Selective Inactivation Strategies for Vegetable Raw Materials: Regulating Microbial Communities to Ensure the Safety and Quality of Fermented Vegetables
Abstract
1. Introduction
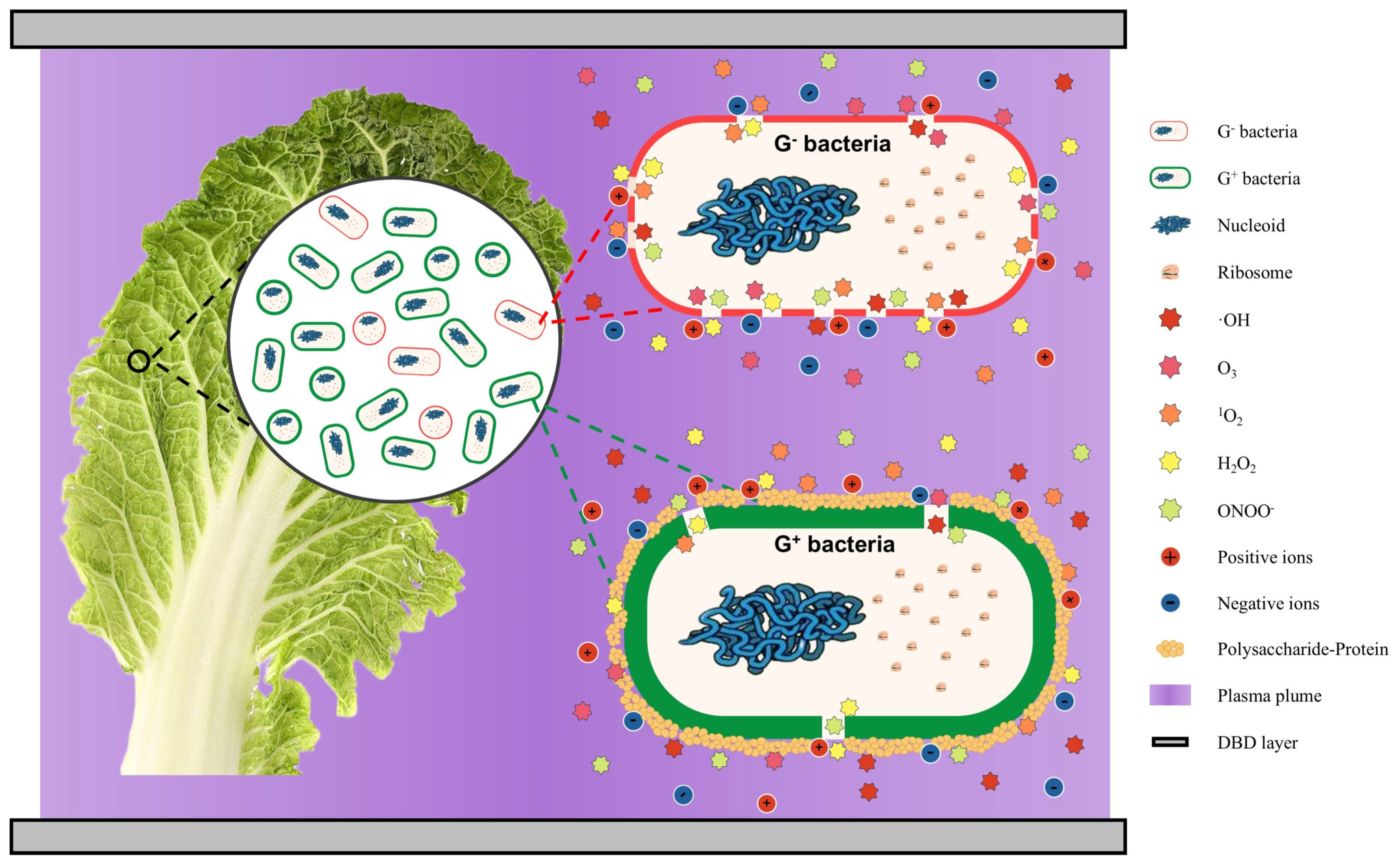
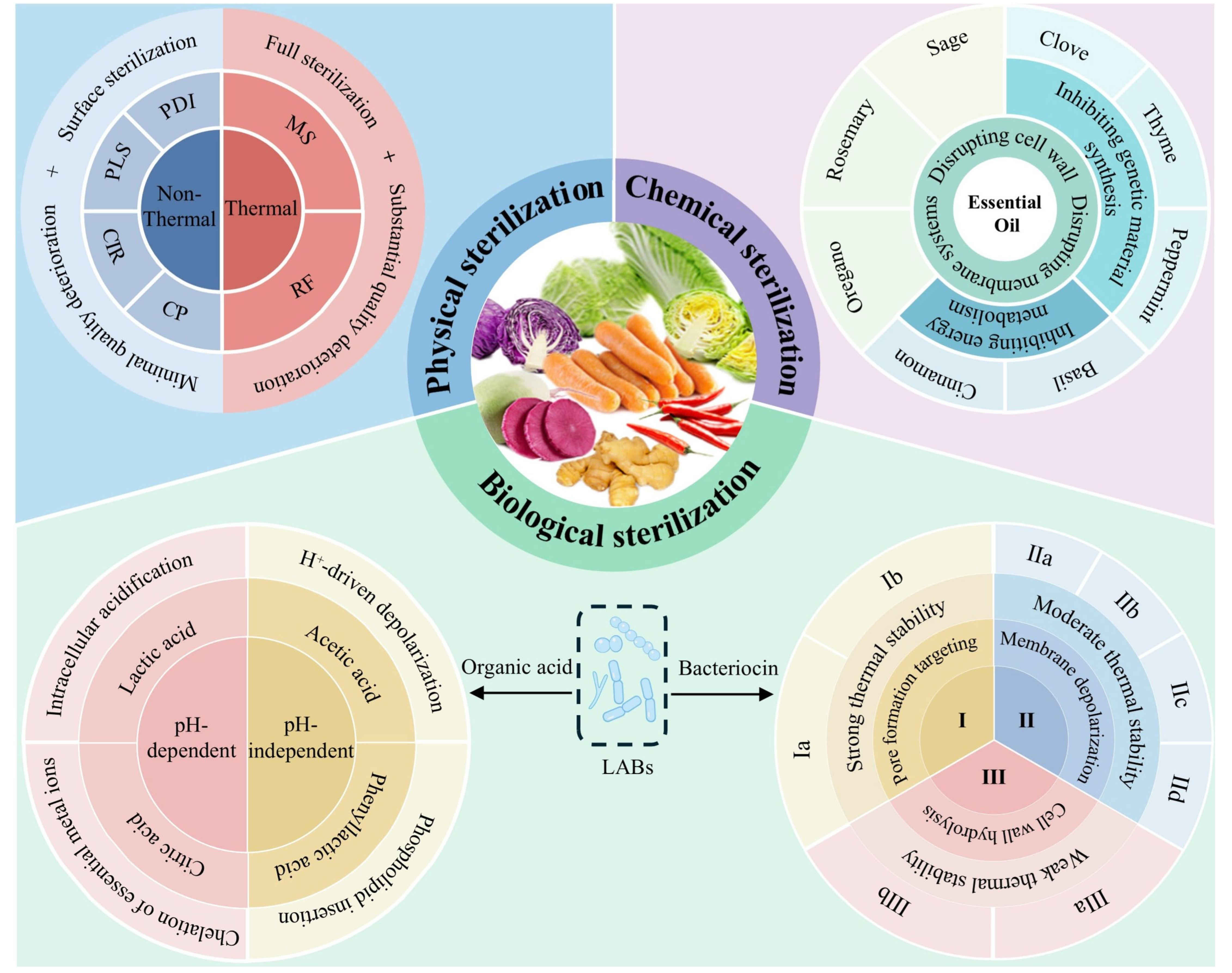
2. Cold Plasma
2.1. Cold Plasma Inactivation on Vegetable Ingredient Surfaces
2.2. Cold Plasma-Activated Water/Ice for Regulating Surface Microorganisms of Fermented Vegetable Ingredients
2.3. In-Package Cold Plasma Technology for Inhibiting Secondary Contamination of Fermented Vegetables
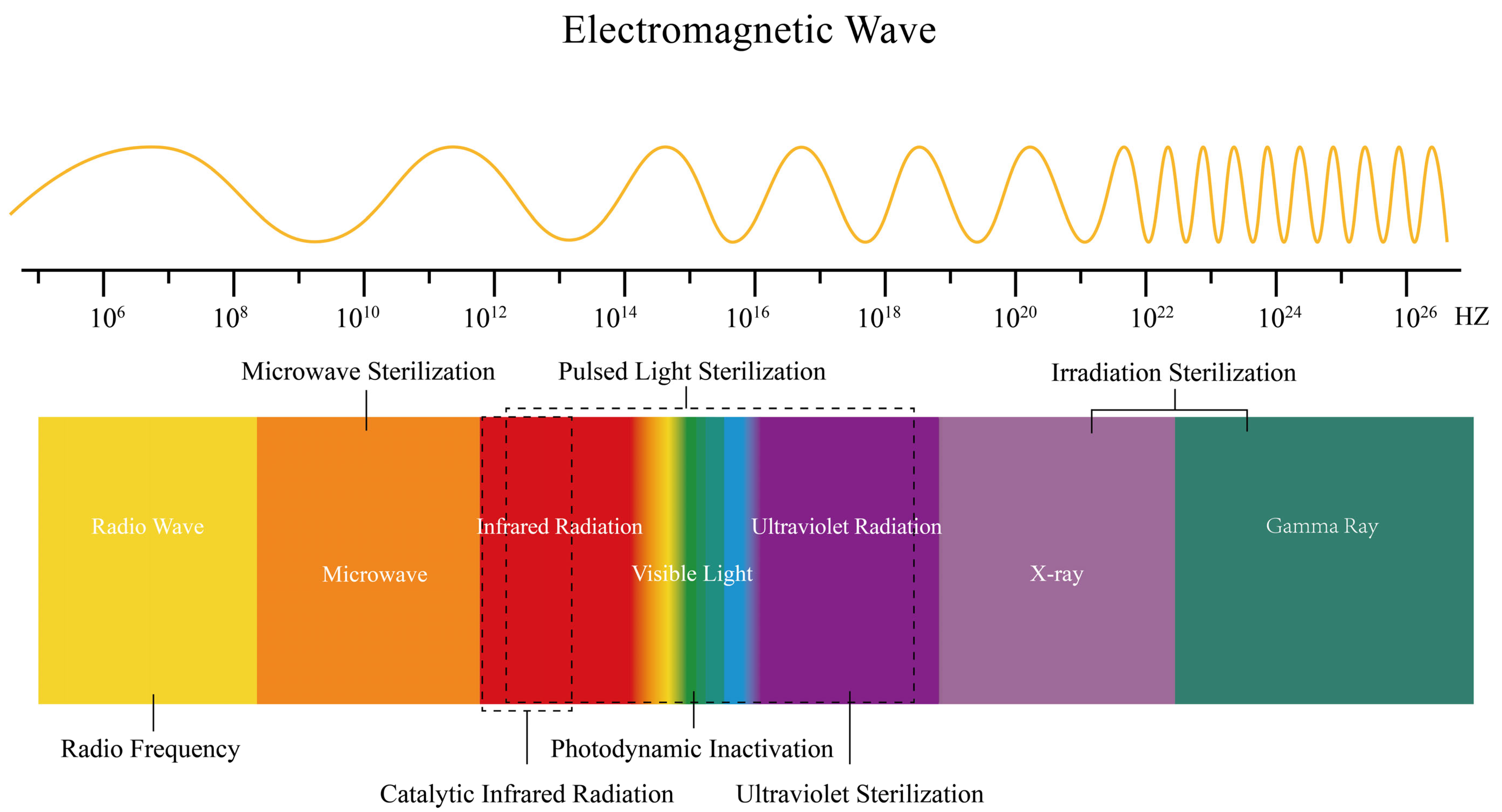
3. Electromagnetic Wave-Based Inactivation
3.1. Photodynamic Inactivation
3.2. Pulsed Light
3.3. Catalytic Infrared Radiation
3.4. Microwave
3.5. Radio Frequency
4. Natural Essential Oil
| Name | Major Active Components | Mode of Action | Applications and Antibacterial Concentration | References |
|---|---|---|---|---|
| Clove Essential Oil | Eugenol, Acetyl Eugenol | Induces cytoplasmic aggregation; Disrupts cell wall and membrane systems; alters DNA structure | Cucumber, 2.5 mg/mL; Tomato paste 500 ppm; | [74,75] |
| Thyme Essential Oil | Thymol, p-Cymene, γ-Terpinene | Disrupts cell wall and membrane systems; Interferes with genetic material synthesis and metabolism | Iceberg Lettuce, Spinach 0.2% (v/v); | [76,77] |
| Cinnamon Essential Oil | Trans-Cinnamaldehyde | Disrupts cell wall and membrane systems; Inhibits cellular energy metabolism | Lettuce, 5 μL/mL; Cucumber 0.1% (v/v); | [78,79] |
| Peppermint Essential Oil | Menthol, Menthone | Disrupts cell wall and membrane systems; Interferes with genetic material synthesis and metabolism | Corn Kernels, Peanut Kernels, 0.343 μL/mL; | [80] |
| Sage Essential Oil | Camphor, β-Caryophyllene, 1,8-Cineole | Disrupts cell wall and membrane systems | Potato, 0.22–0.75 μg/mL; | [81,82] |
| Rosemary Essential Oil | α-Pinene, 1,8-Cineole, Camphor | Disrupts cell wall and membrane systems | Spinach, Red Cabbage, Broccoli, 0.48 mg/mL; | [83,84] |
| Basil Essential Oil | Eugenol, Caryophyllene | Disrupts cell wall and membrane systems; inhibits cellular energy metabolism | Kohlrabi, 500 ppm; | [85,86] |
| Oregano Essential Oil | Carvacrol, Thymol | Disrupts cell wall and membrane systems | Lettuce, Beetroot, 0.6 μL/mL; | [87,88] |
5. LAB Metabolite
5.1. Organic Acids: Synergistic Antibacterial Effects of Constructing an Acidic Barrier
5.2. Bacteriocins: Targeting Membrane Structures and Intracellular Metabolism
- (1)
- Membrane integrity and permeability disruption: Most bacteriocins disrupt bacterial membrane integrity and permeability, forming transmembrane pores. In bacteria, the cell wall consists of a layer of peptidoglycan formed by negatively charged teichoic acids and N-acetylmuramic acid. Lipopolysaccharides, a vital component of the Gram-negative bacterial outer membrane, also carry a negative charge. LAB bacteriocins are generally cationic peptides with strong hydrophobicity and high isoelectric points, allowing them to bind to bacterial cell membranes. Studies have shown that the effectiveness of bacteriocins in targeting bacterial cell membranes and exerting antimicrobial activity is strongly influenced by their positive charge and interaction with cell membranes [114]. The bacteriocin Nisin, produced by Lactococcus lactis, is applied in dairy and canned foods at nanomolar concentrations to inhibit spoilage and pathogenic Gram-positive bacteria such as Listeria monocytogenes, Staphylococcus aureus, and Clostridium botulinum [115]. One of the antimicrobial mechanisms of Nisin is its ability to target the bacterial cell membrane, forming pores that lead to the leakage of intracellular components such as K+ and ATP, disrupting the membrane potential and ultimately impeding cell growth, thereby inhibiting Staphylococcus Koch [116]. The second antimicrobial mechanism of Nisin involves binding to the pyrophosphate group of Lipid II (a crucial precursor for peptidoglycan synthesis in bacterial cell walls), preventing cell wall synthesis and forming transmembrane pores, resulting in cell death [117].
- (2)
- Intracellular targeting: The intracellular action of bacteriocins refers to their interference with normal cellular metabolic activities by binding to cell membrane surface receptors or accumulating within bacterial cells. This can be classified into several types. First, LAB bacteriocins can interfere with bacterial cell growth by inhibiting nucleotide and protein synthesis. Second, they can inhibit bacterial cell wall biosynthesis, such as by disrupting the production and degradation of precursor substances such as Lipid II, thereby obstructing bacterial proliferation and metabolism. Moreover, some LAB bacteriocins, such as RNA polymerase or DNA gyrase, can affect key enzyme activities within bacterial cells [118]. Additionally, certain LAB-derived bacteriocins can inhibit bacterial spore germination and proliferation through nonmembrane-targeting mechanisms. For example, they can nonspecifically degrade bacterial DNA or specifically cleave 16S rRNA, blocking the biosynthesis of related functional proteins [119]. In summary, the antibacterially active substance bacteriocin produced by LAB causes bacterial lysis and death by acting on cell membranes and intracellular substances, effectively inhibiting and killing pathogens and damaging bacteria during fermentation of fermented vegetables while preserving the LAB community, which plays a decisive role in quality and flavor formation.
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunawardena, S.; Nadeeshani, H.; Amarasinghe, V.; Liyanage, R. Bioactive properties and therapeutic aspects of fermented vegetables: A review. Food Prod. Process. Nutr. 2024, 6, 31. [Google Scholar] [CrossRef]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Liu, L.; Li, G.; Cui, L.; Cai, R.; Yuan, Y.; Gao, Z.; Yue, T.; Wang, Z. The health benefits of fermented fruits and vegetables and their underlying mechanisms. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70072. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Che, Z.; Li, H.; Liu, L. Influence of oxygen exposure on fermentation process and sensory qualities of Sichuan pickle (paocai). RSC Adv. 2019, 9, 38520–38530. [Google Scholar] [CrossRef]
- Hu, X.; Gao, F.; Cheng, M.; Zhang, M.; Yang, B.; Wei, W.; Zhu, L.; Xiao, X. Genotype and phenotype-based elucidation of the dominant microbial community and metabolic characteristics responsible for nitrite hazards in fermented vegetable. LWT 2025, 226, 117962. [Google Scholar] [CrossRef]
- Hu, X.; Wei, W.; Zhang, J.; Fan, S.; He, Y.; Bai, J.; Zhu, Y.; Zhao, Y.; Zhu, L.; Xiao, X. Nitrite self-degradation process in radish paocai under the synergistic regulation of prokaryotic microorganisms. Food Biosci. 2024, 57, 103612. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, Y.; Li, T.; Yang, Y.; Zeng, F.; Wang, H.; Suo, H.; Song, J.; Zhang, Y. Microbial composition and correlation between microbiota and quality-related physiochemical characteristics in chongqing radish paocai. Food Chem. 2022, 369, 130897. [Google Scholar] [CrossRef]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Chen, X.; Guo, S.; Xiang, W.; Liu, L.; Du, H.; et al. Characterization of the microbial communities and their correlations with chemical profiles in assorted vegetable Sichuan pickles. Food Control 2020, 113, 107174. [Google Scholar] [CrossRef]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Y.; Li, Y.; Hou, J.; Liang, Y.; Zhang, Z. Investigation of the formation of furfural compounds in apple products treated with pasteurization and high pressure processing. Food Res. Int. 2024, 190, 114546. [Google Scholar] [CrossRef]
- Singh, H.; Ramaswamy, H.S. Thermal Processing of Acidified Vegetables: Effect on Process Time-Temperature, Color and Texture. Processes 2023, 11, 1272. [Google Scholar] [CrossRef]
- Fujiyoshi, T.; Ikami, T.; Kikukawa, K.; Kobayashi, M.; Takai, R.; Kozaki, D.; Yamamoto, A. Direct quantitation of the preservatives benzoic and sorbic acid in processed foods using derivative spectrophotometry combined with micro dialysis. Food Chem. 2018, 240, 386–390. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, J.H.F.; Szilágyi, I.M.; Regdon, G.; Cavalheiro, E.T.G. Thermal behavior of food preservative sorbic acid and its derivates. Food Chem. 2021, 337, 127770. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Shen, H.; Liu, H.; Song, F.; Li, P.; Peng, N.; Liang, Y.; Zhao, S. Unraveling the microbial community and succession during zha-chili fermentation and their relationships with flavor formation. Food Res. Int. 2022, 157, 111239. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Xin, X.; Niu, H.; Liao, X.; Liu, D. Reduced formation of biogenic amines in low-salt Zhacai via fermentation under CO2-modified atmosphere. Food Res. Int. 2023, 163, 112256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation strategies to enhance the antibacterial properties of essential oils in food system. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Wu, P.; Qu, W.; Abdualrahman, M.A.Y.; Guo, Y.; Xu, K.; Ma, H. Study on inactivation mechanisms of Listeria grayi affected by pulse magnetic field via morphological structure, Ca2+ transmembrane transport and proteomic analysis. Int. J. Food Sci. Technol. 2017, 52, 2049–2057. [Google Scholar] [CrossRef]
- Tang, J.; Hong, Y.-K.; Inanoglu, S.; Liu, F. Microwave pasteurization for ready-to-eat meals. Curr. Opin. Food Sci. 2018, 23, 133–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Cui, K.; Wang, H.; Su, J.; Zhang, W.; Jiang, W. The encapsulation strategies of clove essential oil enhance its delivery effect in food preservation applications. Food Chem. 2025, 484, 144465. [Google Scholar] [CrossRef]
- Jung, M.-J.; Kim, J.; Lee, S.H.; Whon, T.W.; Sung, H.; Bae, J.-W.; Choi, Y.-E.; Roh, S.W. Role of combinated lactic acid bacteria in bacterial, viral, and metabolite dynamics during fermentation of vegetable food, kimchi. Food Res. Int. 2022, 157, 111261. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, M.; Zhang, M.; Yang, B.; Wang, R.; Bai, J.; Zhang, J.; Wei, W.; Zhu, L.; Xiao, X. Insights into Leuconostoc-clade as starters in Chinese paocai: Shaping microbial communities for rapid, safe, and flavor-preserving fermentation. Food Biosci. 2025, 68, 106410. [Google Scholar] [CrossRef]
- Niu, C.; Fan, Z.; Zheng, F.; Li, Y.; Liu, C.; Wang, J.; Li, Q. Isolation and identification of gas-producing spoilage microbes in fermented broad bean paste. Food Control 2018, 84, 8–16. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, M.; Wang, Y.; Hu, X.; Wang, R.; Yang, B.; Xiao, X.; Wei, W. A Novel Strategy for Controlling Nitrite Accumulation in Fermented Cabbage by Regulating Microbial Community Structure via Cold Plasma. Food Control 2025, 180, 111628. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, Y.; Zhou, Y.; Yu, H.; Li, B.; Yang, W.; Zhai, X.; Wang, X.; Liu, J.; Wang, J. Air and argon cold plasma effects on lipolytic enzymes inactivation, physicochemical properties and volatile profiles of lightly-milled rice. Food Chem. 2024, 445, 138699. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Yuan, L.; Surendhiran, D.; Lin, L. Sequential effect of phages and cold nitrogen plasma against Escherichia coli O157:H7 biofilms on different vegetables. Int. J. Food Microbiol. 2018, 268, 1–9. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.; Li, C.; Cui, H.; Lin, L. The effects of cold plasma (CP) treatment on the inactivation of yam peroxidase and characteristics of yam slices. J. Food Eng. 2023, 359, 111693. [Google Scholar] [CrossRef]
- Chamorro, J.C.; Denoya, G.I.; Santamaría, B.; Fina, B.; Ferreyra, M.; Cejas, E.; Rodríguez, A.; Vaudagna, S.R.; Prevosto, L. Effects of the Plasma-Activated Water on the Quality and Preservation of Fresh-Cut Lettuce. IEEE Trans. Plasma Sci. 2023, 52, 1936–1946. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Frimpong, E.B.; Muhammad, U.; Qian, J.; Mustapha, A.T.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2626–2659. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Umair, M.; Sultana, T.; Xun, S.; Jabbar, S.; Riaz Rajoka, M.S.; Albahi, A.; Abid, M.; Ranjha, M.M.A.N.; El-Seedi, H.R.; Xie, F.; et al. Advances in the application of functional nanomaterial and cold plasma for the fresh-keeping active packaging of meat. Food Sci. Nutr. 2023, 11, 5753–5772. [Google Scholar] [CrossRef]
- Cui, H.; Ma, C.; Lin, L. Synergetic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157: H7 biofilms on lettuce. Food Control 2016, 66, 8–16. [Google Scholar] [CrossRef]
- Wei, W.; Yang, S.; Yang, F.; Hu, X.; Wang, Y.; Guo, W.; Yang, B.; Xiao, X.; Zhu, L. Cold plasma controls nitrite hazards by modulating microbial communities in pickled radish. Foods 2023, 12, 2550. [Google Scholar] [CrossRef]
- Zhao, N.; Ge, L.; Huang, Y.; Wang, Y.; Wang, Y.; Lai, H.; Wang, Y.; Zhu, Y.; Zhang, J. Impact of cold plasma processing on quality parameters of packaged fermented vegetable (radish paocai) in comparison with pasteurization processing: Insight into safety and storage stability of products. Innov. Food Sci. Emerg. Technol. 2020, 60, 102300. [Google Scholar] [CrossRef]
- Li, M.; Shi, T.; Wang, X.; Bao, Y.; Xiong, Z.; Monto, A.R.; Jin, W.; Yuan, L.; Gao, R. Plasma-activated water promoted the aggregation of Aristichthys nobilis myofibrillar protein and the effects on gelation properties. Curr. Opin. Food Sci. 2022, 5, 1616–1624. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Siyu, L.-P.; Wan, Z.; Keener, K.M.; Misra, N. In-package cold plasma decontamination of fresh-cut carrots: Microbial and quality aspects. J. Phys. D Appl. Phys. 2020, 53, 154002. [Google Scholar] [CrossRef]
- Perinban, S.; Orsat, V.; Lyew, D.; Raghavan, V. Effect of plasma activated water on Escherichia coli disinfection and quality of kale and spinach. Food Chem. 2022, 397, 133793. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, H.; Ryu, S.; Eom, S.; Min, S.C. In-package cold plasma treatment for microbial inactivation in plastic-pouch packaged steamed rice cakes. Int. J. Food Microbiol. 2023, 389, 110108. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhang, Y.; Hu, H.; Luo, S.; Zhang, L.; Zhou, H.; Li, P. Effects of in-package atmospheric cold plasma treatment on the qualitative, metabolic and microbial stability of fresh-cut pears. J. Sci. Food Agric. 2021, 101, 4473–4480. [Google Scholar] [CrossRef]
- Zhou, R.; Rezaeimotlagh, A.; Zhou, R.; Zhang, T.; Wang, P.; Hong, J.; Soltani, B.; Mai-Prochnow, A.; Liao, X.; Ding, T. In-package plasma: From reactive chemistry to innovative food preservation technologies. Trends Food Sci. Technol. 2022, 120, 59–74. [Google Scholar] [CrossRef]
- Gong, C.; Li, Y.; Gao, R.; Xiao, F.; Zhou, X.; Wang, H.; Xu, H.; Wang, R.; Huang, P.; Zhao, Y. Preservation of sturgeon using a photodynamic non-thermal disinfection technology mediated by curcumin. Food Biosci. 2020, 36, 100594. [Google Scholar] [CrossRef]
- Mushtaq, S.; Yasin, T.; Saleem, M.; Dai, T.; Yameen, M.A. Potentiation of Antimicrobial Photodynamic Therapy by Curcumin-loaded Graphene Quantum Dots. Photochem. Photobiol. 2022, 98, 202–210. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, H. Antibacterial Effect and Mechanism of Curcumin Mediated Photodynamic Inactivation Combined with Phage on Foodborne Pathogens. J. Food Sci. Technol. 2025, 43, 36–43. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Khir, R.; Wu, B.; Zhou, C.; Pan, Z.; Ma, H. Degradation kinetics of aflatoxin B(1) and B(2) in solid medium by using pulsed light irradiation. J. Sci. Food Agric. 2018, 98, 5220–5224. [Google Scholar] [CrossRef]
- Wang, B.; Wei, W.; Aputexiakere, J.; Li, Y.; Ma, H. Surface decontamination of whole eggs using pulsed light technology and shelf life study of combined pulsed light and vaseline coating during room temperature storage. Food Control 2022, 137, 108411. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Venkitasamy, C.; Wu, B.; Pan, Z.; Ma, H. Effect of pulsed light on activity and structural changes of horseradish peroxidase. Food Chem. 2017, 234, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kwaw, E.; Osae, R.; Apaliya, M.T.; Alolga, R.N.; Sackey, A.S.; Yongkun, M.; Tchabo, W.; Obikyembi, V. Effect of optimized pulsed light treatment conditions on microbiological safety, phytochemical and sensory properties of lactic-acid-fermented mulberry juice. J. Food Meas. Charact. 2024, 18, 1878–1888. [Google Scholar] [CrossRef]
- Artíguez, M.L.; Lasagabaster, A.; Marañón, I.M.d. Factors affecting microbial inactivation by Pulsed Light in a continuous flow-through unit for liquid products treatment. Procedia Comput. Sci. 2011, 1, 786–791. [Google Scholar] [CrossRef][Green Version]
- Xu, F.; Wang, B.; Hong, C.; Telebielaigen, S.; Nsor-Atindana, J.; Duan, Y.; Zhong, F. Optimization of spiral continuous flow-through pulse light sterilization for Escherichia coli in red grape juice by response surface methodology. Food Control 2019, 105, 8–12. [Google Scholar] [CrossRef]
- Xia, G.; Wang, M.; Li, H.; Ren, M.; Wahia, H.; Zhou, C.; Yang, H. Synergistic principle of catalytic infrared and intense pulsed light for bacteriostasis on green sichuan pepper (Zanthoxylum schinifolium). Food Biosci. 2023, 56, 103177. [Google Scholar] [CrossRef]
- Huang, G.; Sun, W.; Dai, C.; Sun, L.; Tang, Y.; He, R.; Ma, H. Sterilization of Bacillus tequilensis isolated from aerogenic vinegar by intense pulsed light. LWT 2020, 118, 108811. [Google Scholar] [CrossRef]
- Ren, M.; Ren, Z.; Chen, L.; Zhou, C.; Okonkwo, C.E.; Mujumdar, A.S. Comparison of ultrasound and ethanol pretreatments before catalytic infrared drying on physicochemical properties, drying, and contamination of Chinese ginger (Zingiber officinale Roscoe). Food Chem. 2022, 386, 132759. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, C.; ElGasim, A.; Yagoub, A.; Sun, Y.; Owusu-Ansah, P.; Yu, X.; Wang, X.; Xu, X.; Zhang, J.; et al. Improvement of the catalytic infrared drying process and quality characteristics of the dried garlic slices by ultrasound-assisted alcohol pretreatment. LWT 2019, 116, 108577. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, B.; Yu, X.; Yagoub, A.E.A.; Sarpong, F.; Zhou, C. Effect of catalytic infrared dry-blanching on the processing and quality characteristics of garlic slices. Food Chem. 2018, 266, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Li, Y.; Tao, H.; Zhang, L.; Zhang, J.; Yang, H.; Mustapha, A.T.; Zhou, C. Inactivation mechanism of catalytic infrared against Pseudomonas aeruginosa and its decontamination application on dry green Sichuan pepper (Zanthoxylum schinifolium). Food Control 2022, 132, 108483. [Google Scholar] [CrossRef]
- Wu, B.; Ma, Y.; Guo, Y.; Zielinska, M.; Gao, K.; Song, C.; Bouhile, Y.; Qiu, C.; Pan, Z.; Ma, H. Research progress in the application of catalytic infrared technology in fruit and vegetable processing. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13291. [Google Scholar] [CrossRef]
- Xia, G.; Li, H.; Wang, M.; Fu, S.; Wang, P.; Yang, H.; Zhou, C.; Zhang, L. Catalytic Infrared Promoted the Decontamination of Intense Pulsed Light on Green Sichuan Pepper (Zanthoxylum schinifolium). J. Food Saf. 2025, 45, e70005. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Pan, Z.; Ye, X.; Ma, H. Effectiveness of combined catalytic infrared radiation and holding time for decontamination Aspergillus niger on dried shiitake mushrooms (Lentinus edodes) with different moisture contents. LWT 2023, 176, 114503. [Google Scholar] [CrossRef]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research progress and application of ultrasonic- and microwave-assisted food processing technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Wang, L.; Liu, S.; Wang, K.; Sun, J.; Xu, B. Evaluation of the possible non-thermal effect of microwave radiation on the inactivation of wheat germ lipase. J. Food Process Eng. 2017, 40, e12506. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Bassey, A.P.; Singh, M.; Zhu, Y.; Corradini, M.G.; Cui, X.; Zhang, X.; Liu, X. Efficacy and mechanisms of Pseudomonas aeruginosa PAO1 biofilm inactivation using high-power pulsed microwave. LWT 2024, 198, 115982. [Google Scholar] [CrossRef]
- Canumir, J.A.; Celis, J.E.; de Bruijn, J.; Vidal, L.V. Pasteurisation of apple juice by using microwaves. LWT-Food Sci. Technol. 2002, 35, 389–392. [Google Scholar] [CrossRef]
- Afraz, M.T.; Khan, M.R.; Roobab, U.; Noranizan, M.A.; Tiwari, B.K.; Rashid, M.T.; Inam-ur-Raheem, M.; Hashemi, S.M.B.; Aadil, R.M. Impact of novel processing techniques on the functional properties of egg products and derivatives: A review. J. Food Process Eng. 2020, 43, e13568. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, H.; Wang, S. Pasteurization mechanism of S. aureus ATCC 25923 in walnut shells using radio frequency energy at lab level. LWT 2021, 143, 111129. [Google Scholar] [CrossRef]
- Zhong, Q.; Sandeep, K. Continuous flow radio frequency heating of liquid and particulate foods. Innov. Food Sci. Emerg. Technol. 2004, 5, 475–483. [Google Scholar] [CrossRef]
- Zhong, Q.; Sandeep, K.; Swartzel, K. Continuous flow radio frequency heating of water and carboxymethylcellulose solutions. J. Food Sci. 2003, 68, 217–223. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Li, C.; Lin, L. Synergistic effect between Helichrysum italicum essential oil and cold nitrogen plasma against Staphylococcus aureus biofilms on different food-contact surfaces. Int. J. Food Sci. Technol. 2016, 51, 2493–2501. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Loffi, C.; Montalbano, S.; Chiarello, E.; Dellafiora, L.; Picone, G.; Antonelli, G.; Tedeschi, T.; Buschini, A.; Capozzi, F. Cleaning the label of cured meat; Effect of the replacement of nitrates/nitrites on nutrients bioaccessibility, peptides formation, and cellular toxicity of in vitro digested salami. Int. J. Mol. Sci. 2022, 23, 12555. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, W.; Li, M.; Liu, H.; Zhao, X.; Yang, S.; Yang, M. Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice. Ind. Crops Prod. 2016, 80, 186–193. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Shi, C.; Alharbi, M.; Cui, H.; Lin, L. Phosphoproteomics analysis reveals the anti-bacterial and anti-virulence mechanism of eugenol against Staphylococcus aureus and its application in meat products. Int. J. Food Microbiol. 2024, 414, 110621. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Abdel-Samie, M.A.; Cui, H.; Lin, L. Unraveling the inhibitory mechanism of clove essential oil against Listeria monocytogenes biofilm and applying it to vegetable surfaces. LWT 2020, 134, 110210. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Rashed, M.M.A.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Omidbeygi, M.; Barzegar, M.; Hamidi, Z.; Naghdibadi, H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control 2007, 18, 1518–1523. [Google Scholar] [CrossRef]
- Coimbra, A.; Carvalho, F.; Duarte, A.P.; Ferreira, S. Antimicrobial activity of Thymus zygis essential oil against Listeria monocytogenes and its application as food preservative. Innov. Food Sci. Emerg. Technol. 2022, 80, 103077. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Encapsulation of thyme essential oil using electrospun zein fiber for strawberry preservation. Chem. Biol. Technol. Agric. 2022, 9, 2. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Možina, S.S.; Di Mattia, C.; Scuota, S.; Luzzi, I.; Jenič, T.; Paparella, A.; Serio, A. Salmonella enterica adhesion: Effect of Cinnamomum zeylanicum essential oil on lettuce. LWT 2019, 111, 16–22. [Google Scholar] [CrossRef]
- Istúriz-Zapata, M.A.; Hernández-López, M.; Correa-Pacheco, Z.N.; Barrera-Necha, L.L. Quality of cold-stored cucumber as affected by nanostructured coatings of chitosan with cinnamon essential oil and cinnamaldehyde. LWT 2020, 123, 109089. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, R.; Shang, Z.; Wang, K.; Zhang, C.; Wang, Z.; Lou, Y.; Liu, J.; Li, P. Peppermint Essential Oil For Controlling Aspergillus flavus and Analysis of its Antifungal Action Mode. Curr. Microbiol. 2025, 82, 140. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2023, 24, 5179. [Google Scholar] [CrossRef]
- Mouhoub, A.; Guendouz, A.; El Alaoui-Talibi, Z.; Ibnsouda Koraichi, S.; Delattre, C.; El Modafar, C. Utilization of chitosan-based coating enriched with Syzygium aromaticum, Cinnamomum zeylanicum, and Thymus satureioides essential oils mixture for strawberries with extended shelf life. J. Food Meas. Charact. 2024, 18, 3315–3325. [Google Scholar] [CrossRef]
- Alikhani-Koupaei, M. Liposome-entrapped essential oils on in vitro and in vivo antioxidant activity in leafy vegetables. Qual. Assur. Saf. Crops Foods 2015, 7, 369–373. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial, antioxidant, and sensorial impacts of oregano and rosemary essential oils over broccoli florets. J. Food Process. Preserv. 2019, 43, e13889. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Hanus, P.; Bakay, L.; Zagrobelna, E.; Kluz, M.; et al. Assessment of Ocimum basilicum Essential Oil Anti-Insect Activity and Antimicrobial Protection in Fruit and Vegetable Quality. Plants 2022, 11, 1030. [Google Scholar] [CrossRef]
- Cai, M.; Wang, Y.; Wang, R.; Li, M.; Zhang, W.; Yu, J.; Hua, R. Antibacterial and antibiofilm activities of chitosan nanoparticles loaded with Ocimum basilicum L. essential oil. Int. J. Biol. Macromol. 2022, 202, 122–129. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros Barbosa, I.; Da Costa Medeiros, J.A.; De Oliveira, K.Á.R.; Gomes-Neto, N.J.; Tavares, J.F.; Magnani, M.; De Souza, E.L. Efficacy of the combined application of oregano and rosemary essential oils for the control of Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis in leafy vegetables. Food Control 2016, 59, 468–477. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Yang, Z.; Xiong, X.; Song, Q.; Li, B.; Zou, H.; Zhang, L.; Liu, T. Antifungal Effect of Oregano Essential Oil Against Penicillium expansum on Pyrus sinkiangensis. J. Fungi 2024, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, M.; Abdelkarim, E.A.; Hussein, M.A.; Aziz, T.; Al-Asmari, F.; Alabbosh, K.F.; Cui, H.; Lin, L. Essential oils as antibacterials against multidrug-resistant foodborne pathogens: Mechanisms, recent advances, and legal considerations. Food Biosci. 2025, 64, 105937. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Hua, Z.; Aziz, T.; Khojah, E.; Cui, H.; Lin, L. Transcriptomic combined with molecular dynamics simulation analysis of the inhibitory mechanism of clove essential oil against Staphylococcus aureus biofilm and its application on surface of food contact materials. Food Bioprod. Process. 2025, 150, 131–140. [Google Scholar] [CrossRef]
- Rasheed, H.A.; Rehman, A.; Chen, X.; Aziz, T.; Al-Asmari, F.; Alhomrani, M.; Alamri, A.S.; Cui, H.; Lin, L. Unveiling the anti-listerial effect of Citrus bergamia essential oil: Mechanism of membrane disruption and anti-hemolytic activity. Food Biosci. 2024, 61, 104742. [Google Scholar] [CrossRef]
- Bai, M.; Dai, J.; Hu, W.; Li, C.; Cui, H.; Lin, L. The inhibition effect and mechanism of litsea cubeba essential oil on the hemolytic activity of listeriolysin O. Food Biosci. 2023, 56, 103137. [Google Scholar] [CrossRef]
- Hashim, S.B.H.; Tahir, H.E.; Mahdi, A.A.; Al-Maqtari, Q.A.; Shishir, M.R.I.; Mahunu, G.K.; Akpabli-Tsigbe, N.D.K.; Zhang, J.; Xiaobo, Z.; Jiyong, S. Enhancing the functionality of the Origanum compactum essential oil capsules by combining sugarcane wax with various biopolymers. J. Food Meas. Charact. 2025, 19, 833–849. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Cui, H.; Lin, L. Enhancement of antioxidant activity, antifungal activity, and oxidation stability of Citrus reticulata essential oil nanocapsules by clove and cinnamon essential oils. Food Biosci. 2021, 43, 101226. [Google Scholar] [CrossRef]
- Li, C.; Bai, M.; Chen, X.; Hu, W.; Cui, H.; Lin, L. Controlled release and antibacterial activity of nanofibers loaded with basil essential oil-encapsulated cationic liposomes against Listeria monocytogenes. Food Biosci. 2022, 46, 101578. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Lin, L. Antibacterial activity of liposome containing curry plant essential oil against Bacillus cereusin rice. J. Food Saf. 2017, 37, e12302. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Preparation and antibacterial activity of Litsea cubeba essential oil/dandelion polysaccharide nanofiber. Ind. Crop Prod. 2019, 140, 111739. [Google Scholar] [CrossRef]
- Xu, J.; Peng, S.; Xiong, Y.; Zheng, Z.; Liu, M.; Xu, J.; Chen, W.; Liu, M.; Kong, J.; Wang, C.; et al. A review on fermented vegetables: Microbial community and potential upgrading strategy via inoculated fermentation. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13362. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Y.; Li, L.; Yu, Y.; Xu, Z. Dynamic evolution of flavor substances and bacterial communities during fermentation of leaf mustard (Brassica juncea var. multiceps) and their correlation. LWT 2022, 167, 113796. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef]
- Ma, E.; An, Y.; Zhang, G.; Zhao, M.; Iqbal, M.W.; Zabed, H.M.; Qi, X. Enhancing the antibacterial activity of Lactobacillus reuteri against Escherichia coli by random mutagenesis and delineating its mechanism. Food Biosci. 2023, 51, 102209. [Google Scholar] [CrossRef]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Improving anti-listeria activity of cheese packaging via nanofiber containing nisin-loaded nanoparticles. Lwt-food Sci. Technol. 2017, 81, 233–242. [Google Scholar] [CrossRef]
- Chai, Y.; Ma, Q.; Nong, X.; Mu, X.; Huang, A. Dissecting LuxS/AI-2 quorum sensing system-mediated phenyllactic acid production mechanisms of Lactiplantibacillus plantarum L3. Food Res. Int. 2023, 166, 112582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.; Yagoub, A.E.A.; Xia, G.; Zhou, C. Effect of vacuum impregnation assisted probiotics fermentation suspension on shelf life quality of freshly cut lotus root. Food Chem. 2022, 381, 132281. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- El-Garhi, H.-E.M.; Abd-Elghany, A.A.; Ibrahim, A.M.A.; El-Aidie, S.A.M.; Castro-Muñoz, R. Effect of Organic Acids on Pathogenic and Lactic Acid Bacteria in Directly Raw Milk Cheddar Cheese. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Arrioja-Bretón, D.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control 2020, 115, 107286. [Google Scholar] [CrossRef]
- Guo, C.; He, Y.; Wang, Y.; Yang, H. NMR-based metabolomic investigation on antimicrobial mechanism of Salmonella on cucumber slices treated with organic acids. Food Control 2022, 137, 108973. [Google Scholar] [CrossRef]
- Alakomi, H.-L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Z.; Wang, F.; Liu, Y.; Zhu, Y.; Du, L.; Wang, D.; Xu, W. Inhibition of biofilm formation and exopolysaccharide synthesis of Enterococcus faecalis by phenyllactic acid. Food Microbiol. 2020, 86, 103344. [Google Scholar] [CrossRef]
- Guimarães, A.; Venancio, A.; Abrunhosa, L. Antifungal effect of organic acids from lactic acid bacteria on Penicillium nordicum. Food Addit Contam A. 2018, 35, 1803–1818. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Sun, Y.; Xin, X.; Wang, Y.; Ping, W. Purification and partial characterization of a novel bacteriocin synthesized by Lactobacillus paracasei HD1-7 isolated from Chinese sauerkraut juice. Sci. Rep. 2016, 6, 19366. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kim, C.R.; Chang, H.C. Heterofermentative lactic acid bacteria as a starter culture to control kimchi fermentation. LWT 2018, 88, 181–188. [Google Scholar] [CrossRef]
- Lozo, J.; Topisirovic, L.; Kojic, M. Natural bacterial isolates as an inexhaustible source of new bacteriocins. Appl. Microbiol. Biotechnol. 2021, 105, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.A.T.; de Melo, M.R.; da Silva, C.M.R.; Jain, S.; Dolabella, S.S. Nisin resistance in Gram-positive bacteria and approaches to circumvent resistance for successful therapeutic use. Crit. Rev. Microbiol. 2021, 47, 376–385. [Google Scholar] [CrossRef]
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent advances in the application of the antimicrobial peptide nisin in the inactivation of spore-forming bacteria in foods. Molecules. 2021, 26, 5552. [Google Scholar] [CrossRef]
- Medeiros-Silva, J.; Jekhmane, S.; Breukink, E.; Weingarth, M. Towards the native binding modes of antibiotics that target lipid II. ChemBioChem 2019, 20, 1731–1738. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Z.; Liang, Z.; Fu, X.; Li, D.; Zhu, C.; Kong, Q.; Mou, H. Current challenges and development strategies of bacteriocins produced by lactic acid bacteria applied in the food industry. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70038. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, L.; Wang, X.; Lu, Y.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Mining, heterologous expression, purification, antibactericidal mechanism, and application of bacteriocins: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 863–899. [Google Scholar] [CrossRef]
- van der Ploeg, J.R. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 2005, 187, 3980–3989. [Google Scholar] [CrossRef]
- Wei, Z.; Shan, C.; Zhang, L.; Ge, D.e.; Wang, Y.; Xia, X.; Liu, X.; Zhou, J. A novel subtilin-like lantibiotics subtilin JS-4 produced by Bacillus subtilis JS-4, and its antibacterial mechanism against Listeria monocytogenes. LWT 2021, 142, 110993. [Google Scholar] [CrossRef]
- Jain, P.M.; Nellikka, A.; Kammara, R. Understanding bacteriocin heterologous expression: A review. Int. J. Biol. Macromol. 2024, 277, 133916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yue, Q.; Xin, Y.; Ngea, G.L.N.; Dhanasekaran, S.; Luo, R.; Li, J.; Zhao, L.; Zhang, H. The biocontrol potentiality of Bacillus amyloliquefaciens against postharvest soft rot of tomatoes and insights into the underlying mechanisms. Postharvest Biol. Technol. 2024, 214, 112983. [Google Scholar] [CrossRef]
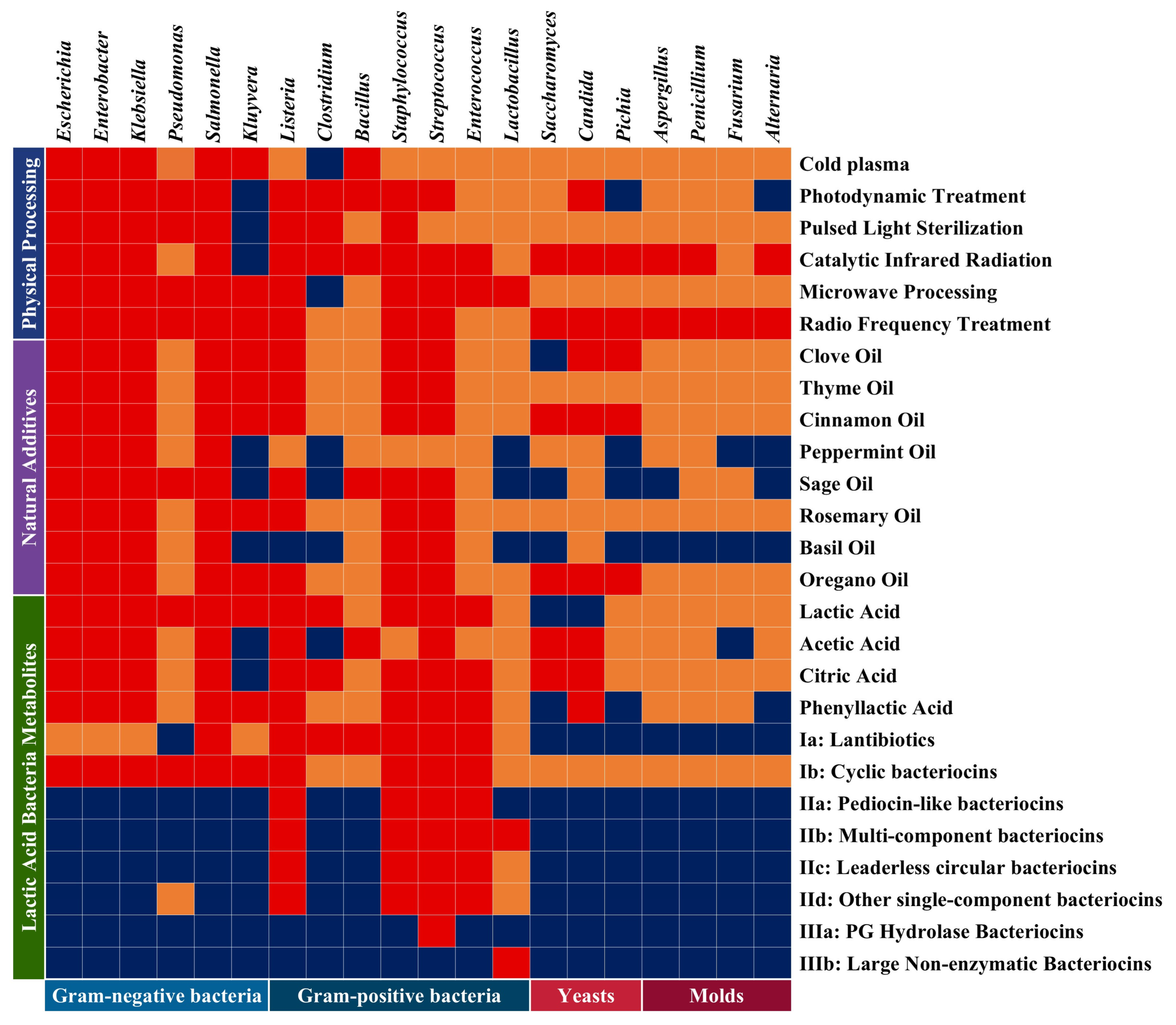
| Class | Example | Characteristics of Bacteriocins |
|---|---|---|
| I | Ia: Lantibiotics (Nisin J) | Thermally stable, Thermostable |
| Ib: Cyclic bacteriocins (Labyrinthopeptin A1) | ||
| II | IIa: Pediocin-like bacteriocins (Acidocin A) | Non-lanthionine-containing thermostable small peptides |
| IIb: Two-component or multicomponent bacteriocins (Plantaricin EF) | Two-peptide bacteriocin requiring synergistic action of both peptides for antimicrobial activity | |
| IIc: Leaderless circular bacteriocins (Gassericin A) | Characterized by covalently linked N- and C-terminal cyclic structures; exhibits enhanced stability under high temperatures, acidic conditions, and digestive enzymes compared to other Class II bacteriocins | |
| IId: Other linear non-pediocin-like single-component bacteriocins (Lacticin Z) | Unmodified, linear, and non-pediocin-like | |
| III | IIIa: PG Hydrolase Bacteriocins (Zoocin A) | Bind to surface receptors of target cells, inducing cell lysis |
| IIIb: Large Nonenzymatic Bacteriocins (Helveticin 34.9) | Disrupt cell membranes; heat-sensitive large proteins produced predominantly by Lactobacillus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Cheng, M.; Xu, C.; Wang, R.; Zhang, M.; Tao, Y.; Qi, S.; Wei, W. Selective Inactivation Strategies for Vegetable Raw Materials: Regulating Microbial Communities to Ensure the Safety and Quality of Fermented Vegetables. Foods 2025, 14, 3291. https://doi.org/10.3390/foods14193291
Zhu L, Cheng M, Xu C, Wang R, Zhang M, Tao Y, Qi S, Wei W. Selective Inactivation Strategies for Vegetable Raw Materials: Regulating Microbial Communities to Ensure the Safety and Quality of Fermented Vegetables. Foods. 2025; 14(19):3291. https://doi.org/10.3390/foods14193291
Chicago/Turabian StyleZhu, Lin, Mengke Cheng, Cuicui Xu, Rong Wang, Meng Zhang, Yufei Tao, Shanshan Qi, and Wei Wei. 2025. "Selective Inactivation Strategies for Vegetable Raw Materials: Regulating Microbial Communities to Ensure the Safety and Quality of Fermented Vegetables" Foods 14, no. 19: 3291. https://doi.org/10.3390/foods14193291
APA StyleZhu, L., Cheng, M., Xu, C., Wang, R., Zhang, M., Tao, Y., Qi, S., & Wei, W. (2025). Selective Inactivation Strategies for Vegetable Raw Materials: Regulating Microbial Communities to Ensure the Safety and Quality of Fermented Vegetables. Foods, 14(19), 3291. https://doi.org/10.3390/foods14193291





