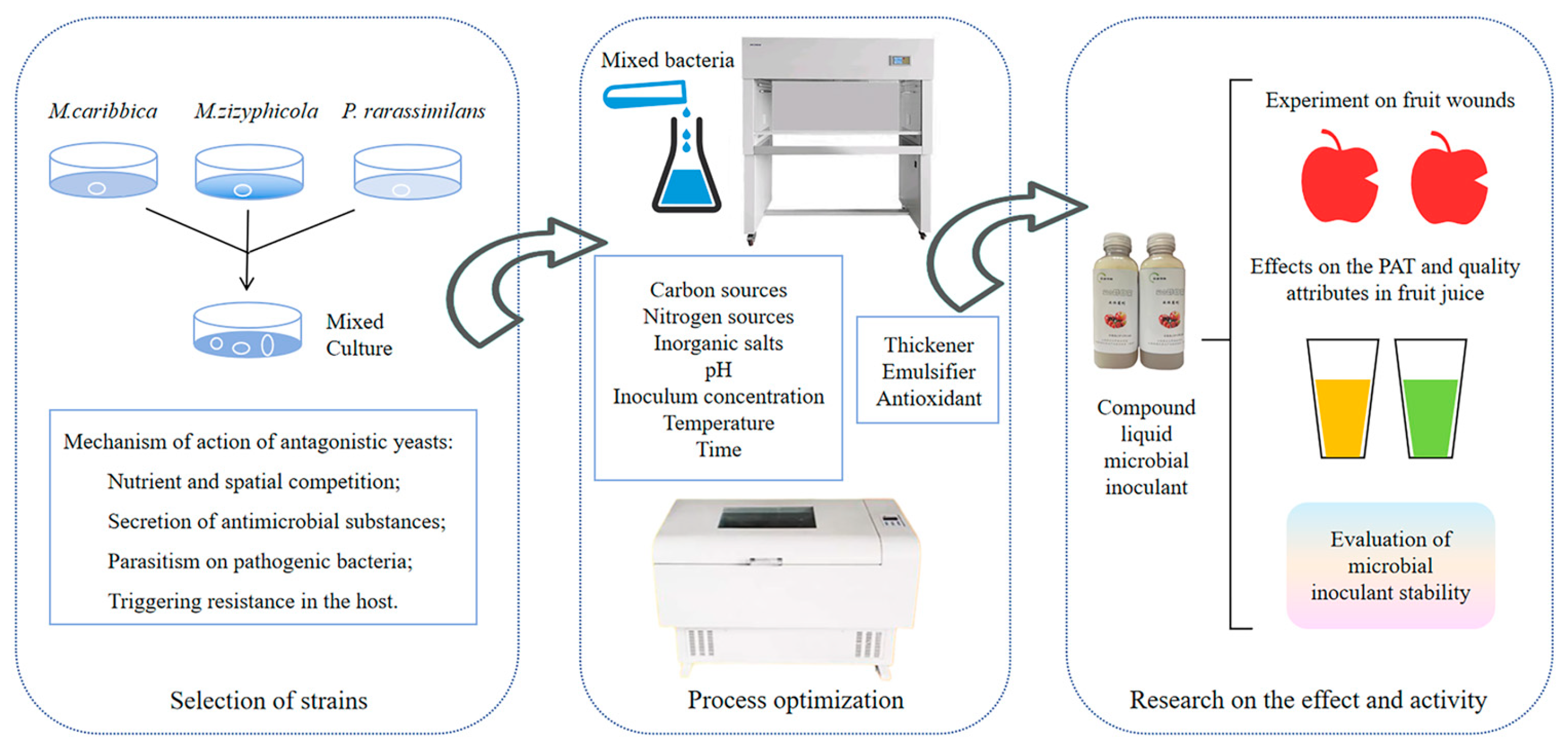
Construction of Composite Biocontrol Agent (BCA): Developing Effective Strategies for Controlling Postharvest Blue Mold and Patulin in Apples
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Biocompatibility Assessment Among Yeast Strains
2.2.2. Efficacy of Multi-Strain Yeast Cultures Against Apple Blue Mold
2.2.3. Optimization of Nutrient Conditions for Mixed Culture
2.2.4. Optimization of Fermentation Conditions for Mixed Culture
2.2.5. Optimization of Protective Additives Formulation for the Biocontrol Agent (BCA)
2.2.6. Evaluation of Biocontrol Efficacy of the Composite BCA
2.2.7. Degradation of PAT in Different Fruit Juices by the Composite BCA and Impact on Juice Quality
2.2.8. Storage Stability of the Liquid Composite BCA
2.3. Statistical Analysis
3. Results
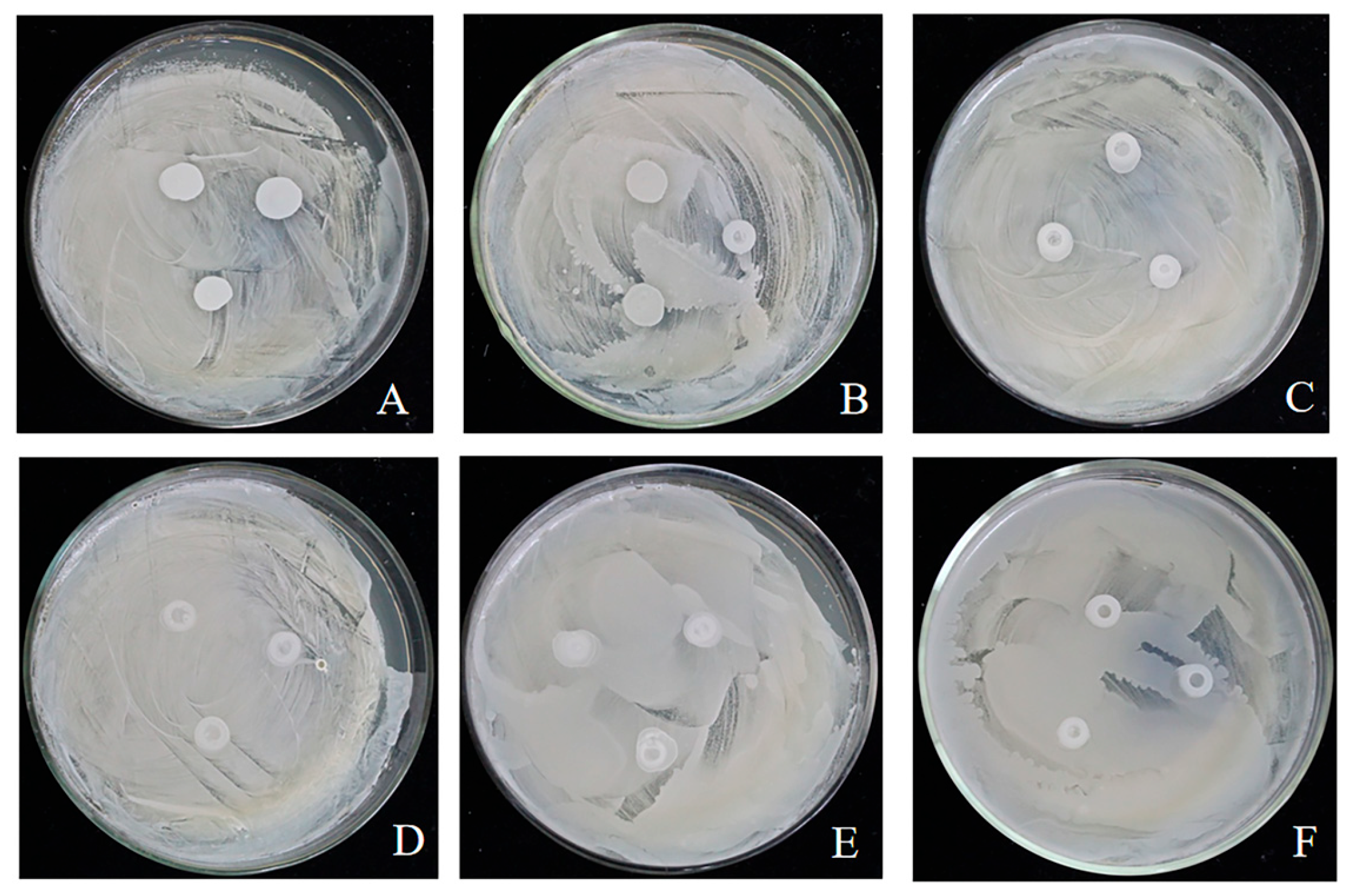
3.1. Biocompatibility Assessment Among Yeast Strains
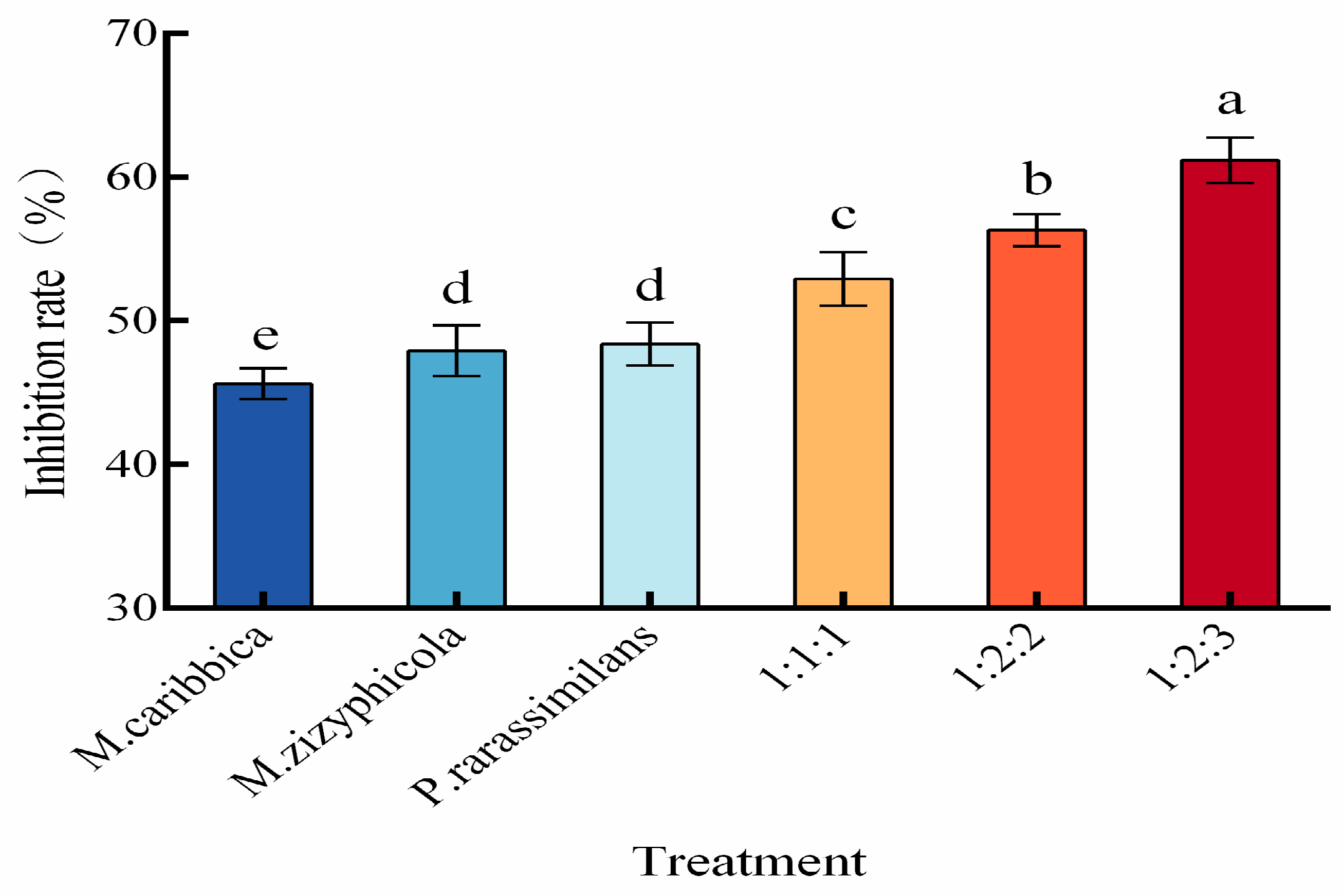
3.2. Efficacy of Multi-Strain Yeast Cultures Against Apple Blue Mold
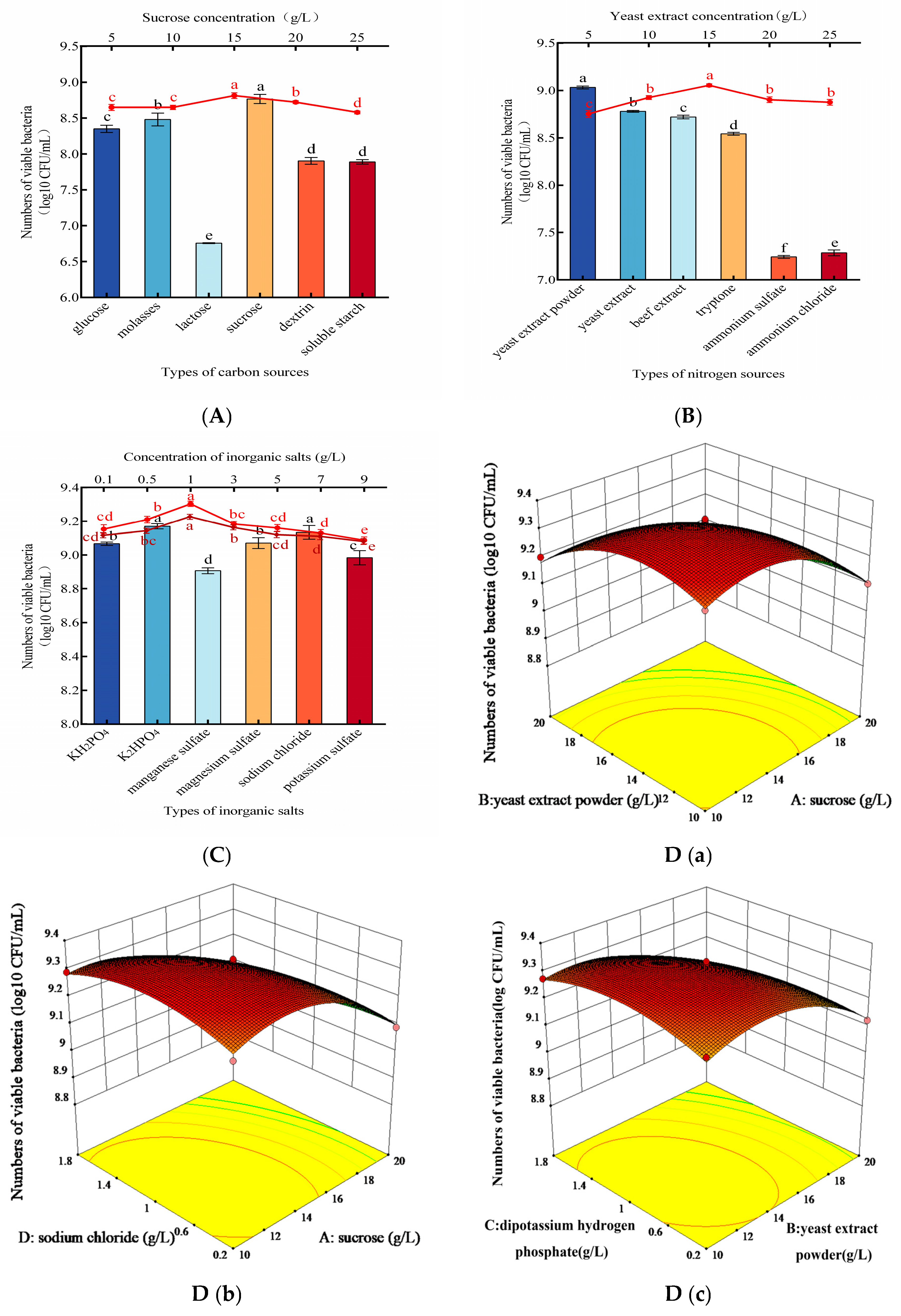
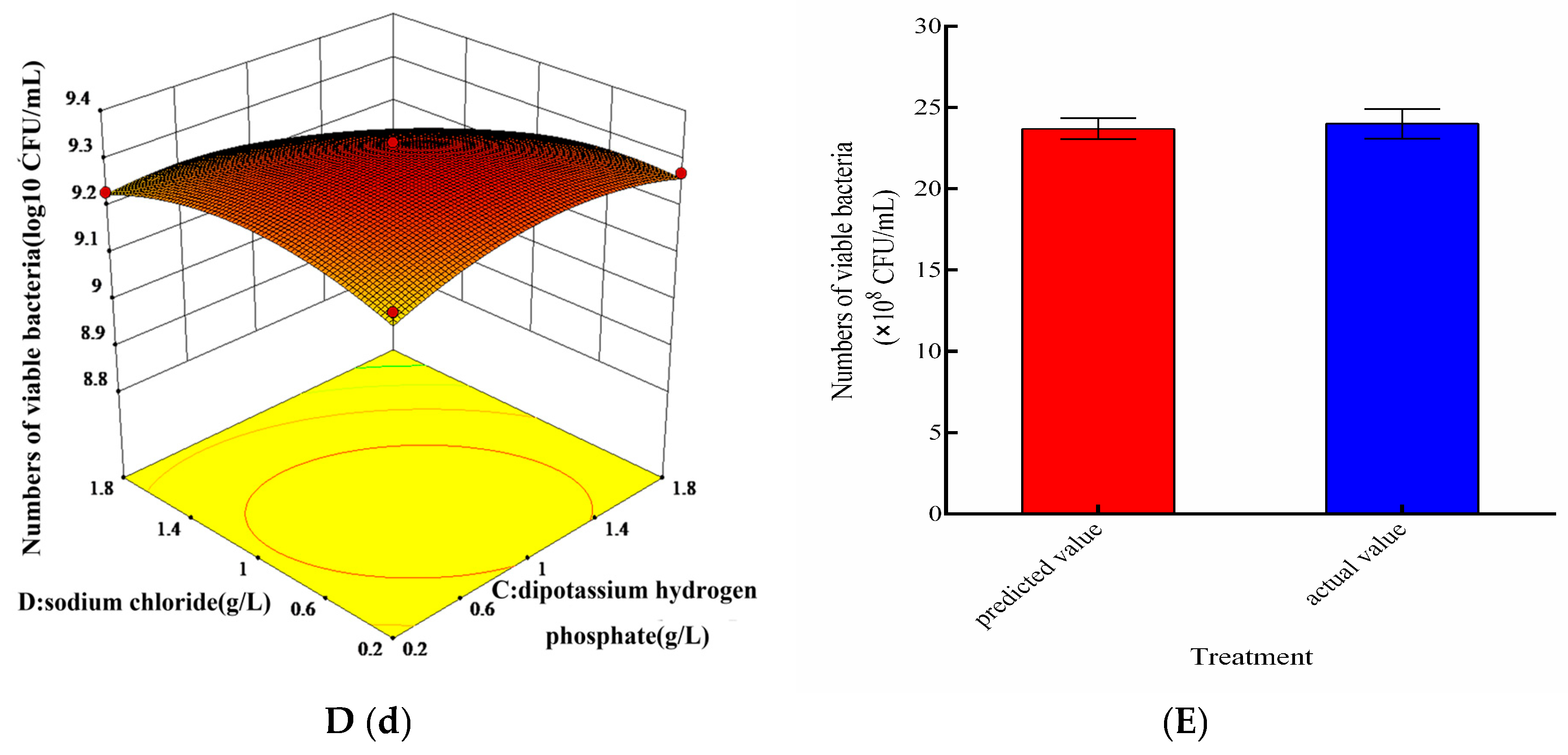
3.3. Optimization of Nutrient Conditions for Mixed Culture
3.4. Effect of Cultivation Conditions on Mixed Culture
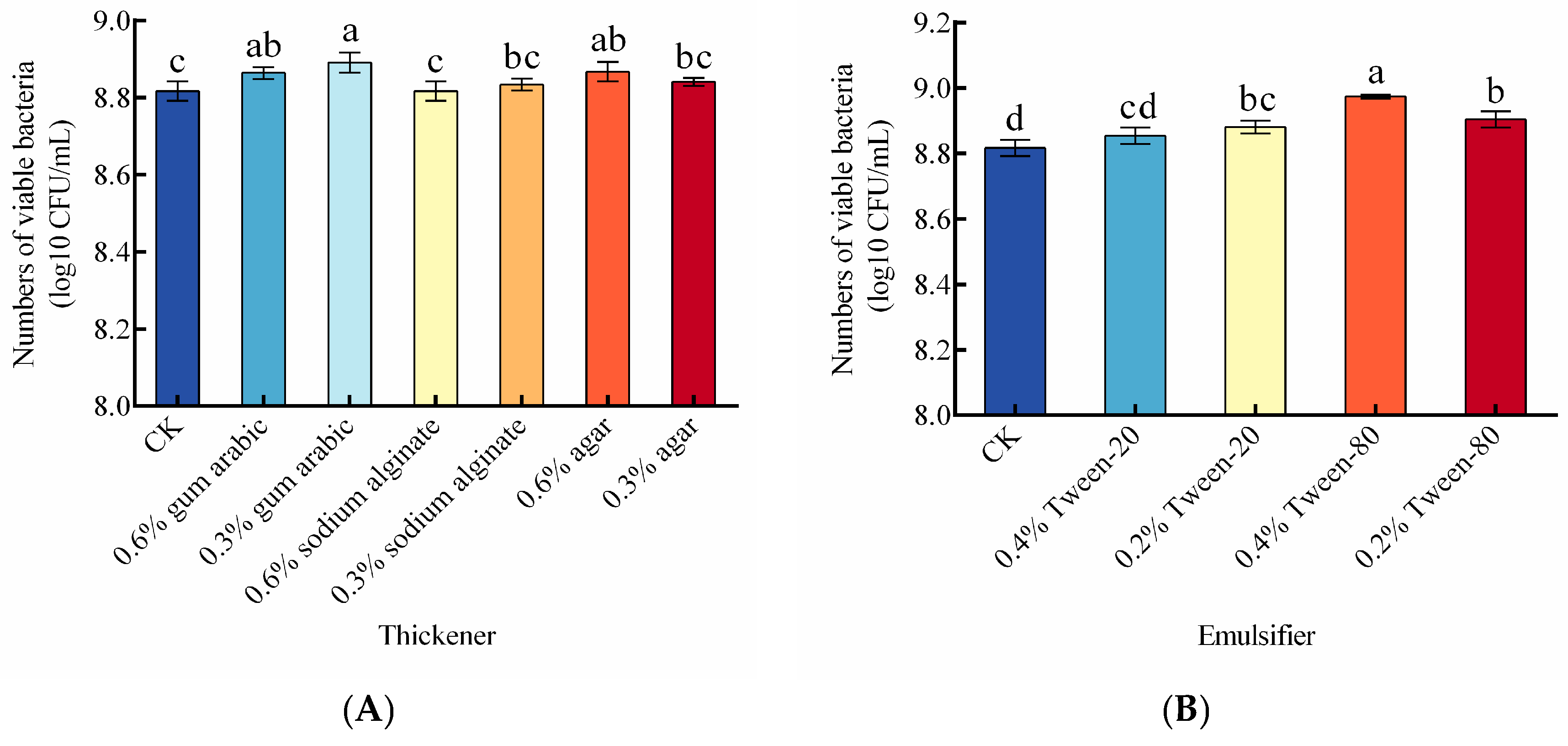
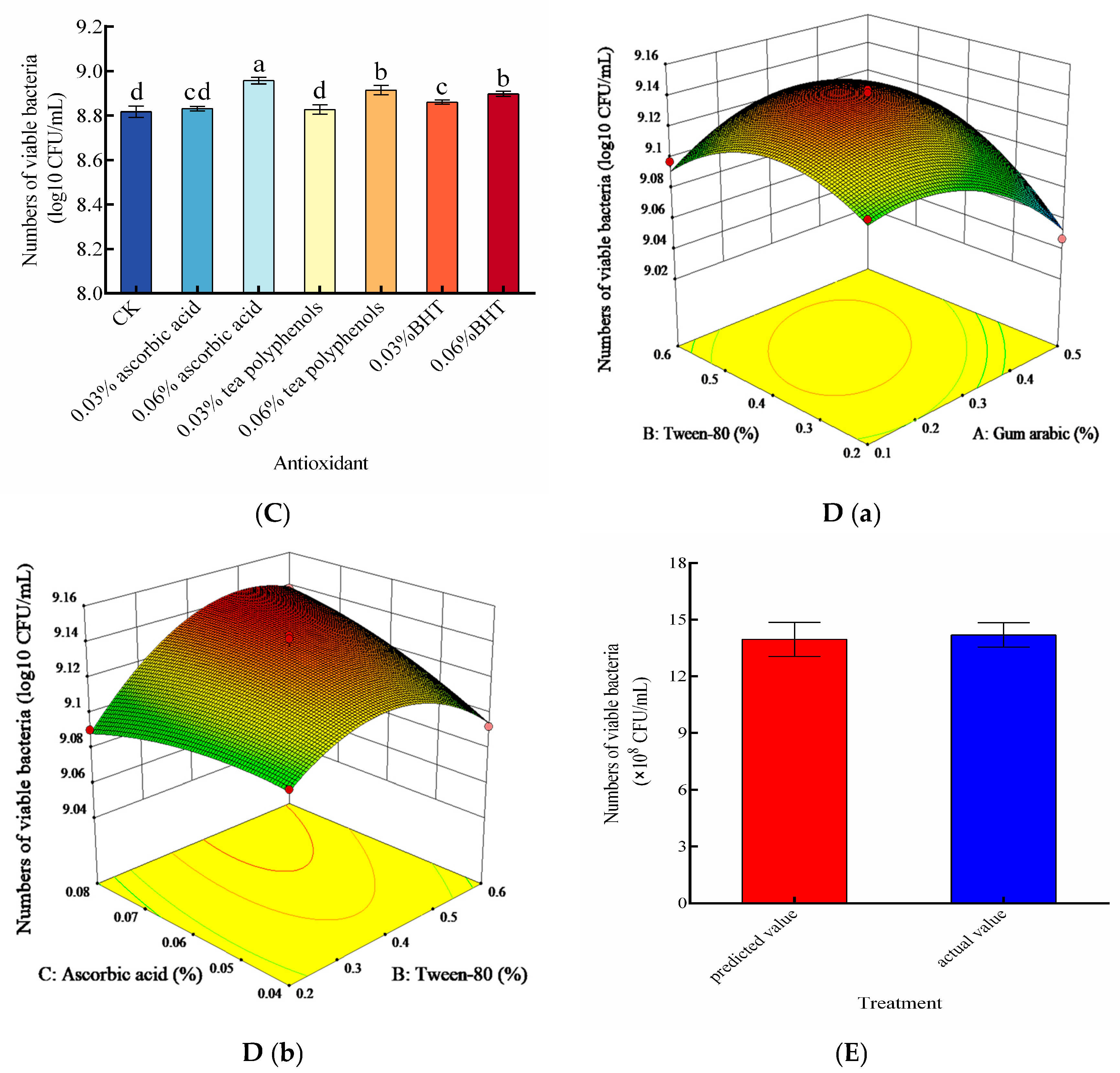
3.5. Effect of Thickeners, Emulsifiers, and Antioxidants on Viable Cell Count of the Liquid Composite BCA
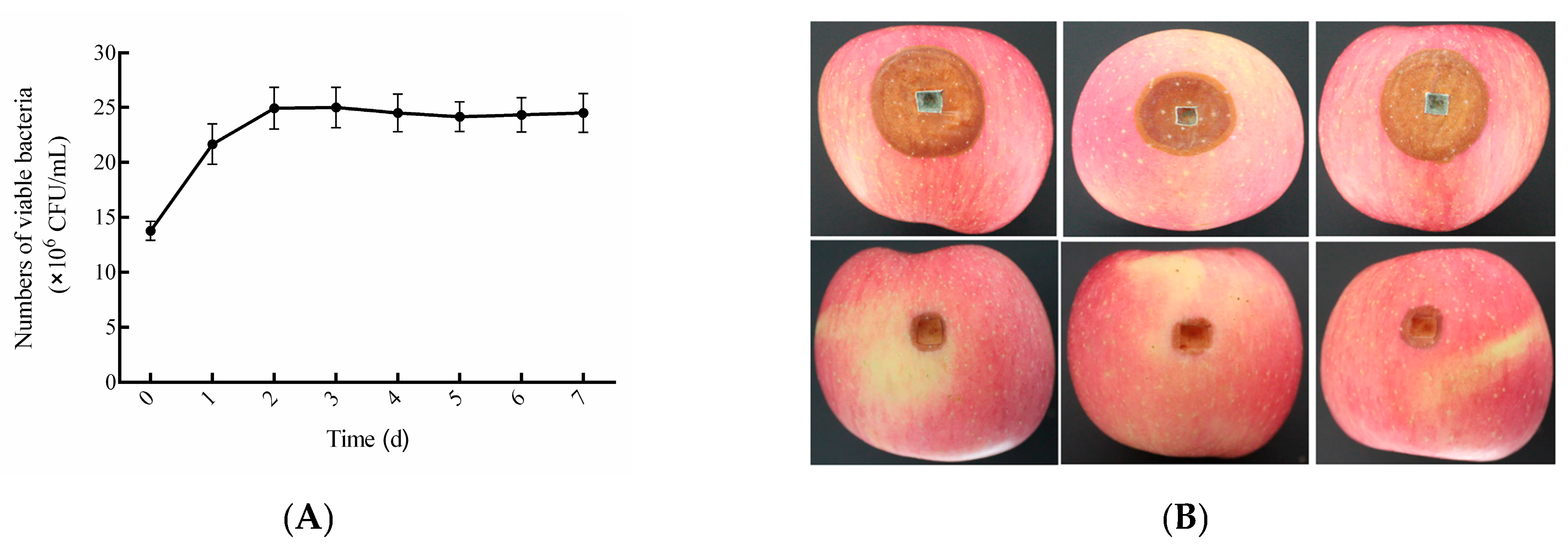
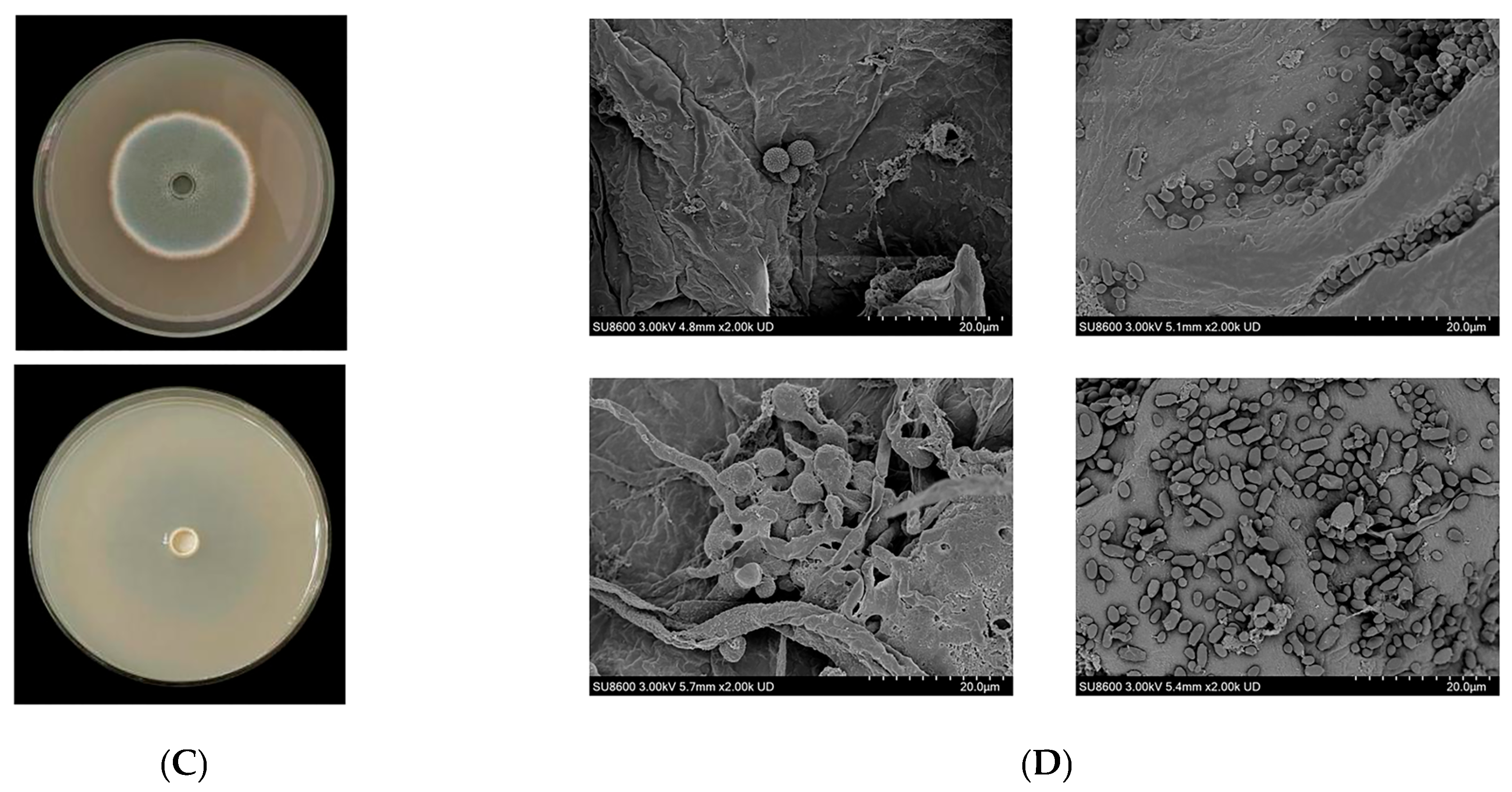
3.6. Biocontrol Efficacy of the Composite BCA Against Blue Mold
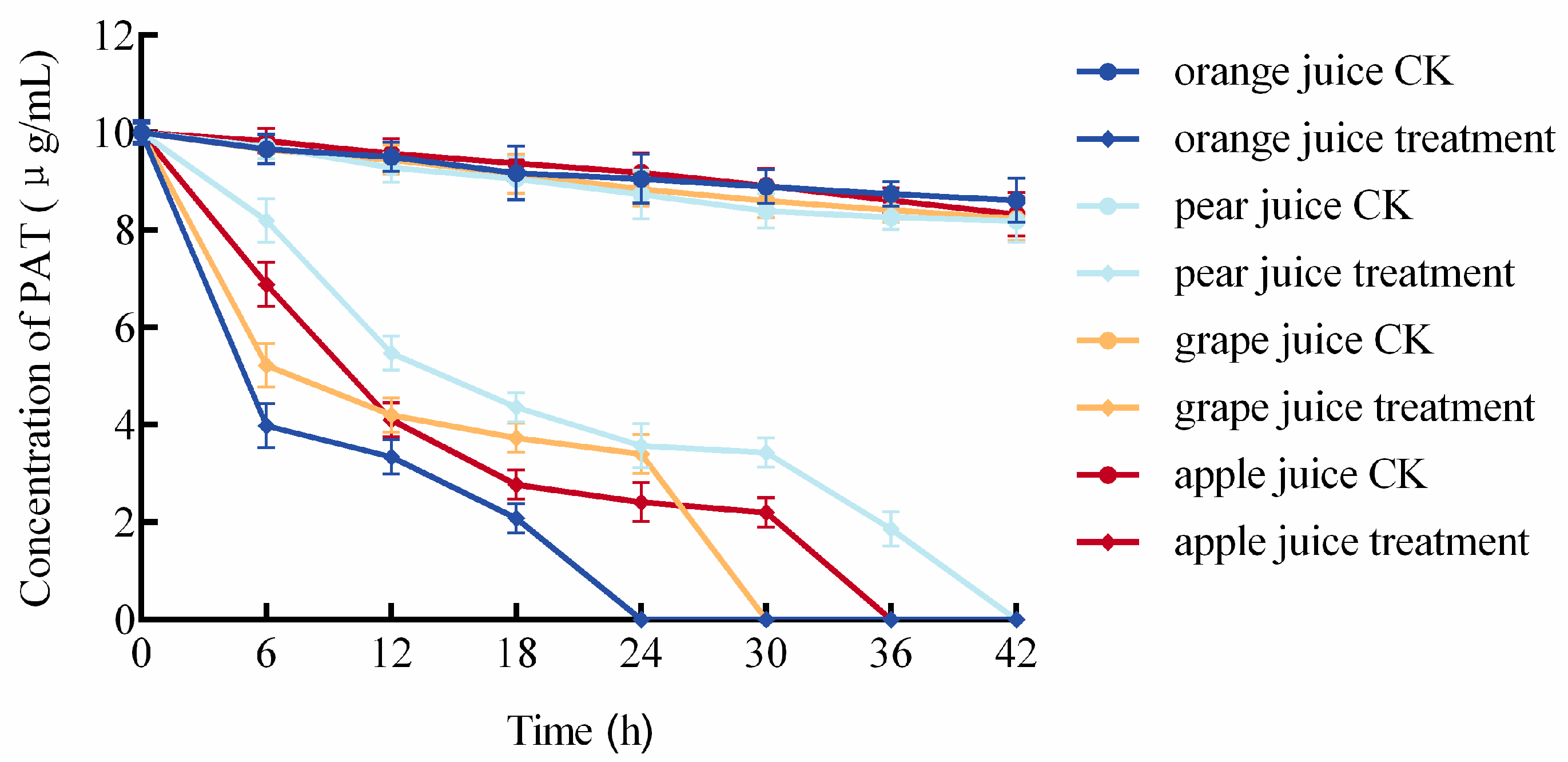
3.7. Degradation of Patulin (PAT) by the Composite BCA in Fruit Juices
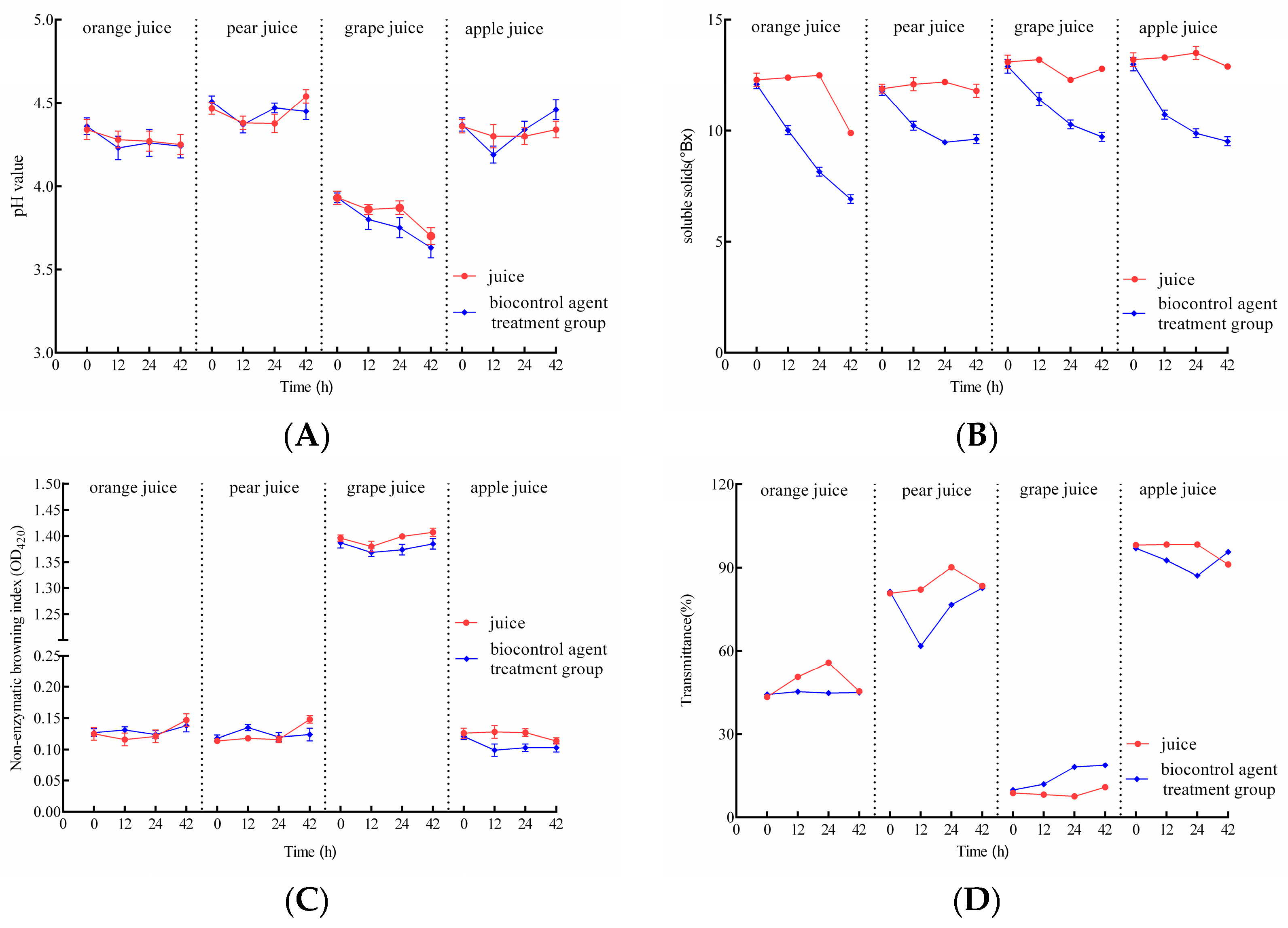
3.8. Effect of the Composite BCA on Juice Quality
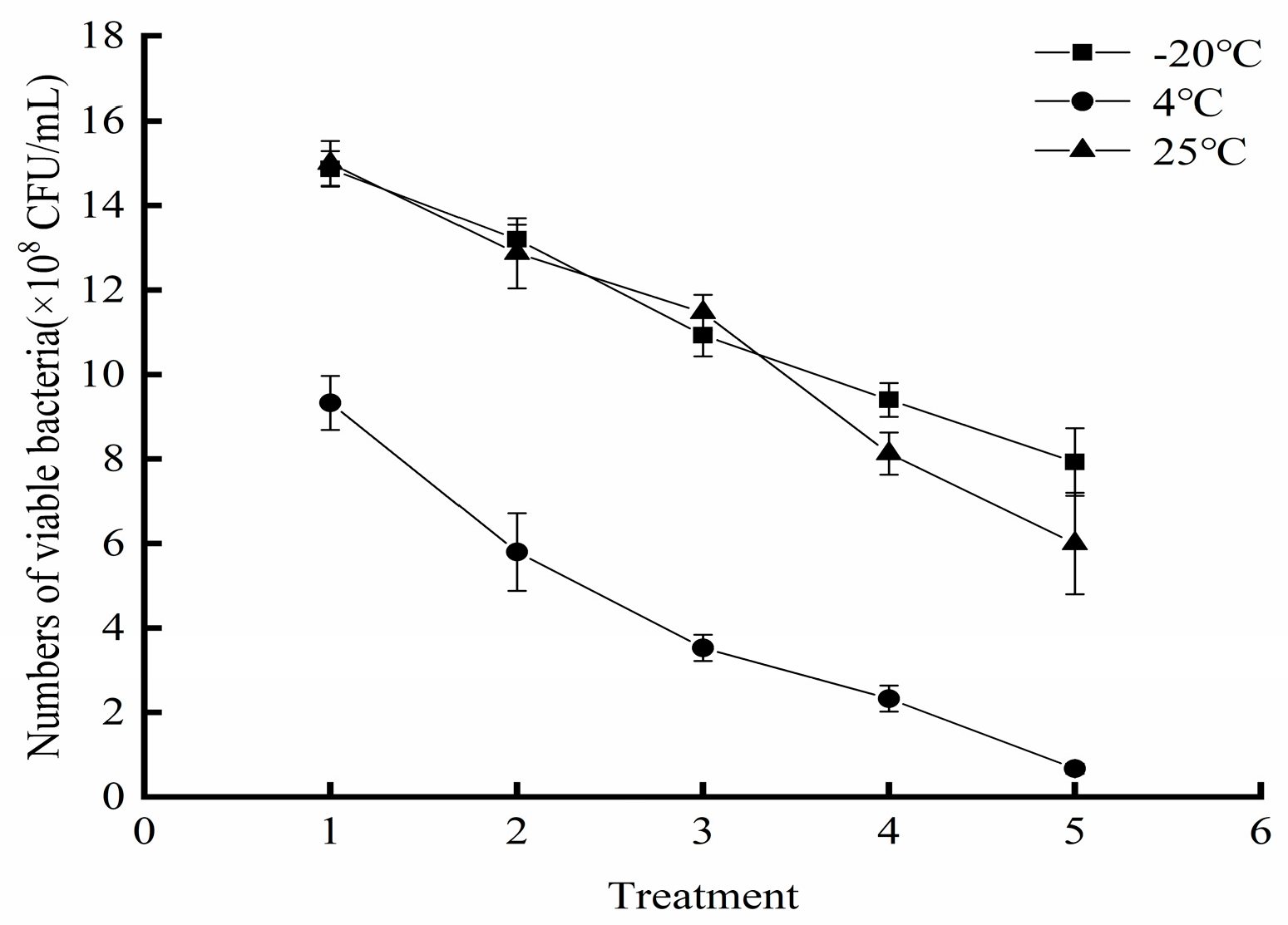
3.9. Storage Stability of the Liquid Composite BCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | Biocontrol agent |
| PAT | Patulin |
| RSM | Response surface methodology |
| HPLC | High-performance liquid chromatography |
| NYDA | Nutrient yeast dextrose agar |
| NYDB | Nutrient yeast dextrose broth |
| BHT | Butylated hydroxytoluene |
| SEM | Scanning electron microscopy |
| SSC | Soluble solids content |
| NEBI | Non-enzymatic browning index |
| ANOVA | Analysis of variance |
| VOCs | Volatile organic compounds |
References
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Mycotoxins in Functional Beverages: A Review. Beverages 2020, 6, 52. [Google Scholar] [CrossRef]
- Oztekin, S.; Dikmetas, D.N.; Devecioglu, D.; Acar, E.G.; Karbancioglu-Guler, F. Recent Insights into the Use of Antagonistic Yeasts for Sustainable Biomanagement of Postharvest Pathogenic and Mycotoxigenic Fungi in Fruits with Their Prevention Strategies against Mycotoxins. J. Agric. Food Chem. 2023, 71, 9923–9950. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Singh, N.; Ansari, K.M. Toxicological effects of patulin mycotoxin on the mammalian system: An overview. Toxicol. Res. 2017, 6, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Bahuguna, A.; Kim, M. The effects of mycotoxin patulin on cells and cellular components. Trends Food Sci. Technol. 2019, 83, 99–113. [Google Scholar] [CrossRef]
- Mandappa, I.M.; Basavaraj, K.; Manonmani, H.K. Chapter 36—Analysis of Mycotoxins in Fruit Juices. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 763–777. [Google Scholar]
- Hsu, S.-S.; Lin, Y.-S.; Chio, L.-M.; Liang, W.-Z. Evaluation of the mycotoxin patulin on cytotoxicity and oxidative stress in human glioblastoma cells and investigation of protective effect of the antioxidant N-acetylcysteine (NAC). Toxicon 2023, 221, 106957. [Google Scholar] [CrossRef]
- WoldemariamYohannes, K.; Wan, Z.; Yu, Q.L.; Li, H.Y.; Wei, X.T.; Liu, Y.L.; Wang, J.; Sun, B.G. Prebiotic, Probiotic, Antimicrobial, and Functional Food Applications of Bacillus amyloliquefaciens. J. Agric. Food Chem. 2020, 68, 14709–14727. [Google Scholar] [CrossRef]
- Zhang, X.K.; Li, B.Q.; Zhang, Z.Q.; Chen, Y.; Tian, S.P. Antagonistic Yeasts: A Promising Alternative to Chemical Fungicides for Controlling Postharvest Decay of Fruit. J. Fungi 2020, 6, 158. [Google Scholar] [CrossRef]
- Montiel, L.G.H.; Droby, S.; Rangel, P.P.; Quezada, G.D.A. A Sustainable Alternative for Postharvest Disease Management and Phytopathogens Biocontrol in Fruit: Antagonistic Yeasts. Plants 2021, 10, 2641. [Google Scholar] [CrossRef]
- Tahir, H.E.; Arslan, M.; Mahunu, G.K.; Hashim, S.B.H.; Shi, J.Y.; Zhang, W.; Huang, X.W.; Mariod, A.A.; Abdalla, I.I.H.; Zou, X.B. Efficacy of biologically active agents and antagonistic yeast to control the incidence of postharvest diseases: A meta-analysis and meta-regression. Biol. Control 2022, 172, 104952. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Mahunu, G.K.; Castoria, R.; Yang, Q.Y.; Apaliya, M.T. Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Pang, B.; Solairaj, D.; Hu, W.C.; Legrand, N.N.G.; Ma, J.F.; Huang, S.Y.; Wu, X.Y.; Zhang, H.Y. Effect of Rhodotorula mucilaginosa on patulin degradation and toxicity of degradation products. Food Addit. Contam. A 2021, 38, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.U.; Thani, R.A.; Atia, F.A.; Alsafran, M.; Jaoua, S. Application of yeasts and yeast derivatives for the biological control of toxigenic fungi and their toxic metabolites. Environ. Technol. Innov. 2021, 22, 101447. [Google Scholar] [CrossRef]
- Zheng, X.F.; Yang, Q.Y.; Zhang, H.Y.; Cao, J.; Zhang, X.Y.; Apaliya, M.T. The Possible Mechanisms Involved in Degradation of Patulin by Pichia caribbica. Toxins 2016, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Alasmar, R.; Ul-Hassan, Z.; Zeidan, R.; Al-Thani, R.; Al-Shamary, N.; Alnaimi, H.; Migheli, Q.; Jaoua, S. Isolation of a Novel Strain QKM-4 and Evidence of Its Volatilome Production and Binding Potentialities in the Biocontrol of Toxigenic Fungi and Their Mycotoxins. ACS Omega 2020, 5, 17637–17645. [Google Scholar] [CrossRef]
- Feng, M.F.; Lv, Y.; Li, T.T.; Li, X.M.; Liu, J.Y.; Chen, X.L.; Zhang, Y.; Chen, X.; Wang, A.X. Postharvest Treatments with Three Yeast Strains and Their Combinations to Control of Snap Beans. Foods 2021, 10, 2736. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Saftner, R.A.; Conway, W.S.; Yoder, K.S. Control of blue mold decay of apple during commercial controlled atmosphere storage with yeast antagonists and sodium bicarbonate. Postharvest Biol. Technol. 2008, 49, 374–378. [Google Scholar] [CrossRef]
- Öztekin, S.; Karbancioglu-Guler, F. Biological control of green mould on mandarin fruit through the combined use of antagonistic yeasts. Biol. Control 2023, 180, 105186. [Google Scholar] [CrossRef]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Dinoor, A. Combining biocontrol agents to reduce the variability of biological control. Phytopathology 2001, 91, 621–627. [Google Scholar] [CrossRef]
- Shi, J.F.; Sun, C.Q. Isolation, identification, and biocontrol of antagonistic bacterium against Botrytis cinerea after tomato harvest. Braz. J. Microbiol. 2017, 48, 706–714. [Google Scholar] [CrossRef]
- Lin, R.; Yang, Q.; Xiao, J.; Solairaj, D.; Ngea, G.L.N.; Zhang, H. Study on the biocontrol effect and physiological mechanism of Hannaella sinensis on the blue mold decay of apples. Int. J. Food Microbiol. 2022, 382, 109931. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; Zheng, X.; Yu, T.; Yan, F. Enhancement of biocontrol efficacy of Kluyveromyces marxianus induced by N-acetylglucosamine against Penicillium expansum. Food Chem. 2023, 404, 134658. [Google Scholar] [CrossRef]
- Pietrysiak, E.; Ganjyal, G.M. Apple peel morphology and attachment of through aqueous environment as shown by scanning electron microscopy. Food Control 2018, 92, 362–369. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, W.; Rao, S.; Gao, L.; Li, H.; Yang, Z. Degradation of patulin in fruit juice by a lactic acid bacteria strain Lactobacillus casei YZU01. Food Control 2020, 112, 107147. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Wang, X.; Xu, J.; Xie, L.; Hu, H.; Sun, J.; Yue, T.; Wang, J. Aerogel doped by sulfur-functionalized graphene oxide with convenient separability for efficient patulin removal from apple juice. Food Chem. 2021, 338, 127785. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Calvo, J.; Calvente, V.; Orellano, M.E.d.; Benuzzi, D.; Tosetti, M.I.S.D. Improvement in the biocontrol of postharvest diseases of apples with the use of yeast mixtures. Biocontrol 2003, 48, 579–593. [Google Scholar] [CrossRef]
- Li, F.; Ghanizadeh, H.; Cui, G.; Liu, J.; Miao, S.; Liu, C.; Song, W.; Chen, X.; Cheng, M.; Wang, P.; et al. Microbiome—Based agents can optimize composting of agricultural wastes by modifying microbial communities. Bioresour. Technol. 2023, 374, 128765. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Li, Z.; Li, C.; Li, B.; Gu, X.; Zhang, X.; Zhang, H. Screening and identification of an antagonistic yeast controlling postharvest blue mold decay of pears and the possible mechanisms involved. Biol. Control 2019, 133, 26–33. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, Y.; Li, X.; Feng, L.; Li, K. Optimization of an economical medium composition for the coculture of Clostridium butyricum and Bacillus coagulans. AMB Express 2022, 12, 19. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.-S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Amish, M.; Khodja, M.; Jada, A. Development of a New Technique for Emulsifiers Optimization. SPE J. 2022, 27, 1424–1435. [Google Scholar] [CrossRef]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food thickening agents: Sources, chemistry, properties and applications—A review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Marín, S.; Mateo, E.M.; Sanchis, V.; Valle-Algarra, F.M.; Ramos, A.J.; Jiménez, M. Patulin contamination in fruit derivatives, including baby food, from the Spanish market. Food Chem. 2011, 124, 563–568. [Google Scholar] [CrossRef]
- Ji, X.; Li, R.; Yang, H.; Qi, P.; Xiao, Y.; Qian, M. Occurrence of patulin in various fruit products and dietary exposure assessment for consumers in China. Food Control 2017, 78, 100–107. [Google Scholar] [CrossRef]
- Oteiza, J.M.; Khaneghah, A.M.; Campagnollo, F.B.; Granato, D.; Mahmoudi, M.R.; Sant’Ana, A.S.; Gianuzzi, L. Influence of production on the presence of patulin and ochratoxin A in fruit juices and wines of Argentina. LWT 2017, 80, 200–207. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; Wei, D. Biological Detoxification of Mycotoxins: Current Status and Future Advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Guo, P.; Qi, J.; Cui, Y.; Wang, K.; Du, G.; Wang, Z.; Yuan, Y.; Yue, T. Detoxification of Mycotoxin Patulin by the Yeast Kluyveromyces marxianus YG-4 in Apple Juice. J. Agric. Food Chem. 2024, 72, 12. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Yuan, L.; Li, J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci. Technol. 2020, 96, 127–134. [Google Scholar] [CrossRef]
- Ocvirk, M.; Kočar Mlinarič, N.; Košir, I.J. Impact of Successive Exploitation of a Saccharomyces pastorianus Starter Culture on Saccharide Uptake Dynamics from Wort. Food Technol. Biotechnol. 2023, 61, 412, Erratum in Food Technol. Biotechnol. 2021, 59, 16—23. [Google Scholar] [CrossRef]
- Vegara, S.; Martí, N.; Mena, P.; Saura, D.; Valero, M. Effect of pasteurization process and storage on color and shelf-life of pomegranate juices. LWT—Food Sci. Technol. 2013, 54, 592–596. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Ji, J.; Mou, Y.; Chen, F.; Xiao, Z.; Liao, X.; Hu, X.; Ma, L. Polyphenol mediated non-enzymatic browning and its inhibition in apple juice. Food Chem. 2023, 404, 134504. [Google Scholar] [CrossRef]
| Type | SS | DF | MS | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.52 | 14 | 0.037 | 103.70 | <0.0001 | ** |
| A | 0.21 | 1 | 0.21 | 594.67 | <0.0001 | ** |
| B | 0.11 | 1 | 0.11 | 308.60 | <0.0001 | ** |
| C | 0.0075 | 1 | 0.0075 | 21.04 | 0.0004 | ** |
| D | 0.021 | 1 | 0.021 | 57.97 | <0.0001 | ** |
| AB | 0.007 | 1 | 0.007 | 19.79 | 0.0006 | ** |
| AC | 0.0006 | 1 | 0.0006 | 1.68 | 0.2154 | |
| AD | 0.0088 | 1 | 0.0088 | 24.79 | 0.0002 | ** |
| BC | 0.00497 | 1 | 0.00497 | 13.94 | 0.0022 | ** |
| BD | 0.00096 | 1 | 0.00096 | 2.70 | 0.1229 | |
| CD | 0.0062 | 1 | 0.00624 | 17.51 | 0.0009 | ** |
| A2 | 0.090 | 1 | 0.090 | 251.81 | <0.0001 | ** |
| B2 | 0.066 | 1 | 0.066 | 186.13 | <0.0001 | ** |
| C2 | 0.021 | 1 | 0.021 | 58.63 | <0.0001 | ** |
| D2 | 0.024 | 1 | 0.024 | 67.74 | <0.0001 | ** |
| Residual | 0.00499 | 14 | 0.00035 | |||
| Lack of fit | 0.00465 | 10 | 0.00046 | 5.53 | 0.0569 | Not significant |
| Pure error | 0.00033 | 4 | 0.000084 |
| Type | SS | DF | MS | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.00956 | 9 | 0.00106 | 25.02 | 0.0002 | ** |
| A | 0.00061 | 1 | 0.00061 | 14.42 | 0.0067 | ** |
| B | 0.00088 | 1 | 0.00088 | 20.77 | 0.0026 | ** |
| C | 0.00045 | 1 | 0.00045 | 10.60 | 0.0140 | * |
| AB | 0.00122 | 1 | 0.00122 | 28.84 | 0.0010 | ** |
| AC | 0.00012 | 1 | 0.00012 | 2.85 | 0.1353 | |
| BC | 0.00084 | 1 | 0.00084 | 19.80 | 0.0030 | ** |
| A2 | 0.00220 | 1 | 0.00220 | 51.99 | 0.0002 | ** |
| B2 | 0.00261 | 1 | 0.00261 | 61.47 | 0.0001 | ** |
| C2 | 0.00017 | 1 | 0.00017 | 4.06 | 0.0837 | |
| Residual | 0.00029 | 7 | 0.00004 | |||
| Lack of fit | 0.0002 | 3 | 0.00006 | 2.76 | 0.1757 | Not significant |
| Pure error | 0.00009 | 4 | 0.00002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, L.; Li, L.; Zhang, Q.; Hu, J.; Du, J.; Shi, J. Construction of Composite Biocontrol Agent (BCA): Developing Effective Strategies for Controlling Postharvest Blue Mold and Patulin in Apples. Foods 2025, 14, 3378. https://doi.org/10.3390/foods14193378
Cong L, Li L, Zhang Q, Hu J, Du J, Shi J. Construction of Composite Biocontrol Agent (BCA): Developing Effective Strategies for Controlling Postharvest Blue Mold and Patulin in Apples. Foods. 2025; 14(19):3378. https://doi.org/10.3390/foods14193378
Chicago/Turabian StyleCong, Longmei, Limei Li, Qian Zhang, Junyue Hu, Jingting Du, and Junfeng Shi. 2025. "Construction of Composite Biocontrol Agent (BCA): Developing Effective Strategies for Controlling Postharvest Blue Mold and Patulin in Apples" Foods 14, no. 19: 3378. https://doi.org/10.3390/foods14193378
APA StyleCong, L., Li, L., Zhang, Q., Hu, J., Du, J., & Shi, J. (2025). Construction of Composite Biocontrol Agent (BCA): Developing Effective Strategies for Controlling Postharvest Blue Mold and Patulin in Apples. Foods, 14(19), 3378. https://doi.org/10.3390/foods14193378






