Biodegradable Levan/Chitosan Composite Films: Development and Application in Beef Filet Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chitosan Preparation
2.2. Production and Purification of Levan from Bacillus mojavensis
2.3. Edible Film Production
2.4. Film Characterization
2.4.1. Thickness, Moisture Content and Wettability
2.4.2. Water Solubility
2.4.3. Opacity Measurement
2.4.4. Color
2.4.5. Fourier Transform Infrared (FT-IR) Analysis of the Films
2.4.6. Mechanical Properties
2.4.7. Antioxidant Activity of Films
2.4.8. Antibacterial Activity of Films
2.5. Preparation of Beef Filets
2.6. Microbiological Analysis of Beef Filets During Storage
2.7. Biodegradability
2.8. Statistical Study
3. Results
3.1. Levan and Chitosan-Based Films
3.2. Film Characterization
3.2.1. FTIR Analysis
3.2.2. Thickness, Moisture Content, Wettability, Water Solubility, and Optical Properties
3.2.3. Mechanical Properties of the Films
3.2.4. Antioxidant Activity of Films
3.2.5. Antibacterial Properties of Films
3.3. Microbiological Analysis of Beef Filets
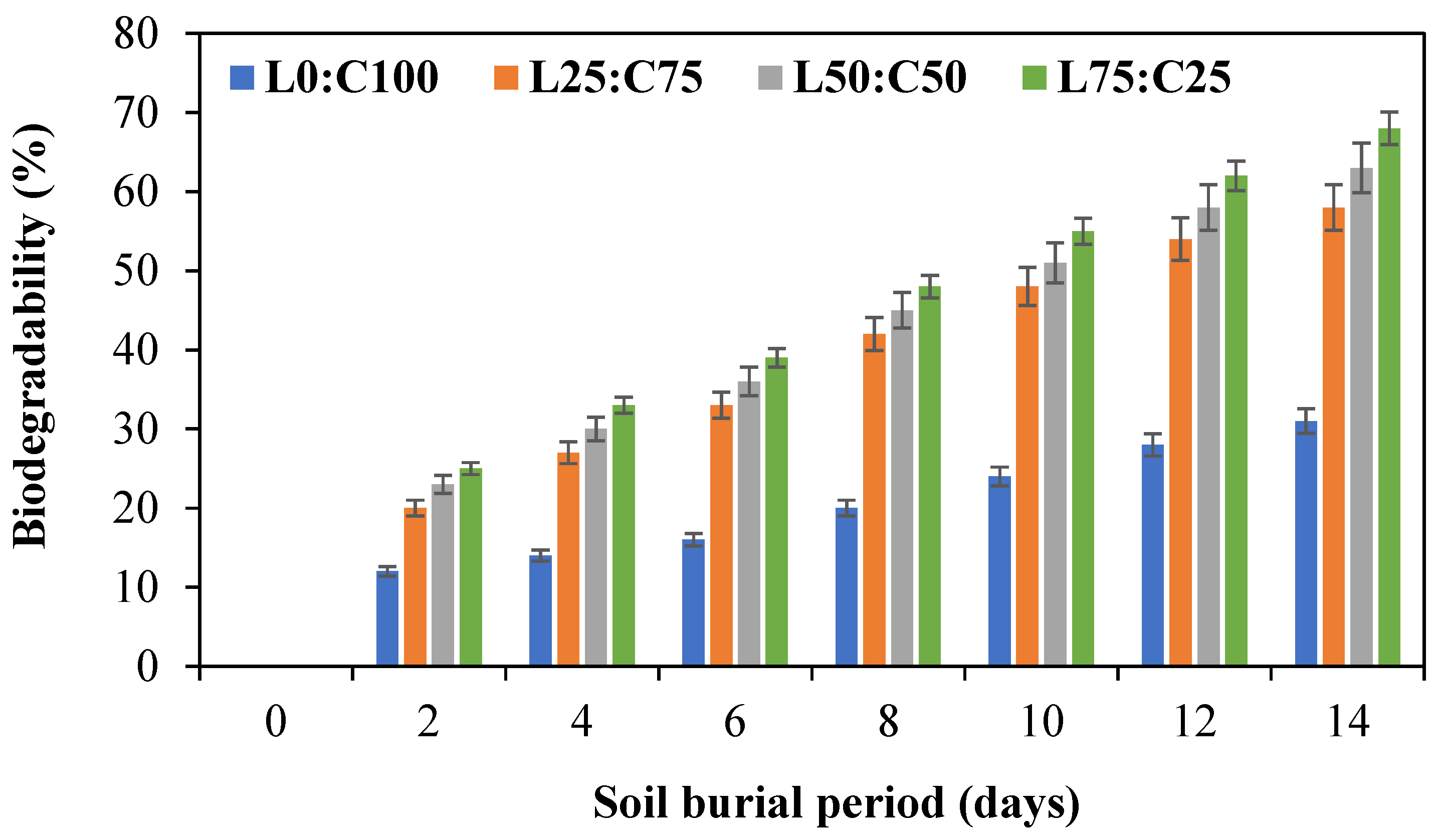
3.4. Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, A. The importance of food packaging. J. Food Sci. 2020, 45, 123–134. [Google Scholar]
- Jones, B. Environmental impact of petroleum-based polymers. Environ. Res. Lett. 2018, 13, 567–579. [Google Scholar]
- Periyasamy, T.; Asrafali, S.P.; Lee, J. Recent Advances in Functional Biopolymer Films with Antimicrobial and Antioxidant Properties for Enhanced Food Packaging. Polymers 2025, 17, 1257. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, J.G.; Rosa, M.; Bahadur, A.N. 3D-Xray-tomography of american lobster shell-structure. An Overview. Fish. Res. 2017, 186, 372–382. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A State-of-the-Art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate yydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.-Y.; Ward, K. Multistage extraction and purification of waste sargassum natans to produce sodium alginate: An optimization approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef]
- Al-Rooqi, M.M.; Masudul Hassan, M.; Moussa, Z.; Obaid, R.J.; Suman, N.H.; Wagner, M.H.; Natto, S.S.A.; Ahmed, S.A. Advancement of chitin and chitosan as promising biomaterials. J. Saudi Chem. Soc. 2022, 22, 101561. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews; Crini, G., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 36, pp. 81–123. [Google Scholar]
- Zhan, Z.; Feng, Y.; Zhao, J.; Qiao, M.; Jin, Q. Valorization of seafood waste for food packaging development. Foods 2024, 13, 2122. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Habib, M.L.; Anwaruzzaman, M.; Kamruzzaman, M.; Khan, M.N.; Rahman, M.M. Processing techniques of chitosan-based interpenetrating polymer networks, gels, blends, composites and nanocomposites. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 61–93. [Google Scholar]
- Mujtaba, M.; Ali, Q.; Yilmaz, B.A.; Kurubas, M.S.; Ustun, H.; Erkan, M.; Kaya, M.; Cicek, M.; Oner, E.T. Understanding the effects of chitosan, chia mucilage, levan based composite coatings on the shelf life of sweet cherry. Food Chem. 2023, 416, 135816. [Google Scholar] [CrossRef] [PubMed]
- Haddar, A.; Hamed, M.; Bouallegue, A.; Bastos, R.; Coelho, E.; Coimbra, M.A. Structural elucidation and interfacial properties of a levan isolated from Bacillus mojavensis. Food Chem. 2021, 343, 128456. [Google Scholar] [CrossRef]
- Erkorkmaz, B.A.; Kırtel, O.; Ates Duru, O.; Toksoy Oner, E. Development of a cost-effective production process for Halomonas levan. Bioprocess Biosyst. Eng. 2018, 41, 1247–1259. [Google Scholar] [CrossRef]
- Haddar, A.; Bouallegue, A.; Methneni, R.; Ellouz-Chaabouni, S. Rheological and thermal properties of levan from Bacillus mojavensis. J. Polym. Environ. 2022, 30, 741–751. [Google Scholar] [CrossRef]
- Srikanth, R.; Reddy, C.H.S.S.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Mantovan, J.; Bersaneti, G.T.; Faria-Tischer, P.C.; Celligoi, M.A.P.C.; Mali, S. Use of microbial levan in edible films based on cassava starch. Food Packag. Shelf Life. 2018, 18, 31–36. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Zhao, F.; Yin, N.; Zhou, Z.; Han, Y. Biosynthesis and structural characterization of levan by a recombinant levansucrase from Bacillus subtilis ZW019. Waste Biomass Valoriz. 2022, 13, 4599–4609. [Google Scholar] [CrossRef]
- Jadaun, J.S.; Narnoliya, L.K.; Agarwal, N.; Singh, S.P. Catalytic biosynthesis of levan and short-chain fructooligosaccharides from sucrosecontaining feedstocks by employing the levansucrase from Leuconostoc mesenteroides MTCC10508. Int. J. Biol. Macromol. 2019, 127, 486–495. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Polysaccharides: Sources, characteristics, properties, and their application in biodegradable films. Polysaccharides 2022, 3, 480–501. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Levan versus fructooligosaccharide synthesis using the levansucrase from Zymomonas mobilis: Effect of reaction conditions. J. Mol. Catal. B Enzym. 2015, 119, 18–25. [Google Scholar] [CrossRef]
- Visnapuu, T.; Mardo, K.; Alamäe, T. Levansucrases of a Pseudomonas syringae pathovar as catalysts for the synthesis of potentially prebiotic oligo- and polysaccharides. New Biotechnol. 2015, 32, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.D.; Caridade, S.G.; Sousa, M.P.; Azevedo, S.; Kandur, M.Y.; Öner, E.T.; Alves, N.M.; Mano, J.F. Adhesive free-standing multilayer films containing sulfated levan for biomedical applications. Acta. Biomater. 2018, 69, 183–195. [Google Scholar] [CrossRef]
- Bostan, M.S.; Mutlu, E.C.; Kazak, H.; Keskin, S.S.; Oner, E.T.; Eroglu, M.S. Comprehensive characterization of chitosan/PEO/levan ternary blend films. Carbohydr. Polym. 2014, 102, 993–1000. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Han, Y. Levan-Chitosan Blend Films: Preparation, Structural, Physical Properties and Application in Pork Packaging. Int. J. Biol. Macromol. 2022, 217, 624–632. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Ploehn, H.J. Montmorillonite-levan nanocomposites with improved thermal and mechanical properties. Carbohydr. Polym. 2014, 101, 565–573. [Google Scholar] [CrossRef]
- Dos Santos, L.F.; Pineda, E.A.G.; Celligoi, M.A.P.C.; Cavalcanti, O.A. Levan as a new additive for colon-specific films: A new approach in the use of exopolysaccharides in time-dependent free films (aminoalkyl methacrylate copolymer RS. Pak. J. Pharm. Sci. 2013, 26, 943–948. [Google Scholar]
- Chelminiak-Dudkiewicz, D.; Rybczynski, P.; Smolarkiewicz-Wyczachowski, A.; Mlynarczyk, D.T.; Wegrzynowska-Drzymalska, K.; Ilnicka, A.; Goslinski, T.; Marszałł, M.P.; Ziegler-Borowska, M. Photosensitizing potential of tailored magnetite hybrid nanoparticles functionalized with levan and zinc (II) phthalocyanine. Appl. Surf. Sci. 2020, 524, 146602. [Google Scholar] [CrossRef]
- Ağçeli, G.K.; Hammamchi, H.; Cihangir, N. Novel levan/bentonite/essential oil films: Characterization and antimicrobial activity. J. Food Sci. Technol. 2021, 59, 249–256. [Google Scholar] [CrossRef]
- Ağçeli, G.K. Development of ostrich eggshell and nano-levan-based edible biopolymer composite films: Characterization and bioactivity. Polym. Bull. 2022, 79, 11201–11215. [Google Scholar] [CrossRef]
- Ağçeli, G.K. A new approach to nanocomposite carbohydrate polymer films: Levan and chia seed mucilage. Int. J. Biol. Macromol. 2022, 218, 751–759. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, E.C.; Rebouças, J.d.S.; Pinheiro, I.O.; Formiga, F.R. Levan-Based Nanostructured Systems: An Overview. Int. J. Pharm. 2020, 580, 119242. [Google Scholar] [CrossRef] [PubMed]
- Haddar, A.; Ben Ayed, E.; Sila, A.; Putaux, J.-L.; Bougatef, A.; Boufi, S. Hybrid levan-Ag/AgCl nanoparticles produced by UV-irradiation: Properties, antibacterial efficiency and application in bioactive poly(vinyl alcohol) films. RSC Adv. 2021, 11, 38990–39003. [Google Scholar] [CrossRef]
- Bertuzzi, M.; Vidaurre, E.C.; Armada, M.; Gottifredi, J. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Vanin, F.; Sobral, P.; Menegalli, F.; Carvalho, R.; Habitante, A. Effects of plasticizers and their concentrations on thermal and functional properties of gelatin-based films. Food Hydrocol. 2005, 19, 899–907. [Google Scholar] [CrossRef]
- Foroughi-Dahr, M.; Mostoufi, N.; Sotudeh-Gharebagh, R.; Chaouki, J. Particle coating in fluidized beds. Ref. Modul. Chem. Mol. Sci. Chem. Eng. 2017, 1–89. [Google Scholar]
- Fedorko, P.; Djurado, D.; Trznadel, M.; Dufour, B.; Rannou, P.; Travers, J.P. Insulator-Metal transition in Polyaniline induced by plasticizers. Synth. Met. 2003, 135, 327–328. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizersand research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic plasticizer-based films: Preparation, physicochemical and antimicrobial properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Santacruz, S.; Rivadeneira, C.; Castro, M. Edible films based on starch and chitosan. Effect of starch source and concentration, plasticizer, surfactant’s hydrophobic tail and mechanical treatment. Food Hydrocol. 2015, 49, 89–94. [Google Scholar] [CrossRef]
- Patel, A.K.; Michaud, P.; de Baynast, H.; Grédiac, M.; Mathias, J.-D. Preparation of chitosan-based adhesives and assessment of their mechanical properties. J. Appl. Polym. Sci. 2013, 127, 3869–3876. [Google Scholar] [CrossRef]
- Razali, M.H.; Ismail, N.A.; Osman, U.M.; Amin, K.A.M. Mechanical and physical properties of gellan gum (GG) biofilm: Effect of glycerol. ASM Sci. J. 2018, 1, 158–165. [Google Scholar]
- Sila, A.; Mlaik, N.; Sayari, N.; Balti, R.; Bougatef, A. Chitin and chitosan extracted from shrimp waste using fish proteases aided process: Efficiency of chitosan in the treatment of unhairing effluents. J. Polym. Environ. 2013, 22, 78–87. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Zhan, X.; Xie, W.; Yang, X.; Cui, S.W.; Xia, W. Development and properties of new kojic acid and chitosan composite biodegradable films for active packaging materials. Int. J. Biol. Macromol. 2020, 144, 483–490. [Google Scholar] [CrossRef]
- ISO 527-3:2018; Plastics — Determination of Tensile Properties — Part 3: Test Conditions for Films and Sheets. ISO: Geneva, Switzerland, 2018.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Alqahtani, N.; Alnemr, T.; Ali, S. Development of low-cost biodegradable films from corn starch and date palm pits (Phoenix dactylifera). Food Biosci. 2021, 42, 101199. [Google Scholar] [CrossRef]
- González-Torres, M.; Hernández-Rosas, F.; Pacheco, N.; Salinas-Ruiz, J.; Herrera-Corredor, J.A.; Hernández-Martínez, R. Levan Production by Suhomyces kilbournensis Using Sugarcane Molasses as a Carbon Source in Submerged Fermentation. Molecules 2024, 29, 1105. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocol. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Esti, M. Papain covalently immobilized on chitosan–clay nanocomposite films: Application in synthetic and real white wine. Nanomaterials 2020, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Lombardelli, C.; Benucci, I.; Esti, M. Clay/chitosan biocomposite systems as novel green carriers for covalent immobilization of food enzymes. J. Mater. Res Technol. 2019, 8, 3644–3652. [Google Scholar] [CrossRef]
- Agarwal, N.; Jyoti, J.; Thakur, M.; Mishra, B.B. Preparation and characterization of biodegradable films based on levan polysaccharide blended with gellan gum. Environ. Technol. Innov. 2023, 31, 103231. [Google Scholar] [CrossRef]
- Jamroz, E.; Janik, M.; Juszczak, L.; Kruk, T.; Kulawik, P.; Szuwarzynski, M.; Kawecka, A.; Khachatryan, K. Composite biopolymer films based on a polyelectrolyte complex of furcellaran and chitosan. Carbohydr. Polym. 2021, 274, 118627. [Google Scholar] [CrossRef]
- Ejaz, S.; Ihsan, A.; Noor, T.; Shabbir, S.; Imran, M. Mannose functionalized chitosan nanosystems for enhanced antimicrobial activity against multidrug resistant pathogens. Polym. Test. 2020, 91, 106814. [Google Scholar] [CrossRef]
- Xu, W.; Peng, J.; Ni, D.; Zhang, W.; Wu, H.; Mu, W. Preparation, characterization and application of levan/montmorillonite biocomposite and levan/BSA nanoparticle. Carbohydr. Polym. 2020, 234, 115921. [Google Scholar] [CrossRef]
- Santos, M.I.; Araujo-Andrade, C.; Tymczyszyn, E.E.; Gómez-Zavaglia, A. Determination of amorphous/rubbery states in freeze-dried prebiotic sugars using a combined approach of near-infrared spectroscopy and multivariate analysis. Food Res. Inter. 2014, 64, 514–519. [Google Scholar] [CrossRef]
- Romano, N.; Santos, M.; Mobili, P.; Vega, R.; Gómez-Zavaglia, A. Effect of sucrose concentration on the composition of enzymatically synthesized short-chain fructo-oligosaccharides as determined by FTIR and multivariate analysis. Food Chem. 2016, 202, 467–475. [Google Scholar] [CrossRef]
- Barone, J.R.; Medynets, M. Thermally processed levan polymers. Carbohydr. Polym. 2007, 69, 554–561. [Google Scholar] [CrossRef]
- Bersaneti, G.T.; Mantovan, J.; Magri, A.; Mali, S.; Celligoi, M.A.P.C. Edible films based on cassava starch and fructooligosaccharides produced by Bacillus subtilis natto CCT 7712. Carbohydr. Polym. 2016, 151, 1132–1138. [Google Scholar] [CrossRef]
- Romero-Bastida, C.A.; Bello-Pérez, L.A.; García, M.A.; Martino, M.N.; Solorza-Feria, J.; Zaritzky, N.E. Physicochemical and microstructural characterization of films prepared by thermal and cold gelatinization from non-conventional sources of starches. Carbohydr. Polym. 2005, 60, 235–244. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, Q.; Yu, L.; Xie, F. Thermomechanically processed chitosan:gelatin films being transparent, mechanically robust and less hygroscopic. Carbohydr. Polym. 2021, 272, 118522. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Garcia-Serna, E.; Martínez-Abad, A.; Vilaplana, F.; Jimenez, A.; Garrigós, M.C. Gelatin-based antimicrobial films incorporating pomegranate (Punica granatum L.) seed juice by-product. Molecules 2020, 25, 166. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kopel, P. Polysaccharide and protein films with antimicrobial/antioxidant activity in the food industry: A Review. Polymers 2020, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Palupi, N.W.; Maryanto, M.; Purwowibowo, E. Relationship antioxidant concentration and volume headspace on the rancidity of fish oil during storage. J. Teknol. 2016, 78, 159–165. [Google Scholar]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Inter. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Jim’enez, A.; Cacciotti, I.; Garrig’os, M.C. Encapsulation of bioactive compounds from aloe vera agrowastes in electrospun poly (ethylene oxide) nanofibers. Polymers 2020, 12, 1323. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Jridi, M.; Hajji, S.; Ben Ayed, H.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. Inter. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Hajji, S.; Kchaou, H.; Bkhairia, I.; Ben Slama-Ben Salem, R.; Boufi, S.; Debeaufort, F.; Nasri, M. Conception of active food packaging films based on crab chitosan and gelatin enriched with crustacean protein hydrolysates with improved functional and biological properties. Food Hydrocol. 2021, 116, 106639. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L.; Gong, X.; Chi, F.; Ma, Z. Fabrication and comparison of active films from chitosan incorporating different spice extracts for shelf life extension of refrigerated pork. LWT Food Sci. Technol. 2021, 135, 110181. [Google Scholar] [CrossRef]
- Tsai, G.J.; Su, W.H.; Chen, H.C.; Pan, C.L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Park, P.J.; Kim, S.K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Inter. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan-polyvinyl alcohol films with natural extracts. Food Hydrocol. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Möller, H.; Grelier, S.; Pardon, P.; Coma, V. Antimicrobial and physicochemical properties of chitosan–HMPC based films. J. Agr. Food Chem. 2004, 52, 6585–6591. [Google Scholar] [CrossRef]
- Honary, S.; Ghajar, K.; Khazaeli, P.; Shalchian, P. Preparation, characterization and antibacterial properties of silver-chitosan nanocomposites using different molecular weight grades of chitosan. Trop. J. Pharm. Res. 2011, 10, 69–74. [Google Scholar] [CrossRef]
- Hadwiger, L.A.; Kendra, D.F.; Fristensky, B.W.; Wagoner, W. Chitosan both activates genes in plants and inhibits RNA synthesis in fungi. In Chitin in Nature and Technology; Muzzarelli, R., Jeuniaux, C., Gooday, G.W., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 209–214. [Google Scholar]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Cuero, R.G.; Osuji, G.; Washington, A.N.C. N-Carboxymethylchitosan inhibition of aflatoxin production: Role of zinc. Biotechnol. Lett. 1991, 13, 441–444. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of Chitosan for Improvement of Quality and Shelf Life of Foods: A Review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin-chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, G.; Yang, Y.; Zheng, B.; Zhang, S.; Li, X.; Tian, Y.; Peng, B. Development and characterization of Levan/Pullulan/Chitosan edible films enriched with ε-Polylysine for active food packaging. Food Chem. 2022, 388, 132989. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pirouzi, S.; Yaghoubi, M.; Maryam Karimi-Dehkordi, M.; Shima Jafarzadeh, S.; Mousavi Khaneghah, A. Packaging of beef fillet with active chitosan film incorporated with ε-polylysine: An assessment of quality indices and shelf life. Meat Sci. 2021, 176, 108475. [Google Scholar] [CrossRef]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Liu, D.; Fu, M.; Yang, X.; Guo, Y. The preservative effects of chitosan film incorporated with thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) fillets during cold storage: Correlation between the preservative effects and the active properties of the. Food Packag. Shelf Life 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Domżał-Kędzia, M.; Ostrowska, M.; Lewińska, A.; Łukaszewicz, M. Recent Developments and Applications of Microbial Levan, A Versatile Polysaccharide-Based Biopolymer. Molecules 2023, 28, 5407. [Google Scholar] [CrossRef]
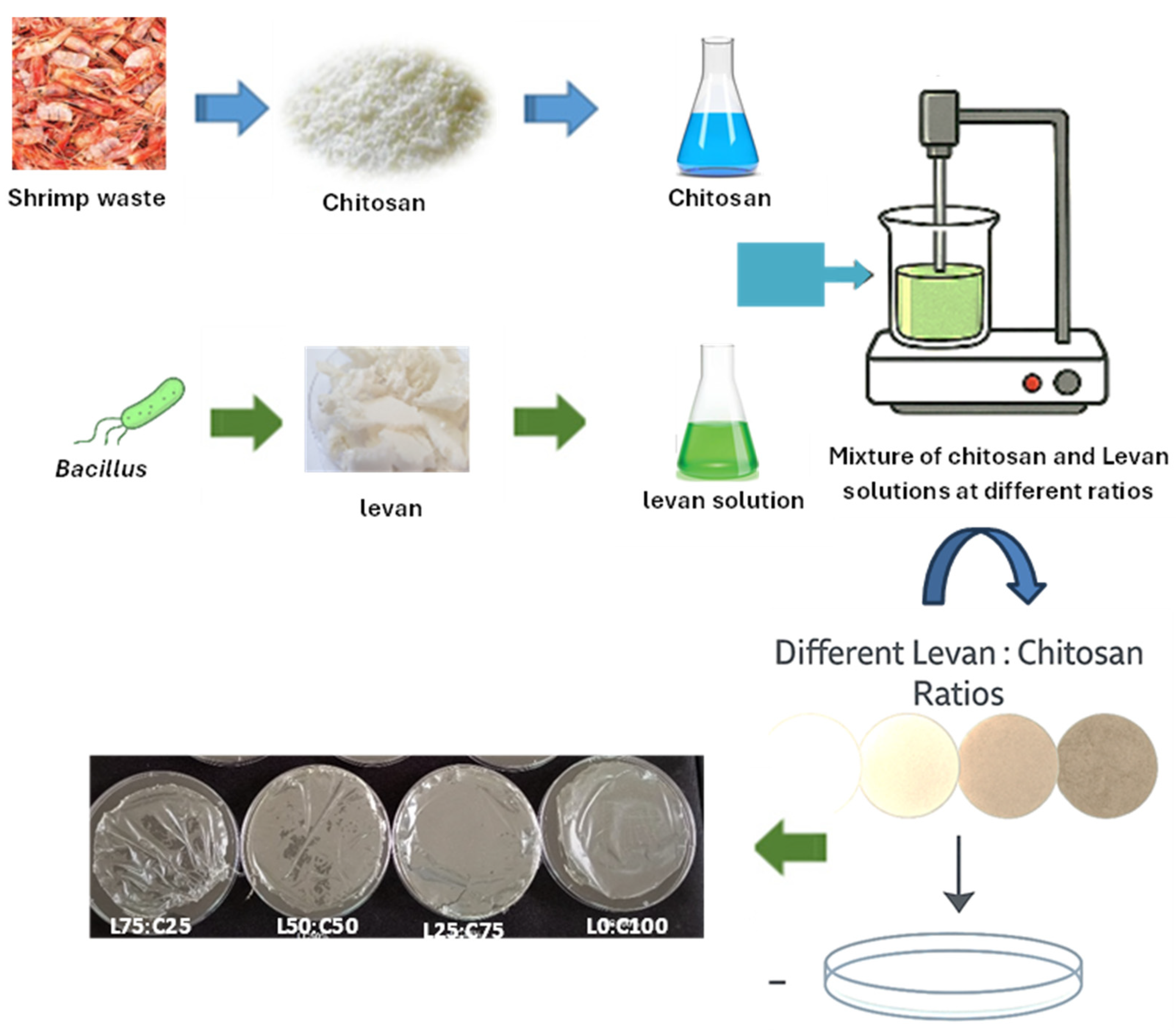
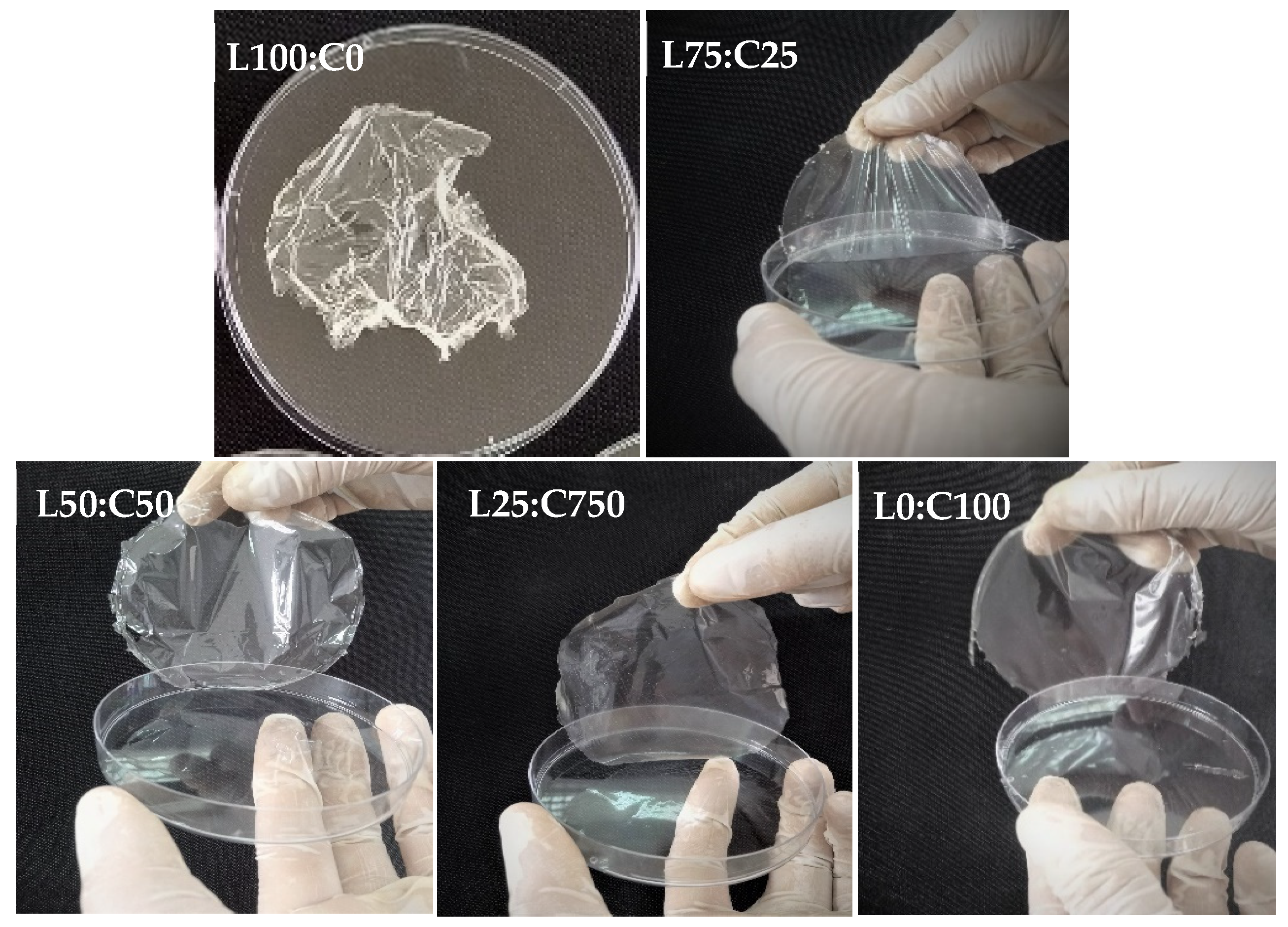
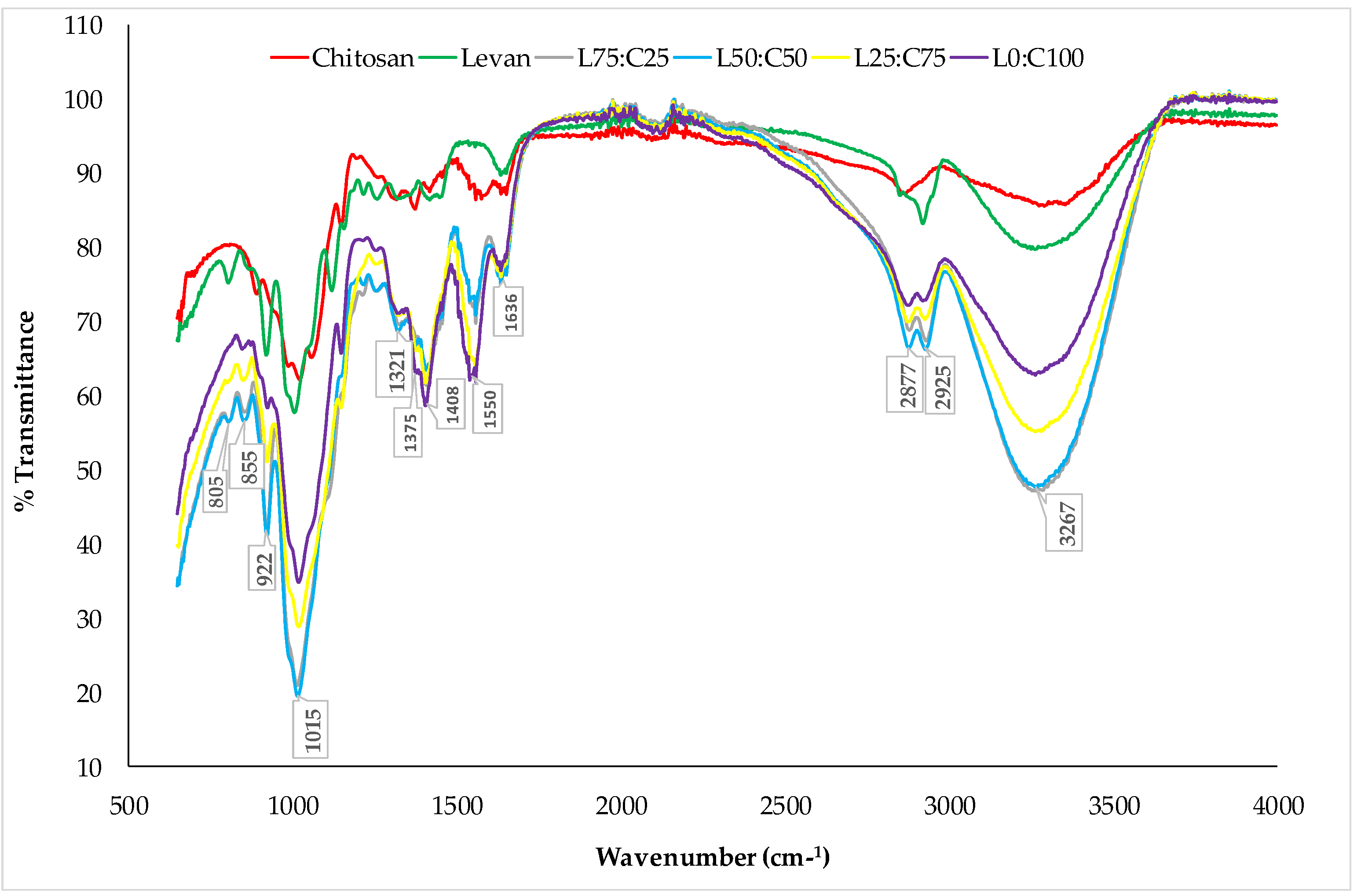
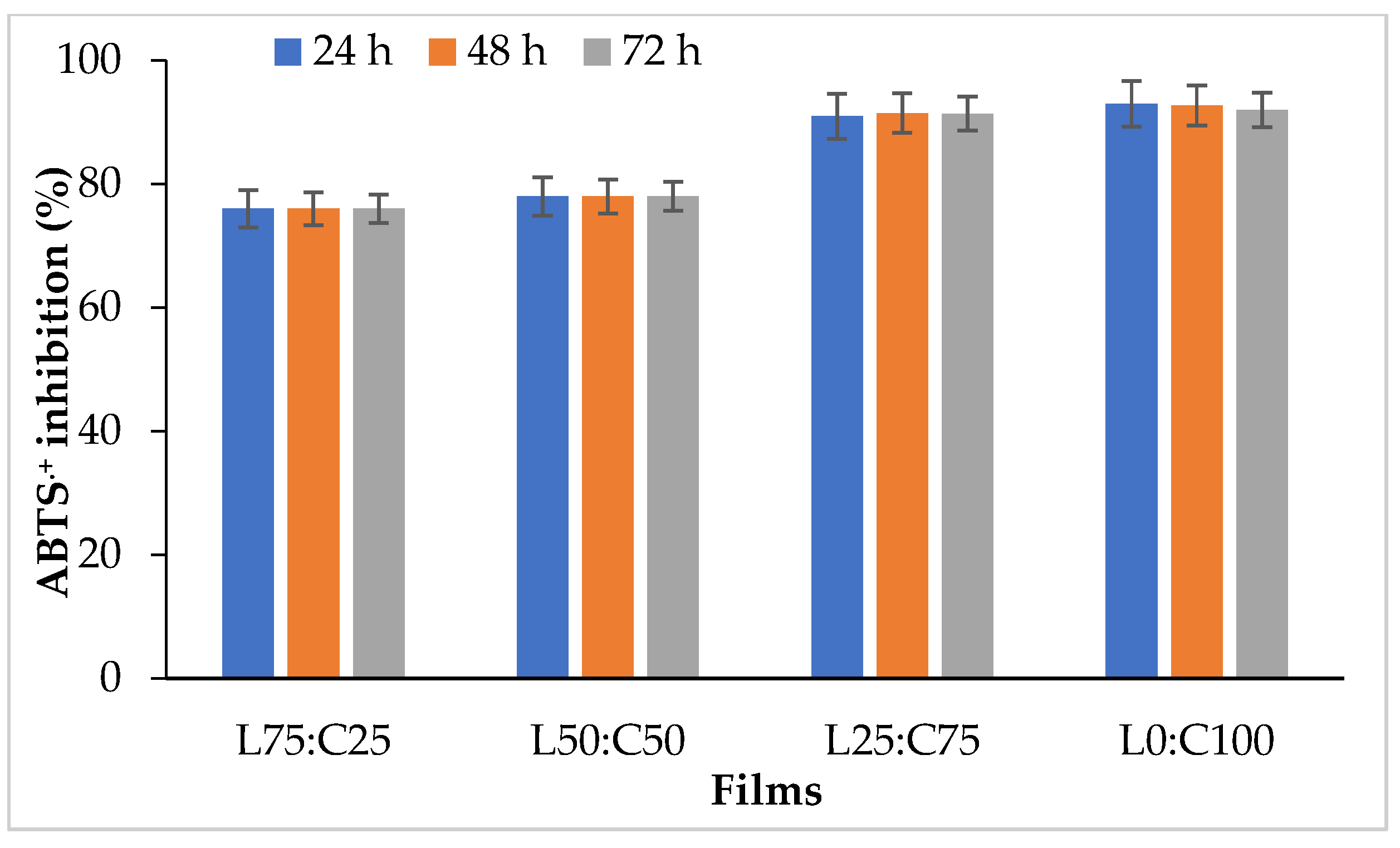
| S. No. | Levan (g) | Chitosan (g) | Glycerol (g) | Water (g) | Film Properties |
|---|---|---|---|---|---|
| 1 (L100:C0) | 3 | 0 | 0.9 | 96.1 | The film could not be removed from the Petri dish. |
| 2 (L75:C25) | 2.25 | 0.75 | 0.9 | 96.1 | Quite flexible and not easily lifted from the Petri dish. |
| 3 (L50:C50) | 1.5 | 1.5 | 0.9 | 96.1 | Easily handled, flexible, transparent without bubbles or cracks. |
| 4 (L25:C75) | 0.75 | 2.25 | 0.9 | 96.1 | Easily handled, flexible, transparent uniformly smooth, and clear. |
| 5 (L0:C100) | 0 | 3 | 0.9 | 96.1 | Easily lifted from the Petri dish and flexible. |
| Film Sample | Thickness (μm) | Moisture Content (%) | Water Contact Angle (°) | Opacity (%) | Solubility (%) | Color Parameters | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| L75:C25 | 131 ± 0.09 | 25.53 ± 0.35 | 62.5 ± 0.6 * | 19.2 ± 2 * | 73.36 ± 0.25 * | 86.33 ± 0.21 | 2.03 ± 0.03 | 10.70 ± 0.05 |
| L50:C50 | 132 ± 0.05 | 24.95 ± 0.24 | 63.9 ± 1.2 * | 18.7 ± 1.5 * | 55.03 ± 0.09 * | 88.54 ± 0.15 | 1.54 ± 0.01 | 11.21 ± 0.01 |
| L25:C75 | 133 ± 0.04 | 23.05 ± 0.36 *¥ | 65.3 ± 0.4 *¥ | 18.25 ± 1.5 *¥ | 46.64 ± 0.37 * | 89.45 ± 0.12 * | 0.87 ± 0.02 * | 12.26 ± 0.10 * |
| L0:C100 | 133 ± 0.04 | 21.87 ± 0.19 | 67.7 ± 0.2 | 17.5 ± 1.25 | 40.94 ± 0.24 | 90.35 ± 0.25 | 0.38 ± 0.05 | 14.27 ± 0.05 |
| Film Sample | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| L75:C25 | 0.41 ± 0.06 * | 99.97 ± 1.02 * | 0.34 ± 0.04 * |
| L50:C50 | 2.57 ± 0.06 * | 54.03 ± 3.36 * | 6.00 ± 0.05 * |
| L25:C75 | 15.43 ± 0.04 * | 35.37 ± 1.12 *¥ | 87.85 ± 0.02 *¥ |
| L0:C100 | 17.01 ± 0.03 | 40.53 ± 2.60 | 114.03 ± 0.03 |
| Indicator Organism | Inhibition (mm) | ||||
|---|---|---|---|---|---|
| L75:C25 | L50:C50 | L25:C75 | L0:C100 | ||
| Gram − | E. coli | 15 ± 0.30 | 17 ± 0.20 * | 18 ± 0.30 *¥ | 18 ± 0.20 |
| K. pneumoniea | 13 ± 0.25 | 15 ± 0.20 | 16 ± 0.40 * | 16 ± 0.25 | |
| P. aeruginosa | 16 ± 0.30 * | 18 ± 0.15 * | 19 ± 0.25 *¥ | 19 ± 0.15 | |
| S. enterica | 14 ± 0.20 | 15 ± 0.25 * | 15 ± 0.30 | 16 ± 0.20 | |
| Gram + | S. aureus | 12 ± 0.25 | 12 ± 0.30 | 14 ± 0.20 | 14 ± 0.20 |
| B. subtilis | 13 ± 0.25 | 14 ± 0.20 | 16 ± 0.20 * | 16 ± 0.30 | |
| M luteus | 10 ± 0.3 | 10 ± 0.15 | 12 ± 0.25 *¥ | 13 ± 0.30 | |
| E. faecalis | 11 ± 0.25 | 13 ± 0.40 * | 13 ± 0.30 *¥ | 14 ± 0.30 | |
| L. monocytogenes | 10 ± 0.40 | 10 ± 0.15 * | 11 ± 0.25 * | 11 ± 0.35 | |
| Microbes | Packaging Films | Storage Time (Day) | |||
|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | ||
| Total viable counts (log UFC g−1) | LDPE films | 3.25 ± 0.25 | 3.89 ± 0.20 | 4.75 ± 0.15 | 6.30 ± 0.30 |
| L25:C75 films | 3.20 ± 0.15 | 3.50 ± 0.15 | 3.85 ± 0.10 | 4.50 ± 0.25 * | |
| Psychrophilic bacteria (log UFC g−1) | LDPE films | 2.50 ± 0.10 | 3.20 ± 0.25 | 4.15 ± 0.25 | 5.64 ± 0.15 |
| L25:C75 films | 2.35 ± 0.10 * | 2.75 ± 0.20 | 3.10 ± 0.20 | 3.95 ± 0.30 * | |
| Total coliform (log UFC g−1) | LDPE films | 1.95 ± 0.15 | 2.35 ± 0.20 | 3.10 ± 0.15 | 3.55 ± 010 |
| L25:C75 films | 1.75 ± 0.10 | 2.00 ± 0.25 | 2.17 ± 0.15 | 2.90 ± 0.10 * | |
| Fecal coliform (log UFC g−1) | LDPE films | 1.55 ± 0.20 | 2.35 ± 0.25 | 2.95± 0.15 | 3.20 ± 0.25 |
| L25:C75 films | 1.36 ± 0.25 | 1.75 ± 0.20 | 2.10 ± 0.20 | 2.28 ± 0.25 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddar, A.; Sellami, E.; Bouazizi, O.; Sila, A.; Bougatef, A. Biodegradable Levan/Chitosan Composite Films: Development and Application in Beef Filet Packaging. Foods 2025, 14, 2133. https://doi.org/10.3390/foods14122133
Haddar A, Sellami E, Bouazizi O, Sila A, Bougatef A. Biodegradable Levan/Chitosan Composite Films: Development and Application in Beef Filet Packaging. Foods. 2025; 14(12):2133. https://doi.org/10.3390/foods14122133
Chicago/Turabian StyleHaddar, Anissa, Emna Sellami, Oumayma Bouazizi, Assaad Sila, and Ali Bougatef. 2025. "Biodegradable Levan/Chitosan Composite Films: Development and Application in Beef Filet Packaging" Foods 14, no. 12: 2133. https://doi.org/10.3390/foods14122133
APA StyleHaddar, A., Sellami, E., Bouazizi, O., Sila, A., & Bougatef, A. (2025). Biodegradable Levan/Chitosan Composite Films: Development and Application in Beef Filet Packaging. Foods, 14(12), 2133. https://doi.org/10.3390/foods14122133







