Gelatin and Carboxymethyl Chitosan Edible Coating Incorporated with Carvacrol: Development and Application in Strawberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Development
2.3. Physico-Mechanical Characteristics of the GL/CMCS/CA Films
2.3.1. Opacity and Water Vapor Permeability (WVP)
2.3.2. Tensile Strength (TS) and Elongation-at-Break (EB)
2.3.3. Moisture Content (MC), Swelling Degree (SD), and Water Solubility (WS)
2.3.4. Color and Thickness
2.4. Film Characterization
2.4.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. X-Ray Diffraction (XRD) Analysis
2.5. Application of GL/CMCS/CA Coatings on Strawberries
2.6. Physicochemical Analyses of the Strawberries
2.6.1. Weight Loss (WL) and Decay Rate
2.6.2. Firmness and pH
2.6.3. Color
2.7. Biochemical and Antioxidant Activities of Coated Strawberries
2.7.1. Total Soluble Solids (TSS) and Titratable Acidity (TA)
2.7.2. Total Phenolic Content (TPC)
2.7.3. Total Anthocyanin Content (TAC)
2.7.4. Vitamin C Content
2.7.5. Antioxidant Activity
2.8. Microbiological Enumeration
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results
3.1. Physical and Mechanical Characteristics of the GL/CMCS/CA-Based Films
3.1.1. Opacity and WVP
3.1.2. TS and EB
3.1.3. MC, SD, and WS
3.1.4. Color and Thickness
3.2. Film Characterization
3.2.1. FTIR Spectra
3.2.2. XRD
3.2.3. SEM
3.3. Physicochemical Analyses of the Strawberries
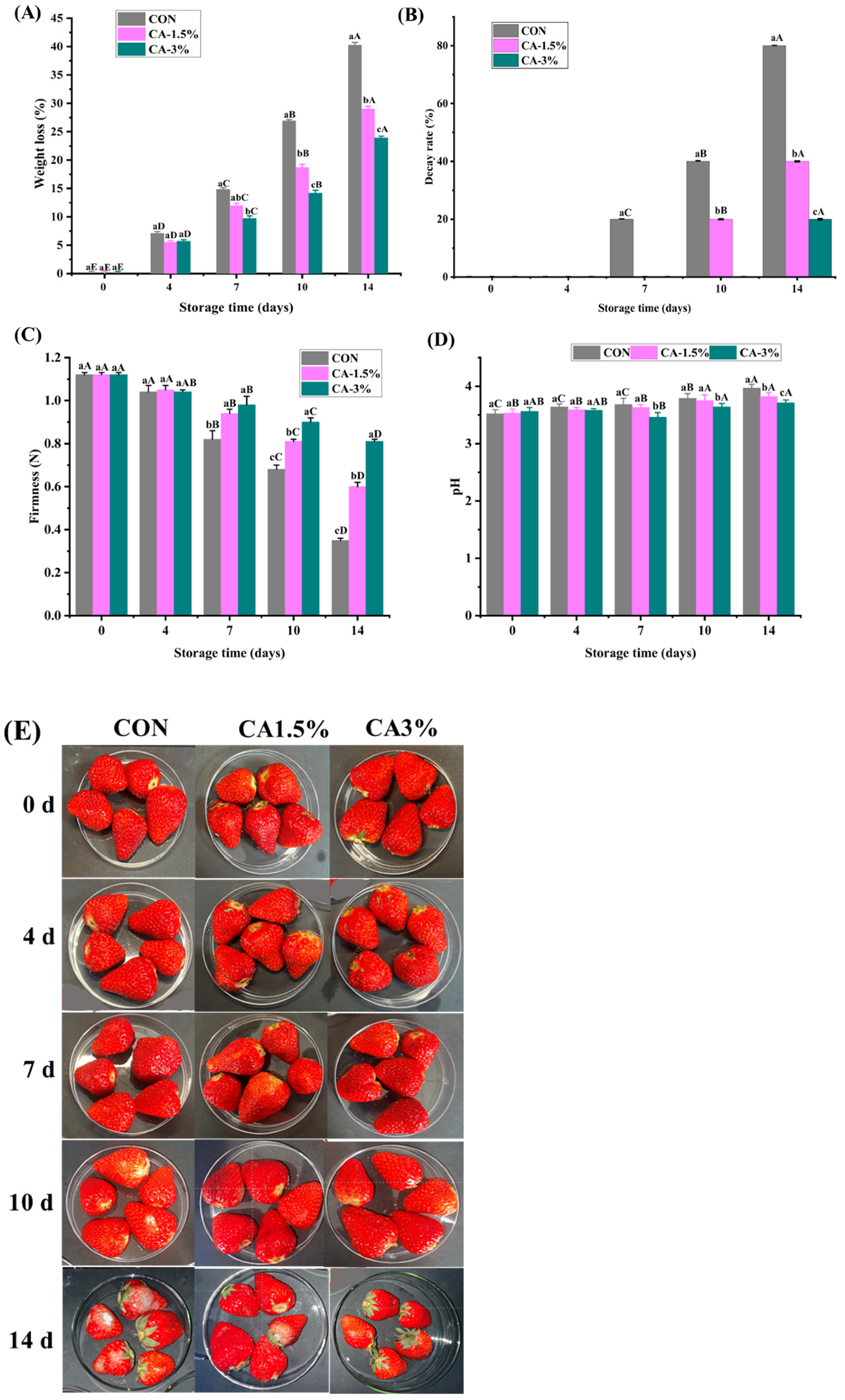
3.3.1. WL and Decay Rate
3.3.2. Firmness and pH
3.3.3. Color
3.4. Biochemical and Antioxidant Activities of Coated Strawberries
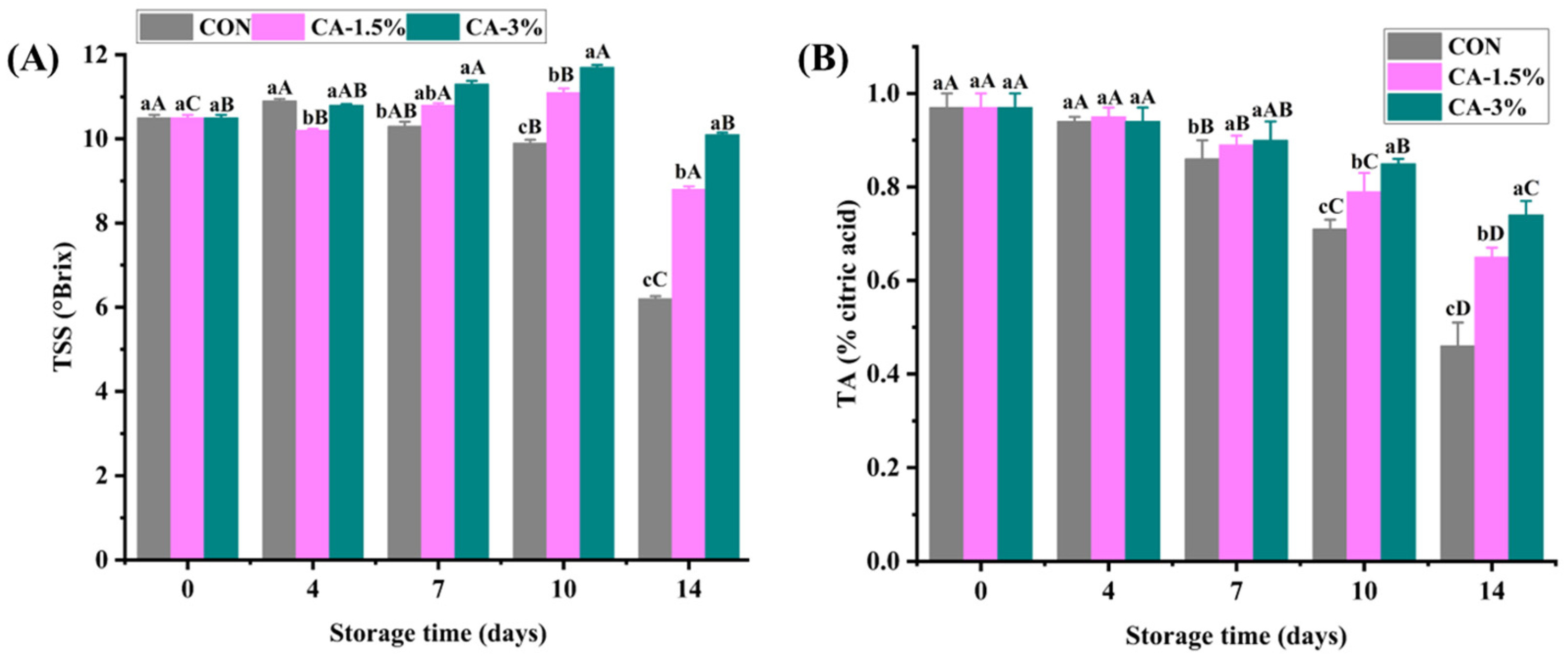
3.4.1. Total Soluble Solids (TSS) and Titratable Acidity (TA)
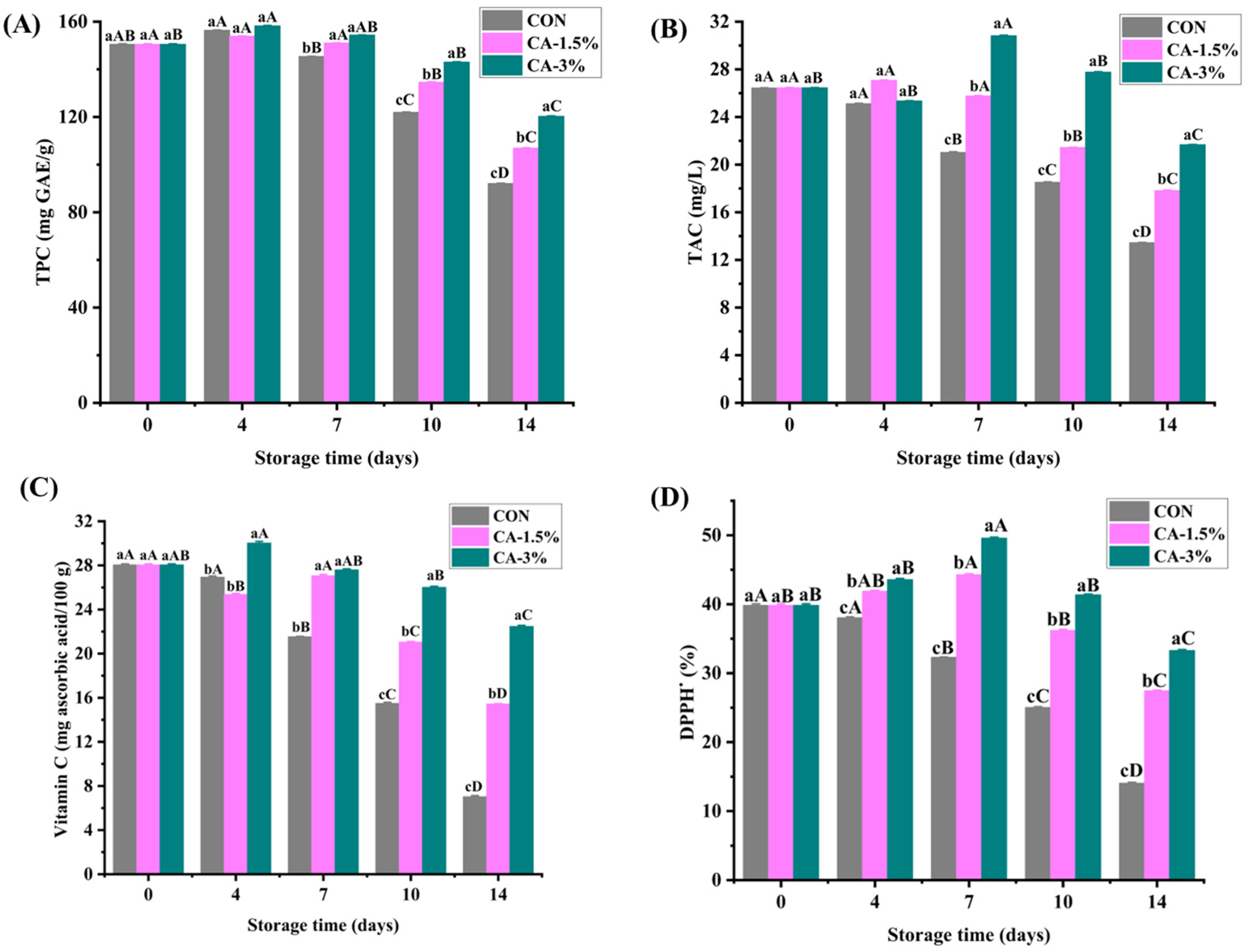
3.4.2. TPC
3.4.3. TAC
3.4.4. Vitamin C
3.4.5. Antioxidant Activity
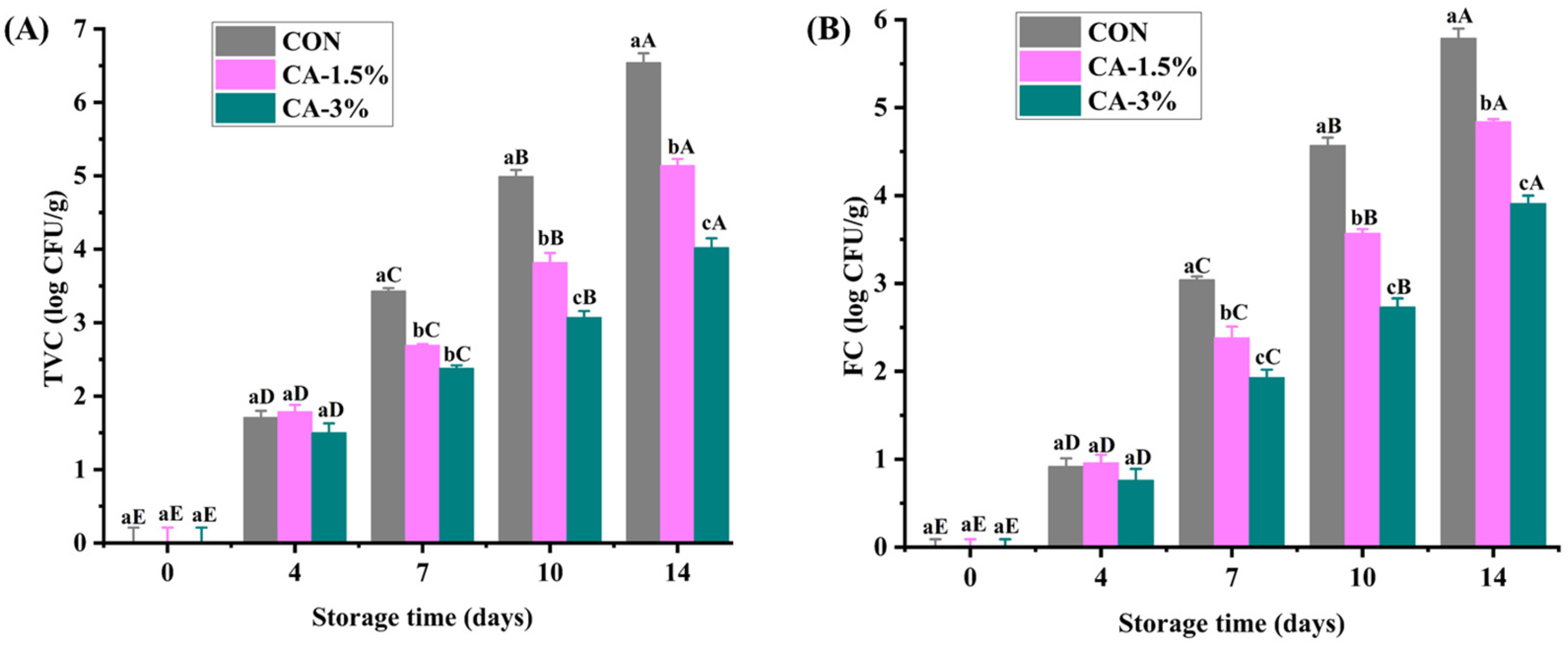
3.5. Microbiological Assessment
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khodaei, D.; Hamidi-Esfahani, Z. Influence of Bioactive Edible Coatings Loaded with Lactobacillus plantarum on Physicochemical Properties of Fresh Strawberries. Postharvest Biol. Technol. 2019, 156, 110944. [Google Scholar] [CrossRef]
- GIR Global Info Research. Global Fresh Strawberry Market 2024 by Manufacturers, Regions, Type and Application, Forecast to 2030. 2024. Available online: https://www.globalinforesearch.com/reports/1655749/fresh-strawberry (accessed on 28 July 2025).
- Bertolo, M.R.V.; de Oliveira Filho, J.G.; Lamonica, G.C.; de Oliveira Nobre Bezerra, C.C.; da Conceição Amaro Martins, V.; Ferreira, M.D.; de Guzzi Plepis, A.M.; Bogusz Junior, S. Improvement of the Physical-Chemical, Microbiological, Volatiles and Sensory Quality of Strawberries Covered with Chitosan/Gelatin/Pomegranate Peel Extract-Based Coatings. Food Chem. 2025, 471, 142755. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ding, Y.; Sun, Y.; Li, X.; Sun, C.; Guo, F.; Zeng, X.; Gong, H.; Fan, X. Chitosan/Pullulan Edible Coatings Containing Thyme Essential Oil Nanoemulsion: Preparation, Characterization and Application in Strawberry Preservation. Int. J. Biol. Macromol. 2025, 309, 143043. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Moreno, M.; González-González, R.B. Effect of Gelatin and Salicylic Acid Incorporated in Chitosan Coatings on Strawberry Preservation. Int. J. Biol. Macromol. 2025, 305, 140918. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ali, R.M.; Dinh, L.N.M.; Yao, Y.; Agarwal, V. Development of Hyaluronic Acid Based Polysaccharide-Protein Composite Edible Coatings for Preservation of Strawberry Fruit. Int. J. Biol. Macromol. 2024, 259, 128932. [Google Scholar] [CrossRef]
- César de Albuquerque Sousa, T.; de Lima Costa, I.H.; Gandra, E.A.; Meinhart, A.D. Use of Edible Coatings as a New Sustainable Alternative to Extend the Shelf Life of Strawberries (Fragaria ananassa): A Review. J. Stored Prod. Res. 2024, 108, 102375. [Google Scholar] [CrossRef]
- Bassey, A.P.; Cui, X.; Ibeogu, I.H.; Wang, F.; Nasiru, M.M.; Bako, H.K.; Fan, L.; Liu, X. Fabrication and Characterization of Gelatin/Carboxymethyl Chitosan Composite Film Incorporated with Carvacrol and Its Preservation Efficacy in Chinese Mitten Crab (Eriocheir sinensis). Food Hydrocoll. 2025, 160, 110723. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Zhang, M.; Yang, Z.; Chen, X.; Wu, X. Evolution and Expression Analysis of Carotenoid Cleavage Oxygenase Gene Family in Chinese Mitten Crab Eriocheir Sinensis. Int. J. Biol. Macromol. 2024, 257, 128475. [Google Scholar] [CrossRef]
- Radan, N.; Ghobadi Nejad, Z.; Ghasemi, S.; Yaghmaei, S. Boosting Antibacterial Efficiency of Gelatin/Chitosan Composite Films through Synergistic Interaction of Ag Nanoparticles and ZIF-8 Metal-Organic Frameworks for Food Packaging. Int. J. Biol. Macromol. 2025, 305, 141175. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, L.; Huang, H.; Xu, J.; Li, Y.; Yu, W.; Peng, S.; Zou, L.; Liu, W. The Improvement of Water Barrier Property in Gelatin/Carboxymethyl Cellulose Composite Film by Electrostatic Interaction Regulation and Its Application in Strawberry Preservation. Food Chem. 2024, 450, 139352. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Liu, Y.-P.; Liu, J.-L.; Nie, G.-W. Effect of Carboxymethyl Chitosan-Gelatin-Based Edible Coatings on the Quality and Antioxidant Properties of Sweet Cherry during Postharvest Storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
- Chakraborty, P.; Hati, S.; Mishra, B.K. Biocomposite Films from Banana Flour/Halloysite Nanoclay/Carvacrol: Preparation, Characterization, and Application on Capsicum (Capsicum annuum) Fruits. Sustain. Chem. Pharm. 2023, 36, 101304. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Yang, K.; Guo, L.; Yang, J.; Lan, Z.; Guo, Y.; Xiao, L.; Wang, X. Fabrication and Characterization of Carvacrol Encapsulated Gelatin/Chitosan Composite Nanofiber Membrane as Active Packaging Material. Int. J. Biol. Macromol. 2024, 282, 137114. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, F.; Wang, K.; Xie, D. Green and Facile Fabrication of Multifunctional Cellulose Nanocrystal and Carvacrol Together Reinforced Chitosan Bio-Nanocomposite Coatings for Fruit Preservation. Int. J. Biol. Macromol. 2024, 265, 130651. [Google Scholar] [CrossRef]
- Wu, H.; Ma, L.; Li, S.; Wang, J.; Li, T.; Peng, L.; Li, S.; Li, Q.; Yuan, X.; Zhou, M.; et al. Sustained-Release Antibacterial Gelatin Films: Effects of Diatomite/Carvacrol Complex on Their Structure, Physicochemical and Antibacterial Properties. Food Packag. Shelf Life 2023, 35, 101019. [Google Scholar] [CrossRef]
- Lei, K.; Wang, X.; Li, X.; Wang, L. The Innovative Fabrication and Applications of Carvacrol Nanoemulsions, Carboxymethyl Chitosan Microgels and Their Composite Films. Colloids Surf. B Biointerfaces 2019, 175, 688–696. [Google Scholar] [CrossRef]
- Xiao, L.; Lapu, M.; Cui, L.; Li, J.; Wang, X.; Li, X.; Liu, M.; Liu, D. Impacts of Chitosan/Pullulan/Carvacrol Film on the Quality and Microbial Diversity of Refrigerated Goat Meat. Meat Sci. 2025, 220, 109704. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, B.-G.; Chun, Y.-G.; Kim, H.R.; Woo, S.-H.; Choi, Y.-S.; Kim, B.-K. Effect of Astaxanthin and Carvacrol Co-Encapsulated Emulsion and Chitosan on the Physicochemical, Rheological, and Antimicrobial Properties in Nitrite-Free Meat Spread. Food Chem. 2025, 469, 142605. [Google Scholar] [CrossRef]
- Di Matteo, A.; Stanzione, M.; Orlo, E.; Russo, C.; Lavorgna, M.; Buonocore, G.G.; Isidori, M. Biodegradable Films Enriched with Thymol and Carvacrol with Antioxidant and Antibacterial Properties to Extend Cheese Shelf-Life. Food Control 2026, 179, 111541. [Google Scholar] [CrossRef]
- Dong, Q.; Dai, Y.; Wang, W.; Ma, Y.; Li, L. Fabrication of Carvacrol Loaded Cellulose Acetate Phthalate/Shellac Composite Film and Its Application to Mackerel Fillets Preservation. Int. J. Biol. Macromol. 2024, 262, 129904. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.-W. Gelatin/Carrageenan-Based Smart Packaging Film Integrated with Cu-Metal Organic Framework for Freshness Monitoring and Shelf-Life Extension of Shrimp. Food Hydrocoll. 2023, 145, 109180. [Google Scholar] [CrossRef]
- Nong, W.; Luo, H.; Wang, G.; Chen, Q.; Zou, X.; Miao, W.; Wu, J.; Guan, W.; Qu, S. β-CD-MOF-Based Edible Antimicrobial Packaging Film with Humidity-Controlled Carvacrol Release for Preserving Fresh Strawberry. Carbohydr. Polym. 2025, 351, 123133. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Lei, Y.; Cheng, C.; Luo, Y.; Zhang, Y.; Yue, J. Highly Efficient Anchoring of γ-Cyclodextrin-MOFs on Chitosan/Cellulose Film by in Situ Growth to Enhance Encapsulation and Controlled Release of Carvacrol. Food Hydrocoll. 2024, 150, 109633. [Google Scholar] [CrossRef]
- Qian, L.; Jia, R.; Zhao, Q.; Sun, N.; Yang, J.; Wen, J.; Li, H.; Yang, J.; Mo, L.; Gao, W.; et al. Tough, Antibacterial, and Antioxidant Chitosan-Based Composite Films Enhanced with Proanthocyanidin and Carvacrol Essential Oil for Fruit Preservation. Food Res. Int. 2025, 208, 116269. [Google Scholar] [CrossRef] [PubMed]
- Ibeogu, I.H.; Bako, H.K.; Alnadari, F.; Bassey, A.P.; Jibril, A.N.; Zhou, T.; Nasiru, M.M.; Yar, M.S.; Xie, Y.; Zhou, G.; et al. Development and Characterization of Edible Packaging Films Based on Pork Gelatin Integrated with Serum Plasma for Pork Packaging and Preservation. Food Biosci. 2024, 58, 103662. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis. Official Methods of Analysis of AOAC International; Latimer, G.W., Ed.; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- de Oliveira Filho, J.G.; Albiero, B.R.; Calisto, Í.H.; Bertolo, M.R.V.; Oldoni, F.C.A.; Egea, M.B.; Bogusz Junior, S.; de Azeredo, H.M.C.; Ferreira, M.D. Bio-Nanocomposite Edible Coatings Based on Arrowroot Starch/Cellulose Nanocrystals/Carnauba Wax Nanoemulsion Containing Essential Oils to Preserve Quality and Improve Shelf Life of Strawberry. Int. J. Biol. Macromol. 2022, 219, 812–823. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Rahmati, E. Effect of Edible Coatings on the Shelf-Life of Fresh Strawberries: A Comparative Study Using TOPSIS-Shannon Entropy Method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- Cui, X.; Bassey, A.P.; Wang, F.; Xie, S.; Li, D.; Fan, L.; Zhu, Y.; Bin, L.; Liu, X. Inactivation of Penicillium ochrochloron Spores by Pulsed Light: Mechanism and Preservation Efficacy in Strawberries. Food Control 2026, 179, 111569. [Google Scholar] [CrossRef]
- Hassani, B.; Ebrahimi, F.; Najafi, A.; Ziaolhagh, S. Investigation of the Effect of Aloe Vera Gel Coating Combined with Free and Encapsulated Savory Essential Oil on the Shelf Life of Strawberries. Appl. Food Res. 2025, 5, 101269. [Google Scholar] [CrossRef]
- Bian, H.; Luo, D.; Shi, J.; Zhou, X.; Xu, T.; Xiao, H.; Dai, H.; Li, T.; Huang, C.; Ragauskas, A.J. Cellulose Nanofibrils-Based Packaging Films Synergistically Reinforced by Lignin and Tea Polyphenols for Strawberry Preservation. Chem. Eng. J. 2024, 502, 157843. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.-T.T.; Van Tran, T.; Van Tan, L.; Danh, L.T.; Than, V.T. Development of Antibacterial, Antioxidant, and UV-Barrier Chitosan Film Incorporated with Piper Betle Linn Oil as Active Biodegradable Packaging Material. Coatings 2021, 11, 351. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, Physicochemical and Biological Evaluation of Quercetin Based Chitosan-Gelatin Film for Food Packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhu, J.; Qiao, S.; Yang, Y.; Ma, L.; Dai, H.; Chen, H.; Zhang, Y.; Wang, H. Improved Mechanical, Thermal and Barrier Properties of Sericin/Gelatin/Carboxymethyl Chitosan Based Films by Laccase for Packaging Application in Fruits and Food Cooking. Food Hydrocoll. 2026, 171, 111839. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, J.; Zhou, C.; Huang, K.; Fan, X.; Zhou, M.; Xu, B.; Huang, C.; Li, H.; Ma, H.; et al. Synergistic Enhancement of Gelatin-Chitosan Films with Vanillin Schiff Base and ZnO for Effective Strawberry Preservation. Food Control 2026, 180, 111647. [Google Scholar] [CrossRef]
- Alnadari, F.; Al-Dalali, S.; Nasiru, M.M.; Frimpong, E.B.; Hu, Y.; Abdalmegeed, D.; Dai, Z.; AL-Ammari, A.; Chen, G.; Zeng, X. A New Natural Drying Method for Food Packaging and Preservation Using Biopolymer-Based Dehydration Film. Food Chem. 2023, 404, 134689. [Google Scholar] [CrossRef] [PubMed]
- Tagrida, M.; Nilsuwan, K.; Gulzar, S.; Prodpran, T.; Benjakul, S. Fish Gelatin/Chitosan Blend Films Incorporated with Betel (Piper betle L.) Leaf Ethanolic Extracts: Characteristics, Antioxidant and Antimicrobial Properties. Food Hydrocoll. 2023, 137, 108316. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef]
- Sarraf-Ov, N.; Awlqadr, F.H.; Abdalla, K.R.; Hashemi, H.; Rouhi, M.; Mohammadi, R.; Ebrahimi, B. Characterization of Gelatin-Chitosan Films Incorporated with Nicotiana tabacum Extract Nanoliposomes for Food Packaging Applications. Int. J. Biol. Macromol. 2025, 311, 143701. [Google Scholar] [CrossRef]
- Li, S.; Wei, N.; Wei, J.; Fang, C.; Feng, T.; Liu, F.; Liu, X.; Wu, B. Curcumin and Silver Nanoparticles Loaded Antibacterial Multifunctional Pectin/Gelatin Films for Food Packaging Applications. Int. J. Biol. Macromol. 2024, 266, 131248. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and Characterization of Pectin Films Activated by Nanoemulsion and Pickering Emulsion Stabilized Marjoram (Origanum majorana L.) Essential Oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Yan, J.; He, S.; Chen, L.; Chen, H.; Wang, W. Characterization, Antioxidant and Antibacterial Activities of Gelatin-Chitosan Edible Coated Films Added with Cyclocarya paliurus Flavonoids. Int. J. Biol. Macromol. 2023, 253, 127664. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Li, H.; Cai, Z.; Wang, H.; Dong, M.; Huang, L.; Li, C. Preparation and Characterization of Edible Gelatin-Chitosan Films Incorporated with Finger Millet (Eleusine coracana L.) Polyphenols and Its Application in Pork. Int. J. Biol. Macromol. 2025, 307, 142178. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vazquez, A.; Barciela, P.; Carpena, M.; Prieto, M. Edible Coatings as a Natural Packaging System to Improve Fruit and Vegetable Shelf Life and Quality. Foods 2023, 12, 3570. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shan, P.; Yu, F.; Li, H.; Peng, L. Fabrication and Characterization of Waste Fish Scale-Derived Gelatin/Sodium Alginate/Carvacrol Loaded ZIF-8 Nanoparticles Composite Films with Sustained Antibacterial Activity for Active Food Packaging. Int. J. Biol. Macromol. 2023, 230, 123192. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Chai, Y.; Guo, C.; Luo, C.; Chen, D.; Cheng, X.; Wang, F.; Huang, C. Chitosan–Pullulan Films Enriched with Artemisia annua Essential Oil: Characterization and Application in Grape Preservation. Int. J. Biol. Macromol. 2023, 243, 125216. [Google Scholar] [CrossRef]
- Rad, S.S.; Sayyari, M.; Torabi, M.; Zolfigol, M.A. Fabrication and Investigation of Chitosan-Based Edible Coating Derived Mushroom Substrates: Efficient Performance on Storage and Improving Postharvest Quality of Strawberry. Int. J. Biol. Macromol. 2025, 309, 142731. [Google Scholar] [CrossRef]
- Guo, B.; Liu, G.; Ye, W.; Xu, Z.; Li, W.; Zhuang, J.; Zhang, X.; Wang, L.; Lei, B.; Hu, C.; et al. Multifunctional Carbon Dots Reinforced Gelatin-Based Coating Film for Strawberry Preservation. Food Hydrocoll. 2024, 147, 109327. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing Shelf Life of Strawberries (Fragaria ssp.) by Using a Banana Starch-chitosan-Aloe Vera Gel Composite Edible Coating. Int. J. Food Sci. Technol. 2020, 55, 92–98. [Google Scholar] [CrossRef]
- Paniagua, C.; Santiago-Doménech, N.; Kirby, A.R.; Gunning, A.P.; Morris, V.J.; Quesada, M.A.; Matas, A.J.; Mercado, J.A. Structural Changes in Cell Wall Pectins during Strawberry Fruit Development. Plant Physiol. Biochem. 2017, 118, 55–63. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Han, S.; Hua, S.; Zhang, H.; Wang, J.; Xia, N.; Liu, Y.; Meng, D. Edible Chitosan-Based Pickering Emulsion Coatings: Preparation, Characteristics, and Application in Strawberry Preservation. Int. J. Biol. Macromol. 2024, 264, 130672. [Google Scholar] [CrossRef]
- Shahbazi, Y. Application of Carboxymethyl Cellulose and Chitosan Coatings Containing Mentha Spicata Essential Oil in Fresh Strawberries. Int. J. Biol. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; Sarreal, S.B.L.; Hua, S.S.T.; Sambo, P.; McHugh, T.H. Increasing Strawberry Shelf-Life with Carvacrol and Methyl Cinnamate Antimicrobial Vapors Released from Edible Films. Postharvest Biol. Technol. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Qaderi, R.; Mezzetti, B.; Capocasa, F.; Mazzoni, L. Stability of Strawberry Fruit (Fragaria x ananassa Duch.) Nutritional Quality at Different Storage Conditions. Appl. Sci. 2022, 13, 313. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Miranda, M.; Ferreira, M.D.; Plotto, A. Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation. Foods 2021, 10, 2438. [Google Scholar] [CrossRef]
- Lee, D.; Shayan, M.; Gwon, J.; Picha, D.H.; Wu, Q. Effectiveness of Cellulose and Chitosan Nanomaterial Coatings with Essential Oil on Postharvest Strawberry Quality. Carbohydr. Polym. 2022, 298, 120101. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Tosif, M.M.; Bains, A.; Goksen, G.; Nagraik, R.; Dhull, S.B.; Ali, N.; Muzaffar, N.; Chawla, P. Impact of Organic Acid Cross-Linking on the Structure and Functional Properties of Gum Arabic and Guar Gum: Formulation of an Edible Coating for Enhancing Strawberry Shelf Life. Food Chem. X 2025, 28, 102527. [Google Scholar] [CrossRef]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of Edible Coating Enriched with Natural Antioxidant Extract and Bergamot Essential Oil on the Shelf Life of Strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Zebua, D.N.; Prima, E.C.; Yelliantty; Garnida, Y. Effect of a Pectin Edible Coating with Lemon Peel Extract to Maintain Strawberry Fruit’s Quality during Cold Storage. Food Humanit. 2025, 4, 100541. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, P.; Das, K.; Katiyar, V. Chitosan/Water Caltrop Pericarp Extract Reinforced Active Edible Film and Its Efficacy as Strawberry Coating for Prolonging Shelf Life. Int. J. Biol. Macromol. 2025, 307, 142115. [Google Scholar] [CrossRef]
- Gautam, A.; Gill, P.P.S.; Singh, N.; Jawandha, S.K.; Arora, R.; Singh, A. Ajay Composite Coating of Xanthan Gum with Sodium Nitroprusside Alleviates the Quality Deterioration in Strawberry Fruit. Food Hydrocoll. 2024, 155, 110208. [Google Scholar] [CrossRef]
- Muley, A.B.; Kedia, P.; Pegu, K.; Kausley, S.B.; Rai, B. Analyzing the Physical and Biochemical Changes in Strawberries during Storage at Different Temperatures and the Development of Kinetic Models. J. Food Meas. Charact. 2022, 16, 222–247. [Google Scholar] [CrossRef]
- Yildiz, S.; Turan, S.; Kiralan, M.; Ramadan, M.F. Antioxidant Properties of Thymol, Carvacrol, and Thymoquinone and Its Efficiencies on the Stabilization of Refined and Stripped Corn Oils. J. Food Meas. Charact. 2021, 15, 621–632. [Google Scholar] [CrossRef]
- Zahra, N.I.; Songtipya, P.; Songtipya, L.; Prodpran, T.; Sengsuk, T.; Utami, T. Xyloglucan Based Edible Coating in Combination with Borassus flabellifer Seed Coat Extract for Extending Strawberry Postharvest Shelf Life. Int. J. Biol. Macromol. 2025, 285, 138288. [Google Scholar] [CrossRef]


| Films | Opacity (mm) | WVP (×10−11 g−1 s−1 Pa−1) | TS (MPa) | EB (%) | MC (%) | SD (%) | WS |
|---|---|---|---|---|---|---|---|
| GL/CMCS/CA-0% | 1.64 ± 0.13 c | 4.07 ± 0.11 a | 28.38 ± 0.20 c | 28.08 ± 0.09 c | 14.62 ± 0.10 a | 58.52 ± 0.11 a | 52.50 ± 0.18 a |
| GL/CMCS/CA-1.5% | 3.15 ± 0.17 b | 2.84 ± 0.07 b | 36.52 ± 0.05 b | 31.26 ± 0.11 b | 13.03 ± 0.03 b | 55.27 ± 0.08 b | 42.57 ± 0.10 b |
| GL/CMCS/CA-3% | 3.74 ± 0.31 a | 2.51 ± 0.08 c | 42.70 ± 0.08 a | 33.04 ± 0.05 a | 12.41 ± 0.07 c | 49.53 ± 0.09 c | 38.05 ± 0.07 c |
| Films | L* | a* | b* | ΔE* | Thickness (mm) |
|---|---|---|---|---|---|
| GL/CMCS/CA-0% | 82.19 ± 0.06 a | 1.38 ± 0.18 a | 2.58 ± 1.06 c | 12.84 ± 0.11 a | 0.10 ± 0.07 c |
| GL/CMCS/CA-1.5% | 83.71 ± 0.11 a | 1.35 ± 1.03 ab | 3.27 ± 0.50 b | 11.05 ± 0.38 b | 0.15 ± 0.06 b |
| GL/CMCS/CA-3% | 83.62 ± 0.19 a | 1.33 ± 0.37 b | 3.58 ± 0.51 a | 10.24 ± 0.09 b | 0.17 ± 0.09 a |
| Storage Time (Days) | Group | Sensory Characteristics | |||
|---|---|---|---|---|---|
| Appearance | Firmness | Taste | Overall Acceptance | ||
| 0 | CON | 9.00 ± 0.00 aA | 9.00 ± 0.00 aA | 9.00 ± 0.00 aA | 9.00 ± 0.00 aA |
| GL/CMCS/CA-1.5% | 9.00 ± 0.00 aA | 9.00 ± 0.00 aA | 8.92 ± 0.16 aA | 8.83 ± 0.09 aA | |
| GL/CMCS/CA-3% | 9.00 ± 0.00 aA | 9.00 ± 0.00 aA | 9.00 ± 0.00 aA | 9.00 ± 0.00 aA | |
| 4 | CON | 8.75 ± 0.18 aA | 8.92 ± 0.16 aA | 8.92 ± 0.16 aA | 8.92 ± 0.16 aA |
| GL/CMCS/CA-1.5% | 8.75 ± 0.18 aAB | 8.92 ± 0.16 aA | 8.83 ± 0.09 aA | 8.75 ± 0.18 aA | |
| GL/CMCS/CA-3% | 8.83 ± 0.09 aA | 8.92 ± 0.16 aA | 8.92 ± 0.16 aA | 8.83 ± 0.09 aA | |
| 7 | CON | 7.92 ± 0.14 bB | 7.25 ± 0.28 bB | 7.92 ± 0.14 aB | 7.33 ± 0.10 cB |
| GL/CMCS/CA-1.5% | 8.25 ± 0.09 aB | 8.25 ± 0.09 aB | 8.00 ± 0.10 abB | 8.25 ± 0.09 bB | |
| GL/CMCS/CA-3% | 8.83 ± 0.09 aA | 8.67 ± 0.12 aA | 8.67 ± 0.12 bA | 8.67 ± 0.12 aA | |
| 10 | CON | 6.58 ± 0.18 cC | 5.92 ± 0.15 cC | 6.17 ± 0.12 bC | 6.17 ± 0.12 cC |
| GL/CMCS/CA-1.5% | 7.17 ± 0.36 bC | 6.58 ± 0.18 bC | 5.83 ± 0.20 aC | 6.67 ± 0.19 bC | |
| GL/CMCS/CA-3% | 7.50 ± 0.11 aB | 7.17 ± 0.36 aB | 5.83 ± 0.20 aB | 6.91 ± 0.08 aB | |
| 14 | CON | 3.75 ± 0.07 cD | 3.58 ± 0.30 cD | 3.67 ± 0.32 cD | 3.83 ± 0.16 cD |
| GL/CMCS/CA-1.5% | 4.58 ± 0.24 bD | 5.16 ± 0.15 bD | 4.33 ± 0.27 bD | 4.58 ± 0.24 bD | |
| GL/CMCS/CA-3% | 6.91 ± 0.08 aC | 6.42 ± 0.20 aC | 5.16 ± 0.15 aC | 6.17 ± 0.12 aC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassey, A.P.; Meng, C.; Zhang, Y.; Wang, F.; Nasiru, M.M.; Wu, H.; Ibeogu, I.H.; Fan, L.; Liu, X. Gelatin and Carboxymethyl Chitosan Edible Coating Incorporated with Carvacrol: Development and Application in Strawberries. Foods 2025, 14, 3297. https://doi.org/10.3390/foods14193297
Bassey AP, Meng C, Zhang Y, Wang F, Nasiru MM, Wu H, Ibeogu IH, Fan L, Liu X. Gelatin and Carboxymethyl Chitosan Edible Coating Incorporated with Carvacrol: Development and Application in Strawberries. Foods. 2025; 14(19):3297. https://doi.org/10.3390/foods14193297
Chicago/Turabian StyleBassey, Anthony Pius, Chaoxiong Meng, Yin Zhang, Fan Wang, Mustapha Muhammad Nasiru, Han Wu, Isaiah Henry Ibeogu, Linlin Fan, and Xiaoli Liu. 2025. "Gelatin and Carboxymethyl Chitosan Edible Coating Incorporated with Carvacrol: Development and Application in Strawberries" Foods 14, no. 19: 3297. https://doi.org/10.3390/foods14193297
APA StyleBassey, A. P., Meng, C., Zhang, Y., Wang, F., Nasiru, M. M., Wu, H., Ibeogu, I. H., Fan, L., & Liu, X. (2025). Gelatin and Carboxymethyl Chitosan Edible Coating Incorporated with Carvacrol: Development and Application in Strawberries. Foods, 14(19), 3297. https://doi.org/10.3390/foods14193297










