Susceptibility of Conventional and Organic Chicken Breast and Thigh Meat to Lipid and Protein Oxidation During Heating and In Vitro Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Meat Sampling and Experimental Design
2.3. In Vitro Digestion
2.4. Meat Composition Analyses
2.4.1. Fatty Acids
2.4.2. Heme-Fe
2.4.3. α-Tocopherol
2.4.4. Carnosine and Anserine
2.5. Oxidation Products in Meats and Digests
2.5.1. Lipid Oxidation Products
2.5.2. Protein Oxidation Products
2.6. Statistical Analysis
3. Results
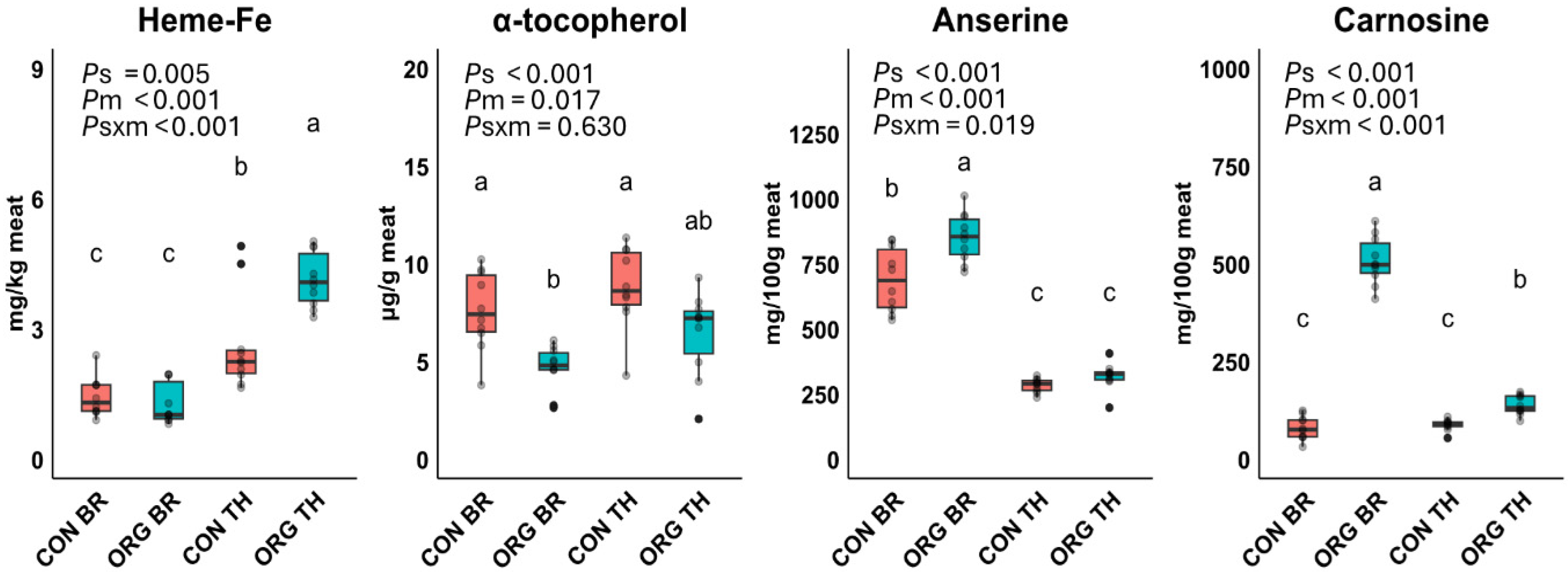
3.1. Experiment 1: Meat Composition
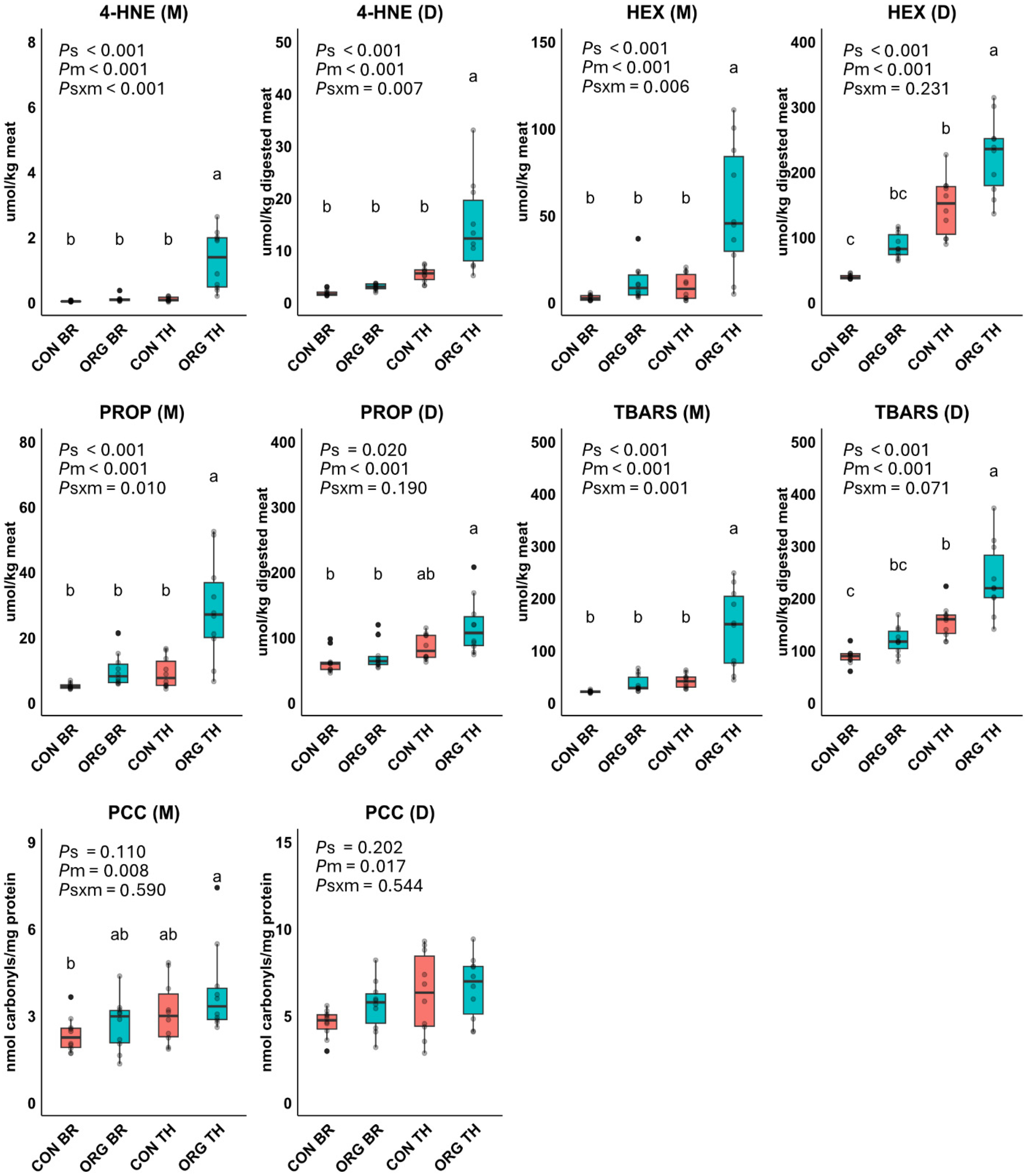
3.2. Experiment 1: Lipid and Protein Oxidation Products
3.3. Experiment 1: Relationships Among Compositional Variables and Oxidation Products
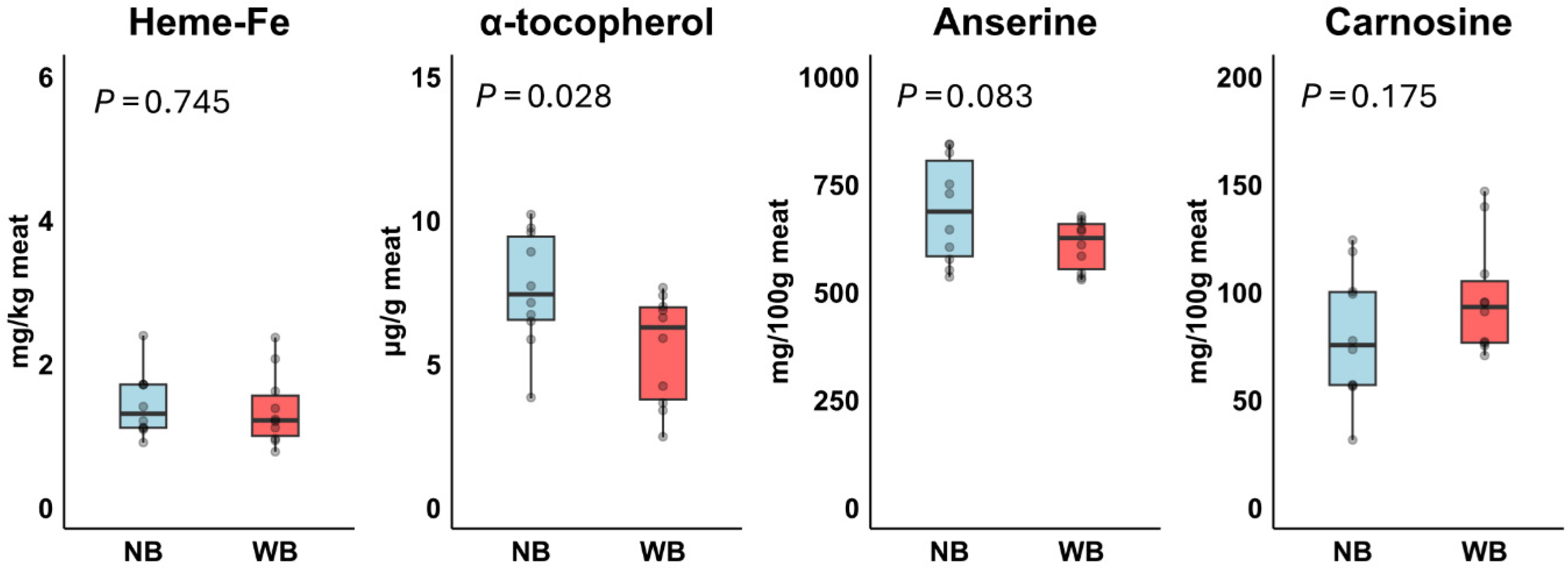
3.4. Experiment 2: Meat Composition
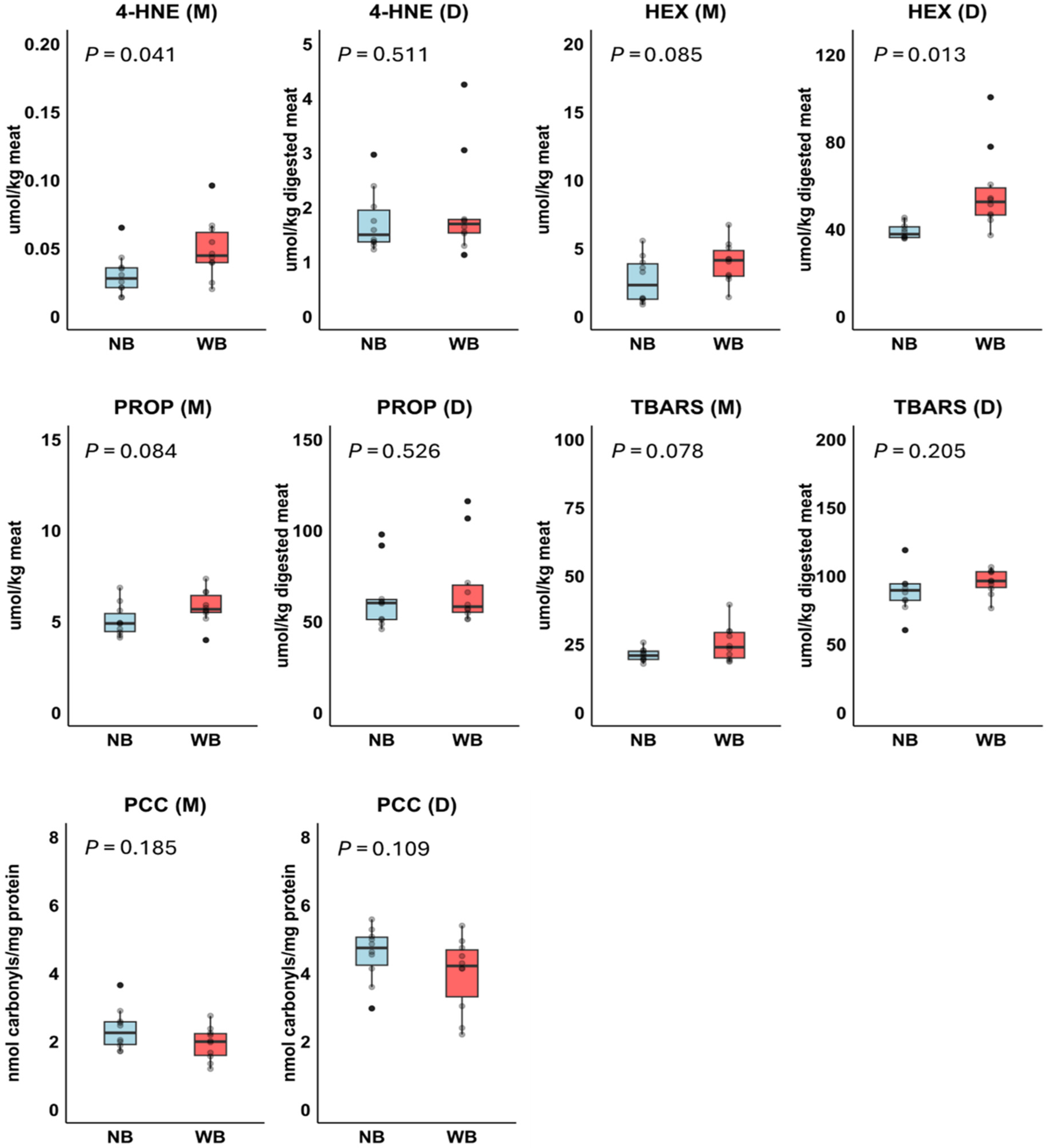
3.5. Experiment 2: Lipid and Protein Oxidation Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| CON | conventional production system |
| DNPH | 2,4-dinitrophenylhydrazine |
| FA | fatty acid |
| HCD | histidine-containing dipeptides |
| HEX | hexanal |
| LDL | low-density lipoproteins |
| MDA | malondialdehyde |
| MUFA | monounsaturated fatty acids |
| ORG | organic production system |
| PCC | protein carbonyl compounds |
| PROP | propanal |
| PUFA | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| SFA | saturated fatty acids |
| TBA | thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
References
- FAO. Meat and meat products: Market summaries. In Food Outlook—June 2024; Markets and Trade Division, Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024; pp. 7–8. [Google Scholar] [CrossRef]
- Albrecht, A.; Hebel, M.; Mittler, M.; Hurck, C.; Kustwan, K.; Heitkönig, B.; Bitschinski, D.; Kreyenschmidt, J. Influence of Different Production Systems on the Quality and Shelf Life of Poultry Meat: A Case Study in the German Sector. J. Food Qual. 2019, 2019, 3718057. [Google Scholar] [CrossRef]
- European Parliament; Directorate General for Parliamentary Research Services. The EU Poultry Meat and Egg Sector: Main 626 Features, Challenges and Prospects. In Depth Analysis; Publications Office: Luxembourg, 2019. [Google Scholar]
- Bordignon, F.; Xiccato, G.; Bošković Cabrol, M.; Birolo, M.; Trocino, A. Factors Affecting Breast Myopathies in Broiler Chickens and Quality of Defective Meat: A Meta-Analysis. Front. Physiol. 2022, 13, 933235. [Google Scholar] [CrossRef]
- Sihvo, H.-K.; Immonen, K.; Puolanne, E. Myodegeneration with Fibrosis and Regeneration in the Pectoralis Major Muscle of Broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Che, S.; Hall, P. Spaghetti Meat and Woody Breast Myopathies in Broiler Chickens: Similarities and Differences. Front. Physiol. 2024, 15, 1453322. [Google Scholar] [CrossRef]
- Gálvez, F.; Domínguez, R.; Maggiolino, A.; Pateiro, M.; Carballo, J.; De Palo, P.; Barba, F.J.; Lorenzo, J.M. Meat Quality of Commercial Chickens Reared in Different Production Systems: Industrial, Range and Organic. Ann. Anim. Sci. 2020, 20, 263–285. [Google Scholar] [CrossRef]
- Escobedo Del Bosque, C.I.; Risius, A.; Spiller, A.; Busch, G. Consumers’ Opinions and Expectations of an “Ideal Chicken Farm” and Their Willingness to Purchase a Whole Chicken from This Farm. Front. Anim. Sci. 2021, 2, 682477. [Google Scholar] [CrossRef]
- Baéza, E.; Guillier, L.; Petracci, M. Review: Production Factors Affecting Poultry Carcass and Meat Quality Attributes. Animal 2022, 16, 100331. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, P.; Ehsani, A.; Vaez Torshizi, R.; Masoudi, A.A. A Meta-Analysis Comparing the Composition and Quality Differences between Chicken Meats Produced under the Free-Range and Conventional Systems. World’s Poult. Sci. J. 2022, 78, 353–375. [Google Scholar] [CrossRef]
- Michalczuk, M.; Zdanowska-Sąsiadek, Ż.; Damaziak, K.; Niemiec, J. Influence of Indoor and Outdoor Systems on Meat Quality of Slow-Growing Chickens. CyTA-J. Food 2017, 15, 15–20. [Google Scholar] [CrossRef]
- Barbaresi, S.; Maertens, L.; Claeys, E.; Derave, W.; De Smet, S. Differences in Muscle Histidine-containing Dipeptides in Broilers. J. Sci. Food Agric. 2019, 99, 5680–5686. [Google Scholar] [CrossRef]
- Bošković Cabrol, M.; Xiccato, G.; Petracci, M.; Hernández Pérez, P.; Mayr Marangon, C.; Trocino, A. Nutritional Composition, Technological Quality, and Sensory Attributes of Chicken Breast Meat Affected by White Striping, Wooden Breast, and Spaghetti Meat: A Comprehensive Evaluation. Foods 2024, 13, 4007. [Google Scholar] [CrossRef]
- Min, B.; Nam, K.C.; Cordray, J.; Ahn, D.U. Endogenous Factors Affecting Oxidative Stability of Beef Loin, Pork Loin, and Chicken Breast and Thigh Meats. J. Food Sci. 2008, 73, C439–C446. [Google Scholar] [CrossRef]
- Van Hecke, T.; Van Camp, J.; De Smet, S. Oxidation During Digestion of Meat: Interactions with the Diet and Helicobacter pylori Gastritis, and Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2017, 16, 214–233. [Google Scholar] [CrossRef]
- Van Hecke, T.; Vanden Bussche, J.; Vanhaecke, L.; Vossen, E.; Van Camp, J.; De Smet, S. Nitrite Curing of Chicken, Pork, and Beef Inhibits Oxidation but Does Not Affect N-Nitroso Compound (NOC)-Specific DNA Adduct Formation during in Vitro Digestion. J. Agric. Food Chem. 2014, 62, 1980–1988. [Google Scholar] [CrossRef]
- Steppeler, C.; Haugen, J.-E.; Rødbotten, R.; Kirkhus, B. Formation of Malondialdehyde, 4-Hydroxynonenal, and 4-Hydroxyhexenal during in Vitro Digestion of Cooked Beef, Pork, Chicken, and Salmon. J. Agric. Food Chem. 2016, 64, 487–496. [Google Scholar] [CrossRef]
- Estévez, M. Protein Carbonyls in Meat Systems: A Review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Gorelik, S.; Kanner, J.; Schurr, D.; Kohen, R. A Rational Approach to Prevent Postprandial Modification of LDL by Dietary Polyphenols. J. Funct. Foods 2013, 5, 163–169. [Google Scholar] [CrossRef]
- Nègre-Salvayre, A.; Garoby-Salom, S.; Swiader, A.; Rouahi, M.; Pucelle, M.; Salvayre, R. Proatherogenic Effects of 4-Hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Luna, C. Dietary Protein Oxidation: A Silent Threat to Human Health? Crit. Rev. Food Sci. Nutr. 2017, 57, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- European Union. Council Directive 2007/43/EC of 28 June 2007 Laying down Minimum Rules for the Protection of Chickens Kept for Meat Production. Off. J. Eur. Union 2007, L 182, 19–28. [Google Scholar]
- European Commission. Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control. Off. J. Eur. Union 2008, L 250, 1–84. [Google Scholar]
- Tijare, V.V.; Yang, F.L.; Kuttappan, V.A.; Alvarado, C.Z.; Coon, C.N.; Owens, C.M. Meat Quality of Broiler Breast Fillets with White Striping and Woody Breast Muscle Myopathies. Poult. Sci. 2016, 95, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Versantvoort, C.H.M.; Oomen, A.G.; Van De Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in Vitro Digestion Model in Assessing the Bioaccessibility of Mycotoxins from Food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Basso, V.; De Smet, S. Lipid and Protein Oxidation during in Vitro Gastrointestinal Digestion of Pork under Helicobacter Pylori Gastritis Conditions. J. Agric. Food Chem. 2018, 66, 13000–13010. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Raes, K.; Smet, S.D.; Demeyer, D. Effect of Double-Muscling in Belgian Blue Young Bulls on the Intramuscular Fatty Acid Composition with Emphasis on Conjugated Linoleic Acid and Polyunsaturated Fatty Acids. Anim. Sci. 2001, 73, 253–260. [Google Scholar] [CrossRef]
- Hornsey, H.C. The Colour of Cooked Cured Pork. I.—Estimation of the Nitric oxide-Haem Pigments. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- Claeys, E.; Vossen, E.; De Smet, S. Determination of A-tocopherol by Reversed-phase HPLC in Feed and Animal-derived Foods without Saponification. J. Sci. Food Agric. 2016, 96, 522–529. [Google Scholar] [CrossRef]
- Kobe, R.; Ishihara, Y.; Takano, J.; Kitami, H. Simultaneous Determination of Anserine and Carnosine in Chicken Meat by Hydrophilic Interaction Chromatography on an Aminopropyl Bonded Silica Gel Column. Bunseki Kagaku 2011, 60, 859–863. [Google Scholar] [CrossRef]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The Potential of Herbs and Spices to Reduce Lipid Oxidation during Heating and Gastrointestinal Digestion of a Beef Product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Grotto, D.; Santa Maria, L.D.; Boeira, S.; Valentini, J.; Charão, M.F.; Moro, A.M.; Nascimento, P.C.; Pomblum, V.J.; Garcia, S.C. Rapid Quantification of Malondialdehyde in Plasma by High Performance Liquid Chromatography–Visible Detection. J. Pharm. Biomed. Anal. 2007, 43, 619–624. [Google Scholar] [CrossRef]
- Ganhão, R.; Morcuende, D.; Estévez, M. Protein Oxidation in Emulsified Cooked Burger Patties with Added Fruit Extracts: Influence on Colour and Texture Deterioration during Chill Storage. Meat Sci. 2010, 85, 402–409. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of Freezing Temperature and Duration of Frozen Storage on Lipid and Protein Oxidation in Chicken Meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Pellattiero, E.; Tasoniero, G.; Cullere, M.; Gleeson, E.; Baldan, G.; Contiero, B.; Dalle Zotte, A. Are Meat Quality Traits and Sensory Attributes in Favor of Slow-Growing Chickens? Animals 2020, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mugnai, C.; Mattioli, S.; Rosati, A.; Ruggeri, S.; Ranucci, D.; Castellini, C. Transfer of Bioactive Compounds from Pasture to Meat in Organic Free-Range Chickens. Poult. Sci. 2016, 95, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; Tagliabue, M.M.; Akbarian, A.; Ovyn, A.; De Smet, S. Oxidative Status, Meat Quality and Fatty Acid Profile of Broiler Chickens Reared under Free-Range and Severely Feed-Restricted Conditions Compared with Conventional Indoor Rearing. Avian Biol. Res. 2014, 7, 74–82. [Google Scholar] [CrossRef]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of Organic Production System on Broiler Carcass and Meat Quality. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef]
- Mattioli, S.; Mancinelli, A.C.; Dal Bosco, A.; Ciarelli, C.; Amato, M.G.; Angelucci, E.; Chiattelli, D.; Castellini, C. Intake of Nutrients (Polyunsaturated Fatty Acids, Tocols, and Carotenes) and Storage Efficiency in Different Slow-Growing Chickens Genotypes Reared in Extensive Systems. PLoS ONE 2022, 17, e0275527. [Google Scholar] [CrossRef]
- Bonnefous, C.; Collin, A.; Guilloteau, L.A.; Germain, K.; Ravon, L.; Bordeau, T.; Chartrin, P.; Godet, E.; Cailleau-Audouin, E.; Couroussé, N.; et al. Performance, Meat Quality and Blood Parameters in Four Strains of Organic Broilers Differ According to Range Use. Sci. Rep. 2024, 14, 30854. [Google Scholar] [CrossRef]
- Mattioli, S.; Angelucci, E.; Castellini, C.; Cartoni Mancinelli, A.; Chenggang, W.; Di Federico, F.; Chiattelli, D.; Dal Bosco, A. Effect of Genotype and Outdoor Enrichment on Productive Performance and Meat Quality of Slow Growing Chickens. Poult. Sci. 2024, 103, 104131. [Google Scholar] [CrossRef]
- Perini, F.; Wu, Z.; Cartoni Mancinelli, A.; Soglia, D.; Schiavone, A.; Mattioli, S.; Mugnai, C.; Castellini, C.; Smith, J.; Lasagna, E. RNAseq Reveals Modulation of Genes Involved in Fatty Acid Biosynthesis in Chicken Liver According to Genetic Background, Sex, and Diet. Anim. Genet. 2023, 54, 338–354. [Google Scholar] [CrossRef]
- Coudert, E.; Baéza, E.; Chartrin, P.; Jimenez, J.; Cailleau-Audouin, E.; Bordeau, T.; Berri, C. Slow and Fast-Growing Chickens Use Different Antioxidant Pathways to Maintain Their Redox Balance during Postnatal Growth. Animals 2023, 13, 1160. [Google Scholar] [CrossRef]
- Jung, S.; Bae, Y.S.; Kim, H.J.; Jayasena, D.D.; Lee, J.H.; Park, H.B.; Heo, K.N.; Jo, C. Carnosine, Anserine, Creatine, and Inosine 5′-Monophosphate Contents in Breast and Thigh Meats from 5 Lines of Korean Native Chicken. Poult. Sci. 2013, 92, 3275–3282. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Charoensin, S.; Laopaiboon, B.; Boonkum, W.; Phetcharaburanin, J.; Villareal, M.O.; Isoda, H.; Duangjinda, M. Thai Native Chicken as a Potential Functional Meat Source Rich in Anserine, Anserine/Carnosine, and Antioxidant Substances. Animals 2021, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhuang, H.; Zhou, G.; Zhang, J. Investigation of Inhibition of Lipid Oxidation by L-Carnosine Using an Oxidized-Myoglobin-Mediated Washed Fish Muscle System. LWT-Food Sci. Technol. 2018, 97, 703–710. [Google Scholar] [CrossRef]
- Van Hecke, T.; Goethals, S.; Vossen, E.; De Smet, S. Long-Chain n-3 PUFA Content and n-6/n-3 PUFA Ratio in Mammal, Poultry, and Fish Muscles Largely Explain Differential Protein and Lipid Oxidation Profiles Following In Vitro Gastrointestinal Digestion. Mol. Nutr. Food Res. 2019, 63, 1900404. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by Polyphenols of Postprandial Human Plasma and Low-Density Lipoprotein Modification: The Stomach as a Bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef]
- Kenmogne-Domguia, H.B.; Meynier, A.; Boulanger, C.; Genot, C. Lipid Oxidation in Food Emulsions Under Gastrointestinal-Simulated Conditions: The Key Role of Endogenous Tocopherols and Initiator. Food Dig. 2012, 3, 46–52. [Google Scholar] [CrossRef]
- Larsson, K.; Cavonius, L.; Alminger, M.; Undeland, I. Oxidation of Cod Liver Oil during Gastrointestinal in Vitro Digestion. J. Agric. Food Chem. 2012, 60, 7556–7564. [Google Scholar] [CrossRef]
- Kenmogne-Domguia, H.B.; Moisan, S.; Viau, M.; Genot, C.; Meynier, A. The Initial Characteristics of Marine Oil Emulsions and the Composition of the Media Inflect Lipid Oxidation during in Vitro Gastrointestinal Digestion. Food Chem. 2014, 152, 146–154. [Google Scholar] [CrossRef]
- Mahecha, L.; Dannenberger, D.; Nuernberg, K.; Nuernberg, G.; Hagemann, E.; Martin, J. Relationship between Lipid Peroxidation and Antioxidant Status in the Muscle of German Holstein Bulls Fed n-3 and n-6 PUFA-Enriched Diets. J. Agric. Food Chem. 2010, 58, 8407–8413. [Google Scholar] [CrossRef]
- Van Hecke, T.; Wouters, A.; Rombouts, C.; Izzati, T.; Berardo, A.; Vossen, E.; Claeys, E.; Van Camp, J.; Raes, K.; Vanhaecke, L.; et al. Reducing Compounds Equivocally Influence Oxidation during Digestion of a High-Fat Beef Product, Which Promotes Cytotoxicity in Colorectal Carcinoma Cell Lines. J. Agric. Food Chem. 2016, 64, 1600–1609. [Google Scholar] [CrossRef]
- Barski, O.A.; Xie, Z.; Baba, S.P.; Sithu, S.D.; Agarwal, A.; Cai, J.; Bhatnagar, A.; Srivastava, S. Dietary Carnosine Prevents Early Atherosclerotic Lesion Formation in Apolipoprotein E–Null Mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1162–1170. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yaylayan, V.; Palin, M.; Ngapo, T.M.; Cliche, S.; Sabik, H.; Gariépy, C. Dual Effects of Dietary Carnosine during in Vitro Digestion of a Western Meal Model with Added Ascorbic Acid. J. Food Sci. 2024, 89, 710–726. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yaylayan, V.; Palin, M.; Sullivan, B.; Fortin, F.; Cliche, S.; Sabik, H.; Gariépy, C. Protective Effects of Dietary Carnosine during In-vitro Digestion of Pork Differing in Fat Content and Cooking Conditions. J. Food Biochem. 2021, 45, e13624. [Google Scholar] [CrossRef]
- Hu, Y.; Cui, W.; Zhou, H.; Wang, Z.; Xu, B. Co-oxidation Behavior between Lipid and Protein in Muscle Food: A Review. Food Sci. Anim. Prod. 2025, 3, 9240110. [Google Scholar] [CrossRef]
- Santé-Lhoutellier, V.; Engel, E.; Aubry, L.; Gatellier, P. Effect of Animal (Lamb) Diet and Meat Storage on Myofibrillar Protein Oxidation and in Vitro Digestibility. Meat Sci. 2008, 79, 777–783. [Google Scholar] [CrossRef]
- Soglia, F.; Laghi, L.; Canonico, L.; Cavani, C.; Petracci, M. Functional Property Issues in Broiler Breast Meat Related to Emerging Muscle Abnormalities. Food Res. Int. 2016, 89, 1071–1076. [Google Scholar] [CrossRef]
- Thanatsang, K.V.; Malila, Y.; Arayamethakorn, S.; Srimarut, Y.; Tatiyaborworntham, N.; Uengwetwanit, T.; Panya, A.; Rungrassamee, W.; Visessanguan, W. Nutritional Properties and Oxidative Indices of Broiler Breast Meat Affected by Wooden Breast Abnormality. Animals 2020, 10, 2272. [Google Scholar] [CrossRef] [PubMed]
- Abasht, B.; Mutryn, M.F.; Michalek, R.D.; Lee, W.R. Oxidative Stress and Metabolic Perturbations in Wooden Breast Disorder in Chickens. PLoS ONE 2016, 11, e0153750. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Zhao, X.; Xu, X.; Li, J.; Zhang, L.; Gao, F. Physiochemical Properties, Protein and Metabolite Profiles of Muscle Exudate of Chicken Meat Affected by Wooden Breast Myopathy. Food Chem. 2020, 316, 126271. [Google Scholar] [CrossRef] [PubMed]
- Sihvo, H.-K.; Airas, N.; Lindén, J.; Puolanne, E. Pectoral Vessel Density and Early Ultrastructural Changes in Broiler Chicken Wooden Breast Myopathy. J. Comp. Pathol. 2018, 161, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, X.; Puolanne, E.; Ertbjerg, P. Effect of Wooden Breast Degree on Lipid and Protein Oxidation and Citrate Synthase Activity of Chicken Pectoralis Major Muscle. LWT-Food Sci. Technol. 2022, 154, 112884. [Google Scholar] [CrossRef]
- Estévez, M. Oxidative Damage to Poultry: From Farm to Fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Rocha, T.C.; Delgado, J.; Díaz-Velasco, S.; Madruga, M.S.; Estévez, M. Deciphering the Underlying Mechanisms of the Oxidative Perturbations and Impaired Meat Quality in Wooden Breast Myopathy by Label-Free Quantitative MS-Based Proteomics. Food Chem. 2023, 423, 136314. [Google Scholar] [CrossRef]
- Prache, S.; Lebret, B.; Baéza, E.; Martin, B.; Gautron, J.; Feidt, C.; Médale, F.; Corraze, G.; Raulet, M.; Lefèvre, F.; et al. Review: Quality and Authentication of Organic Animal Products in Europe. Animal 2022, 16, 100405. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]

| Production System | ||
|---|---|---|
| Characteristics | Conventional | Organic |
| Breed | Ross 308 | Sasso (Ruby XL) |
| Age at slaughter (days) | 39–42 | 73–76 |
| Finishing phase (days) | 14 | 21 |
| Live weight at slaughter (kg) | 2.63–2.85 | 2.30–2.50 |
| Stocking density (kg/m2) | 42 | 21 |
| Outdoor access | No | Yes |
| Indoor enrichments | No | Yes |
| Diet composition (finisher phase) | ||
| Protein % | 21.33 | 20.56 |
| Fat % | 7.40 | 4.00 |
| Ash % | 5.60 | 5.40 |
| Fiber % | 2.60 | 2.90 |
| Methionine % | 0.75 | 0.30 |
| Lysine % | 1.25 | 0.94 |
| Calcium % | 0.73 | 0.80 |
| Phosphorus % | 0.43 | 0.60 |
| Na % | 0.16 | 0.20 |
| Vitamin A (IU/kg) | 13,200 | 10,000 |
| Vitamin D3 (IU/kg) | 3630 | 3000 |
| Vitamin E (mg/kg) | 50 | 55 |
| Se (mg/kg) | 0.52 | 0.30 |
| Iron (mg/kg) | 19.80 | 70 |
| Zn (mg/kg) | 66 | 70 |
| Cu (mg/kg) | 19.84 | 15.40 |
| Trait | Unit | Breast | Thigh | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| CON | ORG | CON | ORG | RMSE | S | M | S × M | ||
| Total FA | g/100 g | 1.06 ± 0.23 b | 0.77 ± 0.07 b | 2.79 ± 0.80 a | 3.05 ± 0.62 a | 0.49 | 0.921 | <0.001 | 0.102 |
| SFA | mg/100 g | 343 ± 80.2 b | 244 ± 23.8 b | 896 ± 262 a | 875 ± 188 a | 158.5 | 0.263 | <0.001 | 0.458 |
| MUFA | mg/100 g | 316 ± 91.0 b | 183± 35.1 b | 906 ± 287 a | 878 ± 235 a | 182.3 | 0.191 | <0.001 | 0.398 |
| PUFA | mg/100 g | 267 ± 51.3 c | 235 ± 24.4 c | 685 ± 193 b | 981 ± 164 a | 123.1 | 0.002 | <0.001 | <0.001 |
| n-3 PUFA | mg/100 g | 22.7 ± 5.63 c | 21.8 ± 2.55 c | 56.9 ± 16.4 b | 85.5 ± 17.1 a | 11.60 | <0.001 | <0.001 | <0.001 |
| ALA | mg/100 g | 10.7 ± 4.02 c | 7.68 ± 1.19 c | 41.9 ± 13.7 b | 63.4 ± 16.7 a | 10.44 | 0.011 | <0.001 | 0.001 |
| EPA | mg/100 g | 2.21 ± 0.87 ab | 1.39 ± 0.45 b | 2.68 ± 1.27 a | 2.28 ± 0.64 ab | 0.81 | 0.031 | 0.017 | 0.447 |
| DHA | mg/100 g | 2.84 ± 0.88 c | 4.94 ± 1.15 b | 3.42 ± 1.18 c | 7.52 ± 1.39 a | 1.10 | <0.001 | <0.001 | 0.010 |
| n-6 PUFA | mg/100 g | 245 ± 49.8 c | 214 ± 22.36 c | 627 ± 184 b | 894 ± 149 a | 116 | 0.004 | <0.001 | <0.001 |
| LA | mg/100 g | 197 ± 46.7 c | 157 ± 18.9 c | 565 ± 177 b | 803 ± 145 a | 111 | 0.011 | <0.001 | <0.001 |
| AA | mg/100 g | 33.2 ± 3.70 c | 45.3 ± 4.20 b | 44.9 ± 9.38 b | 71.6 ± 10.1 a | 7.08 | <0.001 | <0.001 | 0.004 |
| n-6/n-3 PUFA | ratio | 11.4 ± 3.79 | 9.8 ± 0.69 | 11.5 ± 3.20 | 10.5 ± 0.71 | 2.40 | 0.126 | 0.596 | 0.741 |
| Trait | Unit | Normal Breast | Wooden Breast | RMSE | p-Value |
|---|---|---|---|---|---|
| Total FA | g/100 g | 1.06 ± 0.23 | 1.42 ± 0.70 | 0.49 | 0.156 |
| SFA | mg/100 g | 343 ± 80.2 | 476 ± 244 | 173 | 0.131 |
| MUFA | mg/100 g | 316 ± 91.0 | 430 ± 233 | 167 | 0.173 |
| PUFA | mg/100 g | 267 ± 51.3 | 353 ± 163 | 115 | 0.140 |
| n-3 PUFA | mg/100 g | 22.7 ± 5.63 | 32.9 ± 17.1 | 12.3 | 0.090 |
| ALA | mg/100 g | 10.7 ± 4.02 | 21.7 ± 14.8 | 10.2 | 0.043 |
| EPA | mg/100 g | 2.21 ± 0.87 | 2.15 ± 0.81 | 0.79 | 0.878 |
| DHA | mg/100 g | 2.84 ± 0.88 | 2.63 ± 0.78 | 0.78 | 0.592 |
| n-6 PUFA | mg/100 g | 245 ± 49.8 | 320 ± 146 | 103 | 0.149 |
| LA | mg/100 g | 197 ± 46.7 | 277 ± 138 | 96.9 | 0.110 |
| AA | mg/100 g | 33.2 ± 3.70 | 29.5 ± 8.40 | 6.15 | 0.247 |
| n-6/n-3 PUFA | ratio | 11.4 ± 3.79 | 10.2 ± 2.18 | 2.93 | 0.389 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, Z.; Van Hecke, T.; Vossen, E.; Petracci, M.; Berri, C.; Kowalski, E.; De Smet, S. Susceptibility of Conventional and Organic Chicken Breast and Thigh Meat to Lipid and Protein Oxidation During Heating and In Vitro Digestion. Foods 2025, 14, 3375. https://doi.org/10.3390/foods14193375
Ali Z, Van Hecke T, Vossen E, Petracci M, Berri C, Kowalski E, De Smet S. Susceptibility of Conventional and Organic Chicken Breast and Thigh Meat to Lipid and Protein Oxidation During Heating and In Vitro Digestion. Foods. 2025; 14(19):3375. https://doi.org/10.3390/foods14193375
Chicago/Turabian StyleAli, Zeshan, Thomas Van Hecke, Els Vossen, Massimiliano Petracci, Cécile Berri, Eline Kowalski, and Stefaan De Smet. 2025. "Susceptibility of Conventional and Organic Chicken Breast and Thigh Meat to Lipid and Protein Oxidation During Heating and In Vitro Digestion" Foods 14, no. 19: 3375. https://doi.org/10.3390/foods14193375
APA StyleAli, Z., Van Hecke, T., Vossen, E., Petracci, M., Berri, C., Kowalski, E., & De Smet, S. (2025). Susceptibility of Conventional and Organic Chicken Breast and Thigh Meat to Lipid and Protein Oxidation During Heating and In Vitro Digestion. Foods, 14(19), 3375. https://doi.org/10.3390/foods14193375









