.2. Technofunctional Properties
3.2.1. Apparent Viscosity (ηapp)
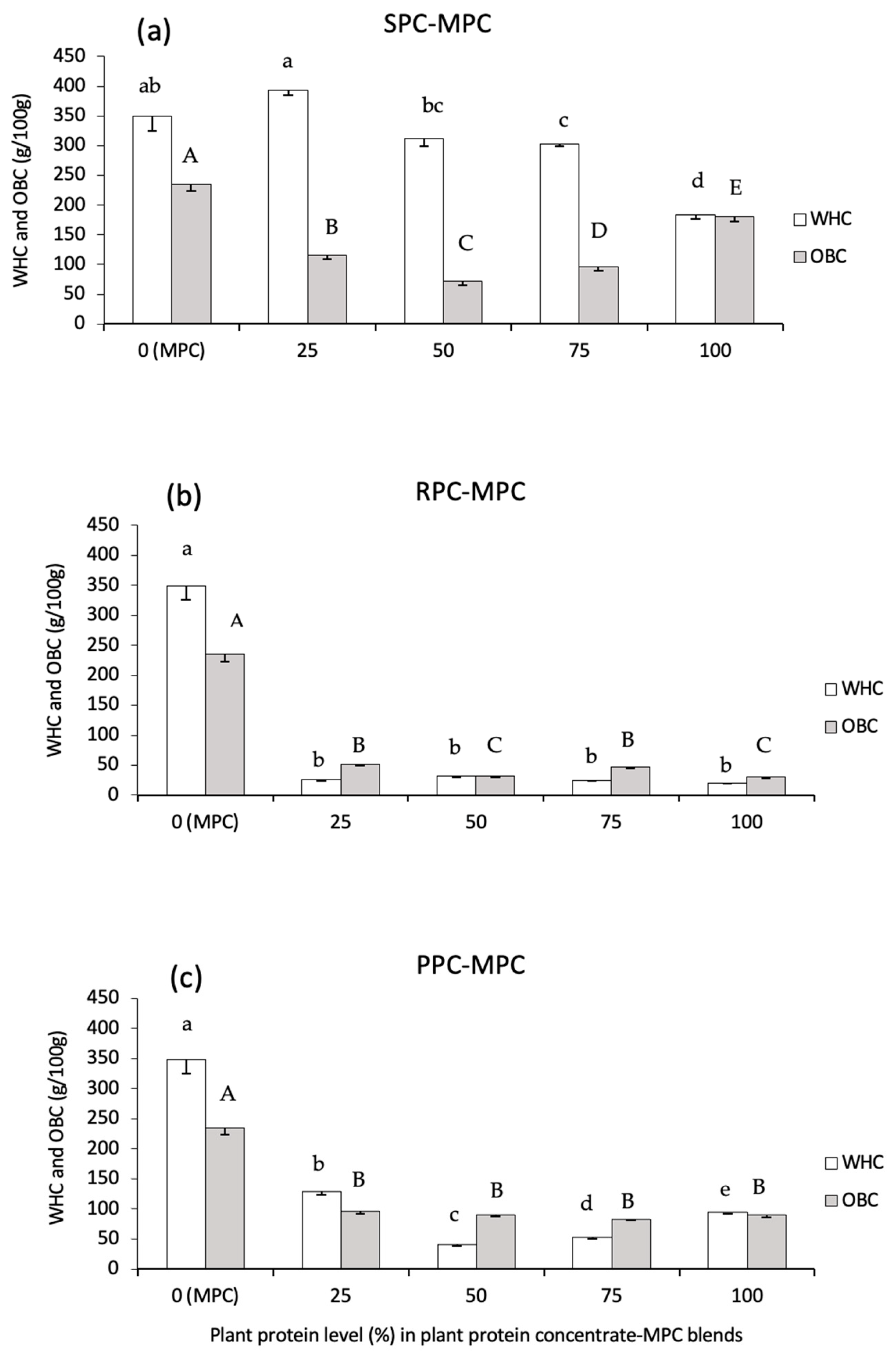
3.2.2. WHC and OBC
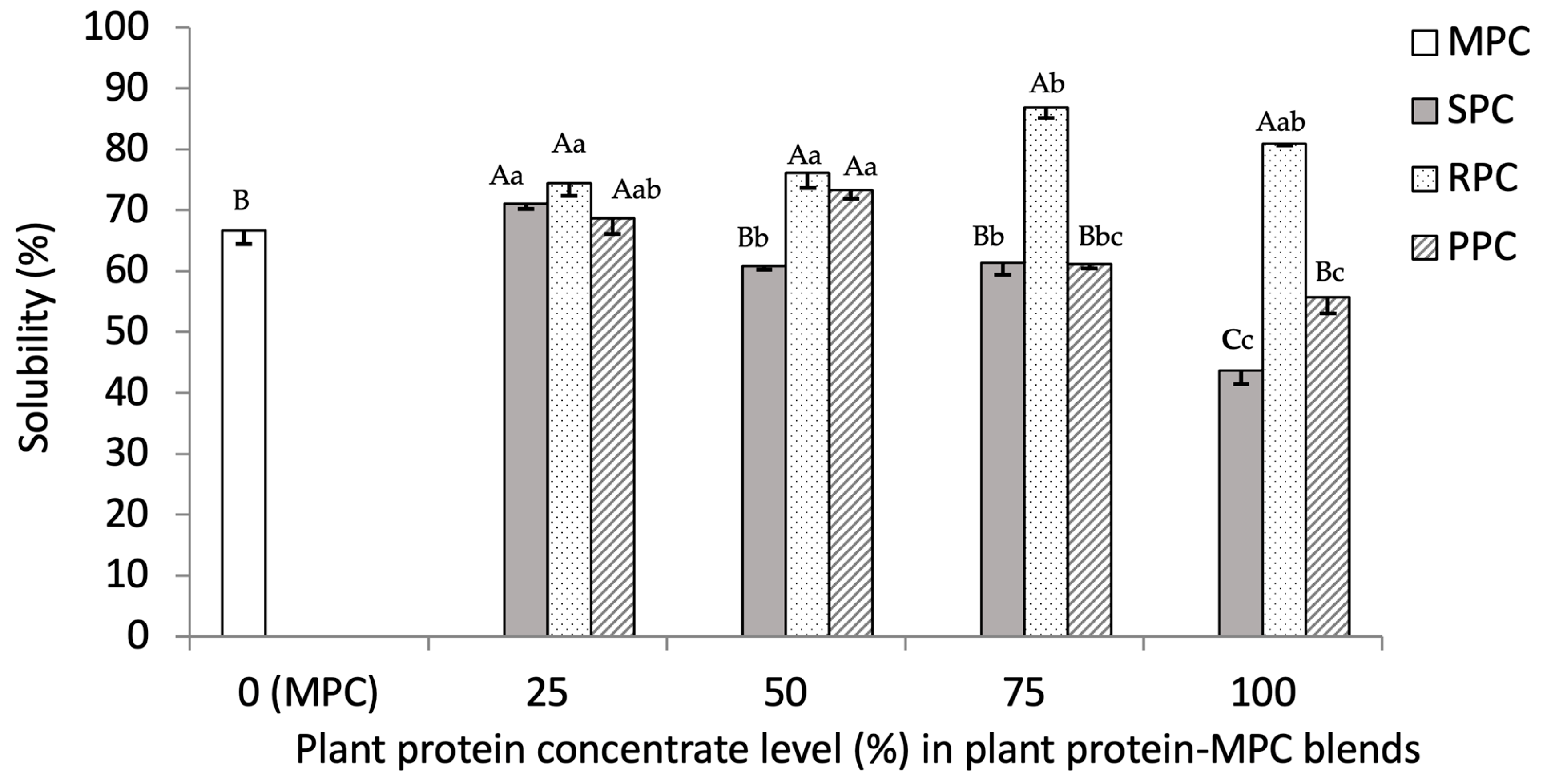
3.2.3. Solubility (%)
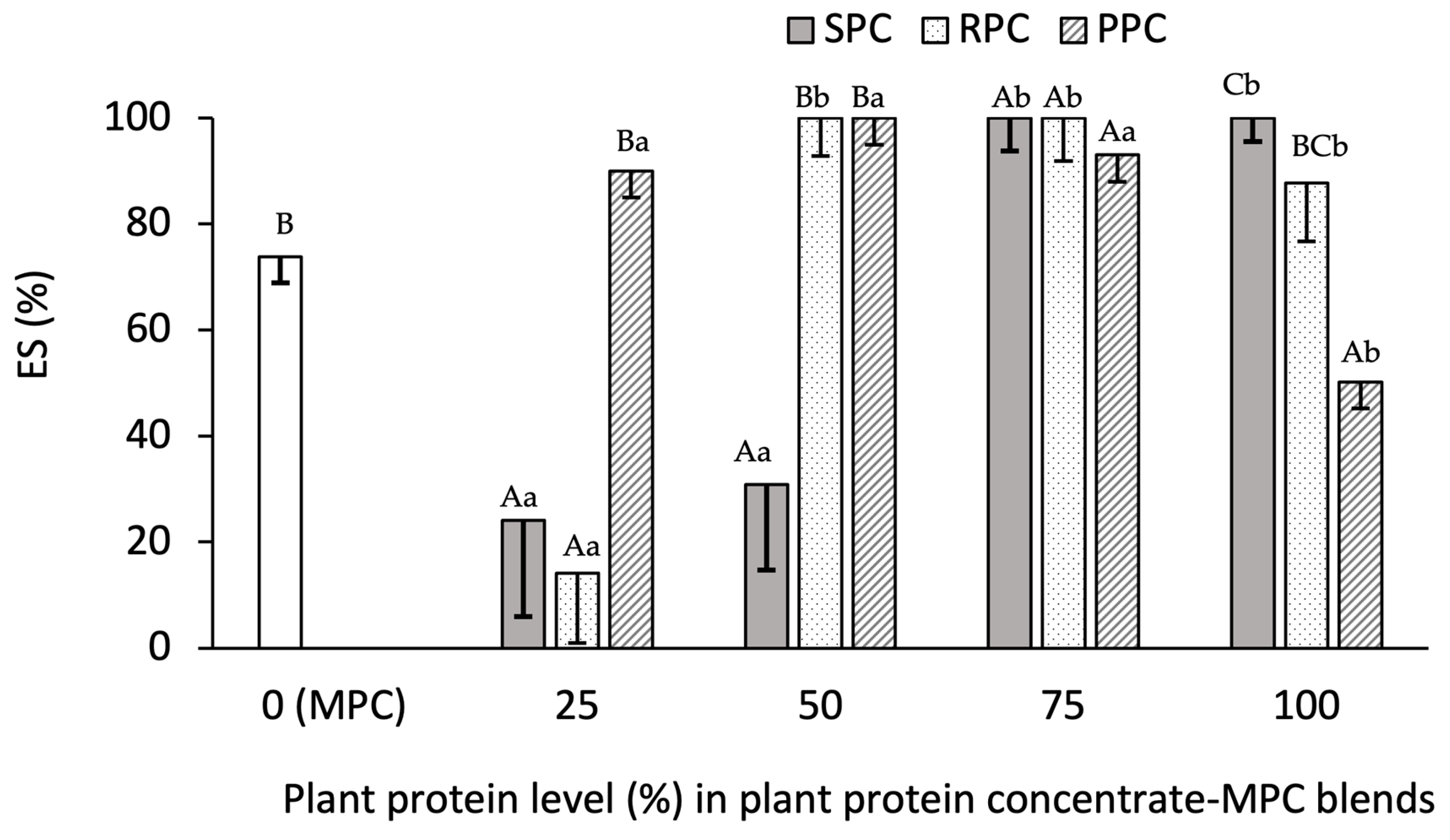
3.2.4. Emulsion Stability (ES%)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | amino acid |
| ηapp | apparent viscosity |
| CN | casein |
| DF | diafiltration |
| ES | emulsion stability |
| EAA | essential amino acids |
| MPC | milk protein concentrate |
| MPI | milk protein isolate |
| MW | molecular weight |
| OBC | oil binding capacity |
| PS | particle size |
| PP | pea protein |
| PPC | pea protein concentrate |
| PPI | pea protein isolate |
| PAGE | polyacrylamide gel electrophoresis |
| RP | rice protein |
| RPC | rice protein concentrate |
| RPI | rice protein isolate |
| SDS | sodium dodecyl sulfate |
| SP | soy protein |
| SPC | soy protein concentrate |
| SPI | soy protein isolate |
| SSA | specific surface area |
| TS | total solids |
| UF | ultrafiltration |
| WHC | water holding capacity |
| WP | whey protein |
| WPC | whey protein concentrate |
| WPH | whey protein hydrolysate |
| WPI | whey protein isolate |
References
- United Nations. World Population Prospects. 2024. Available online: https://population.un.org/wpp/ (accessed on 1 July 2025).
- Khalesi, M.; FitzGerald, R.J. Insolubility in milk protein concentrates: Potential causes and strategies to minimize its occurrence. Crit. Rev. Food Sci. Nutr. 2022, 62, 6973–6989. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Davis, J.P. Food protein functionality: A comprehensive approach. Food Hydrocolloid. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789–106811. [Google Scholar] [CrossRef]
- Khalesi, M.; FitzGerald, R.J. In vitro digestibility and antioxidant activity of plant protein isolate and milk protein concentrate blends. Catalysts 2021, 11, 787. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Fry, C.S.; Borack, M.S. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J. Nutr. 2013, 143, 410–416. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Kristensen, H.T.; Denon, Q.; Tavernier, I.; Gregersen, S.B.; Hammershøj, M.; Van der Meeren, P.; Dewettinck, K.; Dalsgaard, T.K. Improved food functional properties of pea protein isolate in blends and co-precipitates with whey protein isolate. Food Hydrocoll. 2021, 113, 106556. [Google Scholar] [CrossRef]
- Le Roux, L.; Mejean, S.; Chacon, R.; Lopez, C.; Dupont, D.; Deglaire, A.; Nau, F.; Jeantet, R. Plant proteins partially replacing dairy proteins greatly influence infant formula functionalities. LWT Food Sci. Technol. 2020, 120, 108891. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Schroen, K.; Martín-Gonzalez, M.F.S.; Berton-Carabin, C.C. Synergistic and antagonistic effects of plant and dairy protein blends on the physicochemical stability of lycopene-loaded emulsions. Food Hydrocoll. 2018, 81, 180–190. [Google Scholar] [CrossRef]
- Beliciu, C.M.; Moraru, C.I. Physico-chemical changes in heat treated micellar casein–Soy protein mixtures. LWT Food Sci. Technol. 2013, 54, 469–476. [Google Scholar] [CrossRef]
- Mao, C.; Xin, M.; Wu, W.; Wang, S.; Wu, J.; Cheng, Y.; Ma, H. Interfacial characteristics and digestive kinetics of emulsions fabricated by composite potato and whey protein. Int. J. Biol. Macromol. 2025, 308, 142446. [Google Scholar] [CrossRef]
- Wu, M.; He, X.; Feng, D.; Li, H.; Han, D.; Li, Q.; Zhao, B.; Li, N.; Liu, T.; Wang, J. The emulsifying properties, In vitro digestion characteristics and storage stability of high-pressure-homogenization-modified dual-protein-based emulsions. Foods 2023, 12, 4141. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xie, F.; Han, L.; Li, L.; Jiang, L.; Qi, B.; Li, Y. Soy/whey protein isolates: Interfacial properties and effects on the stability of oil-in-water emulsions. J. Sci. Food Agric. 2021, 101, 262–271. [Google Scholar] [CrossRef]
- Hinderink, E.B. Food Emulsions Stabilised by Blends of Plant and Dairy Proteins. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2021. [Google Scholar]
- Hu, T.; Chen, J.; He, X.; Tang, Y.; Sun, J.; Liu, C.; Dai, T. Complex plant protein prepared from rice protein and pea protein: Improve the thermal stability of betanin. Food Res. Int. 2023, 164, 112341. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, C.; Wu, Z.; Zhang, Z.; Xu, C. Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J. Food Biochem. 2020, 44, 13157. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, M.; FitzGerald, R.J. Physicochemical properties and water interactions of milk protein concentrate with two different levels of undenatured whey protein. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127516. [Google Scholar] [CrossRef]
- Kaspchak, E.; Silveira, J.L.; Igarashi-Mafra, L.; Mafra, M.R. Effect of antinutrients on heat-set gelation of soy, pea, and rice protein isolates. J. Food Sci. Technol. 2020, 57, 4201–4210. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.F.; Naeem, H.A.; Schmidt, K.A. Food protein functionality in a liquid system: A comparison of deamidated wheat protein with dairy and soy proteins. J. Food Sci. 2002, 67, 2896–2902. [Google Scholar] [CrossRef]
- Nadathur, S.R.; Wanasundara, J.P.D.; Scanlin, L. Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tarrega, A.; Ramírez-Sucre, M.O.; Vélez-Ruiz, J.F.; Costell, E. Effect of whey and pea protein blends on the rheological and sensory properties of protein-based systems flavoured with cocoa. J. Food Eng. 2012, 109, 467–474. [Google Scholar] [CrossRef]
- Yerramilli, M.; Longmore, N.; Ghosh, S. Improved stabilization of nanoemulsions by partial replacement of sodium caseinate with pea protein isolate. Food Hydrocolloid. 2017, 64, 99–111. [Google Scholar] [CrossRef]
- Dapčević-Hadnađev, T.; Dizdar, M.; Pojić, M.; Krstonošić, V.; Zychowsk, L.M.; Hadnađev, M. Emulsifying properties of hemp proteins: Effect of isolation technique. Food Hydrocoll. 2019, 89, 912–920. [Google Scholar] [CrossRef]
- Onwulata, C.I.; Tunick, M.H.; Mukhopadhyay, S. Flow behavior of mixed-protein incipient gels. Int. J. Food Prop. 2014, 17, 1283–1302. [Google Scholar] [CrossRef]
- Chew, J.H.; Liu, W.; Fu, N.; Gengenbach, T.; Chen, X.D.; Selomulya, C. Exploring the drying behaviour and particle formation of high solids milk protein concentrate. J. Food Eng. 2014, 143, 186–194. [Google Scholar] [CrossRef]
- Chavana, U.D.; McKenzie, D.B.; Shahidi, F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 2001, 74, 177–187. [Google Scholar] [CrossRef]
- Reinkensmeier, A.; Bußler, S.; Schlüter, O.; Rohn, S.; Rawel, H.M. Characterization of individual proteins in pea protein isolates and air classified samples. Food Res. Int. 2015, 76, 160–167. [Google Scholar] [CrossRef]
- Malaki Nik, A.; Tosh, S.M.; Woodrow, L.; Poysa, V.; Corredig, M. Effect of soy protein subunit composition and processing conditions on stability and particle size distribution of soymilk. LWT Food Sci. Technol. 2009, 42, 1245–1252. [Google Scholar] [CrossRef]
- Sagis, L.M.; Yang, J. Protein-stabilized interfaces in multiphase food: Comparing structure-function relations of plant-based and animal-based proteins. Curr. Opin. Food Sci. 2022, 43, 53–60. [Google Scholar] [CrossRef]
- da Silva, A.M.M.; Almeida, F.S.; Sato, A.C.K. Functional characterization of commercial plant proteins and their application on stabilization of emulsions. J. Food Eng. 2021, 292, 110277. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Sagis, L.; Schroën, K.; Berton-Carabin, C.C. Behavior of plant-dairy protein blends at air-water and oil-water interfaces. Colloids Surf. B. 2020, 192, 111015. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Sagis, L.; Schroën, K.; Berton-Carabin, C.C. Sequential adsorption and interfacial displacement in emulsions stabilized with plant-dairy protein blends. J. Colloid Interface Sci. 2021, 583, 704–713. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Hettiarachchy, N.S.; Rath, N. Extraction, denaturation and hydrophobic properties of rice flour proteins. J. Food Sci. 2006, 66, 229–232. [Google Scholar] [CrossRef]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, J.; Chen, J.; Wang, Y.; Dong, N.; Hu, C.; Chen, H.; Li, G.; Pan, X.; Wu, C. Preparation and stabilization of emulsions stabilized by mixed sodium caseinate and soy protein isolate. Food Hydrocoll. 2015, 51, 156–165. [Google Scholar] [CrossRef]
- Pizones Ruiz-Henestrosa, V.M.; Martinez, M.J.; Carrera Sánchez, C.; Rodríguez Patino, J.M.; Pilosof, A.M.R. Mixed soy globulins and β-lactoglobulin systems behaviour in aqueous solutions and at the air–water interface. Food Hydrocoll. 2014, 35 (Suppl. C), 106–114. [Google Scholar] [CrossRef]


| Protein Type | Plant Protein to Milk Protein Ratio | ||||
|---|---|---|---|---|---|
| 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | |
| Plant protein (g) | 20 | 15 | 10 | 5 | 0 |
| Milk protein (g) | 0 | 5 | 10 | 15 | 20 |
| Total weight of blend (g) | 20 | 20 | 20 | 20 | 20 |
| Sample | Moisture | Protein | Ash | Lipid | pH |
|---|---|---|---|---|---|
| (%) | |||||
| SPC | 5.58 ± 1.57 a | 81.11 ± 0.77 a | 5.47 ± 0.27 a | 1.79 ± 0.11 a | 7.15 ± 0.06 a |
| RPC | 1.64 ± 0.41 b | 80.04 ± 0.88 a | 5.50 ± 0.29 a | 9.70 ± 0.37 b | 6.09 ± 0.04 b |
| PPC | 3.86 ± 0.01 a | 71.01 ± 0.25 b | 6.48 ± 0.47 b | 8.13 ± 0.17 c | 8.00 ± 0.06 c |
| MPC | 4.82 ± 0.02 a | 84.17 ± 0.79 c | 6.96 ± 0.16 b | 1.31 ± 0.07 d | 7.09 ± 0.03 a |
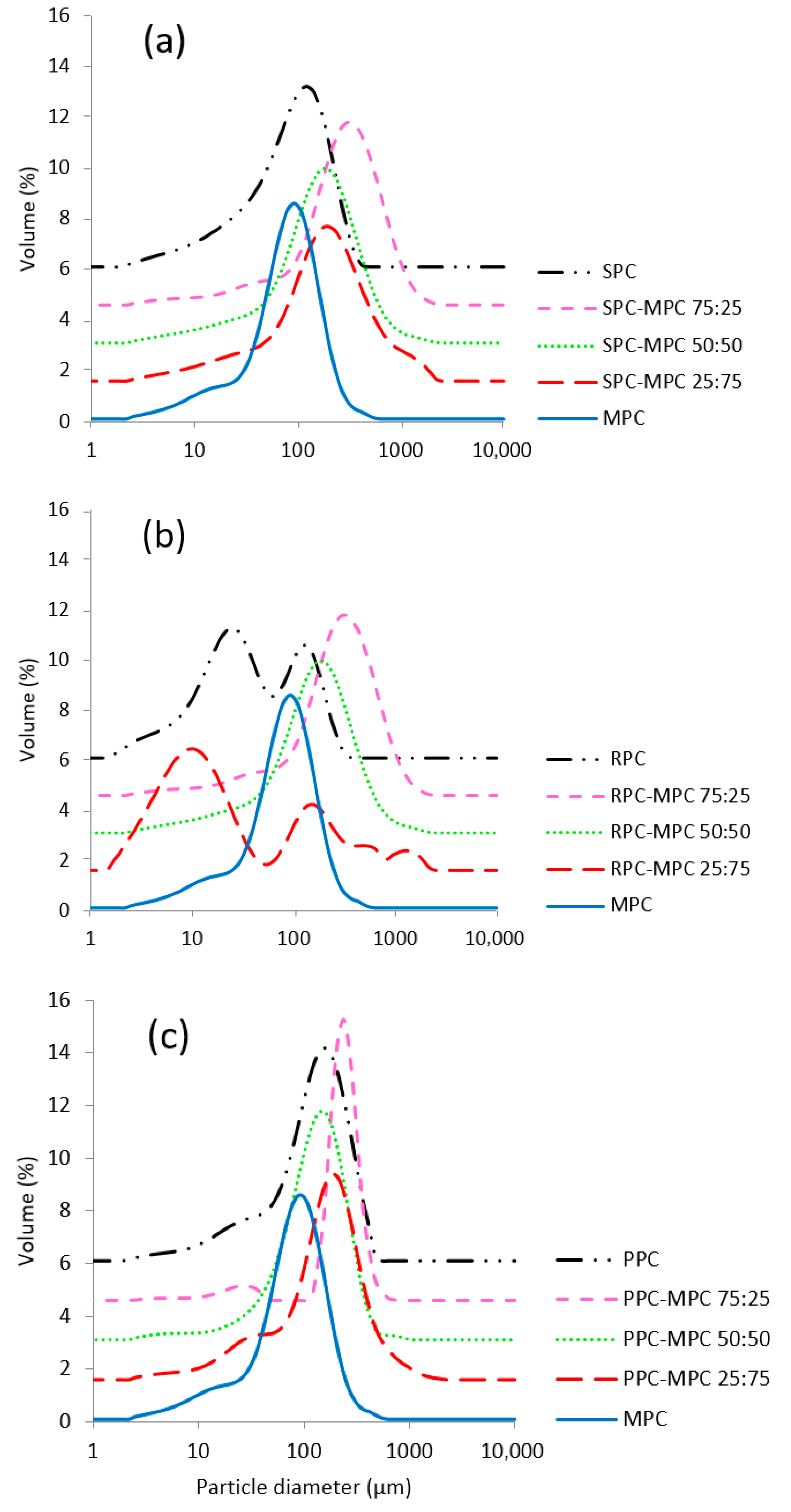
| D[3,2] (µm) | |||
|---|---|---|---|
| Plant protein–MPC | SPC–MPC | RPC–MPC | PPC–MPC |
| 0:100 | 38.55 ± 2.11 a | 38.55 ± 2.11 a | 38.55 ± 2.11 a |
| 25:75 | 59.47 ± 1.67 b | 9.42 ± 1.41 d | 58.60 ± 1.91 b |
| 50:50 | 59.37 ± 1.85 b | 22.07 ± 1.26 e | 61.35 ± 2.20 b |
| 75:25 | 88.36 ± 1.93 c | 12.18 ± 1.44 d | 98.50 ± 3.29 g |
| 100:0 | 34.82 ± 1.90 a | 17.04 ± 1.60 f | 47.89 ± 2.03 h |
| SSA (m2/g) | |||
| 0:100 | 0.16 ± 0.02 AC | 0.16 ± 0.02 AC | 0.16 ± 0.02 AC |
| 25:75 | 0.10 ± 0.02 BC | 0.64 ± 0.01 D | 0.10 ± 0.02 BC |
| 50:50 | 0.10 ± 0.02 BC | 0.27 ± 0.01 E | 0.10 ± 0.02 BC |
| 75:25 | 0.07 ± 0.02 B | 0.49 ± 0.01 F | 0.06 ± 0.03 B |
| 100:0 | 0.17 ± 0.02 A | 0.35 ± 0.02 G | 0.13 ± 0.02 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalesi, M.; Dowling, S.; Comerford, J.; Sweeney, C.; Esteghlal, S.; FitzGerald, R.J. Emulsification Properties of Plant and Milk Protein Concentrate Blends. Foods 2025, 14, 3406. https://doi.org/10.3390/foods14193406
Khalesi M, Dowling S, Comerford J, Sweeney C, Esteghlal S, FitzGerald RJ. Emulsification Properties of Plant and Milk Protein Concentrate Blends. Foods. 2025; 14(19):3406. https://doi.org/10.3390/foods14193406
Chicago/Turabian StyleKhalesi, Mohammadreza, Shauna Dowling, Jack Comerford, Ciara Sweeney, Sara Esteghlal, and Richard J. FitzGerald. 2025. "Emulsification Properties of Plant and Milk Protein Concentrate Blends" Foods 14, no. 19: 3406. https://doi.org/10.3390/foods14193406
APA StyleKhalesi, M., Dowling, S., Comerford, J., Sweeney, C., Esteghlal, S., & FitzGerald, R. J. (2025). Emulsification Properties of Plant and Milk Protein Concentrate Blends. Foods, 14(19), 3406. https://doi.org/10.3390/foods14193406







