Changes in the Volatile Flavor Compounds and Quality Attributes of Tilapia Fillets Throughout the Drying Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Ready-to-Eat Tilapia Fillets
2.2.1. Operation Key Points
2.2.2. Texture Measurement
2.2.3. Determination of pH
2.2.4. Measurement of Thiobarbituric Acid (TBA)
2.2.5. Analysis of Volatile Flavor Components
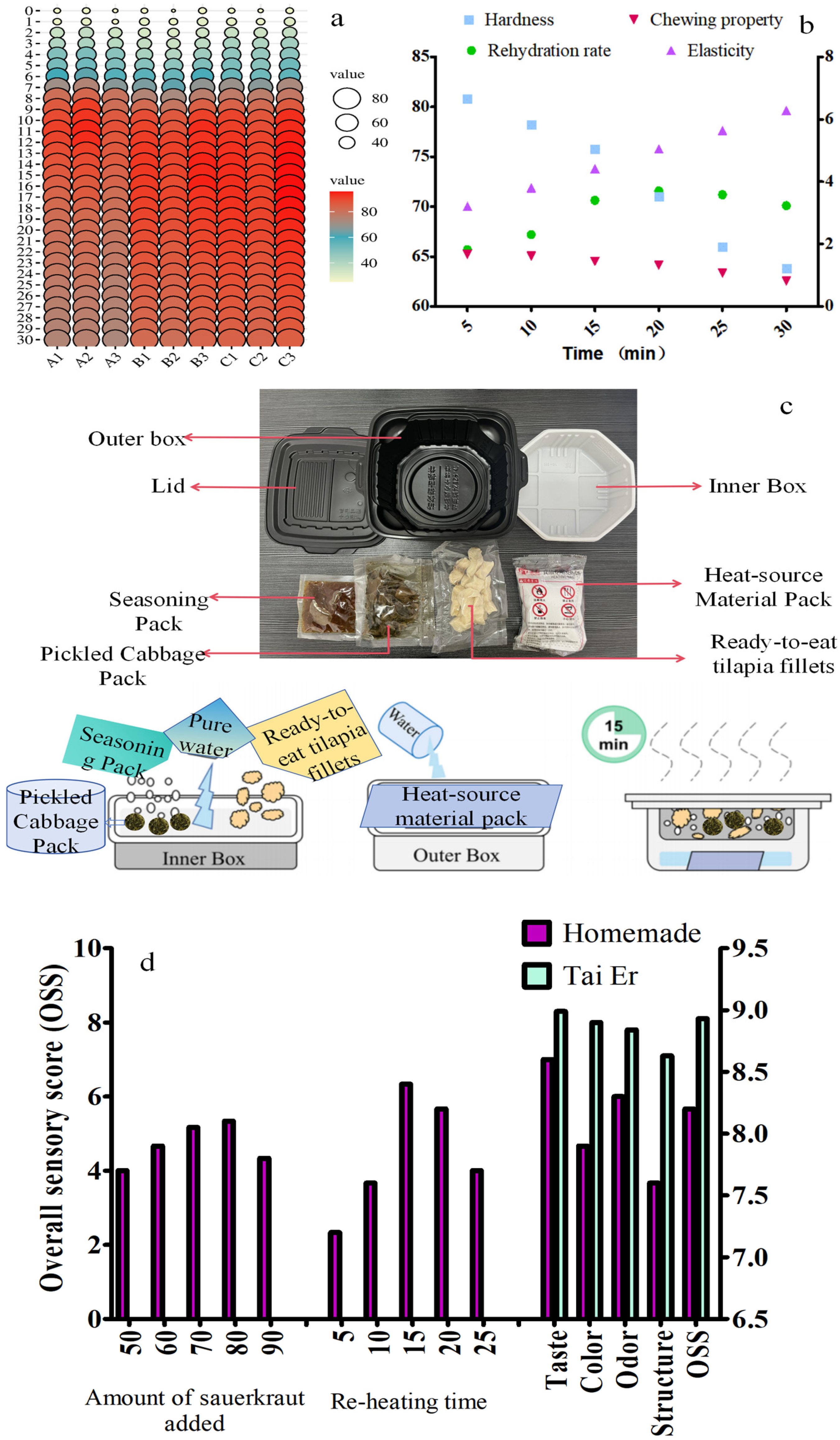
2.3. Influence of Heat Source Material Pack on the Quality of Dried Tilapia Fillets
2.3.1. Effect of Heat Source Material Pack on the Heat Transfer of Dried Tilapia Fillets
2.3.2. Effect of Heat Source Material Pack on the Rehydration Rate and Texture Changes in Dried Tilapia Fillets
2.4. The Sensory Evaluation of Self-Heating Sauerkraut Fish
2.5. Data Analysis
3. Results and Discussion
3.1. Quality of Tilapia Fillets in Different Processing Stages
3.2. Fingerprint Analysis of Flavor Substances in Tilapia Fillets Under Different Processing Stages
3.3. Comparison of the Fingerprints of VOCs in Tilapia Fillets Under Different Processing Stages
3.4. Analysis of Content Change in Volatile Flavor Compounds
3.5. Ready-to-Eat Self-Heating Sauerkraut Fish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Yin, Y.; Zhou, W.; Li, H.; Hu, B.; Cui, Y.; Zhou, R.; Wang, P.; Fu, J. Convenient Self-Heating Instant Food Causes Significant Increasing Human Exposure to Organophosphate Esters. Environ. Health 2023, 2, 52–61. [Google Scholar] [CrossRef]
- Li, M.; Guan, Z.; Ge, Y.; Zhang, X.; Ling, C. Effect of Pretreatment on Water Migration and Volatile Components of Heat Pump Dried Tilapia Fillets. Dry. Technol. 2020, 38, 1828–1842. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid Oxidation in Food Science and Nutritional Health: A Comprehensive Review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, Y.; Li, S.; Shang, S.; Fu, B.; Wang, L.; Dong, X.; Jiang, P. Ready-to-Eat Fish Cake Processing Methods and the Impacts on Quality and Flavor. Foods 2022, 11, 3321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, B.; Cao, J.; Li, C.; Duan, Z. The Impacts of Vacuum Microwave Drying on Osmosis Dehydration of Tilapia Fillets. J. Food Process Eng. 2019, 42, e12956. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Song, S.; Feng, L.; Luo, Y. Influence of Heat Processing on the Volatile Organic Compounds and Microbial Diversity of Salted and Vacuum-Packaged Silver Carp (Hypophthalmichthys molitrix) Fillets during Storage. Food Microbiol. 2018, 72, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Mei, J.; Xie, J. Analysis of Key Volatile Compounds and Quality Properties of Tilapia (Oreochromis mossambicus) Fillets during Cold Storage: Based on Thermal Desorption Coupled with Gas Chromatography-Mass Spectrometry (TD-GC-MS). LWT 2023, 184, 115051. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. Effects of Different Cooking Methods on the Lipids and Volatile Components of Farmed and Wild European Sea Bass (Dicentrarchus labrax). Food Res. Int. 2018, 103, 48–58. [Google Scholar] [CrossRef]
- Li, R.; Sun, Z.; Zhao, Y.; Li, L.; Yang, X.; Chen, S.; Wei, Y.; Li, C.; Wang, Y. Effect of Different Thermal Processing Methods on Water-Soluble Taste Substances of Tilapia Fillets. J. Food Compos. Anal. 2022, 106, 104298. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, C.; Chu, B.; Gu, P.; Zhu, B.; Qian, W.; Chang, X.; Yu, M.; Zhang, Y.; Wang, X. Unraveling the Relationship between Key Aroma Components and Sensory Properties of Fragrant Peanut Oils Based on Flavoromics and Machine Learning. Food Chem. X 2023, 20, 100880. [Google Scholar] [CrossRef]
- Huang, D.; Li, M.; Wang, H.; Fu, M.; Hu, S.; Wan, X.; Wang, Z.; Chen, Q. Combining Gas Chromatography-Ion Mobility Spectrometry and Olfactory Analysis to Reveal the Effect of Filled-N2 Anaerobic Treatment Duration on Variation in the Volatile Profiles of Gabaron Green Tea. LWT 2023, 179, 114630. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Vidyarthi, S.K.; Zhong, C.-S.; Zheng, Z.-A.; An, Y.; Wang, J.; Wei, Q.; Xiao, H.-W. Cold Plasma Enhances Drying and Color, Rehydration Ratio and Polyphenols of Wolfberry via Microstructure and Ultrastructure Alteration. LWT 2020, 134, 110173. [Google Scholar] [CrossRef]
- Jin, W.; Zhao, S.; Li, J.; Cheng, K.; Xi, L.; Pei, J.; Gao, R.; Jiang, P. Unraveling Gender-Specific Lipids and Flavor Volatiles in Giant Salamander (Andrias davidianus) Livers via Lipidomics and GC-IMS. Food Chem. X 2024, 23, 101786. [Google Scholar] [CrossRef]
- Wu, J.; Lin, X.; Cui, C.; Li, J. Comparative Analysis of Kokumi Effects in Ile-Leu, Ile-Ile, Leu-Leu, and Leu-Ile: Sensory, Simulation, and Structural Insights. Food Chem. 2025, 474, 143004. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xu, Y.; Yu, D.; Xia, W.; Jiang, Q. The Impacts of Salt with Chinese Liquor on the Inhibition of Microbial Spoilage and Quality Attributes of Grass Carp (Ctenopharyngodon idellus) Fillets Stored at 4 °C. J. Food Process. Preserv. 2020, 44, e14817. [Google Scholar] [CrossRef]
- Piranavatharsan, U.; Jinadasa, B.K.K.K.; Jayasinghe, C.V.L. Validation of Thiobarbituric Acid Reactive Substances (TBARS) Method for Measuring Secondary Lipid Oxidation Products in Fresh Indian Mackerel (Rastrelliger kanagurta). Food Humanit. 2023, 1, 1194–1199. [Google Scholar] [CrossRef]
- Chen, T.; Li, C.; Huang, H.; Zhao, Y.; Xiang, H.; Wang, D.; Feng, Y.; Yang, S.; Chen, S. Identification of Key Physicochemical Properties and Volatile Flavor Compounds for the Sensory Formation of Roasted Tilapia. Food Chem. 2024, 460, 140636. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Qin, S.; Li, J.; Tang, D.; Xi, B. GC-IMS and Multivariate Analyses of Volatile Organic Components in Different Chinese Breeds of Chickens. Heliyon 2024, 10, e29664. [Google Scholar] [CrossRef]
- Lu, J.; Li, R.; Chen, H.; Sun, D.; Yu, Z.; Liu, Y.; Zhang, B.; Jiang, W. Effect of Brine Concentration on the Quality of Salted Large Yellow Croaker during Processing and Refrigeration. Food Sci. Biotechnol. 2024, 33, 3257–3267. [Google Scholar] [CrossRef]
- Ojangba, T.; Zhang, L.; Boamah, S.; Gao, Y.; Wang, Z.; Amagloh, F.K. Effect of Partial Substitution of Sodium Chloride (NaCl) with Potassium Chloride (KCl) Coupled with High-Pressure Processing (HPP) on Physicochemical Properties and Volatile Compounds of Beef Sausage under Cold Storage at 4 °C. Processes 2022, 10, 431. [Google Scholar] [CrossRef]
- Asefa, B.G. Effect of Temperature Fluctuation on Quality of Frozen Atlantic Salmon (Salmo salar) Fillet. J. Food Technol. 2021, 19, 43–47. Available online: https://www.researchgate.net/publication/361040017_Effect_of_Temperature_Fluctuation_on_Quality_of_Frozen_Atlantic_Salmon_Salmo_Salar_Fillet (accessed on 29 July 2025).
- Qiu, D.; Duan, R.; Wang, Y.; He, Y.; Li, C.; Shen, X.; Li, Y. Effects of Different Drying Temperatures on the Profile and Sources of Flavor in Semi-Dried Golden Pompano (Trachinotus ovatus). Food Chem. 2023, 401, 134112. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Huang, Y.; Zhao, M.; Liu, T.; Wang, J.; Feng, F. Influence of Cooking Techniques on Food Quality, Digestibility, and Health Risks Regarding Lipid Oxidation. Food Res. Int. 2023, 167, 112685. [Google Scholar] [CrossRef]
- Shen, S.; Chen, Y.; Dong, X.; Liu, F.; Cai, W.; Wei, J.; Bai, F.; Shi, Y.; Li, P.; Wang, Y. Changes in Food Quality and Microbial Composition of Russian Sturgeon (Acipenser gueldenstaedti) Fillets Treated with Low Temperature Vacuum Heating Method during Storage at 4 °C. Food Res. Int. 2020, 138, 109665. [Google Scholar] [CrossRef]
- Almansour, A.; Bartlett, D.; Addison, O. Impact of Citric Acid Exposures on the Erosion Susceptibility and Microhardness of Anatomically Different Enamel Surfaces. Dent. Mater. 2024, 40, 53–58. [Google Scholar] [CrossRef]
- Fu, C.; Liu, W.; Chen, Y.; Li, Y.; Li, H.; Zhou, J.; Zhang, K.; Wei, C. Effects of Heating Temperature on Physicochemical Properties, Fatty Acids and Volatile Flavor Compounds of Fat from Horses Raised in Xinjiang. Food Sci. 2021, 42, 54–60. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, L.; Zhang, C.; Feng, T.; Zhuang, H. Analysis of Volatile Flavor Compounds of Corn Under Different Treatments by GC-MS and GC-IMS. Front. Chem. 2022, 10, 725208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, R.; Mi, S.; Chitrakar, B.; Liu, W.; Xu, Z.; Sang, Y.; Yu, W.; Wang, X. Effect of Different Thermal Processing Methods on Flavor Characteristics of Penaeus vannamei. LWT 2024, 191, 115652. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wang, Y.; Luo, N.; Cai, R.; Yu, Y.; Zhang, X.; Zhu, J.; Zhao, G.; Wen, J.; et al. Main Lipid Sources Affecting Key Aroma Volatile Compounds in Chinese Native Chicken. Food Chem. 2025, 474, 142990. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.-Y.; Aheto, J.H.; Huang, X.; Zheng, K.; Dai, C.; Wang, C.; Bai, J.-W. An Evaluation of Biochemical, Structural and Volatile Changes of Dry-Cured Pork Using a Combined Ion Mobility Spectrometry, Hyperspectral and Confocal Imaging Approach. J. Sci. Food Agric. 2021, 101, 5972–5983. [Google Scholar] [CrossRef]
- Zhao, S.; Niu, C.; Wang, Y.; Li, X.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Revealing the Contributions of Sunlight-Expose Process and Core-Microbiota Metabolism on Improving the Flavor Profile during Doubanjiang Fermentation. Food Biosci. 2023, 53, 102522. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Ye, J.; Qian, M.; Li, X.; Zhao, W.; Bai, W. HS-SPME-GC-MS and OAV Analyses of Characteristic Volatile Flavour Compounds in Salt-Baked Drumstick. LWT 2022, 170, 114041. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the Flavour Properties of Cooked Chicken Drumsticks as Affected by Sugar Smoking Times Using an Electronic Nose, Electronic Tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Fiorin, A.; Filipa-Silva, A.; Marques, A.; Castro, C.; Casal, S.; Moreira, P.; Padrão, P.; Valente, L.M.P. Can Culinary Processing Impact the Lipid Composition and Fatty Acid Profile of Turbot (Scophthalmus maximus)? J. Food Compos. Anal. 2024, 133, 106376. [Google Scholar] [CrossRef]
- Jung, Y.; Oh, S.; Kim, D.; Lee, S.; Lee, H.-J.; Shin, D.-J.; Choo, H.-J.; Jo, C.; Nam, K.-C.; Lee, J.-H.; et al. Effect of Cinnamon Powder on Quality Attributes and Off-Flavor in Fried Chicken Drumsticks Made from Long-Term Thawed Korean Native Chicken. Poult. Sci. 2024, 103, 103583. [Google Scholar] [CrossRef] [PubMed]
- Grabež, V.; Bjelanović, M.; Rohloff, J.; Martinović, A.; Berg, P.; Tomović, V.; Rogić, B.; Egelandsdal, B. The Relationship between Volatile Compounds, Metabolites and Sensory Attributes: A Case Study Using Lamb and Sheep Meat. Small Rumin. Res. 2019, 181, 12–20. [Google Scholar] [CrossRef]
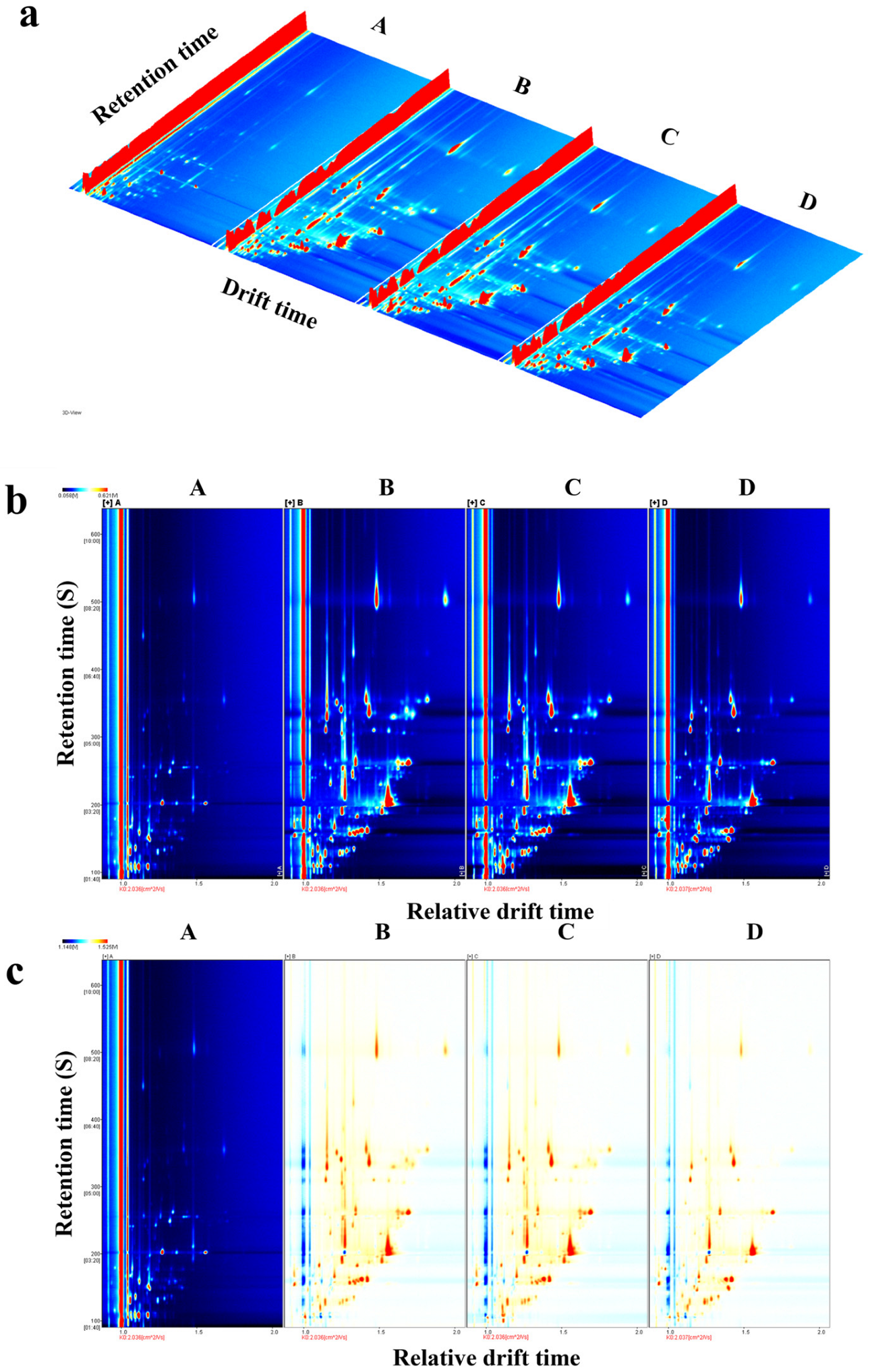


| Sample | Hardness/g | Elasticity/mm | Chewing Property/mJ | pH | TBA (mg/100 g) |
|---|---|---|---|---|---|
| Fresh tilapia fillet (A) | 84.0 ± 1.20 c | 2.87 ± 0.18 c | 1.58 ± 0.35 b | 6.37 ± 0.06 b | 0.39 ± 0.01 b |
| Pickling and blanching tilapia fillet (B) | 81.8 ± 1.40 d | 3.19 ± 0.28 b | 1.59 ± 0.32 b | 7.32 ± 0.07 a | 0.51 ± 0.03 a |
| Freeze-dried fillet (C) | 96.0 ± 0.70 a | 3.70 ± 0.25 a | 2.28 ± 0.04 a | 7.28 ± 0.0.07 a | 0.53 ± 0.03 a |
| Rehydration tilapia fillet (D) | 94.3 ± 0.39 b | 3.44 ± 0.23 a | 1.61 ± 0.06 b | 7.35 ± 0.0.01 a | 0.52 ± 0.01 a |
| Category | NO | Volatile Components | GAS | Rt [s] | Dt [a.u.] | Relative Amount (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| Aldehydes | 1 | Nonanal-M | C9H18O | 503.885 | 1.48432 | 5.68 ± 0.33 | 6.97 ± 0.11 | 5.77 ± 0.62 | 4.81 ± 0.27 |
| 2 | Nonanal-D | C9H18O | 504.809 | 1.94227 | 0.47 ± 0.01 | 1.54 ± 0.09 | 0.96 ± 0.04 | 0.53 ± 0.06 | |
| 3 | Octanal-M | C8H16O | 358.268 | 1.41435 | 1.74 ± 0.12 | 2.76 ± 0.21 | 2.84 ± 0.3 | 2.55 ± 0.05 | |
| 4 | Octanal-D | C8H16O | 355.032 | 1.82142 | 0.38 ± 0.03 | 1.41 ± 0.11 | 1.52 ± 0.6 | 0.69 ± 0.22 | |
| 5 | Benzaldehyde-M | C7H6O | 310.653 | 1.14881 | 1.88 ± 0.25 | 2.07 ± 0.11 | 2.10 ± 0.51 | 2.78 ± 0.95 | |
| 6 | Benzaldehyde-D | C7H6O | 310.653 | 1.46365 | 0.33 ± 0.07 | 0.54 ± 0.07 | 0.58 ± 0.11 | 0.72 ± 0.32 | |
| 7 | Heptanal-M | C7H14O | 264.426 | 1.34439 | 3.60 ± 0.21 | 3.40 ± 0.19 | 3.41 ± 0.48 | 4.20 ± 0.28 | |
| 8 | Heptanal-D | C7H14O | 263.039 | 1.69422 | 0.66 ± 0.08 | 5.39 ± 1.27 | 5.59 ± 0.59 | 4.48 ± 0.71 | |
| 9 | (Z)-4-heptenal | C7H12O | 260.171 | 1.14486 | 0.86 ± 0.05 | 0.38 ± 0.04 | 0.39 ± 0.07 | 0.44 ± 0.06 | |
| 10 | (E)-2-hexenal-M | C6H10O | 230.717 | 1.17856 | 0.62 ± 0.05 | 0.72 ± 0.12 | 0.75 ± 0.25 | 0.46 ± 0.02 | |
| 11 | Hexanal-M | C6H12O | 202.384 | 1.27576 | 12.85 ± 0.88 | 7.35 ± 0.78 | 7.36 ± 0.36 | 8.95 ± 1.42 | |
| 12 | Hexanal-D | C6H12O | 200.623 | 1.56347 | 8.05 ± 0.7 | 25.90 ± 5.47 | 25.47 ± 6.31 | 26.22 ± 4.04 | |
| 13 | (E)-2-octenal | C8H14O | 425.992 | 1.33383 | 0.59 ± 0.01 | 1.23 ± 0.14 | 0.93 ± 0.15 | 0.43 ± 0.05 | |
| 14 | (E)-hept-2-enal-M | C7H12O | 306.12 | 1.25718 | 0.48 ± 0.05 | 1.36 ± 0.17 | 1.09 ± 0.13 | 0.63 ± 0.11 | |
| 15 | (E)-hept-2-enal-D | C7H12O | 305.827 | 1.668 | 0.37 ± 0.04 | 0.32 ± 0.04 | 0.22 ± 0.01 | 0.10 ± 0.02 | |
| 16 | (E)-2-hexenal-D | C6H10O | 230.243 | 1.51311 | 0.29 ± 0.07 | 0.48 ± 0.01 | 0.52 ± 0.01 | 0.13 ± 0.02 | |
| 17 | (E)-2-pentenal-M | C5H8O | 183.709 | 1.10507 | 0.34 ± 0.08 | 1.09 ± 0.12 | 0.97 ± 0.05 | 0.55 ± 0.03 | |
| 18 | 2-methylbutanal-M | C5H10O | 150.641 | 1.1789 | 5.75 ± 0.18 | 1.00 ± 0.08 | 0.95 ± 0.12 | 2.26 ± 0.21 | |
| 19 | 2-methylbutanal-D | C5H10O | 151.36 | 1.39807 | 0.49 ± 0.08 | 0.07 ± 0.00 | 0.06 ± 0.00 | 1.14 ± 0.17 | |
| 20 | 3-methylbutanal-M | C5H10O | 146.807 | 1.19531 | 1.87 ± 0.17 | 0.30 ± 0.05 | 0.31 ± 0.06 | 1.28 ± 0.18 | |
| 21 | 3-methylbutanal-D | C5H10O | 147.046 | 1.40861 | 0.44 ± 0.06 | 0.05 ± 0.00 | 0.05 ± 0.00 | 1.03 ± 0.07 | |
| 22 | (E)-2-pentenal-D | C5H8O | 182.991 | 1.35939 | 0.24 ± 0.02 | 1.08 ± 0.21 | 0.91 ± 0.16 | 0.20 ± 0.01 | |
| 23 | but-(E)-2-enal | C4H6O | 146.807 | 1.03006 | 0.83 ± 0.08 | 0.34 ± 0.03 | 0.29 ± 0.01 | 0.09 ± 0.00 | |
| 24 | 2-methylpropanal | C4H8O | 123.902 | 1.28222 | 0.05 ± 0.00 | 0.08 ± 0.00 | 0.09 ± 0.01 | 0.07 ± 0.00 | |
| Alcohols | 25 | oct-1-en-3-ol-M | C8H16O | 330.994 | 1.15676 | 1.80 ± 0.08 | 4.29 ± 0.35 | 4.20 ± 1.62 | 3.08 ± 0.15 |
| 26 | 3-Heptanol | C7H16O | 248.966 | 1.32889 | 2.10 ± 0.18 | 0.58 ± 0.03 | 0.60 ± 0.03 | 0.68 ± 0.04 | |
| 27 | oct-1-en-3-ol-D | C8H16O | 329.694 | 1.60246 | 0.53 ± 0.02 | 1.44 ± 0.16 | 1.11 ± 0.06 | 0.34 ± 0.01 | |
| 28 | n-Hexanol-M | C6H14O | 241.702 | 1.32791 | 0.25 ± 0.02 | 0.29 ± 0.04 | 0.31 ± 0.02 | 0.10 ± 0.02 | |
| 29 | pentan-1-ol-M | C5H12O | 189.7 | 1.25625 | 1.07 ± 0.06 | 2.60 ± 0.17 | 2.57 ± 0.14 | 2.63 ± 0.09 | |
| 30 | pentan-1-ol-D | C5H12O | 190.179 | 1.51644 | 0.25 ± 0.06 | 1.88 ± 0.11 | 2.01 ± 0.09 | 1.00 ± 0.06 | |
| 31 | 3-pentanol-M | C5H12O | 161.664 | 1.20703 | 2.78 ± 0.11 | 2.32 ± 0.16 | 2.40 ± 0.21 | 3.01 ± 0.17 | |
| 32 | 3-pentanol-D | C5H12O | 161.424 | 1.42151 | 0.57 ± 0.06 | 4.23 ± 0.14 | 4.34 ± 0.57 | 7.32 ± 1.01 | |
| 33 | Isopropyl alcohol | C3H8O | 110.863 | 1.22578 | 0.86 ± 0.12 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.65 ± 0.08 | |
| 34 | pent-1-en-3-ol | C5H10O | 154.248 | 0.94342 | 1.01 ± 0.18 | 2.11 ± 0.12 | 2.20 ± 0.11 | 2.25 ± 0.17 | |
| 35 | n-Hexanol-D | C6H14O | 241.846 | 1.64601 | 0.08 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.03 ± 0.01 | |
| Ketone | 36 | 2-heptanone-M | C7H14O | 255.368 | 1.2615 | 1.43 ± 0.18 | 1.16 ± 0.06 | 1.20 ± 0.02 | 0.87 ± 0.01 |
| 37 | 4-Heptanone | C7H14O | 243.137 | 1.23111 | 1.69 ± 0.52 | 0.18 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | |
| 38 | 3-Octanone | C8H16O | 332.043 | 1.72617 | 0.36 ± 0.01 | 1.21 ± 0.17 | 1.02 ± 0.04 | 0.29 ± 0.01 | |
| 39 | 1-octen-3-one | C8H14O | 325.232 | 1.27654 | 0.28 ± 0.11 | 0.68 ± 0.02 | 0.55 ± 0.01 | 0.34 ± 0.02 | |
| 40 | 2-Butanone | C4H8O | 127.876 | 1.24453 | 3.62 ± 0.71 | 1.59 ± 0.09 | 1.71 ± 0.08 | 3.35 ± 0.22 | |
| 41 | acetone | C3H6O | 109.185 | 1.11679 | 13.30 ± 1.74 | 1.79 ± 0.08 | 5.42 ± 0.31 | 1.92 ± 0.07 | |
| 42 | 1-penten-3-one | C5H8O | 155.673 | 1.07928 | 0.34 ± 0.0 | 0.31 ± 0.02 | 0.26 ± 0.02 | 0.12 ± 0.01 | |
| 43 | 3-Pentanone | C5H10O | 158.069 | 1.12265 | 5.42 ± 0.15 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.20 ± 0.01 | |
| 44 | 2-Hexanone | C6H12O | 197.847 | 1.19062 | 1.17 ± 0.29 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.15 ± 0.01 | |
| 45 | 3-hydroxybutan-2-one | C4H8O2 | 168.234 | 1.06025 | 0.49 ± 0.07 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | |
| 46 | 2-heptanone-D | C7H14O | 253.903 | 1.62724 | 0.08 ± 0.01 | 0.36 ± 0.01 | 0.39 ± 0.01 | 0.09 ± 0.01 | |
| Acid | 47 | 2-methylpentanoic acid | C6H12O2 | 387.456 | 1.2694 | 0.41 ± 0.05 | 0.71 ± 0.05 | 0.66 ± 0.09 | 0.32 ± 0.02 |
| Ester | 48 | Ethyl Acetate-M | C4H8O2 | 135.065 | 1.09686 | 4.51 ± 0.4 | 1.57 ± 0.05 | 1.35 ± 0.13 | 1.90 ± 0.06 |
| 49 | Ethyl Acetate-D | C4H8O2 | 136.024 | 1.33361 | 0.93 ± 0.32 | 0.92 ± 0.03 | 0.79 ± 0.08 | 1.27 ± 0.13 | |
| Sulfide | 50 | Dimethyl sulfide | C2H6S | 115.721 | 0.95802 | 1.78 ± 0.17 | 0.12 ± 0.01 | 0.21 ± 0.05 | 0.15 ± 001 |
| 51 | dimethyl disulfide | C2H6S2 | 177.997 | 0.98139 | 0.46 ± 0.03 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.78 ± 0.22 | |
| Terpenes | 52 | Styrene | C8H8 | 254.568 | 1.41702 | 2.16 ± 0.32 | 0.44 ± 0.06 | 0.58 ± 0.01 | 0.73 ± 0.18 |
| Benzene | 53 | p-xylene | C8H10 | 241.122 | 1.05932 | 0.32 ± 0.03 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.11 ± 0.01 |
| Heterocyclic | 54 | 2-pentyl furan | C9H14O | 342.088 | 1.25057 | 0.40 ± 0.02 | 2.22 ± 0.31 | 1.71 ± 0.29 | 0.85 ± 0.22 |
| 55 | 2-Acetylfuran | C6H6O2 | 275.538 | 1.11764 | 0.29 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.11 | 0.43 ± 0.02 | |
| 56 | 2-n-Butylfuran | C8H12O | 254.181 | 1.1765 | 0.41 ± 0.03 | 0.29 ± 0.27 | 0.23 ± 0.11 | 0.16 ± 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xiang, H.; Hao, S.; Wei, L.; Huang, H.; Wei, Y.; Chen, S.; Zhao, Y. Changes in the Volatile Flavor Compounds and Quality Attributes of Tilapia Fillets Throughout the Drying Process. Foods 2025, 14, 3293. https://doi.org/10.3390/foods14193293
Li J, Xiang H, Hao S, Wei L, Huang H, Wei Y, Chen S, Zhao Y. Changes in the Volatile Flavor Compounds and Quality Attributes of Tilapia Fillets Throughout the Drying Process. Foods. 2025; 14(19):3293. https://doi.org/10.3390/foods14193293
Chicago/Turabian StyleLi, Jun, Huan Xiang, Shuxian Hao, Lina Wei, Hui Huang, Ya Wei, Shengjun Chen, and Yongqiang Zhao. 2025. "Changes in the Volatile Flavor Compounds and Quality Attributes of Tilapia Fillets Throughout the Drying Process" Foods 14, no. 19: 3293. https://doi.org/10.3390/foods14193293
APA StyleLi, J., Xiang, H., Hao, S., Wei, L., Huang, H., Wei, Y., Chen, S., & Zhao, Y. (2025). Changes in the Volatile Flavor Compounds and Quality Attributes of Tilapia Fillets Throughout the Drying Process. Foods, 14(19), 3293. https://doi.org/10.3390/foods14193293









