Depolymerization and Oxidation Events in Used Frying Oils Under Conditions Simulating Gastric Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Digestion Assays
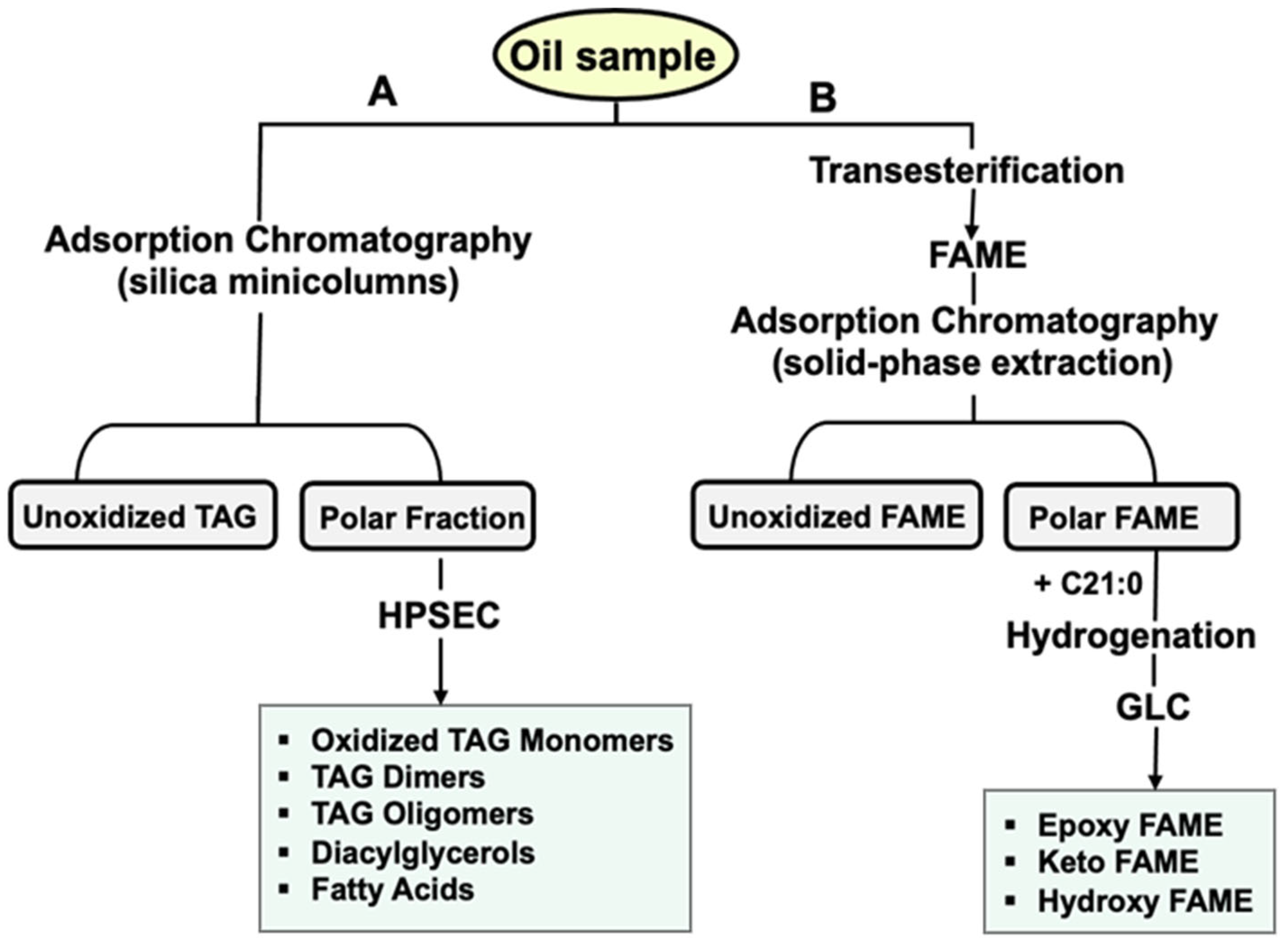
2.4. Lipid Extraction
2.5. Analytical Determinations
2.5.1. Acidity
2.5.2. Peroxide Value
2.5.3. Oil Stability Index
2.5.4. Smoke Point
2.5.5. Fatty Acid Composition
2.5.6. Sterols
2.5.7. Tocopherols
2.5.8. Polar Compounds
2.5.9. Epoxides, Ketones and Hydroxides
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Sunflower Oil
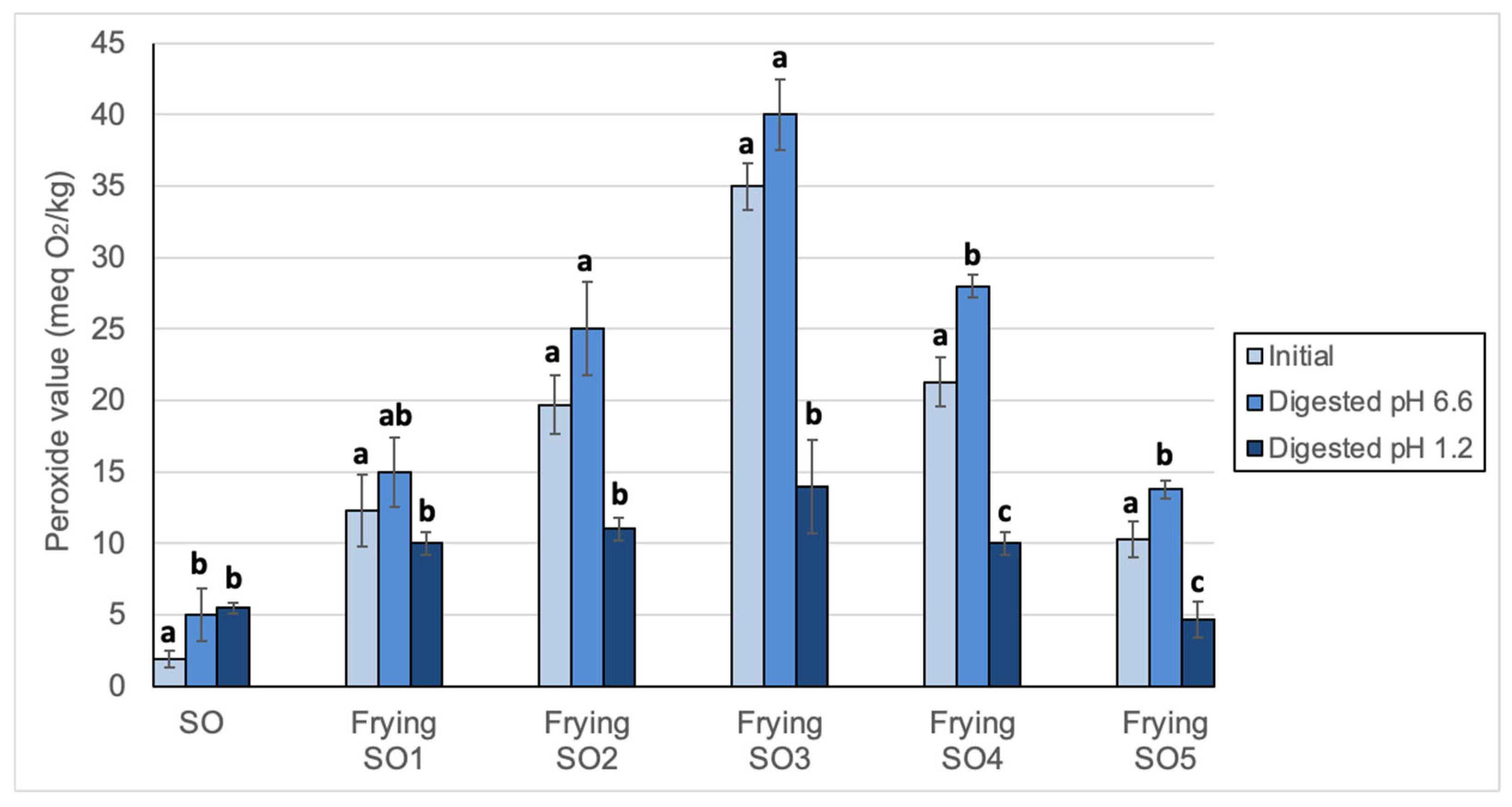
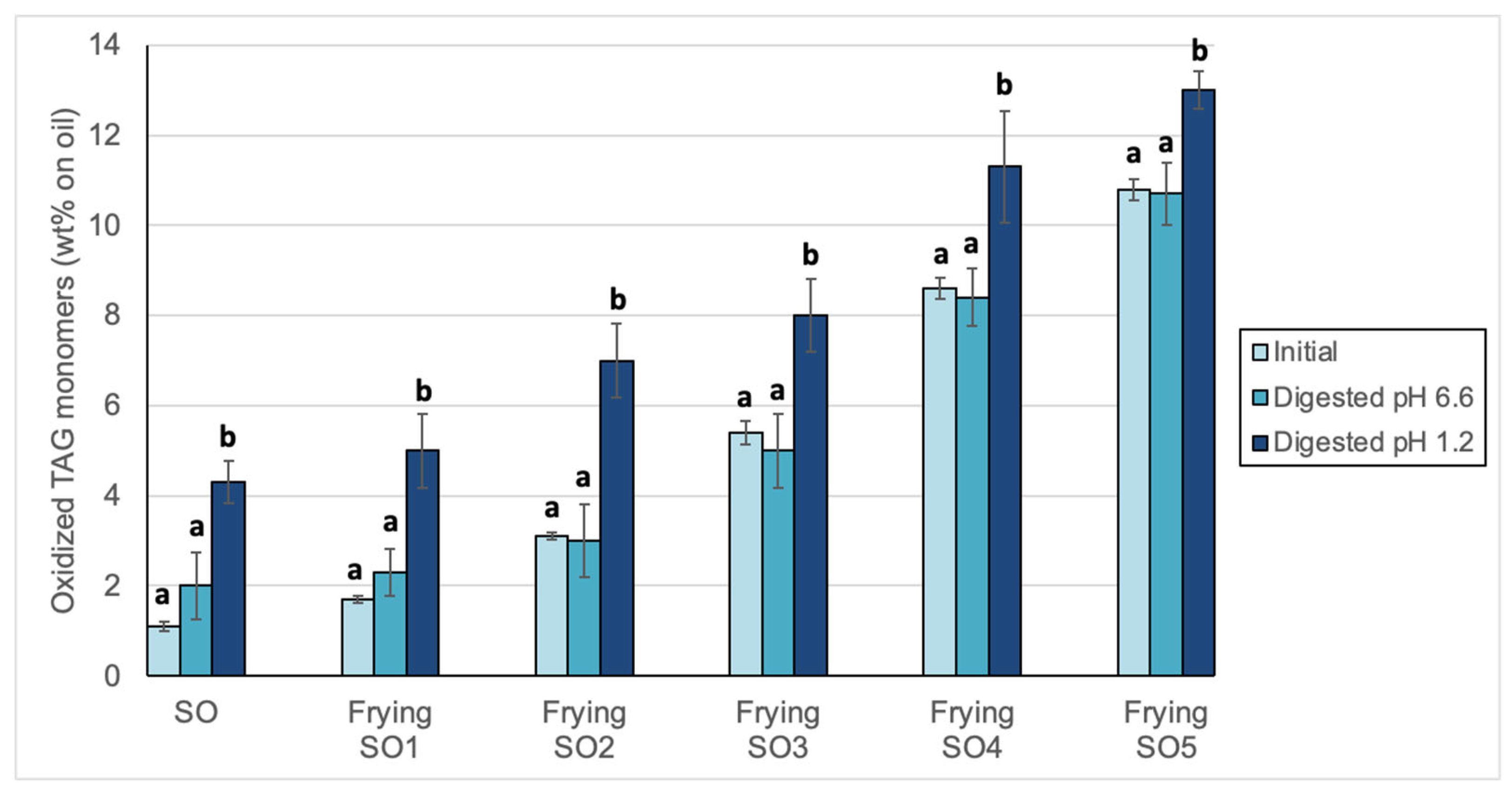
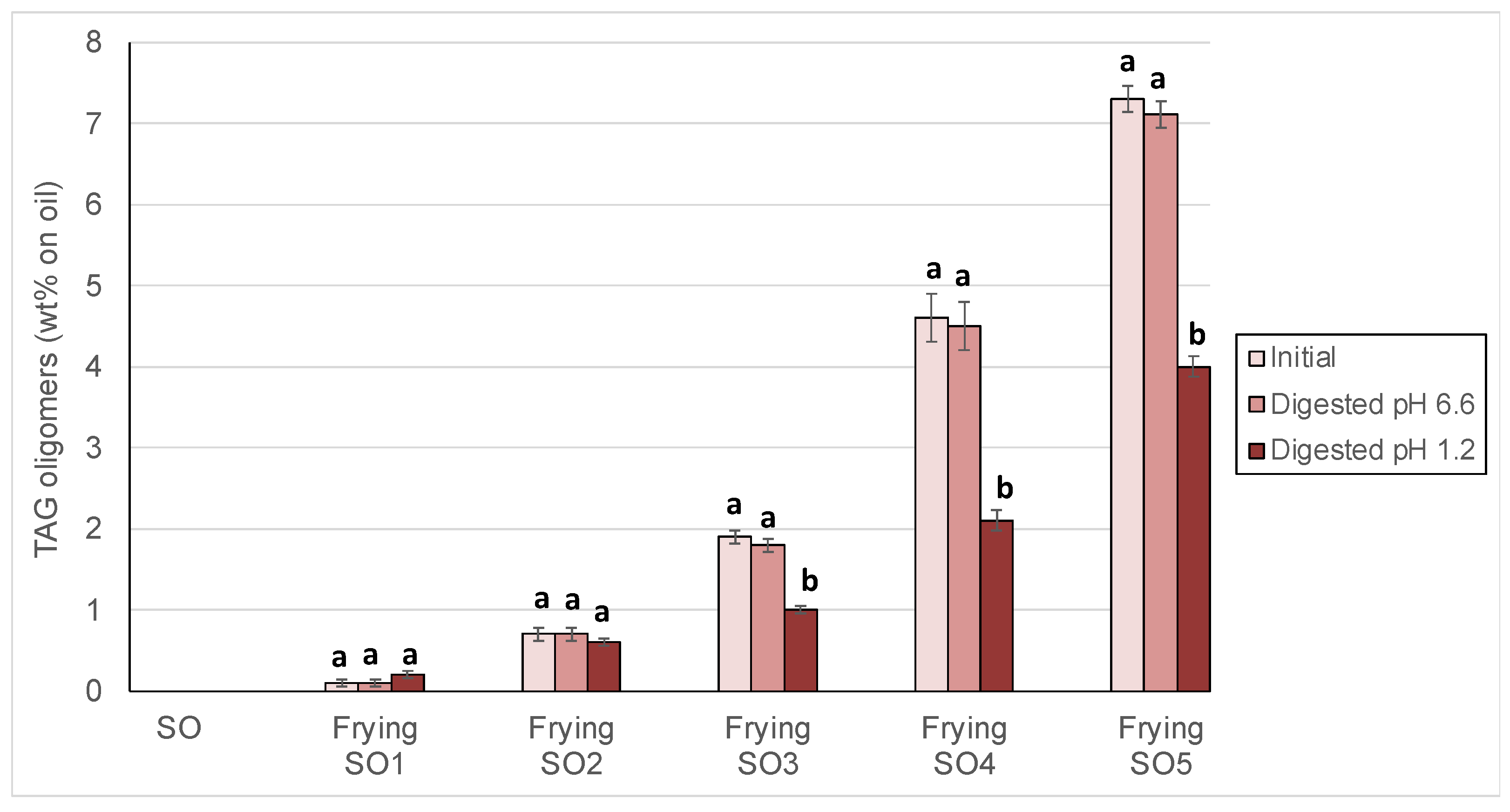
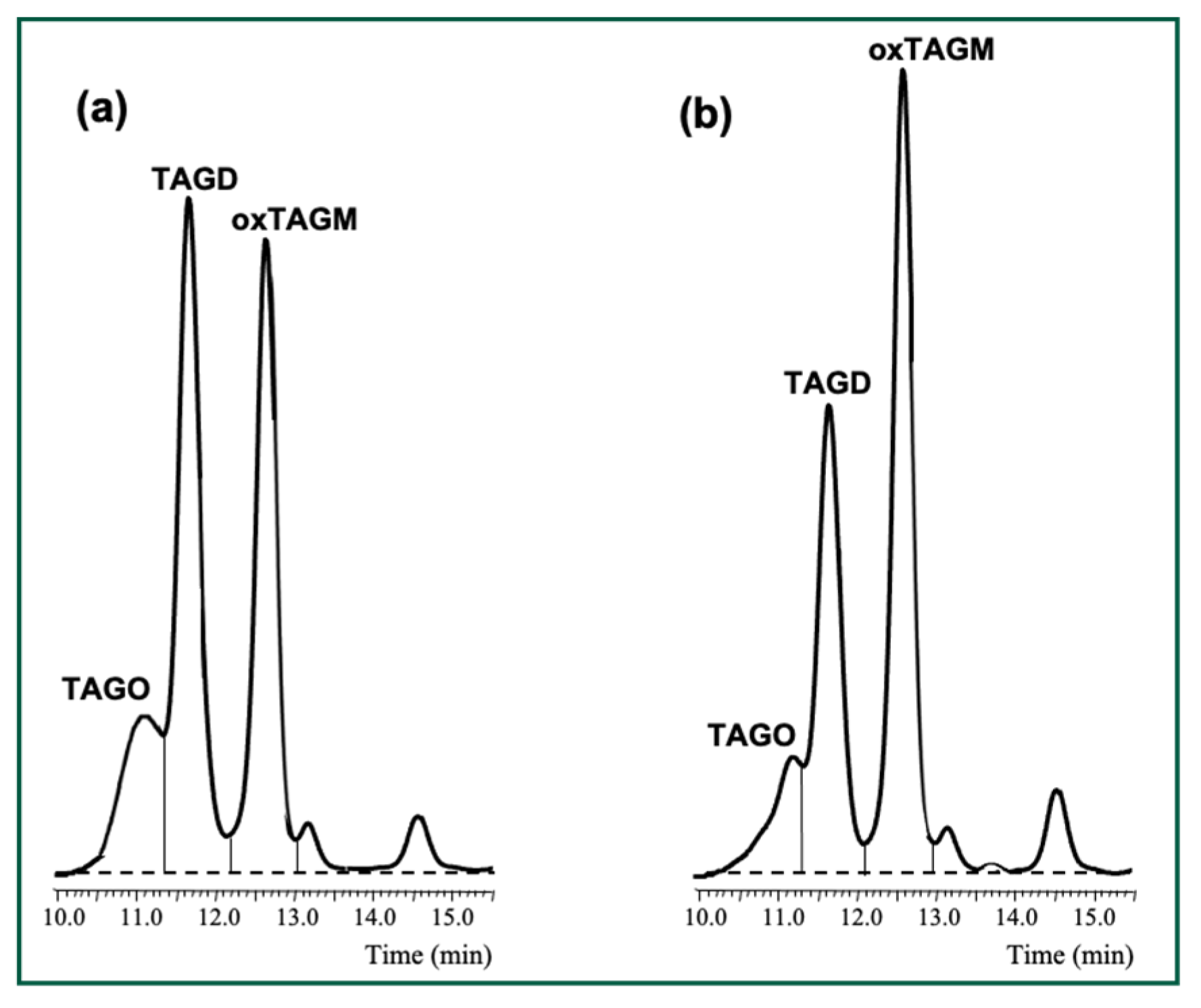
3.2. Changes in Peroxide Values, oxTAGM, TAGD and TAGO After Digestion
3.3. Changes in Unoxidized TAG After Digestion
3.4. Evaluation of Epoxy, Keto and Hydroxyl Fatty Acyls
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Márquez-Ruiz, G.; Holgado, F.; Velasco, J. Mechanisms of oxidation in food lipids. In Food Oxidants and Antioxidants. Chemical, Biological and Functional Properties; Bartosz, G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 79–113. ISBN 978-143988242-9. [Google Scholar] [CrossRef]
- Cao, X.; Li, X.; Shu, N.; Tan, C.P.; Xu, Y.J.; Liu, Y. The characteristics and analysis of polar compounds in deep-frying oil: A mini review. Food Anal. Methods 2022, 15, 2767–2776. [Google Scholar] [CrossRef]
- Leopold, J.; Prabutzki, P.; Engel, K.M.; Schiller, J. From oxidized fatty acids to dimeric species: In vivo relevance, generation and methods of analysis. Molecules 2023, 28, 7850. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, T.; Qiang, Z.; Du, W.; Li, C. Mechanisms of the formation of nonvolatile and volatile oxidation products from methyl linoleic acid at high temperatures. J. Agric. Food Chem. 2023, 72, 704–714. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Guo, X.; Qiang, J.; Cao, Y.; Zhang, S.; Yu, X. Influence of triacylglycerol structure on the formation of lipid oxidation products in different vegetable oils during frying process. Food Chem. 2025, 464, 141783. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Márquez-Ruiz, G. Formation and analysis of oxidized monomeric, dimeric, and higher oligomeric triglycerides. In Deep Frying: Chemistry, Nutrition and Practical Applications, 2nd ed.; Erickson, M.D., Ed.; Academic Press and AOCS Press: Champaign, IL, USA, 2007; pp. 87–110. ISBN 978-1-893997-92-9. [Google Scholar] [CrossRef]
- Firestone, D. Regulation of frying fats and oils. In Deep Frying: Chemistry, Nutrition and Practical Applications; Erickson, M.D., Ed.; Academic Press and AOCS Press: Champaign, IL, USA, 2007; pp. 373–385. ISBN 978-1-893997-92-9. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential adverse public health effects afforded by the ingestion of dietary lipid oxidation product toxins: Significance of fried food sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Li, Y.; Zhang, N.; Gao, Y.; Yu, X. The formation, determination and health implications of polar compounds in edible oils: Current status, challenges and perspectives. Food Chem. 2021, 364, 130451. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.J.; Liu, Y.F.; Xu, Y.J. Deep-frying oil intake and risk of intestinal barrier dysfunction: A systematic review and meta-analyses. Int. J. Food Sci. Tech. 2023, 58, 1720–1727. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Velasco, J.; Holgado, F. Major dietary lipids in nutrition and health. In Advances in Food and Nutrition Research; Gallegos, C., Ruiz-Méndez, M.V., Eds.; Academic Press: London, UK, 2023; Volume 105, pp. 1–49. ISBN 9780443185908. [Google Scholar] [CrossRef]
- Dangal, A.; Tahergorabi, R.; Acharya, D.R.; Timsina, P.; Rai, K.; Dahal, S.; Acharya, P.; Giuffrè, A.M. Review on deep-fat fried foods: Physical and chemical attributes, and consequences of high consumption. Eur. Food Res. Technol. 2024, 250, 1537–1550. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Xie, Z.; Gao, B.; Yu, L. Chemical structures, analytical approaches and toxicological effects of oxidative derivatives of triglycerides as potential hazards in lipid thermal processing: A review. Grain Oil Sci. Technol. 2024, 7, 270–279. [Google Scholar] [CrossRef]
- Ju, J.; Zheng, Z.; Xu, Y.J.; Cao, P.; Li, J.; Li, Q.; Liu, Y. Influence of total polar compounds on lipid metabolism, oxidative stress and cytotoxicity in HepG2 cells. Lipids Health Dis. 2019, 18, 37. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Sun, D.; Li, J.; Wang, Y.; Cao, P.; Liu, Y. Effects of Polar Compounds Generated from the Deep-Frying Process of Palm Oil on Lipid Metabolism and Glucose Tolerance in Kunming Mice. J. Agric. Food Chem. 2016, 65, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Jiang, F.; Cao, X.; Liu, Y.; Xu, Y.J. Metabolomics reveals the toxicological effects of polar compounds from frying palm oil. Food Funct. 2020, 11, 1611–1623. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Pérez-Camino, M.C.; Dobarganes, M.C. Digestibility of fatty acid monomers, dimers and polymers in the rat. J. Am. Oil Chem. Soc. 1992, 69, 930–934. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Dobarganes, M.C. Assessments on the digestibility of oxidized compounds from [1-14C]-linoleic acid using a combination of chromatographic techniques. J. Chromatogr. B 1995, 675, 1–8. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, M.J.; Bastida, S.; Sánchez-Muniz, F.J. Short-term in vivo digestibility of triglyceride polymers, dimers, and monomers of thermoxidized palm olein used in deep-frying. J. Agric. Food Chem. 1998, 46, 5188–5193. [Google Scholar] [CrossRef]
- Olivero David, R.; Sánchez-Muniz, F.J.; Bastida, S.; Benedi, J.; González-Muñoz, M.J. Gastric emptying and short-term digestibility of thermally oxidized sunflower oil used for frying in fasted and nonfasted rats. J. Agric. Food Chem. 2010, 58, 9242–9248. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Guevel, G.; Dobarganes, M.C. Applications of chromatographic techniques to evaluate enzymatic hydrolysis of oxidized and polymeric triglycerides by pancreatic lipase in vitro. J. Am. Oil Chem. Soc. 1998, 75, 119–126. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Cai, W.; Liu, Y. Comparison of different polar compounds-induced cytotoxicity in human hepatocellular carcinoma HepG2 cells. Lipids Health Dis. 2016, 15, 30. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Moran, J.H.; Grant, D.F. Linoleic acid, cis-epoxyoctadecenoic acids, and dihydroxyoctadecadienoic acids are toxic to Sf-21 cells in the absence of albumin. Toxicol. Lett. 2002, 126, 187–196. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Liu, Y. Effects of epoxy stearic acid on lipid metabolism in HepG2 cells. J. Food Sci. 2020, 85, 3644–3652. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.J.; Wang, Y.; Liu, Y.F.; Xu, Y.J. Epoxy Triglyceride Enhances Intestinal Permeability via Caspa-se-1/NLRP3/GSDMD and cGAS-STING Pathways in Dextran Sulfate Sodium-Induced Colitis Mice. J. Agric. Food Chem. 2023, 71, 4371–4381. [Google Scholar] [CrossRef]
- Grootveld, M. Evidence-based challenges to the continued recommendation and use of peroxidatively-susceptible polyunsaturated fatty acid-rich culinary oils for high-temperature frying practises: Experimental revelations focused on toxic aldehydic lipid oxidation products. Front. Nut. 2022, 8, 711640. [Google Scholar] [CrossRef]
- Yamashima, T.; Seike, T.; Mochly-Rosen, D.; Chen, C.H.; Kikuchi, M.; Mizukoshi, E. Implication of the cooking oil-peroxidation product “hydroxynonenal” for Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1211141. [Google Scholar] [CrossRef]
- Yamashima, T. 4-Hydroxynonenal from Mitochondrial and Dietary Sources Causes Lysosomal Cell Death for Lifestyle-Related Diseases. Nutrients 2024, 16, 4171. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Cheng, L.Y.; Chang, C.; Jiang, X.M.; Gao, P.; Zhong, W.; Hu, C.; He, D.; Yin, J.J. Toxic aldehydes in fried foods: Formation, analysis, and reduction strategies. Food Control 2024, 169, 110993. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; García-Martínez, M.C.; Holgado, F. Changes and effects of dietary oxidized lipids in the gastrointestinal tract. Lipids Insight 2008, 2, 11–19. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Food lipid oxidation under gastrointestinal digestion conditions: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 461–478. [Google Scholar] [CrossRef]
- Torbati, M.; Abedinzadeh, S.; Khezri, S.; Hashempour-Baltork, F.; Azadmard-Damirchi, S. A review of lipid and protein oxidation during digestion. Curr. Nutr. Food Sci. 2023, 19, 549–563. [Google Scholar] [CrossRef]
- Kanazawa, K.; Ashida, H. Catabolic fate of dietary trilinoleoylglycerol hydroperoxides in rat gastrointestines. Biochim. Biophys. Acta 1998, 1393, 336–348. [Google Scholar] [CrossRef]
- Kanazawa, K.; Ashida, H. Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochim. Biophys. Acta 1998, 1393, 349–361. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. 1H NMR and SPME-GC/MS study of hydrolysis, oxidation and other reactions occurring during in vitro digestion of non-oxidized and oxidized sunflower oil. Food Res. Int. 2017, 91, 171–182. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Behaviour of non-oxidized and oxidized flaxseed oils, as models of omega-3 rich lipids, during in vitro digestion. Occurrence of epoxidation reactions. Food Res. Int. 2017, 95, 104–115. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. 1H NMR study of the in vitro digestion of highly oxidized soybean oil and the effect of the presence of ovalbumin. Foods 2021, 10, 1573. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Holgado, F.; Ruiz-Méndez, M.V.; Velasco, J. Chemical changes of hydroperoxy-, epoxy-, keto-and hydroxy-model lipids under simulated gastric conditions. Foods 2021, 10, 2035. [Google Scholar] [CrossRef]
- Yoshinaga-Kiriake, A.; Yoshinaga, K.; Miyagawa, S.; Yoshino, K.; Tanaka, S.; Takahashi, T.; Kato, S.; Ito, J.; Otoki, Y.; Nakagawa, K.; et al. Comparison of the catabolic rates of linoleic and oleic acid hydroperoxides using 13CO2 expired from mice. J. Oleo Sci. 2024, 73, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Holgado, F.; Ruiz-Méndez, M.V.; Velasco, J.; Márquez-Ruiz, G. Performance of olive-pomace oils in discontinuous and continuous frying. Comparative behavior with sunflower oils and high-oleic sunflower oils. Foods 2021, 10, 3081. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeia Convention, Inc. United States Pharmacopeia and National Formulary USP38-NF33; The United States Pharmacopeia Convention, Inc.: Rockville, MD, USA, 2015. [Google Scholar]
- Velasco, J.; Dobarganes, M.C.; Márquez-Ruiz, G. Antioxidant activity of phenolic compounds in sunflower oil-in-water emulsions containing sodium caseinate and lactose. Eur. J. Lipid Sci. Technol. 2004, 106, 325–333. [Google Scholar] [CrossRef]
- ISO International Organization for Standardization. Geneva, Switzerland. Available online: https://www.iso.org/standards.html (accessed on 15 January 2025).
- AOCS American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 7th ed.; AOCS Press: Urbana, IL, USA, 2003; ISBN 13 978-0935584899. [Google Scholar]
- IUPAC International Union of Pure and Applied Chemistry. Standard Methods for the Analysis of Oil and Derivatives, 1st Supplement to, 7th ed.; Dieffenbacher, A., Pocklington, W.D., Eds.; Blackwell Scientific Publications: Oxford, UK, 1992; ISBN 0-632-03337-I. [Google Scholar]
- Dobarganes, M.C.; Velasco, J.; Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols and diacylglycerols in oils and fats. Pure Appl. Chem. 2000, 72, 1563–1575. [Google Scholar] [CrossRef]
- Berdeaux, O.; Marquez-Ruiz, G.; Dobarganes, C. Selection of methylation procedures for quantitation of short-chain glycerol-bound compounds formed during thermoxidation. J. Chromatogr. A 1999, 863, 171–181. [Google Scholar] [CrossRef]
- Marmesat, S.; Velasco, J.; Dobarganes, M.C. Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures. J. Chromatogr. A 2008, 1211, 129–134. [Google Scholar] [CrossRef]
- Grompone, M.A. Sunflower and High-Oleic Sunflower Oils. In Bailey’s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2020; Volume 2, pp. 685–738. ISBN 9781-119-25788-2. [Google Scholar] [CrossRef]
- Ovesen, L.; Bendtsen, F.; Tage-Jensen, U.; Pedersen, N.T.; Gram, B.R.; Rune, S.J. Intraluminal pH in the stomach, duodenum, and proximal jejunum in normal subjects and patients with exocrine pancreatic insufficiency. Gastroenterology 1986, 90, 958–962. [Google Scholar] [CrossRef]
- Ekmekcioglu, C. A physiological approach for preparing and conducting intestinal bioavailability studies using experimental systems. Food Chem. 2002, 76, 225–230. [Google Scholar] [CrossRef]
- Gorelik, S.; Lapidot, T.; Shaham, I.; Granit, R.; Ligumsky, M.; Kohen, R.; Kanner, J. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: Health implications. J. Agric. Food Chem. 2005, 53, 3397–3402. [Google Scholar] [CrossRef] [PubMed]
- Kenmogne-Domguia, H.B.; Meynier, A.; Boulanger, C.; Genot, C. Lipid oxidation in food emulsions under gastrointestinal-simulated conditions: The key role of endogenous tocopherols and initiator. Food Dig. 2012, 3, 46–52. [Google Scholar] [CrossRef]
- Kristinova, V.; Storrø, I.; Rustad, T. Influence of human gastric juice on oxidation of marine lipids–in vitro study. Food Chem. 2013, 141, 3859–3871. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. The key role of ovalbumin in lipid bioaccessibility and oxidation product profile during the in vitro digestion of slightly oxidized soybean oil. Food Funct. 2019, 10, 4440–4451. [Google Scholar] [CrossRef]
- Takahashi, T.; Kato, S.; Ito, J.; Shimizu, N.; Parida, I.S.; Itaya-Takahashi, M.; Sakaino, M.; Imagi, J.; Yoshinaga, K.; Yoshinaga-Kiriake, A.; et al. Dietary triacylglycerol hydroperoxide is not absorbed, yet it induces the formation of other triacylglycerol hydroperoxides in the gastrointestinal tract. Redox Biol. 2022, 57, 102471. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Martín-Polvillo, M.; Dobarganes, M.C. Quantitation of oxidized triglyceride monomers and dimers as an useful measurement for early and advanced stages of oxidation. Grasas Aceites 1996, 47, 48–53. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; The Oily Press: Dundee, UK, 2005; ISBN 9780953194988. [Google Scholar]
- Goicoechea, E.; van Twillert, K.; Duits, M.; Brandon, E.D.; Kootstra, P.R.; Blokland, M.H.; Guillén, M.D. Use of an in vitro digestion model to study the bioaccessibility of 4-hydroxy-2-nonenal and related aldehydes present in oxidized oils rich in omega-6 acyl groups. J. Agric. Food Chem. 2008, 56, 8475–8483. [Google Scholar] [CrossRef]
- Goicoechea, E.; Brandon, E.F.; Blokland, M.H.; Guillén, M.D. Fate in digestion in vitro of several food components, including some toxic compounds coming from omega-3 and omega-6 lipids. Food Chem. Toxicol. 2011, 49, 115–124. [Google Scholar] [CrossRef]
- Larsson, K.; Harrysson, H.; Havenaar, R.; Alminger, M.; Undeland, I. Formation of malondialdehyde (MDA), 4-hydroxy-2-hexenal (HHE) and 4-hydroxy-2-nonenal (HNE) in fish and fish oil during dynamic gastrointestinal in vitro digestion. Food Funct. 2016, 7, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Tullberg, C.; Alminger, M.; Havenaar, R.; Undeland, I. Malondialdehyde and 4-hydroxy-2-hexenal are formed during dynamic gastrointestinal in vitro digestion of cod liver oils. Food Funct. 2016, 7, 3458–3467. [Google Scholar] [CrossRef] [PubMed]
- Byrdwell, W.C.; Neff, W.E. Electrospray ionization MS of high MW TAG oligomers. J. Am. Oil Chem. Soc. 2004, 81, 13–26. [Google Scholar] [CrossRef]
- Gardner, H.W. Oxygen radical chemistry of polyunsaturated fatty acids. Free Rad. Biol. Med. 1989, 7, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Patrikios, I.S.; Mavromoustakos, T.M. Monounsaturated fatty acid ether oligomers formed during heating of virgin olive oil show agglutination activity against human red blood cells. J. Agric. Food Chem. 2014, 62, 867–874. [Google Scholar] [CrossRef]
- Bonetti, R.; Parker, W.O., Jr. Insights into polymerization of vegetable oil: Oligomerization of oleic acid. J. Am. Oil Chem. Soc. 2019, 96, 1181–1184. [Google Scholar] [CrossRef]
- Hwang, H.S.; Ball, J.C.; Doll, K.M.; Anderson, J.E.; Vermillion, K. Investigation of polymers and alcohols produced in oxidized soybean oil at frying temperatures. Food Chem. 2020, 317, 126379. [Google Scholar] [CrossRef]
- Picariello, G.; Paduano, A.; Sacchi, R.; Addeo, F. MALDI-TOF mass spectrometry profiling of polar and nonpolar fractions in heated vegetable oils. J. Agric. Food Chem. 2009, 57, 5391–5400. [Google Scholar] [CrossRef]
- Paulose, M.M.; Chang, S.S. Chemical reactions involved in deep fat frying of foods: VI. Characterization of nonvolatile decomposition products of trilinolein. J. Am. Oil Chem. Soc. 1973, 50, 147–154. [Google Scholar] [CrossRef]
- Ottaviani, P.; Graille, J.; Perfetti, P.; Naudet, M. Produits d’alteration thermooxydative des huiles chauffees. II: Composes apolaires ou faiblement polaires. Chem. Phys. Lipids 1979, 24, 57–77. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat, S.; Berdeaux, O.; Márquez-Ruiz, M.G.; Dobarganes, M.C. Formation and evolution of monoepoxy fatty acids in thermoxidized olive and sunflower oils and quantitation in used frying oils from restaurants and fried food outlets. J. Agric. Food Chem. 2004, 52, 4438–4443. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Marmesat, S.; Márquez-Ruiz, G.; Dobarganes, M.C. Formation of short-chain glycerol-bound oxidation products and oxidized monomeric triacylglycerols during deep-frying and occurrence in used frying fats. Eur. J. Lipid Sci. Technol. 2004, 106, 728–735. [Google Scholar] [CrossRef]
- Berdeaux, O.; Márquez-Ruiz, G.; Dobarganes, M.C. Characterization, quantitation and evolution of monoepoxy compounds formed in model systems of fatty acid methyl esters and monoacid triglycerides heated at high temperature. Grasas Aceites 1999, 50, 53–59. [Google Scholar] [CrossRef]
- Machado, E.R.; Marmesat, S.; Dobarganes, M.C.; Abrantes, S. Avaliação quantitativa de monoepoxiácidos, monocetoácidos e monohidroxiácidos em óleos e gorduras provenientes de fritura descontínua de batatas. Food Sci. Technol. 2008, 28, 675–682. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Cao, P.; Liu, Y. Quantitative determination of epoxy stearic acids derived from oxidized frying oil based on solid-phase extraction and gas chromatography. LWT 2018, 92, 250–257. [Google Scholar] [CrossRef]
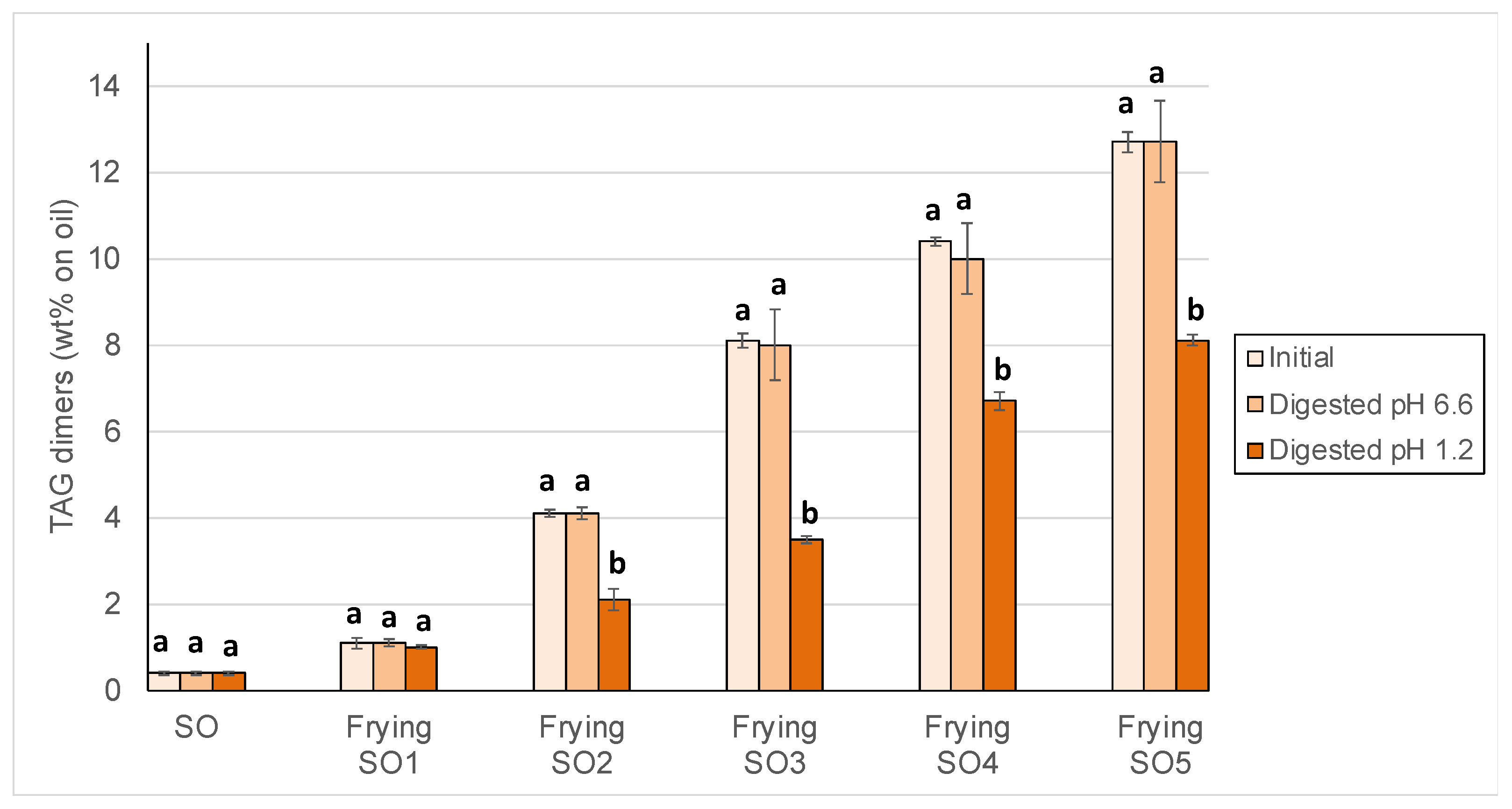
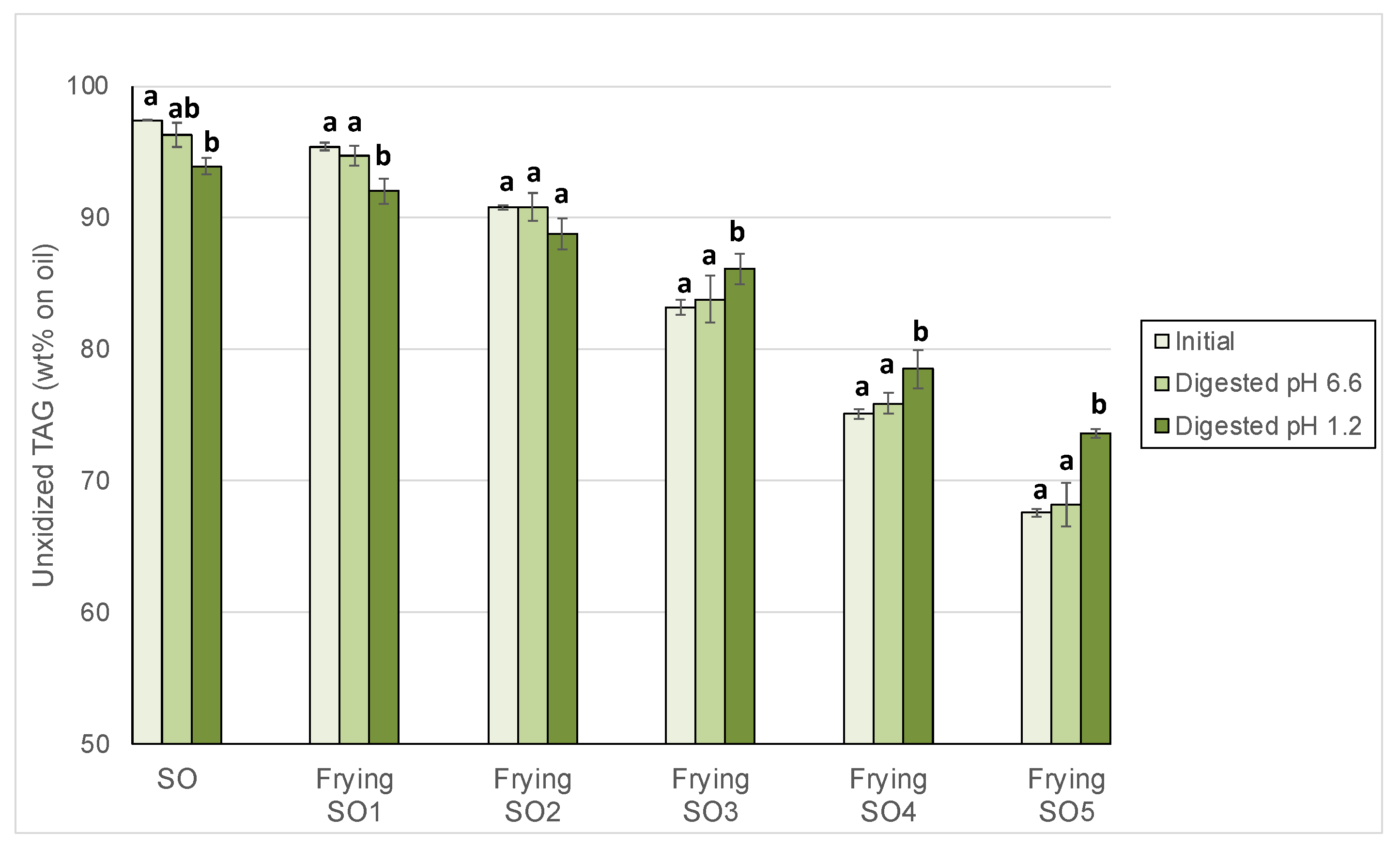
| Analytical Determination | Sunflower Oil |
|---|---|
| Fatty acid composition (%) | |
| C14:0 | 0.08 ± 0.00 |
| C16:0 | 6.02 ± 0.00 |
| C16:1 | 0.09 ± 0.00 |
| C17:0 | 0.03 ± 0.00 |
| C18:0 | 3.73 ± 0.01 |
| C18:1 | 29.31 ± 0.01 |
| C18:2 | 58.83 ± 0.01 |
| Trans C18:2 | 0.10 ± 0.00 |
| C18:3 | 0.06 ± 0.00 |
| C20:0 | 0.22 ± 0.01 |
| C20:1 | 0.14 ± 0.01 |
| C20:4 | 0.01 ± 0.00 |
| C22:0 | 0.57 ± 0.01 |
| C24:0 | 0.11 ± 0.01 |
| Others | 0.70 ± 0.01 |
| Tocopherols (mg/kg) | 627 ± 14 |
| Sterols (mg/kg) | 3219 ± 25 |
| Acidity (% oleic acid) | 0.04 ± 0.01 |
| Peroxide value (meq O2/kg) | 5.7 ± 1.0 |
| Oxidative stability index (h) | 5.3 ± 0.3 |
| Smoke point (°C) | 230 ± 2 |
| Polar Compounds | Total (wt %) | oxTAGM (wt %) | TAGD (wt %) | TAGO (wt %) | DAG (wt %) | FFA (wt %) |
|---|---|---|---|---|---|---|
| Initial SO | 2.7 ± 0.1 a | 1.1 ± 0.1 a | 0.4 ± 0.0 a | nd | 0.8 ± 0.1 a | 0.4 ± 0.0 a |
| Frying SO1 | 4.6 ± 0.3 b | 1.7 ± 0.0 b | 1.1 ± 0.0 b | 0.1 ± 0.0 a | 1.1 ± 0.1 b | 0.5 ± 0.0 a |
| Frying SO2 | 9.4 ± 0.2 c | 3.1 ± 0.4 c | 4.2 ± 0.1 c | 0.7 ± 0.1 b | 1.0 ± 0.0 b | 0.4 ± 0.0 a |
| Frying SO3 | 16.8 ± 0.3 d | 5.6 ± 0.2 d | 7.9 ± 0.2 d | 1.8 ± 0.1 c | 1.1 ± 0.3 b | 0.4 ± 0.1 a |
| Frying SO4 | 24.7 ± 0.4 e | 8.6 ± 0.2 e | 10.5 ± 0.3 e | 4.3 ± 0.1 d | 0.8 ± 0.1 a | 0.5 ± 0.1 a |
| Frying SO5 | 32.3 ± 0.5 f | 10.9 ± 0.3 f | 12.5 ± 0.2 f | 7.3 ± 0.2 e | 1.1 ± 0.2 b | 0.5 ± 0.1 a |
| Oil | Epoxy FAME (mg/g Oil) | Keto FAME (mg/g Oil) | Hydroxy FAME (mg/g Oil) |
|---|---|---|---|
| Initial SO | nd | nd | nd |
| Digested SO | 0.15 ± 0.06 | nd | 0.94 ± 0.19 |
| Initial Frying SO1 | 0.83 ± 0.12 a | 0.24 ± 0.04 a | 0.80 ± 0.08 a |
| Digested Frying SO1 | 0.50 ± 0.22 a | 0.46 ± 0.11 b | 2.07 ± 0.33 b |
| Initial Frying SO2 | 1.58 ± 0.06 a | 0.82 ± 0.06 a | 1.95 ± 0.19 a |
| Digested Frying SO2 | 1.95 ± 0.07 a | 1.53 ± 0.12 b | 3.13 ± 0.37 b |
| Initial Frying SO3 | 2.93 ± 0.09 a | 1.50 ± 0.16 a | 3.17 ± 0.29 a |
| Digested Frying SO3 | 3.57 ± 0.29 a | 1.85 ± 0.35 a | 6.27 ± 0.38 b |
| Initial Frying SO4 | 4.53 ± 0.12 a | 2.43 ± 0.17 a | 5.53 ± 0.25 a |
| Digested Frying SO4 | 5.23 ± 0.56 a | 2.77 ± 0.25 a | 9.23 ± 0.56 b |
| Initial Frying SO5 | 5.67 ± 0.17 a | 3.03 ± 0.21 a | 6.03 ± 0.21 a |
| Digested Frying SO5 | 6.50 ± 0.50 a | 3.50 ± 0.41 a | 9.97 ± 0.56 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Ruiz, G.; Ruiz-Méndez, M.V.; Holgado, F. Depolymerization and Oxidation Events in Used Frying Oils Under Conditions Simulating Gastric Digestion. Foods 2025, 14, 925. https://doi.org/10.3390/foods14060925
Márquez-Ruiz G, Ruiz-Méndez MV, Holgado F. Depolymerization and Oxidation Events in Used Frying Oils Under Conditions Simulating Gastric Digestion. Foods. 2025; 14(6):925. https://doi.org/10.3390/foods14060925
Chicago/Turabian StyleMárquez-Ruiz, Gloria, María Victoria Ruiz-Méndez, and Francisca Holgado. 2025. "Depolymerization and Oxidation Events in Used Frying Oils Under Conditions Simulating Gastric Digestion" Foods 14, no. 6: 925. https://doi.org/10.3390/foods14060925
APA StyleMárquez-Ruiz, G., Ruiz-Méndez, M. V., & Holgado, F. (2025). Depolymerization and Oxidation Events in Used Frying Oils Under Conditions Simulating Gastric Digestion. Foods, 14(6), 925. https://doi.org/10.3390/foods14060925






