Optimizing Crisp Meat Quality with Modified Starches: From Rheological Properties to Post-Freezing Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Batter Preparation
2.3. Meat Preparation
2.4. Rheological Measurements
2.4.1. Dynamic Scanning Tests
2.4.2. Steady-State Flow Tests
2.5. Batter Pick Up
2.6. Gelatinization and Retrogradation Properties
2.7. Frying Conditions
2.8. Determination of Moisture Content
2.9. Determination of Oil Content
2.10. Texture Profile Analysis (TPA)
2.11. Color Analysis
2.12. LF-NMR Analysis
2.12.1. Determination of T2 Relaxation Time
2.12.2. Determination of Magnetic Resonance Imaging (MRI)
2.13. Statistical Analysis
3. Results and Discussion
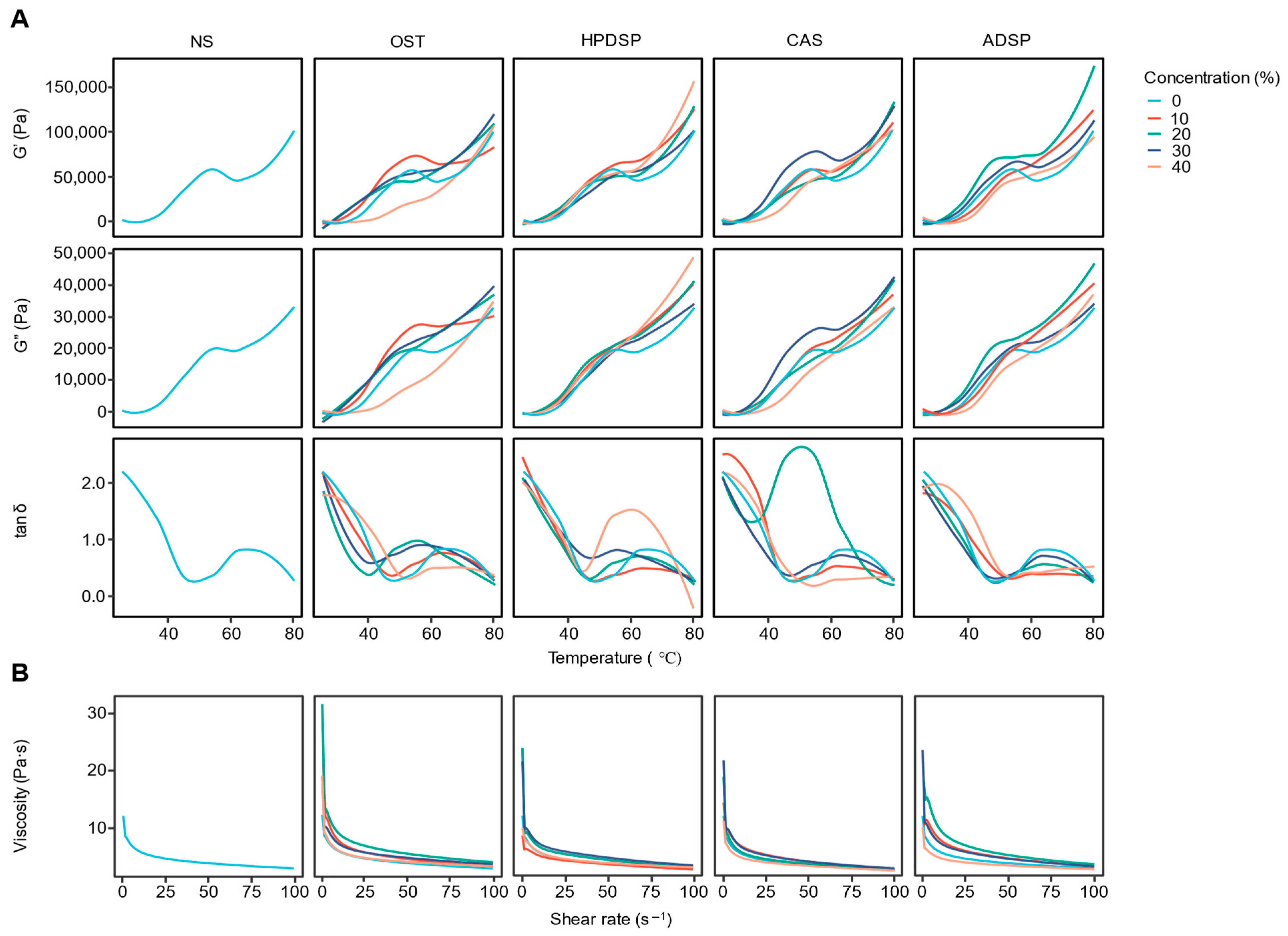
3.1. Rheological Characterization Analysis
3.1.1. Dynamic Temperature Scanning Analysis
3.1.2. Steady-Shear Flow Behavior Analysis
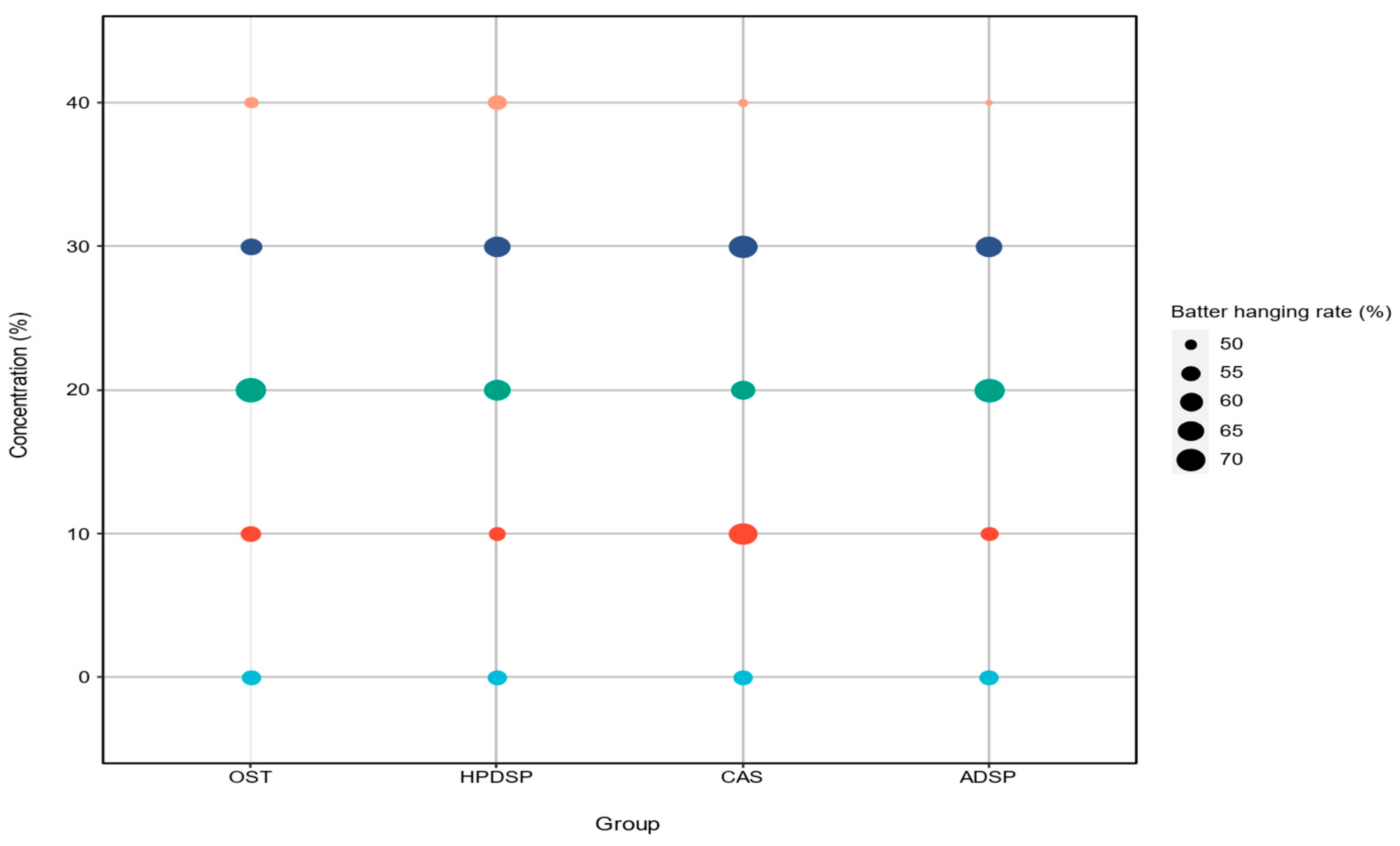
3.2. Batter Pick-Up Analysis
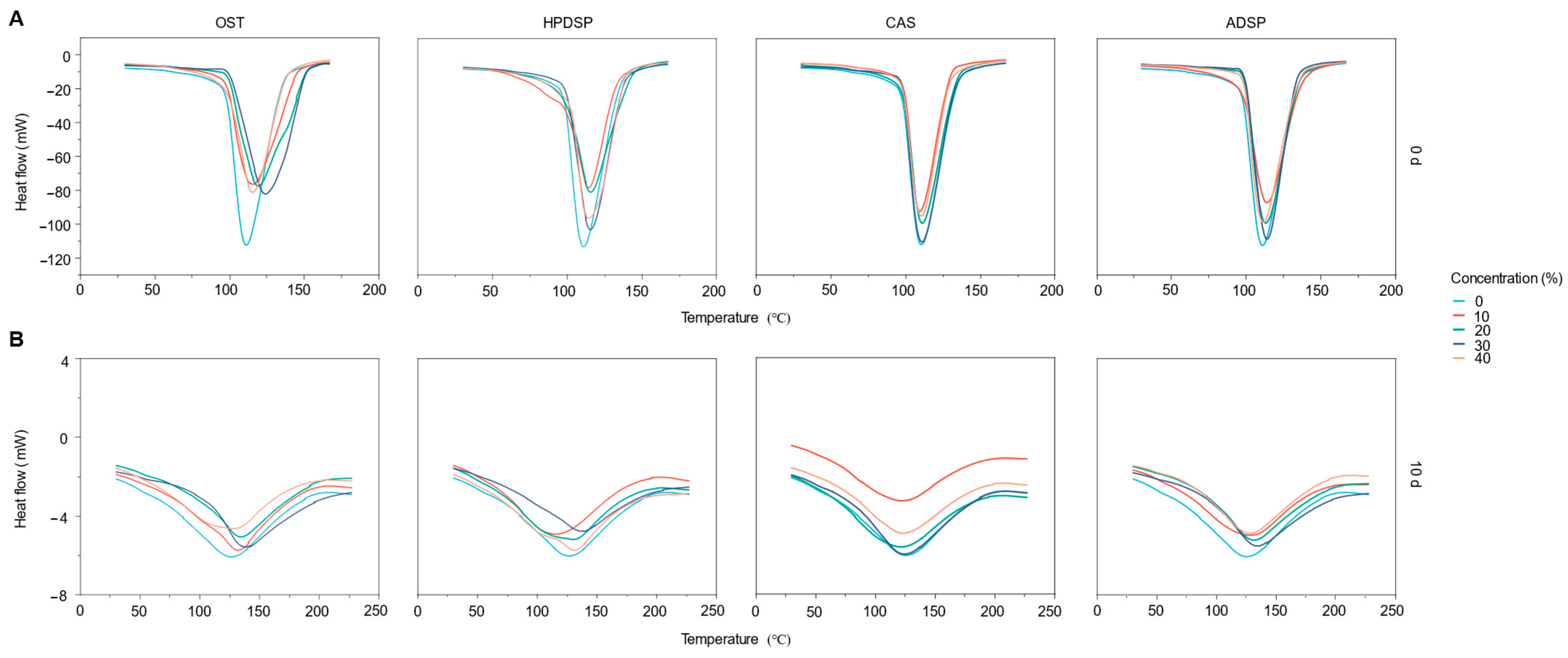
3.3. Gelatinization and Retrogradation Properties Analysis
3.4. Moisture Content of Crisp Meat
3.5. Oil Content of Fried Crisp Meat
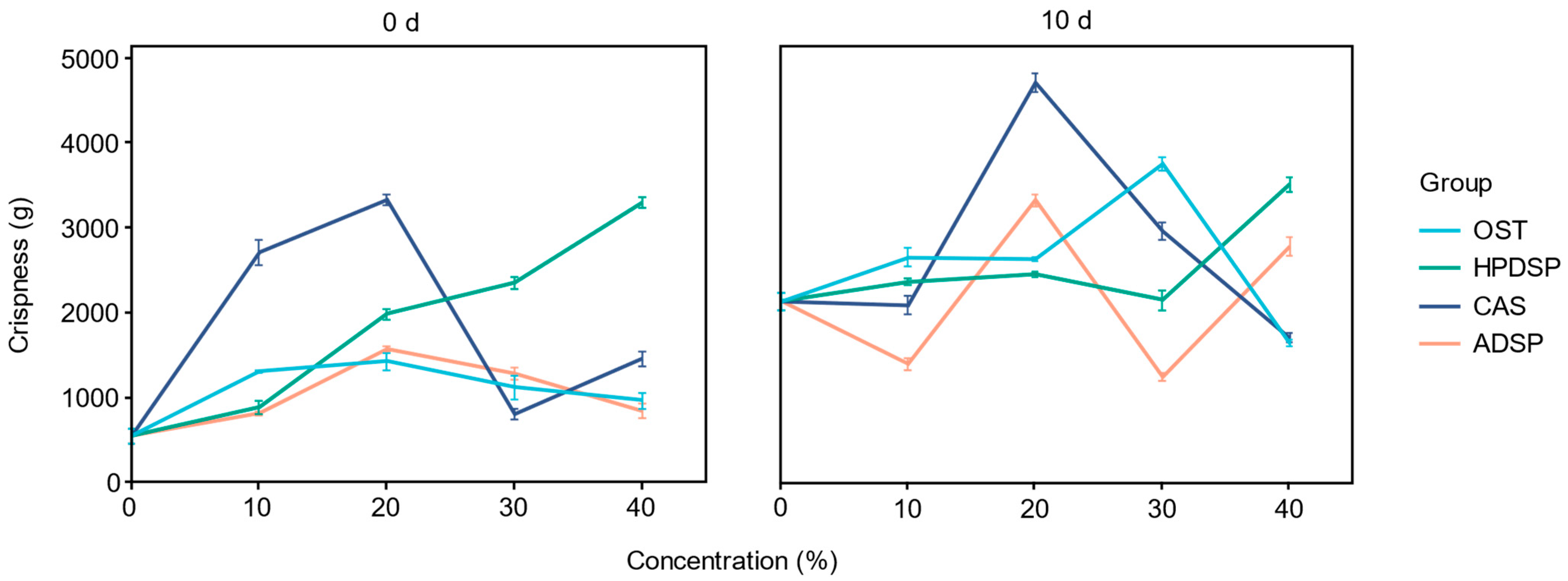
3.6. Crispness of Crisp Meat
3.7. The CIE Color Attributes and Total Color Difference (TCD)
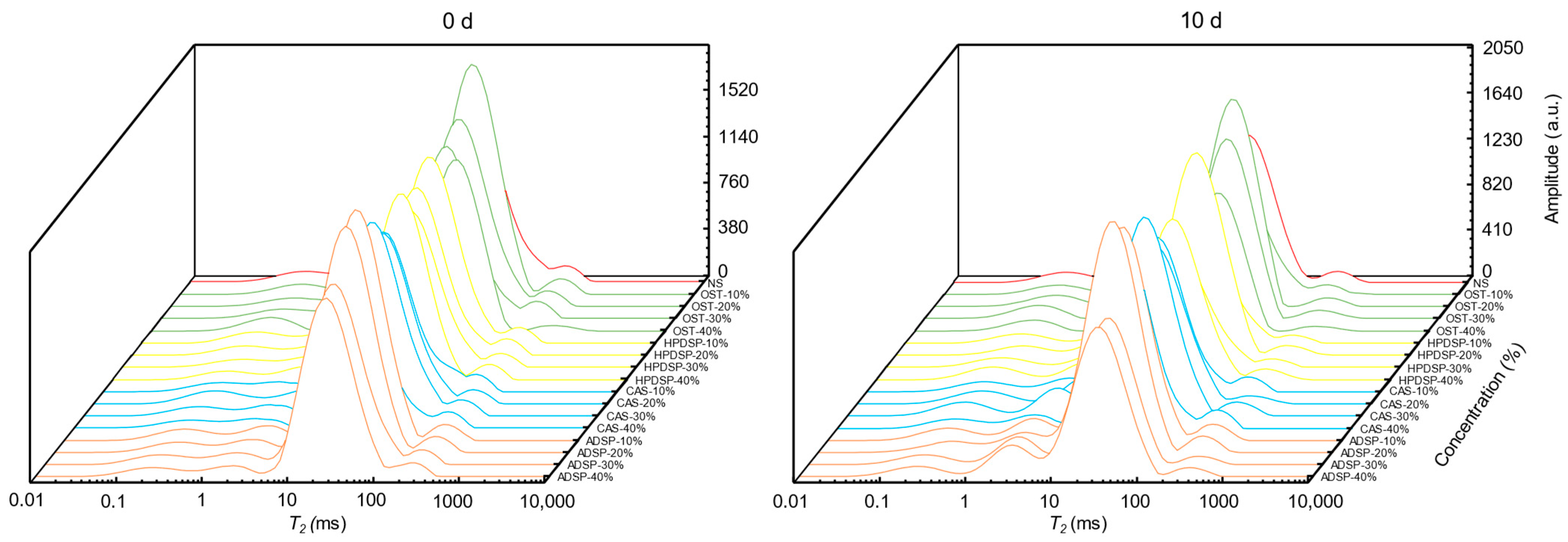
3.8. T2 Relaxation Time Analysis
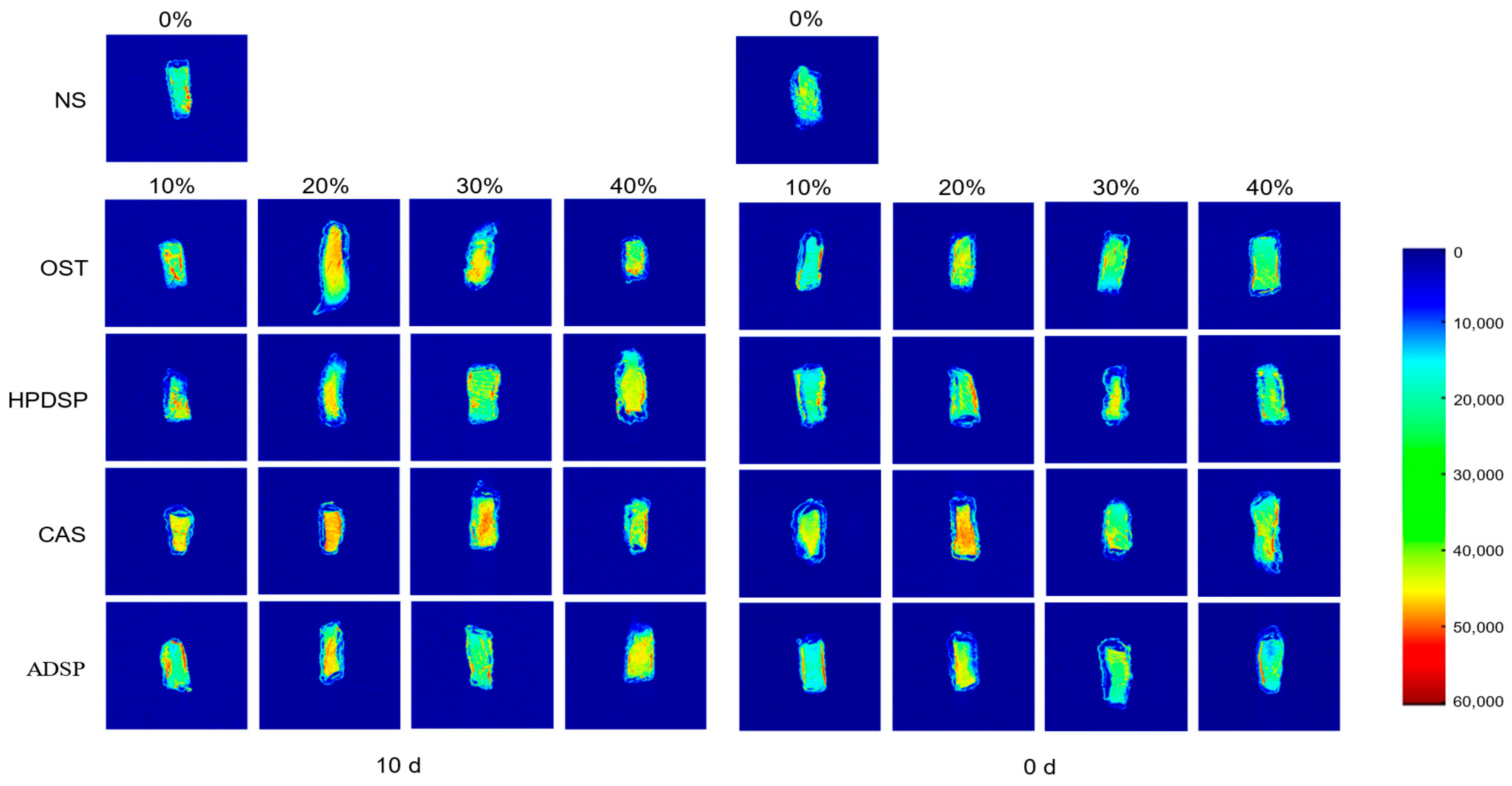
3.9. The MRI Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OST | Oxidized starch |
| HPDSP | hydroxypropyl distarch phosphate |
| CAS | starch acetate |
| ADSP | acetylated distarch phosphate |
| NS | native starch |
References
- Zhang, X.; Zhang, M.; Adhikari, B. Recent developments in frying technologies applied to fresh foods. Trends Food Sci. Technol. 2020, 98, 6881. [Google Scholar] [CrossRef]
- Hafizur, M.R.B.; Michael, O.N. Air-frying of meat analog based parfried frozen batter coated foods. J. Food Eng. 2024, 367, 111844. [Google Scholar]
- Hafizur, M.R.B.; Nushrat, Y.; Michael, N. Restructuring plant-derived composites towards the production of meat-analog based coated fried food. Food Chem. 2024, 443, 138482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; McClements, D.J.; Jin, Z. Recent advances in crispness retention of microwaveable frozen pre-fried foods. Trends Food Sci. Technol. 2023, 132, 54–64. [Google Scholar] [CrossRef]
- Lisa, M.B.; Carsten, B.; Monika, G.; Kurt, H.; Nino, T.; Jochen, W. Effect of manufacturing and frozen meat temperatures on structural and functional properties of hamburgers. J. Food Eng. 2023, 353, 111526. [Google Scholar] [CrossRef]
- Sajad, P.; Kosar, H. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967. [Google Scholar]
- Bhuiyan, M.H.R.; Yeasmen, N.; Ngadi, M.O. Multifractal analysis of meat-analog based coated fried foods texture profile. Food Struct.-Neth. 2024, 39, 100363. [Google Scholar] [CrossRef]
- Qian, H.; Jiukai, Z.; Ranran, X.; Wensheng, H.; Ting, Z.; Ying, C. Comprehensive multiomics analysis reveals the effects of French fries and chicken breast meat on the oxidative degradation of lipids in soybean oil during deep-frying. Food Chem. 2025, 473, 143052. [Google Scholar] [CrossRef]
- Niaz, M.; Joinul, I.; William, O.; Kelvin, A.; Samuel, C.A.; Reza, T. A review of different frying oils and oleogels as alternative frying media for fat-uptake reduction in deep-fat fried foods. Heliyon 2023, 45, 21500. [Google Scholar]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Yuan, Z.; Liao, E.; Xia, W.; Wang, H.; Xiong, Y.L. Effect of the wheat starch/wheat protein ratio in a batter on fat absorption and quality attributes of fried battered and breaded fish nuggets. J. Food Sci. 2020, 85, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Christaki, M.; Verboven, P.; Van Dyck, T.; Nicolaï, B.; Goos, P.; Claes, J. The predictive power of batter rheological properties on cake quality—The effect of pregelatinized flour, leavening acid type and mixing time. J. Cereal Sci. 2017, 77, 219–227. [Google Scholar] [CrossRef]
- Han, A.; Romero, H.M.; Nishijima, N.; Ichimura, T.; Handa, A.; Xu, C.; Zhang, Y. Effect of egg white solids on the rheological properties and bread making performance of gluten-free batter. Food Hydrocoll. 2019, 87, 287–296. [Google Scholar] [CrossRef]
- Hauzoukim; Xavier, K.A.M.; Kannuchamy, N.; Balange, A.; Gudipati, V. Development of enrobed fish products: Improvement of functionality of coated materials by added aquatic polymers. J. Food Process Eng. 2019, 42, 3–14. [Google Scholar] [CrossRef]
- Baixauli, R.; Sanz, T.; Salvador, A.; Fiszman, S.M. Effect of the addition of dextrin or dried egg on the rheological and textural properties of batters for fried foods. Food Hydrocoll. 2003, 17, 305–310. [Google Scholar] [CrossRef]
- Korma, S.A. Chemically Modified Starch and Utilization in Food Stuffs. Int. J. Nutr. Food Sci. 2016, 5, 4–15. [Google Scholar] [CrossRef]
- Muflihati, I.; Marseno, D.W.; Pranoto, Y. Oxidation of Oven-Dried Cassava Starch Using Hydrogen Peroxide and UV-C Irradiation to Improve Frying Expansion. Indones. Food Nutr. Prog. 2019, 16, 135. [Google Scholar] [CrossRef]
- Mehfooz, T.; Ali, T.M.; Hasnain, A. Effect of cross-linking on characteristics of succinylated and oxidized barley starch. J. Food Meas. Charact. 2019, 13, 1058–1069. [Google Scholar] [CrossRef]
- Seet Chi, Y.; Kamilah, H.; Nor Shariffa, Y.; Utra, U. Phosphorylation of sago (Metroxylon sagu) starch cross-linked with sodium triphosphate and the physicochemical properties of the potential application in batter. J. Food Process. Preserv. 2021, 45, 6684. [Google Scholar] [CrossRef]
- Singh, J.; Colussi, R.; McCarthy, O.J.; Kaur, L. Potato Starch and Its Modification. Adv. Potato Chem. Technol. 2016, 21, 195–247. [Google Scholar]
- T/AHFS 003-2022; Prepared Meat Dishes—Crisp Meat. Anhui Food Safety Association: Suzhou, China, 2022.
- Shahzad, S.A.; Hussain, S.; Alamri, M.S.; Mohamed, A.A.; Ahmed, A.S.; Ibraheem, M.A.; Abdo Qasem, A.A. Use of Hydrocolloid Gums to Modify the Pasting, Thermal, Rheological, and Textural Properties of Sweet Potato Starch. Int. J. Polym. Sci. 2019, 2019, 6308591. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Liu, Y.; Cui, B. Fabrication of kappa-carrageenan hydrogels with cinnamon essential oil/hydroxypropyl-beta-cyclodextrin composite: Evaluation of physicochemical properties, release kinetics and antimicrobial activity. Int. J. Biol. Macromol. 2021, 170, 593–601. [Google Scholar] [CrossRef]
- Movahhed, S.; Ahmadi Chenarbon, H. Moisture Content and Oil Uptake in Potatoes (Cultivar Satina) During Deep-Fat Frying. Potato Res. 2018, 61, 261–272. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Pongjaruvat, W.; Methacanon, P.; Seetapan, N.; Fuongfuchat, A.; Klaikherd, A. Effects of cross-linked tapioca starches on batter viscosity and oil absorption in deep-fried breaded chicken strips. J. Food Eng. 2013, 114, 262–268. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, T.; Duan, S.; Qin, Z.; Zhao, H.; Wang, M.; Li, C.; Zhang, Z.; Liu, A.; Han, G.; et al. Applicability of Rice Doughs as Promising Food Materials in Extrusion-Based 3D Printing. Food Bioprocess Technol. 2020, 13, 548–563. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Zhang, D.; Chen, X.; Li, H. Effect of multiple freeze-thaw cycles on protein and lipid oxidation in rabbit meat. Int. J. Food Sci. Technol. 2021, 56, 3004–3015. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Singh, S.; Ahlawat, A.K.; Singh, A.M. Relationship between physicochemical and rheological properties of starches from Indian wheat lines. Int. J. Food Sci. Technol. 2011, 46, 2584–2590. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Z.; Zhao, M.; Milosavljević, V.; Cullen, P.J.; Scally, L.; Sun, D.-W.; Tiwari, B.K. Low-pressure plasma modification of the rheological properties of tapioca starch. Food Hydrocoll. 2022, 125, 516. [Google Scholar] [CrossRef]
- Yashini, M.; Khushbu, S.; Madhurima, N.; Sunil, C.K.; Mahendran, R.; Venkatachalapathy, N. Thermal properties of different types of starch: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 4373–4396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.F.; Jia, Z.Y.; Hou, L.L.; Yang, H.; Xiao, S.S.; Ding, W.P.; Zhang, Y.T.; Wang, X.D.; Fu, Y.; Wu, Y. Interpretation of the effects of hydroxypropyl starch and hydroxypropyl distarch phosphate on frozen raw noodles quality during frozen storage: Studies on water state and starch-gluten network properties. Int. J. Biol. Macromol. 2023, 242, 124783. [Google Scholar] [CrossRef] [PubMed]
- Broekman, J.O.P.; Piersma, W.; Brinksma, J.; Deuss, P.J. Study on physicochemical properties of catalytically oxidised starches from various botanical sources. Food Hydrocoll. 2025, 159, 110622. [Google Scholar] [CrossRef]
- Chi, C.; Lian, S.; Zou, Y.; Chen, B.; He, Y.; Zheng, M.; Zhao, Y.; Wang, H. Preparation, multi-scale structures, and functionalities of acetylated starch: An updated review. Int. J. Biol. Macromol. 2023, 49, 126142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Guo, X.; Zhu, K.; Wu, Z. A Review of the Impact of Starch on the Quality of Wheat-Based Noodles and Pasta: From the View of Starch Structural and Functional Properties and Interaction with Gluten. Foods 2024, 13, 1507. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Bai, Y.; Wang, J.; Jiao, A.; Jin, Z. Effect of frying on the pasting and rheological properties of normal maize starch. Food Hydrocoll. 2018, 77, 85–95. [Google Scholar] [CrossRef]
- Genccelep, H.; Saricaoglu, F.T.; Anil, M.; Agar, B.; Turhan, S. The effect of starch modification and concentration on steady-state and dynamic rheology of meat emulsions. Food Hydrocoll. 2015, 48, 135–148. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Ji, H.; Dong, J.; Li, X.; Liu, J.; Jin, Z. Insights into rice starch degradation by maltogenic α–amylase: Effect of starch structure on its rheological properties. Food Hydrocoll. 2022, 124, 3879. [Google Scholar] [CrossRef]
- Wu, K.; Dai, S.; Gan, R.; Corke, H.; Zhu, F. Thermal and Rheological Properties of Mung Bean Starch Blends with Potato, Sweet Potato, Rice, and Sorghum Starches. Food Bioprocess Technol. 2016, 9, 1408–1421. [Google Scholar] [CrossRef]
- Zhu, F.; Xie, Q. Rheological and thermal properties in relation to molecular structure of New Zealand sweetpotato starch. Food Hydrocoll. 2018, 83, 165–172. [Google Scholar] [CrossRef]
- Bortnowska, G.; Krzemińska, N.; Mojka, K. Effects of waxy maize and potato starches on the stability and physicochemical properties of model sauces prepared with fresh beef meat. Int. J. Food Sci. Technol. 2013, 48, 2668–2675. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.R.; Ngadi, M. Application of batter coating for modulating oil, texture and structure of fried foods: A review. Food Chem. 2024, 453, 139655. [Google Scholar] [CrossRef]
- Chan, H.T.; Bhat, R.; Karim, A.A. Physicochemical and functional properties of ozone-oxidized starch. J. Agric. Food Chem. 2009, 57, 5965–5970. [Google Scholar] [CrossRef]
- Zhou, D.N.; Zhang, B.; Chen, B.; Chen, H.Q. Effects of oligosaccharides on pasting, thermal and rheological properties of sweet potato starch. Food Chem. 2017, 230, 516–523. [Google Scholar] [CrossRef]
- Chen, H.; Fu, X.; Luo, Z. Effect of gum arabic on freeze-thaw stability, pasting and rheological properties of tapioca starch and its derivatives. Food Hydrocoll. 2015, 51, 355–360. [Google Scholar] [CrossRef]
- Primo-Martín, C. Cross-linking of wheat starch improves the crispness of deep-fried battered food. Food Hydrocoll. 2012, 28, 53–58. [Google Scholar] [CrossRef]
- López, O.V.; Zaritzky, N.E.; García, M.A. Physicochemical characterization of chemically modified corn starches related to rheological behavior, retrogradation and film forming capacity. J. Food Eng. 2010, 100, 160–168. [Google Scholar] [CrossRef]
- Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Characterization of physicochemical properties of hypochlorite- and peroxide-oxidized cassava starches. Carbohydr. Polym. 2010, 82, 446–453. [Google Scholar] [CrossRef]
- Obadi, M.; Xu, B. Review on the physicochemical properties, modifications, and applications of starches and its common modified forms used in noodle products. Food Hydrocoll. 2021, 112, 5652. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, S.; Ravi, N.; Chauhan, O.P.; Patki, P.E. Use of partial drying and freezing pre-treatments for development of vacuum fried papaya (Carica papaya L.) chips. J. Food Sci. Technol. 2020, 57, 2310–2320. [Google Scholar] [CrossRef]
- Chen, L.; McClements, D.J.; Yang, T.; Ma, Y.; Ren, F.; Tian, Y.; Jin, Z. Effect of annealing and heat-moisture pretreatments on the oil absorption of normal maize starch during frying. Food Chem. 2021, 353, 129468. [Google Scholar] [CrossRef]
- Hu, G.; Chen, J.; Gao, J. Preparation and characteristics of oxidized potato starch films. Carbohydr. Polym. 2009, 76, 291–298. [Google Scholar] [CrossRef]
- Sharma, V.; Kaur, M.; Sandhu, K.S.; Godara, S.K. Effect of cross-linking on physico-chemical, thermal, pasting, in vitro digestibility and film forming properties of Faba bean (Vicia faba L.) starch. Int. J. Biol. Macromol. 2020, 159, 243–249. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Ding, Z.; Fan, L. Effects of Initial Moisture Content on the Oil Absorption Behavior of Potato Chips During Frying Process. Food Bioprocess Technol. 2015, 9, 331–340. [Google Scholar] [CrossRef]
- Cortés, P.; Badillo, G.; Segura, L.; Bouchon, P. Experimental evidence of water loss and oil uptake during simulated deep-fat frying using glass micromodels. J. Food Eng. 2014, 140, 19–27. [Google Scholar] [CrossRef]
- Yang, D.; Wu, G.; Li, P.; Zhang, H.; Qi, X. Comparative analysis of the oil absorption behavior and microstructural changes of fresh and pre-frozen potato strips during frying via MRl, SEM, and XRD. Food Res. Int. 2019, 122, 295–302. [Google Scholar] [CrossRef]
- Han, J.-A.; Lee, M.-J.; Lim, S.-T. Utilization of Oxidized and Cross-Linked Corn Starches in Wheat Flour Batter. Cereal Chem. 2007, 84, 582–586. [Google Scholar] [CrossRef]
- Salvador, A.; Sanz, T.; Fiszman, S. Effect of the addition of different ingredients on the characteristics of a batter coating for fried seafood prepared without a pre-frying step. Food Hydrocoll. 2005, 19, 703–708. [Google Scholar] [CrossRef]
- Sanz, T.; Salvador, A.; Fiszman, S.M. Resistant starch (RS) in battered fried products: Functionality and high-fibre benefit. Food Hydrocoll. 2008, 22, 543–549. [Google Scholar] [CrossRef]
- Ren, A.; Pan, S.; Li, W.; Chen, G.; Duan, X. Effect of Various Pretreatments on Quality Attributes of Vacuum-Fried Shiitake Mushroom Chips. J. Food Qual. 2018, 2018, 4510126. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, M.; Ji, H.; Ma, H. Some physicochemical properties of starches and their influence on color, texture, and oil content in crusts using a deep-fat-fried model. CyTA—J. Food 2014, 12, 347–354. [Google Scholar] [CrossRef]
- Nawaz, A.; Xiong, Z.; Xiong, H.; Irshad, S.; Chen, L.; Wang, P.K.; Ahsan, H.M.; Walayat, N.; Qamar, S.H. The impact of hydrophilic emulsifiers on the physico-chemical properties, microstructure, water distribution and in vitro digestibility of proteins in fried snacks based on fish meat. Food Funct. 2019, 10, 6927–6935. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Li, E.; Khalifa, I.; Walayat, N.; Liu, J.; Irshad, S.; Zahra, A.; Ahmed, S.; Simirgiotis, M.J.; Pateiro, M.; et al. Effect of Different Processing Methods on Quality, Structure, Oxidative Properties and Water Distribution Properties of Fish Meat-Based Snacks. Foods 2021, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Yang, S.; Kuang, Y.Y.; Shan, C.S.; Lu, Q.Q.; Chen, Z.G. Effects of different modified starches and gums on the physicochemical, functional, and microstructural properties of tapioca pearls. Int. J. Biol. Macromol. 2022, 206, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, S.; Wang, S.; Yi, K.; Sun, S.; Lin, J.; Tan, M.; Li, D. Effect of pre-frying on distribution of protons and physicochemical qualities of mackerel. J. Sci. Food Agric. 2021, 101, 4838–4846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liang, Y.; Tan, C.; Lu, Y.; Cui, B. Research on the water-holding capacity of pork sausage with acetate cassava starch. Starch-Stärke 2014, 66, 1033–1040. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]

| Concentration (%) | OST | HPDSP | CAS | ADSP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K (Pa sn) | N (−) | R2 | Between-Type Comparisons (p-Value) | K (Pa sn) | N (−) | R2 | Between-Type Comparisons (p-Value) | K (Pa·sn) | N (−) | R2 | Between-Type Comparisons (p-Value) | K (Pa·sn) | N (−) | R2 | Between-Type Comparisons (p-Value) | |
| 0 | 10.682 ± 1.221 dA | 0.731 ± 0.017 aA | 0.9977 | Reference | 10.682 ± 1.221 bcA | 0.731 ± 0.017 bcA | 0.9977 | 1.000 (K), 1.000 (n) | 10.682 ± 1.221 bA | 0.731 ± 0.017 bA | 0.9977 | 1.000 (K), 1.000 (n) | 10.682 ± 1.221 cA | 0.731 ± 0.017 bA | 0.9977 | 1.000 (K), 1.000 (n) |
| 10 | 15.982 ± 0.934 bA | 0.671 ± 0.017 bB | 0.9961 | 8.361 ± 1.171 cC | 0.781 ± 0.038 aA | 0.9941 | <0.001 (K), <0.001 (n) | 13.915 ± 0.750 aB | 0.694 ± 0.012 cB | 0.9895 | 0.012 (K), 0.342 (n) | 15.660 ± 2.037 bA | 0.686 ± 0.0185 cB | 0.986 | 0.843 (K), 0.521 (n) | |
| 20 | 19.077 ± 1.505 aAB | 0.669 ± 0.020 bB | 0.9969 | 13.797 ± 1.237 abBC | 0.701 ± 0.019 cAB | 0.9942 | <0.001 (K), 0.187 (n) | 11.866 ± 0.989 bC | 0.710 ± 0.021 bcA | 0.9989 | <0.001 (K), 0.072 (n) | 21.473 ± 3.716 aA | 0.638 ± 0.033 dC | 0.9876 | 0.432 (K), 0.421 (n) | |
| 30 | 13.382 ± 1.255 cC | 0.726 ± 0.019 aA | 0.9982 | 14.264 ± 0.495 aB | 0.712 ± 0.008 cB | 0.9952 | 0.342 (K), 0.521 (n) | 14.052 ± 1.294 aB | 0.688 ± 0.022 cC | 0.9965 | 0.621 (K), 0.112 (n) | 15.716 ± 0.705 bA | 0.677 ± 0.009 cC | 0.9949 | 0.087 (K), 0.034 (n) | |
| 40 | 11.107 ± 0.679 dA | 0.733 ± 0.020 aA | 0.9996 | 9.964 ± 1.822 cB | 0.758 ± 0.032 abA | 0.9977 | 0.421 (K), 0.521 (n) | 8.800 ± 0.104 cC | 0.765 ± 0.008 aA | 0.9989 | 0.012 (K), 0.342 (n) | 7.893 ± 0.421 cC | 0.776 ± 0.021 aA | 0.9988 | <0.001 (K), 0.087 (n) | |
| Concentration (%) | OST | HPDSP | CAS | ADSP | ||||
|---|---|---|---|---|---|---|---|---|
| 0 d | 10 d | 0 d | 10 d | 0 d | 10 d | 0 d | 10 d | |
| 0 | 58.92 ± 0.55 bA | 56.72 ± 2.71 bA | 58.92 ± 0.55 bA | 56.72 ± 2.71 cdA | 58.92 ± 0.55 cdA | 56.72 ± 2.71 cA | 58.92 ± 0.55 cA | 56.72 ± 2.71 abA |
| 10 | 58.87 ± 0.45 bC | 56.22 ± 0.38 bC | 58.27 ± 0.49 bC | 57.90 ± 0.94 bcB | 60.82 ± 0.96 bA | 60.33 ± 0.74 aA | 59.98 ± 0.72 bB | 56.17 ± 0.94 abC |
| 20 | 60.46 ± 0.70 aB | 59.36 ± 0.25 aA | 60.62 ± 0.81 aB | 59.66 ± 0.31 aA | 62.58 ± 0.92 aA | 58.42 ± 0.70 bB | 61.08 ± 0.17 aB | 58.29 ± 0.43 aB |
| 30 | 58.31 ± 0.72 bAB | 53.14 ± 0.34 cC | 59.07 ± 0.66 bA | 58.54 ± 0.45 bA | 58.22 ± 0.97 dB | 56.30 ± 0.82 cB | 58.80 ± 0.52 cAB | 55.58 ± 0.82 bB |
| 40 | 55.08 ± 0.74 cD | 53.72 ± 0.45 cC | 58.99 ± 0.51 bC | 56.26 ± 0.35 dA | 59.68 ± 0.34 cB | 55.03 ± 0.83 dB | 61.62 ± 0.43 aA | 54.92 ± 0.42 bB |
| Concentration (%) | OST | HPDSP | CAS | ADSP | ||||
|---|---|---|---|---|---|---|---|---|
| 0 d | 10 d | 0 d | 10 d | 0 d | 10 d | 0 d | 10 d | |
| 0 | 17.90 ± 0.26 aA | 19.94 ± 1.21 cA | 17.90 ± 0.26 aA | 19.94 ± 1.21 bA | 17.90 ± 0.26 abA | 19.94 ± 1.21 cA | 17.90 ± 0.26 abA | 19.94 ± 1.21 aA |
| 10 | 15.50 ± 1.31 bB | 20.51 ± 0.11 bcA | 16.54 ± 2.54 abAB | 18.82 ± 1.34 cB | 17.85 ± 1.29 abA | 18.74 ± 1.43 cB | 17.05 ± 1.44 abAB | 21.04 ± 1.48 aA |
| 20 | 11.86 ± 0.71 cC | 17.88 ± 1.58 dB | 14.51 ± 0.23 bB | 18.55 ± 0.38 cB | 16.44 ± 1.70 bA | 19.86 ± 2.22 cA | 16.57 ± 1.53 bA | 19.49 ± 0.16 aA |
| 30 | 18.68 ± 1.34 aA | 23.28 ± 1.34 aA | 16.61 ± 1.29 abB | 19.51 ± 0.75 bcC | 19.49 ± 0.69 aA | 22.97 ± 0.31 aA | 19.51 ± 2.08 aA | 21.33 ± 0.40 aB |
| 40 | 19.01 ± 1.70 aA | 21.42 ± 0.23 bA | 16.96 ± 2.06 abB | 21.30 ± 0.13 aA | 17.48 ± 1.14 bAB | 21.56 ± 0.24 bA | 17.20 ± 0.85 abAB | 20.91 ± 2.50 aA |
| (A) | ||||||||||||
| Concentration (%) | OST | HPDSP | CAS | ADSP | ||||||||
| L* | a* | b* | L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| 0 | 51.40 ± 1.08 aA | 7.82 ± 0.51 cA | 26.74 ± 1.22 abA | 51.40 ± 1.08 aA | 7.82 ± 0.51 bA | 26.74 ± 1.22 bcA | 51.40 ± 1.08 aA | 7.82 ± 0.51 cA | 26.74 ± 1.22 cA | 51.40 ± 1.08 aA | 7.82 ± 0.51 cA | 26.74 ± 1.22 dA |
| 10 | 47.03 ± 0.81 bcBC | 9.71 ± 0.85 bB | 25.89 ± 0.42 bC | 50.45 ± 2.54 abA | 9.69 ± 0.64 aB | 29.14 ± 1.55 aAB | 48.98 ± 1.30 bAB | 11.21 ± 0.99 aA | 30.30 ± 1.25 abA | 46.73 ± 1.03 cC | 10.77 ± 0.78 bAB | 27.70 ± 0.96 cBC |
| 20 | 48.39 ± 0.62 bB | 10.86 ± 1.06 aAB | 27.53 ± 1.47 aB | 50.53 ± 1.75 abA | 9.57 ± 1.07 aB | 26.78 ± 0.70 bcB | 50.59 ± 1.15 abA | 10.43 ± 0.86 bAB | 30.02 ± 1.90 bA | 48.81 ± 2.16 bB | 11.60 ± 1.37 aA | 30.14 ± 0.97 aA |
| 30 | 46.12 ± 0.75 cB | 10.53 ± 0.75 aA | 27.44 ± 1.06 aC | 50.14 ± 0.89 abA | 9.98 ± 0.85 aA | 27.06 ± 1.08 bC | 49.94 ± 1.43 bAB | 10.46 ± 0.86 bA | 31.21 ± 1.32 aA | 48.61 ± 2.33 bAB | 10.5 ± 0.73 bA | 28.94 ± 0.89 bB |
| 40 | 47.15 ± 0.96 bcB | 9.53 ± 0.65 bB | 26.47 ± 1.11 abC | 49.08 ± 0.83 bAB | 7.95 ± 1.12 bC | 26.19 ± 0.89 cC | 49.83 ± 1.25 bA | 10.39 ± 0.50 bA | 30.25 ± 1.08 abA | 48.58 ± 1.23 bAB | 7.95 ± 0.75 cC | 27.72 ± 1.11 cB |
| (B) | ||||||||||||
| Concentration (%) | OST | HPDSP | CAS | ADSP | ||||||||
| L * | a * | b * | L * | a * | b * | L * | a * | b * | L * | a * | b * | |
| 0 | 53.92 ± 0.37 aA | 7.34 ± 0.78 cA | 26.86 ± 0.66 bA | 53.92 ± 0.37 aA | 7.34 ± 0.78 bA | 26.86 ± 0.66 bA | 53.92 ± 0.37 aA | 7.34 ± 0.78 dA | 26.86 ± 0.66 cA | 53.92 ± 0.37 aA | 7.34 ± 0.78 dA | 26.86 ± 0.66 cA |
| 10 | 51.40 ± 0.93 bA | 9.27 ± 0.75 bA | 27.34 ± 0.38 abAB | 50.92 ± 1.99 bA | 7.43 ± 0.34 bB | 25.47 ± 0.43 cC | 49.93 ± 1.22 bcAB | 8.40 ± 1.22 cAB | 26.38 ± 1.22 cBC | 47.72 ± 1.45 cB | 9.57 ± 0.91 bA | 28.37 ± 0.89 bA |
| 20 | 50.49 ± 0.80 bcA | 10.68 ± 0.48 aAB | 28.06 ± 0.86 aC | 50.45 ± 1.92 bA | 8.47 ± 1.05 aC | 28.42 ± 0.99 aBC | 50.78 ± 1.12 bA | 9.32 ± 0.97 bBC | 29.43 ± 1.25 aAB | 50.51 ± 0.88 bA | 11.11 ± 1.27 aA | 30.36 ± 1.42 aA |
| 30 | 47.13 ± 0.76 dB | 10.45 ± 0.60 aA | 24.75 ± 1.32 cB | 50.28 ± 1.19 bcA | 7.58 ± 1.22 bC | 23.67 ± 1.48 dB | 50.13 ± 2.01 bcA | 9.98 ± 0.89 aAB | 28.35 ± 0.85 bA | 51.04 ± 1.89 bA | 9.44 ± 0.81 bB | 27.36 ± 0.86 bcA |
| 40 | 49.33 ± 1.44 cdAB | 9.28 ± 0.78 bA | 27.36 ± 0.62 abA | 50.29 ± 1.18 bAB | 5.32 ± 0.64 cC | 21.48 ± 1.29 eB | 49.73 ± 1.64 cB | 9.90 ± 0.69 aA | 28.52 ± 0.81 bA | 51.32 ± 1.97 bA | 8.30 ± 0.80 cB | 28.25 ± 1.07 bA |
| (A) | ||||||||
| Concentration (%) | OST | HPDSP | CAS | ADSP | ||||
| T22(ms) | A22(%) | T22(ms) | A22(%) | T22(ms) | A22(%) | T22(ms) | A22(%) | |
| 0 | 21.54 ± 04 cA | 85.74 ± 1.13 cA | 21.54 ± 06 bcA | 85.74 ± 1.13 cA | 21.54 ± 03 abA | 85.74 ± 1.13 dA | 21.54 ± 01 dA | 85.74 ± 1.13 cA |
| 10 | 26.01 ± 2.14 aA | 88.38 ± 0.88 aA | 22.62 ± 1.86 abB | 86.65 ± 0.89 bB | 18.74 ± 1.61 cC | 88.09 ± 0.88 bcA | 26.01 ± 2.14 aC | 87.93 ± 1.28 abA |
| 20 | 23.16 ± 2.28 bAB | 88.57 ± 2.01 aB | 20.61 ± 1.62 cB | 87.63 ± 0.66 aB | 22.35 ± 02 aAB | 90.70 ± 0.41 aA | 24.77 ± 04 bA | 88.49 ± 0.80 aB |
| 30 | 21.54 ± 05 cA | 87.67 ± 0.64 abA | 23.16 ± 1.86 aA | 87.07 ± 1.18 abA | 22.62 ± 1.86 aA | 88.84 ± 1.43 bA | 23.16 ± 1.86 cA | 87.37 ± 1.31 bA |
| 40 | 20.14 ± 1.98 dB | 86.39 ± 0.45 bA | 23.16 ± 1.86 aA | 87.48 ± 0.99 aA | 19.67 ± 1.62 bB | 86.92 ± 1.09 cA | 23.70 ± 1.86 bcA | 87.97 ± 0.99 bA |
| (B) | ||||||||
| Concentration (%) | OST | HPDSP | CAS | ADSP | ||||
| T22(ms) | A22(%) | T22(ms) | A22(%) | T22(ms) | A22(%) | T22(ms) | A22(%) | |
| 0 | 33.97 ± 2.45 aA | 79.55 ± 0.94 cA | 33.97 ± 2.45 aA | 79.55 ± 0.94 dA | 33.97 ± 2.45 aA | 79.55 ± 0.94 dA | 33.97 ± 2.45 aA | 79.55 ± 0.94 cA |
| 10 | 26.35 ± 5.77 cAB | 80 ± 4.45 bcB | 31.32 ± 2.46 abA | 84.87 ± 1.13 bA | 23.96 ± 1.61 cB | 85.28 ± 0.37 bA | 27.24 ± 2.14 cAB | 84.87 ± 1.13 bA |
| 20 | 29.90 ± 2.46 bA | 86.25 ± 1.80 aB | 30.61 ± 2.46 bA | 87.71 ± 2.20 aB | 28.62 ± 3.26 bcA | 91.47 ± 0.60 aA | 25.70 ± 1.85 dB | 86.17 ± 2.62 aB |
| 30 | 34.38 ± 2.83 aA | 82.27 ± 0.98 bA | 28.48 ± 01 cB | 82.99 ± 0.97 Ac | 29.55 ± 2.13 bB | 82.22 ± 0.90 cA | 30.61 ± 2.46 bB | 78.98 ± 3.09 cB |
| 40 | 26.01 ± 2.14 cBC | 77.72 ± 2.69 cB | 28.62 ± 3.26 bcAB | 84.20 ± 1.02 bA | 23.70 ± 1.86 cC | 85.57 ± 0.91 bA | 30.61 ± 2.46 bA | 78.70 ± 0.94 cB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, C.; Zeng, Z.; Qin, Z.; Ding, J.; Liu, Y. Optimizing Crisp Meat Quality with Modified Starches: From Rheological Properties to Post-Freezing Performance. Foods 2025, 14, 2947. https://doi.org/10.3390/foods14172947
Ouyang C, Zeng Z, Qin Z, Ding J, Liu Y. Optimizing Crisp Meat Quality with Modified Starches: From Rheological Properties to Post-Freezing Performance. Foods. 2025; 14(17):2947. https://doi.org/10.3390/foods14172947
Chicago/Turabian StyleOuyang, Can, Zhen Zeng, Zhizhi Qin, Jiaqi Ding, and Yuntao Liu. 2025. "Optimizing Crisp Meat Quality with Modified Starches: From Rheological Properties to Post-Freezing Performance" Foods 14, no. 17: 2947. https://doi.org/10.3390/foods14172947
APA StyleOuyang, C., Zeng, Z., Qin, Z., Ding, J., & Liu, Y. (2025). Optimizing Crisp Meat Quality with Modified Starches: From Rheological Properties to Post-Freezing Performance. Foods, 14(17), 2947. https://doi.org/10.3390/foods14172947







