Effect of Probiotics Containing Lactobacillus plantarum on Blood Lipids: Systematic Review, Meta-Analysis, and Network Pharmacological Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment of the Studie
2.5. Network Pharmacological Analysis
2.6. Statistical Analysis
3. Results
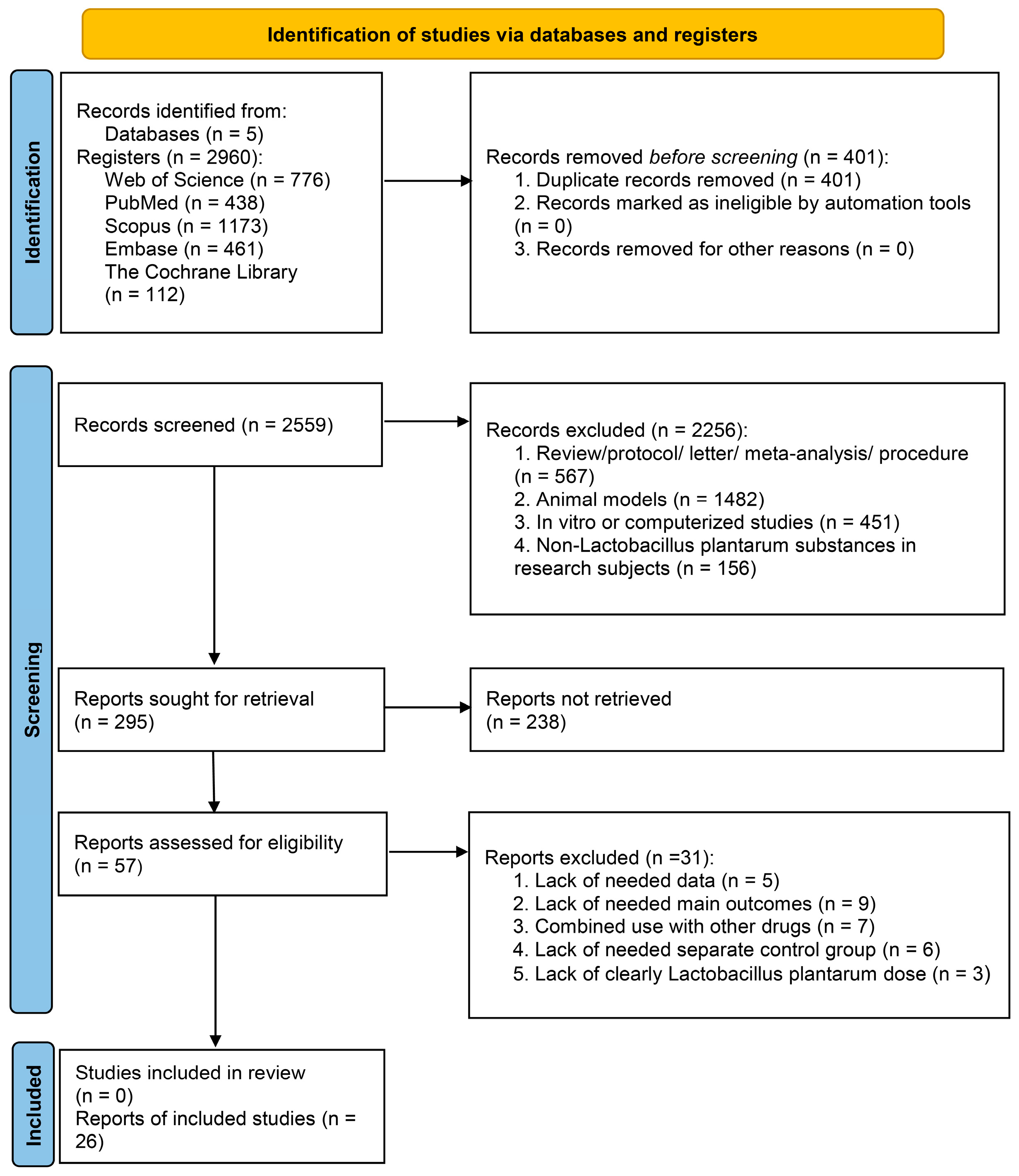
3.1. Search Results
3.2. Characteristics of Participants and Studies
3.3. Quality Assessment
3.4. Pooled Effect Size of L. plantarum Supplementation on TC
3.5. Pooled Effect Size of L. plantarum Supplementation on TG
3.6. Pooled Effect Size of L. plantarum Supplementation on HDL-C
3.7. Pooled Effect Size of L. plantarum Supplementation on LDL-C
3.8. Subgroup Analysis
3.9. Meta-Regression Analysis
3.10. Dose–Response Meta-Analysis
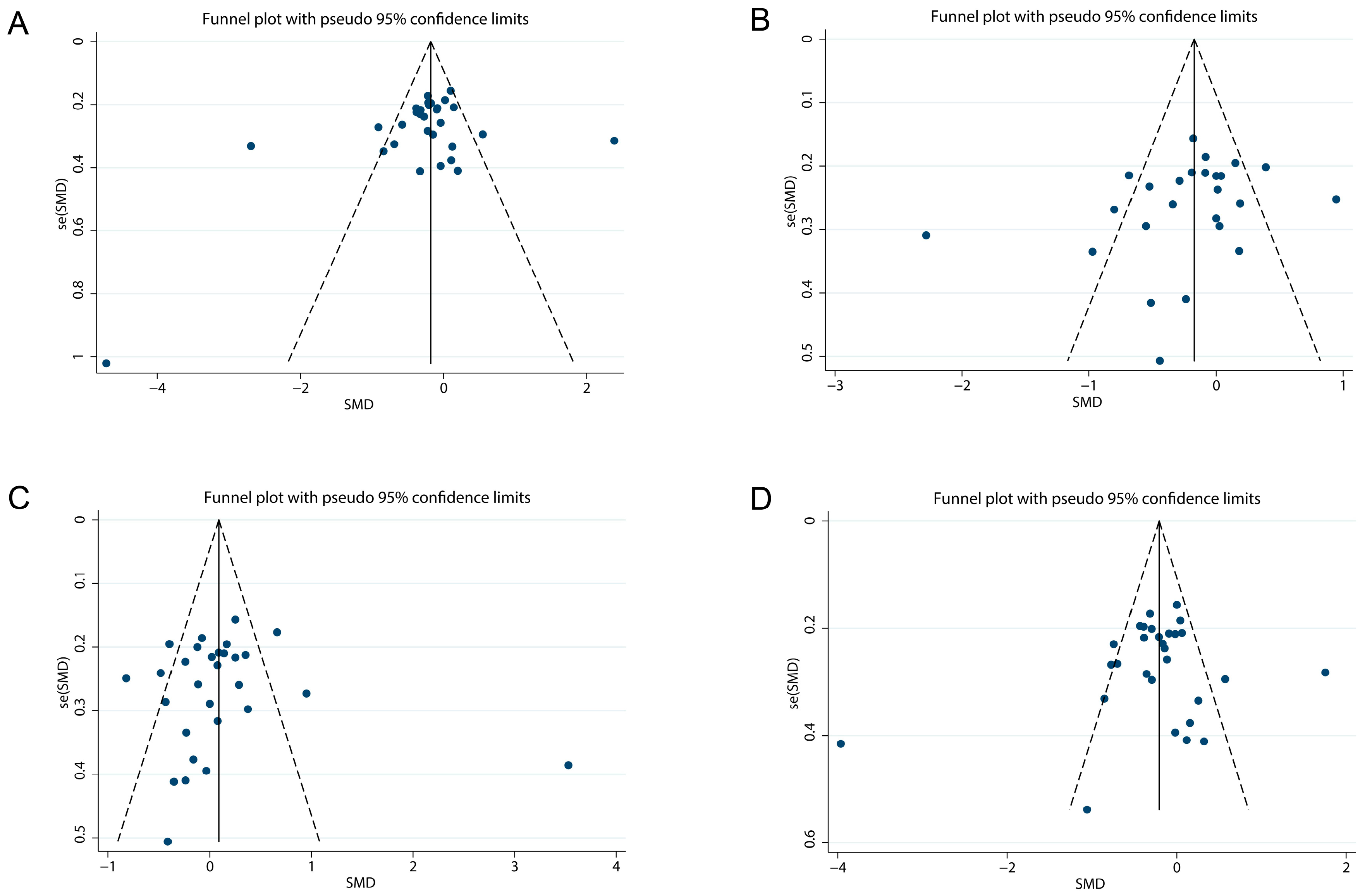
3.11. Publication Bias Assessment
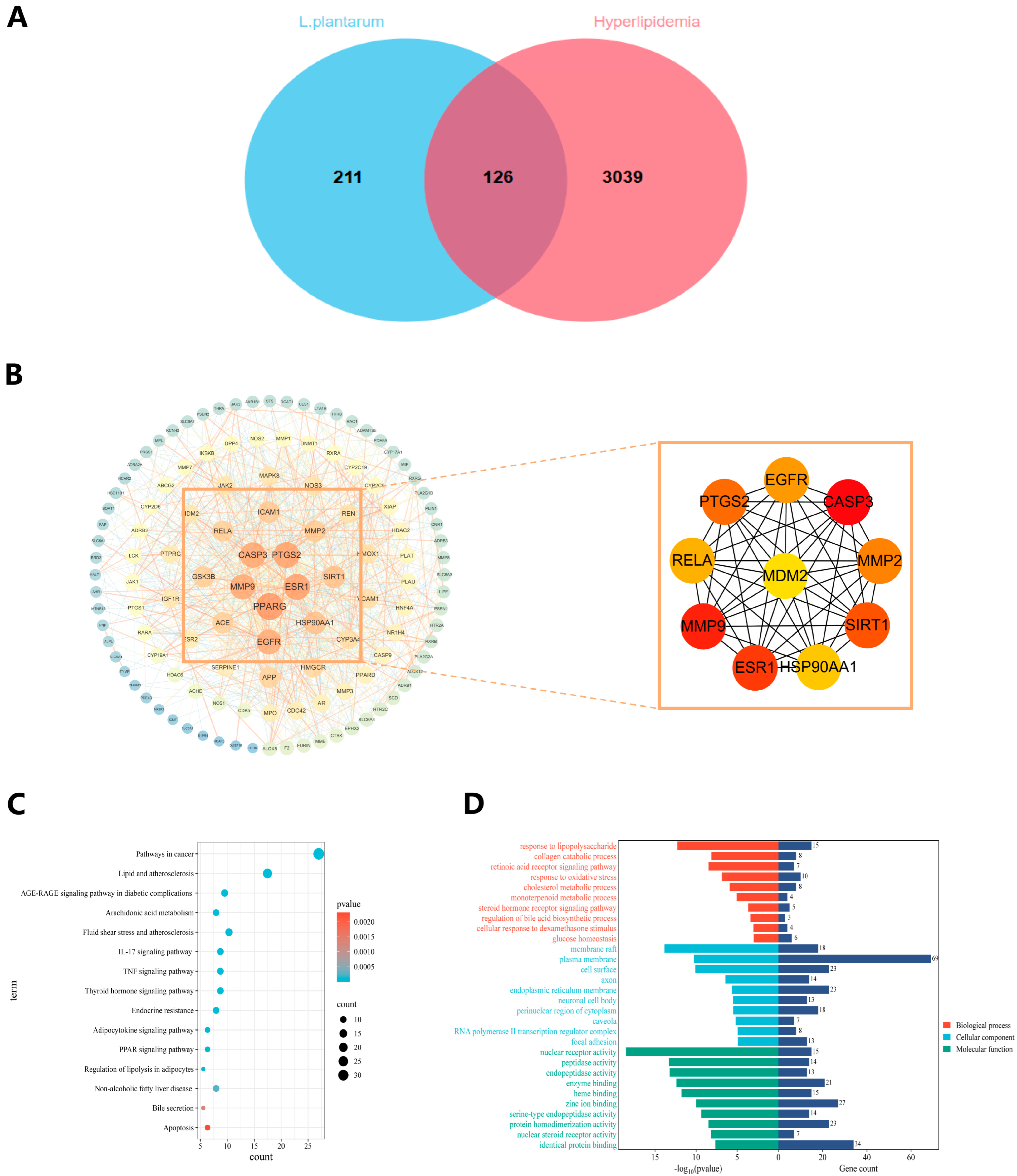
3.12. Information on Mechanisms and Targets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Cokkinos, D.V. Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad. Med. J. 2005, 81, 358–366. [Google Scholar] [CrossRef]
- Hendrani, A.D.; Adesiyun, T.; Quispe, R.; Jones, S.R.; Stone, N.J.; Blumenthal, R.S.; Martin, S.S. Dyslipidemia management in primary prevention of cardiovascular disease: Current guidelines and strategies. World J. Cardiol. 2016, 8, 201–210. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Sahebkar, A.; Cosentino, T.; Pirro, M. Cholesterol-Lowering Nutraceuticals Affecting Vascular Function and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2018, 20, 53. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendía, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef]
- Pirro, M.; Vetrani, C.; Bianchi, C.; Mannarino, M.R.; Bernini, F.; Rivellese, A.A. Joint position statement on “Nutraceuticals for the treatment of hypercholesterolemia” of the Italian Society of Diabetology (SID) and of the Italian Society for the Study of Arteriosclerosis (SISA). Nutr. Metab. Cardiovasc. Dis. NMCD 2017, 27, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Scicali, R.; Di Pino, A.; Ferrara, V.; Urbano, F.; Piro, S.; Rabuazzo, A.M.; Purrello, F. New treatment options for lipid-lowering therapy in subjects with type 2 diabetes. Acta Diabetol. 2018, 55, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J. Metabolic syndrome and type 2 diabetes: Lipid and physiological consequences. Diabetes Vasc. Dis. Res. 2007, 4 (Suppl. 3), S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Ghanavati, M.; Hosseini, S.A.; Alipour, M.; Ashtari-larky, D.; Cheraghpour, K. The role of probiotics in the management of cardiovascular disease risk factors. Clin. Excell. 2015, 4, 140–156. [Google Scholar]
- Lee, S.J.; Bose, S.; Seo, J.G.; Chung, W.S.; Lim, C.Y.; Kim, H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: A randomized double-blind controlled clinical trial. Clin. Nutr. 2014, 33, 973–981. [Google Scholar] [CrossRef]
- Håkansson, Å.; Andrén Aronsson, C.; Brundin, C.; Oscarsson, E.; Molin, G.; Agardh, D. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the Peripheral Immune Response in Children with Celiac Disease Autoimmunity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 1925. [Google Scholar] [CrossRef]
- Ma, Y.; Fei, Y.; Han, X.; Liu, G.; Fang, J. Lactobacillus plantarum Alleviates Obesity by Altering the Composition of the Gut Microbiota in High-Fat Diet-Fed Mice. Front. Nutr. 2022, 9, 947367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Y.; Ma, F.; Sun, M.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum Y44 oral administration on inflammation and lipid metabolism in obese mice fed with a high fat diet. Food Funct. 2020, 11, 5024–5039. [Google Scholar] [CrossRef]
- Chen, M.; Guo, W.L.; Li, Q.Y.; Xu, J.X.; Cao, Y.J.; Liu, B.; Yu, X.D.; Rao, P.F.; Ni, L.; Lv, X.C. The protective mechanism of Lactobacillus plantarum FZU3013 against non-alcoholic fatty liver associated with hyperlipidemia in mice fed a high-fat diet. Food Funct. 2020, 11, 3316–3331. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, F.; Lu, J.; Shi, J.; Guan, J.; Yan, F.; Li, B.; Huo, G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef]
- Wang, G.; Huang, W.; Xia, Y.; Xiong, Z.; Ai, L. Cholesterol-lowering potentials of Lactobacillus strain overexpression of bile salt hydrolase on high cholesterol diet-induced hypercholesterolemic mice. Food Funct. 2019, 10, 1684–1695. [Google Scholar] [CrossRef]
- Hütt, P.; Songisepp, E.; Rätsep, M.; Mahlapuu, R.; Kilk, K.; Mikelsaar, M. Impact of probiotic Lactobacillus plantarum TENSIA in different dairy products on anthropometric and blood biochemical indices of healthy adults. Benef. Microbes 2015, 6, 233–243. [Google Scholar] [CrossRef]
- Kerlikowsky, F.; Müller, M.; Greupner, T.; Amend, L.; Strowig, T.; Hahn, A. Distinct Microbial Taxa Are Associated with LDL-Cholesterol Reduction after 12 Weeks of Lactobacillus plantarum Intake in Mild Hypercholesterolemia: Results of a Randomized Controlled Study. Probiotics Antimicrob. Proteins 2025, 17, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Park, J.S.; Kim, S.B.; Cho, Y.H.; Seo, H.; Lee, H.S. A 12-Week, Single-Centre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Design Clinical Trial for the Evaluation of the Efficacy and Safety of Lactiplantibacillus plantarum SKO-001 in Reducing Body Fat. Nutrients 2024, 16, 1137. [Google Scholar] [CrossRef]
- Štšepetova, J.; Rätsep, M.; Gerulis, O.; Jõesaar, A.; Mikelsaar, M.; Songisepp, E. Impact of Lactiplantibacillus plantarum Inducia on metabolic and antioxidative response in cholesterol and BMI variable indices: Randomised, double-blind, placebo-controlled trials. Benef. Microbes 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Keleszade, E.; Kolida, S.; Costabile, A. The cholesterol lowering efficacy of Lactobacillus plantarum ECGC 13110402 in hypercholesterolemic adults: A double-blind, randomized, placebo controlled, pilot human intervention study. J. Funct. Foods 2022, 89, 104939. [Google Scholar] [CrossRef]
- Rahayu, E.S.; Mariyatun, M.; Manurung, N.E.P.; Hasan, P.N.; Therdtatha, P.; Mishima, R.; Komalasari, H.; Mahfuzah, N.A.; Pamungkaningtyas, F.H.; Yoga, W.K.; et al. Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J. Gastroenterol. 2021, 27, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Rustanti, N.; Murdiati, A.; Juffrie, M.; Rahayu, E.S. Effect of Probiotic Lactobacillus plantarum Dad-13 on Metabolic Profiles and Gut Microbiota in Type 2 Diabetic Women: A Randomized Double-Blind Controlled Trial. Microorganisms 2022, 10, 1806. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Mikhael, A.M.; Davoodvandi, A.; Jafarnejad, S. Effect of Lactobacillusplantarum containing probiotics on blood pressure: A systematic review and meta-analysis. Pharmacol. Res. 2020, 153, 104663. [Google Scholar] [CrossRef]
- Li, C.P.; Chen, C.C.; Hsiao, Y.; Kao, C.H.; Chen, C.C.; Yang, H.J.; Tsai, R.Y. The Role of Lactobacillus plantarum in Reducing Obesity and Inflammation: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 7608. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2011, 343, d5928. [Google Scholar] [CrossRef]
- Kong, X.-L.; Wu, X.-Y.; Xu, X.-X. Research progress on metabolites and bacteriostasis mechanism of Lactobacillus plantarum. J. Food Saf. Qual. Test. 2021, 12, 3131–3140. [Google Scholar] [CrossRef]
- Feng, R.N.; Zhao, C.; Sun, C.H.; Li, Y. Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes mellitus. PLoS ONE 2011, 6, e18480. [Google Scholar] [CrossRef]
- Mo, S.J.; Lee, K.; Hong, H.J.; Hong, D.K.; Jung, S.H.; Park, S.D.; Shim, J.J.; Lee, J.L. Effects of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 on Overweight and the Gut Microbiota in Humans: Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 2484. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Na, G.Y.; Chu, J.; Joung, H.; Kim, B.K.; Lim, S. Efficacy and Safety of Lactobacillus plantarum K50 on Lipids in Koreans with Obesity: A Randomized, Double-Blind Controlled Clinical Trial. Front. Endocrinol. 2021, 12, 790046. [Google Scholar] [CrossRef]
- Park, Y.E.; Kim, M.S.; Shim, K.W.; Kim, Y.I.; Chu, J.; Kim, B.K.; Choi, I.S.; Kim, J.Y. Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.Y.; Kim, M.; Ahn, Y.T.; Sim, J.H.; Choi, I.D.; Lee, S.H.; Lee, J.H. The triglyceride-lowering effect of supplementation with dual probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032: Reduction of fasting plasma lysophosphatidylcholines in nondiabetic and hypertriglyceridemic subjects. Nutr. Metab. Cardiovasc. Dis. NMCD 2015, 25, 724–733. [Google Scholar] [CrossRef]
- Songisepp, E.; Hütt, P.; Rätsep, M.; Shkut, E.; Kõljalg, S.; Truusalu, K.; Stsepetova, J.; Smidt, I.; Kolk, H.; Zagura, M.; et al. Safety of a probiotic cheese containing Lactobacillus plantarum Tensia according to a variety of health indices in different age groups. J. Dairy Sci. 2012, 95, 5495–5509. [Google Scholar] [CrossRef]
- Costabile, A.; Buttarazzi, I.; Kolida, S.; Quercia, S.; Baldini, J.; Swann, J.R.; Brigidi, P.; Gibson, G.R. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS ONE 2017, 12, e0187964. [Google Scholar] [CrossRef]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: A randomized double blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar] [CrossRef]
- Abbasi, B.; Mirlohi, M.; Daniali, M.; Ghiasvand, R. Effects of probiotic soy milk on lipid panel in type 2 diabetic patients with nephropathy: A double-blind randomized clinical trial. Prog. Nutr. 2018, 20, 70–78. [Google Scholar]
- Nishimura, M.; Ohkawara, T.; Tetsuka, K.; Kawasaki, Y.; Nakagawa, R.; Satoh, H.; Sato, Y.; Nishihira, J. Effects of yogurt containing Lactobacillus plantarum HOKKAIDO on immune function and stress markers. J. Tradit. Complement. Med. 2016, 6, 275–280. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Nomura, K.; Oku, H.; Sugiyama, M. Improvement of constipation and liver function by plant-derived lactic acid bacteria: A double-blind, randomized trial. Nutrition 2010, 26, 367–374. [Google Scholar] [CrossRef]
- Okuka, N.; Milinkovic, N.; Velickovic, K.; Polovina, S.; Sumarac-Dumanovic, M.; Minic, R.; Korčok, D.; Djordjevic, B.; Ivanovic, N.D. Beneficial effects of a new probiotic formulation on adipocytokines, appetite-regulating hormones, and metabolic parameters in obese women. Food Funct. 2024, 15, 7658–7668. [Google Scholar] [CrossRef] [PubMed]
- Zikou, E.; Dovrolis, N.; Dimosthenopoulos, C.; Gazouli, M.; Makrilakis, K. The Effect of Probiotic Supplements on Metabolic Parameters of People with Type 2 Diabetes in Greece-A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 4663. [Google Scholar] [CrossRef]
- Neverovskyi, A.; Chernyavskyi, V.; Shypulin, V.; Hvozdetska, L.; Tishchenko, V.; Nechypurenko, T.; Mikhn Ova, N. Probiotic Lactobacillus plantarum may reduce cardiovascular risk: An experimental study. ARYA Atheroscler. 2021, 17, 1–10. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 655–663. [Google Scholar] [CrossRef]
- Culpepper, T.; Rowe, C.C.; Rusch, C.T.; Burns, A.M.; Federico, A.P.; Girard, S.A.; Tompkins, T.A.; Nieves, C., Jr.; Dennis-Wall, J.C.; Christman, M.C.; et al. Three probiotic strains exert different effects on plasma bile acid profiles in healthy obese adults: Randomised, double-blind placebo-controlled crossover study. Benef. Microbes 2019, 10, 497–509. [Google Scholar] [CrossRef]
- Barreto, F.M.; Simão, A.N.C.; Morimoto, H.K.; Batisti Lozovoy, M.A.; Dichi, I.; Helena da Silva Miglioranza, L. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 2014, 30, 939–942. [Google Scholar] [CrossRef]
- Sharafedtinov, K.K.; Plotnikova, O.A.; Alexeeva, R.I.; Sentsova, T.B.; Songisepp, E.; Stsepetova, J.; Smidt, I.; Mikelsaar, M. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients--a randomized double-blind placebo-controlled pilot study. Nutr. J. 2013, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuñé, J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br. J. Nutr. 2013, 109, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.R.; Ahire, J.J.; Jayanthi, N.; Tripathi, A.; Nanal, S. Effect of multi-strain probiotic (UB0316) in weight management in overweight/obese adults: A 12-week double blind, randomised, placebo-controlled study. Benef. Microbes 2019, 10, 855–866. [Google Scholar] [CrossRef]
- Farwell, W.R.; Sesso, H.D.; Buring, J.E.; Gaziano, J.M. Non-high-density lipoprotein cholesterol versus low-density lipoprotein cholesterol as a risk factor for a first nonfatal myocardial infarction. Am. J. Cardiol. 2005, 96, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Liu, S.F.; Shen, Y.C.; Chen, C.L.; Huang, C.N.; Pan, T.M.; Wang, C.K. A randomized, double-blind clinical study to determine the effect of ANKASCIN 568 plus on blood glucose regulation. J. Food Drug Anal. 2017, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Liu, D.-M.; Guo, J.; Zeng, X.-A.; Sun, D.-W.; Brennan, C.S.; Zhou, Q.-X.; Zhou, J.-S. The probiotic role of Lactobacillus plantarum in reducing risks associated with cardiovascular disease. Int. J. Food Sci. Technol. 2017, 52, 127–136. [Google Scholar] [CrossRef]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.; Rosado, E.; Soares-Mota, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Ma, Y.; Yang, Y.; Cheng, Y.; Ma, H.; Ren, D.; Chen, P. Regulation of viable/inactivated/lysed probiotic Lactobacillus plantarum H6 on intestinal microbiota and metabolites in hypercholesterolemic mice. NPJ Sci. Food 2022, 6, 50. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Ren, Y.; Ruan, Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PloS ONE 2017, 12, e0178868. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, L.; Jia, F.; Yan, Y.; Xiong, F.; Li, Y.; Hidayat, K.; Guan, R. Effects of Lactobacillus plantarum supplementation on glucose and lipid metabolism in type 2 diabetes mellitus and prediabetes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2024, 61, 377–384. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Chapman, M.J.; Fazio, S.; Hussain, M.M.; Kontush, A.; Krauss, R.M.; Otvos, J.D.; Remaley, A.T.; Schaefer, E.J. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 2011, 57, 392–410. [Google Scholar] [CrossRef]
- Khera, A.V.; Rader, D.J. Future therapeutic directions in reverse cholesterol transport. Curr. Atheroscler. Rep. 2010, 12, 73–81. [Google Scholar] [CrossRef]
- Kelly, R.B. Diet and exercise in the management of hyperlipidemia. Am. Fam. Physician 2010, 81, 1097–1102. [Google Scholar] [PubMed]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jiang, M.; Chen, B.; Jiang, K.; Ma, N.; Li, Y.; Wang, M.; Bao, M.; Wang, C.; Yang, X. Study the effect of Lactobacillus plantarum ATCC 14917 for caries prevention and anti-obesity. Front. Nutr. 2024, 11, 1511660. [Google Scholar] [CrossRef]
- Pan, R.; Wang, T.; Bai, J.; Zhang, J.; Gu, Y.; Zhao, Z.; Tang, R.; Qian, Z.; Yan, L.; Xiao, X.; et al. Lactobacillus plantarum fermented barley extract attenuates obesity in HFD-induced obese rats by regulating gut microbiota. Lipids 2025, 60, 237–248. [Google Scholar] [CrossRef]
- Cai, Q.; Song, Y.; Wang, S.; Wang, W.; Sun, X.; Yu, J.; Wei, Y. Functional yogurt fermented by two-probiotics regulates blood lipid and weight in a high-fat diet mouse model. J. Food Biochem. 2022, 46, e14248. [Google Scholar] [CrossRef]
- Majithia, A.R.; Flannick, J.; Shahinian, P.; Guo, M.; Bray, M.A.; Fontanillas, P.; Gabriel, S.B.; Rosen, E.D.; Altshuler, D. Rare variants in PPARG with decreased activity in adipocyte differentiation are associated with increased risk of type 2 diabetes. Proc. Natl. Acad. Sci. USA 2014, 111, 13127–13132. [Google Scholar] [CrossRef]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. TEM 2015, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ordovas, J.M. Genetic links between diabetes mellitus and coronary atherosclerosis. Curr. Atheroscler. Rep. 2007, 9, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Evans, R.M. Peroxisome proliferator-activated receptor-gamma in macrophage lipid homeostasis. Trends Endocrinol. Metab. TEM 2002, 13, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.P.; Meisel, S.R.; Ong, J.M.; Kaul, S.; Cercek, B.; Rajavashisth, T.B.; Sharifi, B.; Shah, P.K. Oxidized low-density lipoprotein regulates matrix metalloproteinase-9 and its tissue inhibitor in human monocyte-derived macrophages. Circulation 1999, 99, 993–998. [Google Scholar] [CrossRef]
- Robbesyn, F.; Augé, N.; Vindis, C.; Cantero, A.V.; Barbaras, R.; Negre-Salvayre, A.; Salvayre, R. High-density lipoproteins prevent the oxidized low-density lipoprotein-induced endothelial growth factor receptor activation and subsequent matrix metalloproteinase-2 upregulation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1206–1212. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Author | Year | References | Design of Studies | Country | No. of Subjects in Case Group | No. of Controls | Gender | Age (Mean) | Follow-up Duration | Clinical Condition | Dosage (Daily) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Okuka | 2024 | [43] | Double-blind placebo-controlled supplementary intervention study | Australia | 25 | 23 | F | 39.33 | 12 weeks | Obese women | One probiotic capsule containing 7 × 1010 cfu of Lactobacillus plantarum (L. plantarum) 299v | TC, TG, HDL-C, LDL-C |
| Shin | 2024 | [23] | Double-blind randomized clinical trial | Korea | 50 | 50 | M F | 48.90 | 12 weeks | Healthy adults | One probiotic capsule containing 2 × 1010 cfu of L. plantarum SKO-001 | TC, TG, HDL-C, LDL-C |
| Kerlikowsky | 2023 | [22] | Double-blind randomized placebo-controlled nutritional intervention trial | Germany | 43 | 43 | M F | 63.6 | 12 weeks | Hypercholesterolemia | One probiotic capsule containing 1.2 × 109 cfu of L. plantarum CECT7527 (KABP011), L. plantarum CECT7528 (KABP012), and L. plantarum CECT7529 (KABP013) | TC, TG, HDL-C, LDL-C |
| Zikou | 2023 | [44] | Single-center double-blind placebo-controlled randomized clinical trial | Greece | 46 | 45 | M F | 64.54 | 6 months | Type 2 diabetes mellitus | One probiotic capsule containing 1.75 × 109 cfu of Lactobacillus acidophilus, 0.5 × 109 cfu of L. plantarum 1.75 × 109 cfu of Bifidobacterium lactis, and 1.5 × 109 cfu of Saccharomyces boulardii | TC, TG, HDL-C, LDL-C |
| Štšepetova 1 (JOG 4BC) | 2023 | [24] | Two parallel two-armed double-blind placebo-controlled (DBPC) human intervention trials | Estonia | 73 | 63 | M F | 42.14 | 8 weeks | Healthy adults | 150 g of probiotic yoghurt containing 5.9 × 109 cfu of L. plantarum Inducia, 6.6 × 1010 cfu of Lactobacillus delbrueckii subsp, and 6.75 × 109 cfu of Streptococcus thermophilus | TC, TG, HDL-C, LDL-C |
| Štšepetova 2 (JOG5) | 2023 | [24] | Two parallel two-armed double-blind placebo-controlled (DBPC) human intervention trials | Estonia | 53 | 52 | M F | 46.74 | 8 weeks | Healthy adults | 150 g of probiotic yoghurt containing 2.0 × 109 cfu of L. plantarum Inducia, 6.6 × 1010 cfu of Lactobacillus delbrueckii subsp, and 6.75 × 109 cfu of Streptococcus thermophilus | TC, TG, HDL-C, LDL-C |
| Mo | 2022 | [33] | Randomized double-blind placebo-controlled study | Korea | 30 | 29 | M F | 35.7 | 12 weeks | Healthy obese and overweight adults | One probiotic capsule containing 5 × 109 cfu of L. plantarum KY1032 | TC, TG, HDL-C, LDL-C |
| Sohn | 2022 | [34] | Randomized double-blind placebo-controlled clinical trial | Korea | 41 | 36 | M F | 47.8 | 12 weeks | Healthy adults | Two probiotic capsules containing 4 × 109 cfu of L. plantarum K50 (LPK) | TC, TG, HDL-C, LDL-C |
| Neverovskyi | 2021 | [45] | The present open comparative randomized parallel investigation | Ukraine | 41 | 40 | M F | 57.7 | 12 weeks | Dyslipidemia | One probiotic capsule containing 2 × 109 cfu of L. plantarum | TC, TG, HDL-C, LDL-C |
| Cicero | 2020 | [46] | Double-blind randomized placebo-controlled parallel-group clinical trial | Italy | 30 | 30 | M F | 72 | 2 months | Elderly patients with a diagnosis of MetS | One liquid vial containing 2 × 109 cfu of L. plantarum PBS067—DSM 24,937, 2 × 109 cfu of Lactobacillus acidophilus PBS066—DSM 24,936, and 2 × 109 cfu of Lactobacillus reuteri PBS072—DSM 25,175 | TC, TG, HDL-C, LDL-C |
| Rahayu | 2021 | [26] | Randomized double-blind placebo-controlled study | Indonesia | 30 | 30 | M F | 44.07 | 3months | BMI ≥ 25 | 1 g of skimmed milk powder containing 2 × 109 cfu of L. plantarum Dad-13 | TC, TG, HDL-C, LDL-C |
| Park | 2020 | [35] | Randomized double-blind placebo-controlled parallel trial | Korea | 35 | 35 | M F | 48.3 | 12 weeks | TG < 200 mg/dL | Two 400 mg probiotic capsules containing 4.0 × 109 cfu of L. plantarum (LPQ180) | TC, TG, HDL-C, LDL-C |
| Culpepper | 2019 | [47] | Randomized double-blind placebo controlled crossover study | USA | 35 | 35 | M F | 54.3 | 18 weeks | Healthy adults | One probiotic capsule containing 5 × 109 cfu of L. plantarum HA-119, 2.5 × 109 cfu of B. subtilis R0179, and 5 × 109 cfu of B. lactis B94 | TC, TG, HDL-C, LDL-C |
| Nishimura 1 (Placebo 1) | 2015 | [41] | Randomized double-blind placebo-controlled study | Japan | 57 | 55 | M F | 49.58 | 8 weeks | NK cell activity below 50% | 90 g of yogurt containing L. plantarum HOKKAIDO ≥ 5.0 × 109 cfu | TC, TG, HDL-C, LDL-C |
| Nishimura 2 (Placebo 2) | 2015 | [41] | Randomized double-blind placebo-controlled study | Japan | 57 | 59 | M F | 49.58 | 8 weeks | NK cell activity below 50% | 90 g of yogurt containing L. plantarum HOKKAIDO ≥ 5.0 × 109 cfu | TC, TG, HDL-C, LDL-C |
| Ahn | 2015 | [36] | Randomized double-blind placebo-controlled study | South Korea | 46 | 46 | M F | 54.1 | 12 weeks | Nondiabetic and hypertriglyceridemic subjects | 2 g of powder containing 5 × 109 cfu of L. curvatus HY7601 and 5 × 109 cfu of L. plantarum KY1032 | TC, TG, HDL-C, LDL-C |
| Hütt 1 (Chess trial) | 2015 | [21] | Two double-blind randomized placebo-controlled exploratory trials | Estonia | 82 | 82 | M F | 37.7 | 3 weeks | Healthy adults | 50 g of probiotic cheese containing 1010 cfu of L. plantarum TENSIA | TC, TG, HDL-C, LDL-C |
| Hütt 2 (Yoghurt trial) | 2015 | [21] | Two double-blind randomized placebo-controlled exploratory trials | Estonia | 43 | 43 | M F | 34.2 | 3 weeks | Healthy adults | 150 g of probiotic yoghurt containing 6 × 109 cfu of L. plantarum TENSIA | TC, TG, HDL-C, LDL-C |
| Barreto | 2014 | [48] | Randomized placebo-controlled study | Brazil | 12 | 12 | M | 62 | 3 months | Postmenopausal women with MetS | 80 mL of fermented milk [FM])containing 109 cfu of L. plantarum (LP115) | HDL-C, LDL-C |
| Sharafedtinov | 2013 | [49] | Randomized double-blind placebo-controlled parallel pilot study | Russia | 25 | 15 | M F | 52.0 | 3 weeks | Metabolic syndrome characterized by obesity accompanied by arterial hypertonia | 50 g of probiotic product (semi-hard cheese) containing 7.5 × 1012 cfu of L. plantarum TENSIA | TC, TG, HDL-C, LDL-C |
| Fuentes | 2012 | [50] | Single-center prospective randomized double-blind placebo-controlled parallel-group trial | Spain | 30 | 30 | M F | NA | 12 weeks | Healthy adults | One probiotic capsule containing 1.2 × 109 cfu of L. plantarum (CECT 7527, CECT 7528 and CECT 7529) | TC, HDL-C, LDL-C |
| Higashikawa 1(Group A) | 2010 | [42] | Double-blind randomized design with three parallel groups | Japan | 24 | 20 | M F | 37.3 | 6 weeks | Healthy adults | 100 g of probiotic yoghurt containing 1.9 × 1010 cfu of L. plantarum SN35N and 109 cfu of L. plantarum SN13T | TC, HDL-C, LDL-C |
| Higashikawa 2(Group B) | 2010 | [42] | Double-blind randomized design with three parallel groups | Japan | 18 | 20 | M F | 35.1 | 6 weeks | Healthy adults | 100 g of probiotic yoghurt containing 1.96× 1010 cfu of L. plantarum SN13T and 4 × 108 cfu of L. plantarum SN35N | TC, HDL-C, LDL-C |
| Costabile | 2017 | [38] | Single-center prospective randomized placebo-controlled parallel-group design | United Kingdom | 23 | 23 | M F | 52.3 | 12 weeks | Normal to mildly hypercholesterolemic adults | Two probiotic capsules containing 4 × 109 cfu of L. plantarum ECGC 13110402 | TC, HDL-C, LDL-C |
| Nabhani | 2018 | [39] | Double-blind placebo-controlled randomized clinical trial | Iran | 45 | 45 | F | 29.4 | 6 weeks | Pregnant women | One symbiotic capsule containing 2.5 × 1010 cfu of L. acidophilus, 7.5 × 109 cfu of L. plantarum, 3.5 × 109 cfu of L. fermentum, and 1010 cfu of L. gasseri | TC, TG, HDL-C, LDL-C |
| Keleszade | 2022 | [25] | Parallel double-blind placebo-controlled randomized pilot study | United Kingdom | 8 | 8 | M F | NA | 6 weeks | Hypercholesterolemic adults | One probiotic capsule containing 4 × 109 cfu of L. plantarum ECGC 13,110,402 | TC, TG, HDL-C, LDL-C |
| Abbasi | 2018 | [40] | Parallel design double-blind randomized clinical trial | Iran | 20 | 20 | M F | 56.9 | 8 weeks | Type 2 diabetic patients with nephropathy | 200 mL of probiotic soy milk containing 4 × 109 cfu of L. plantarum A7 | TC, TG, HDL-C, LDL-C |
| Rustanti | 2022 | [27] | Randomized double-blind controlled trial | Indonesia | 10 | 8 | F | 44.11 | 12 weeks | T2D patients | 1 g skim milk powder containing 1010 cfu of L. plantarum Dad-13 | TC |
| Songisepp (Study 1) | 2012 | [37] | Double-blind placebo-controlled (DBPC) crossover study | Estonia | 12 | 12 | M F | 29.1 | 8 weeks | Healthy adults | 50 g of the test cheese containing 1010.4 cfu of L. plantarum Tensia | TC, TG, HDL-C, LDL-C |
| Songisepp (Study 2) | 2012 | [37] | Double-blind placebo-controlled (DBPC) crossover study | Estonia | 18 | 18 | M F | 69.8 | 8 weeks | Healthy elderly participants | 50 g of the test cheese containing 108.17 cfu of L. plantarum Tensia | TC, TG, HDL-C, LDL-C |
| Sudha | 2019 | [51] | Double-blind randomized parallel group placebo-controlled clinical trial | India | 35 | 36 | M F | 43.5 | 12 weeks | Body mass index (BMI) 25–32 kg/m2 | Two multi-strain probiotic capsules containing Lactobacillus salivarius UBLS-22, Lactobacillus casei UBLC-42, L. plantarum, UBLP-40, Lactobacillus acidophilus UBLA-34, Bifidobacterium breve UBBr-01, and Bacillus coagulans Unique IS 5 × 109 cfu each | TC, TG, HDL-C, LDL-C |
| Subgroup | ||||||
|---|---|---|---|---|---|---|
| SMD (95% CI) | Test for Overall Effect | Test for Heterogeneity | I2 (%) | |||
| Duration of study, weeks | ≤8 weeks | |||||
| TC | −0.073 (−0.404,0.257) | 0.663 | <0.001 | 84.60 | ||
| TG | −0.080 (−0.324,0.165) | 0.525 | 0.003 | 62.50 | ||
| HDL-C | 0.212 (−0.138,0.562) | 0.235 | <0.001 | 86.60 | ||
| LDL-C | −0.050 (−0.314,0.214) | 0.709 | <0.001 | 76.60 | ||
| >8 weeks | ||||||
| TC | −0.386 (−0.686,−0.058) | 0.012 | <0.001 | 81.70 | ||
| TG | −0.334 (−0.647,−0.021) | 0.036 | <0.001 | 82.70 | ||
| HDL-C | −0.012 (−0.217,0.193) | 0.911 | 0.002 | 60.80 | ||
| LDL-C | −0.466 (−0.834,−0.098) | 0.013 | <0.001 | 87.20 | ||
| Supplement | L. plantarum | |||||
| TC | −0.406 (−0.678,−0.135) | 0.003 | <0.001 | 80.20 | ||
| TG | −0.270 (−0.527,−0.013) | 0.040 | <0.001 | 77.00 | ||
| HDL-C | −0.057 (−0.193,0.078) | 0.407 | 0.164 | 24.10 | ||
| LDL-C | −0.406 (−0.700,−0.111) | 0.007 | <0.001 | 83.00 | ||
| Multi-strain probiotic | ||||||
| TC | 0.086 (−0.310,0.482) | 0.672 | <0.001 | 87.90 | ||
| TG | −0.145 (−0.507,0.218) | 0.434 | <0.001 | 80.40 | ||
| HDL-C | 0.457 (−0.025,0.938) | 0.063 | <0.001 | 91.50 | ||
| LDL-C | 0.008 (−0.348,0.364) | 0.965 | <0.001 | 85.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Huang, D.; Liu, J.; Wang, Z.; Ren, Y.; Su, Y.; Ma, Y. Effect of Probiotics Containing Lactobacillus plantarum on Blood Lipids: Systematic Review, Meta-Analysis, and Network Pharmacological Analysis. Foods 2025, 14, 3300. https://doi.org/10.3390/foods14193300
Zuo J, Huang D, Liu J, Wang Z, Ren Y, Su Y, Ma Y. Effect of Probiotics Containing Lactobacillus plantarum on Blood Lipids: Systematic Review, Meta-Analysis, and Network Pharmacological Analysis. Foods. 2025; 14(19):3300. https://doi.org/10.3390/foods14193300
Chicago/Turabian StyleZuo, Jinshi, Dan Huang, Jie Liu, Zidan Wang, Yuerong Ren, Yang Su, and Yuxia Ma. 2025. "Effect of Probiotics Containing Lactobacillus plantarum on Blood Lipids: Systematic Review, Meta-Analysis, and Network Pharmacological Analysis" Foods 14, no. 19: 3300. https://doi.org/10.3390/foods14193300
APA StyleZuo, J., Huang, D., Liu, J., Wang, Z., Ren, Y., Su, Y., & Ma, Y. (2025). Effect of Probiotics Containing Lactobacillus plantarum on Blood Lipids: Systematic Review, Meta-Analysis, and Network Pharmacological Analysis. Foods, 14(19), 3300. https://doi.org/10.3390/foods14193300







