Exploring the Novel Pancreatic Lipase-Inhibitory Peptides in Chlorella pyrenoidosa: Preparation, Purification, Identification, and Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Chlorella Pyrenoidosa Protein Hydrolysates (CPPHs)
2.3. Ultrafiltration
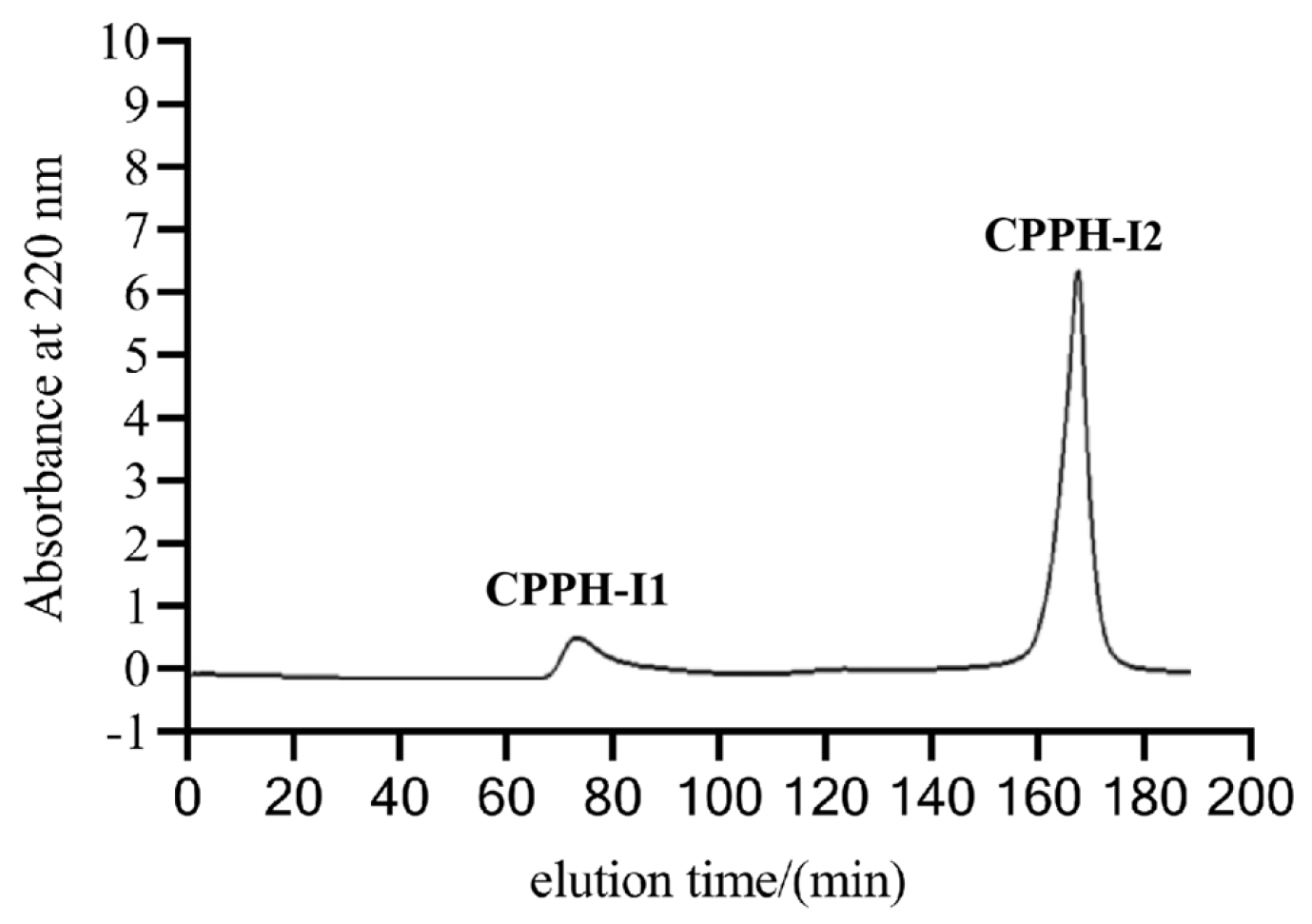
2.4. Sephadex G-25 Gel Chromatography
2.5. PL Inhibition Assay
2.6. Evaluation of Antioxidant Activity
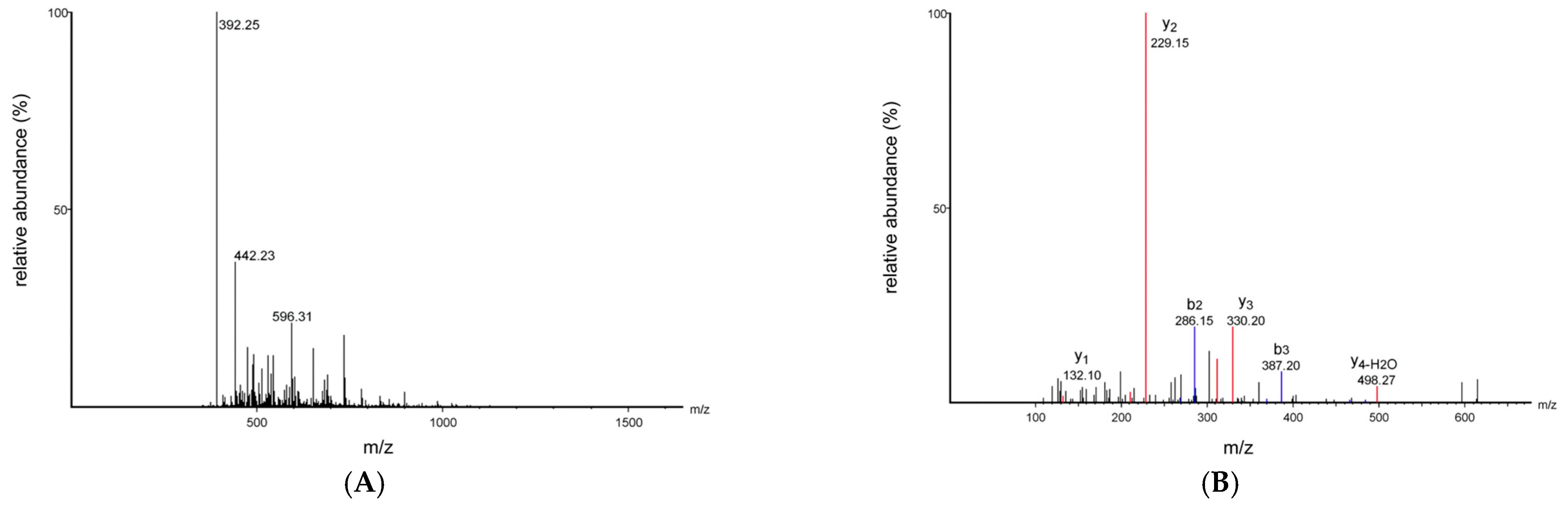
2.7. Peptide Sequencing and Analysis
2.8. Molecular Docking
2.9. Solid-Phase Synthesis of Peptides
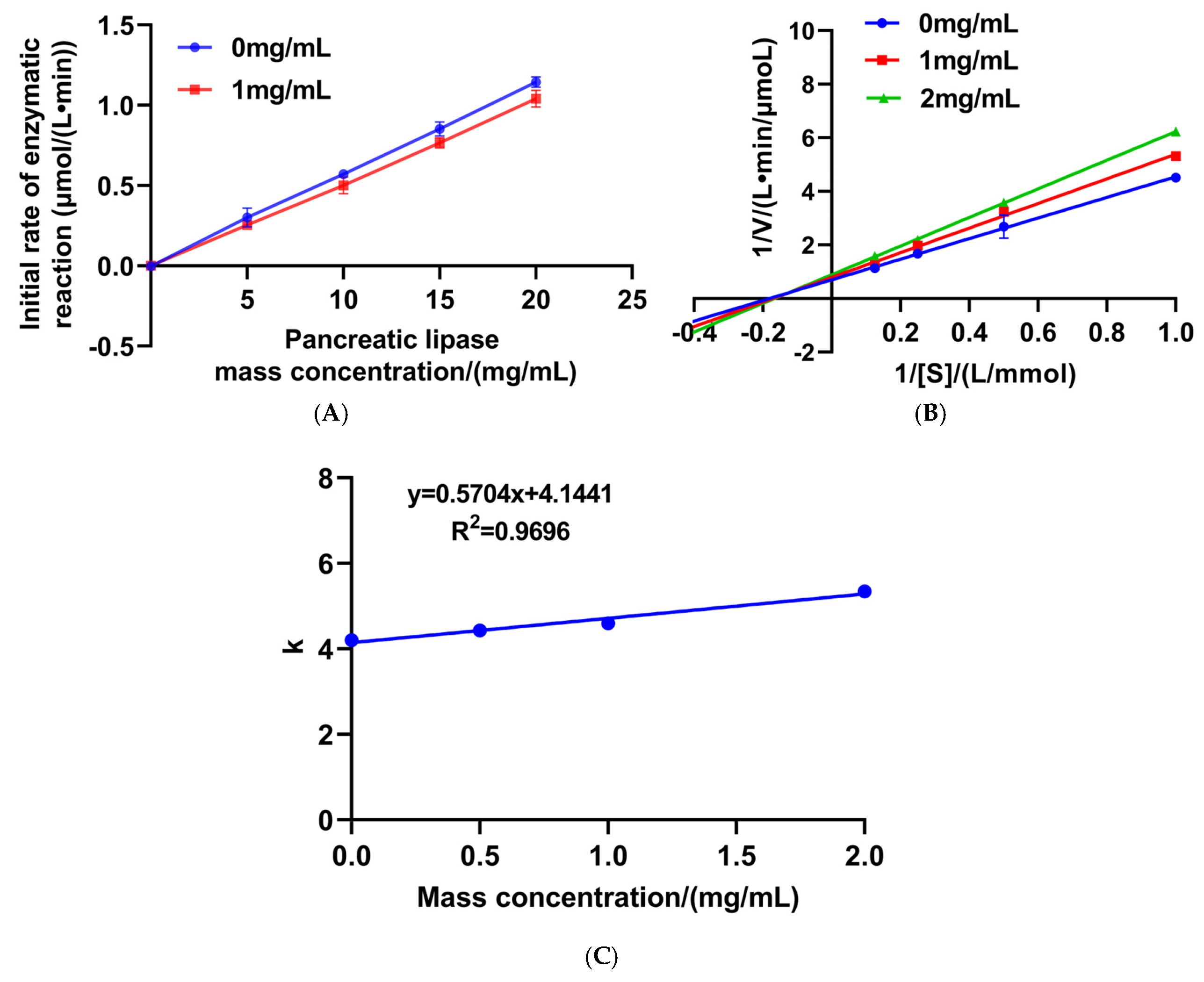
2.10. Kinetic Study of PL Inhibition
2.11. Statistical Analysis
3. Results and Discussion
3.1. PL-I Activity of Ultrafiltration Fractions
3.2. Antioxidant Activity Evaluation of Ultrafiltration Fractions
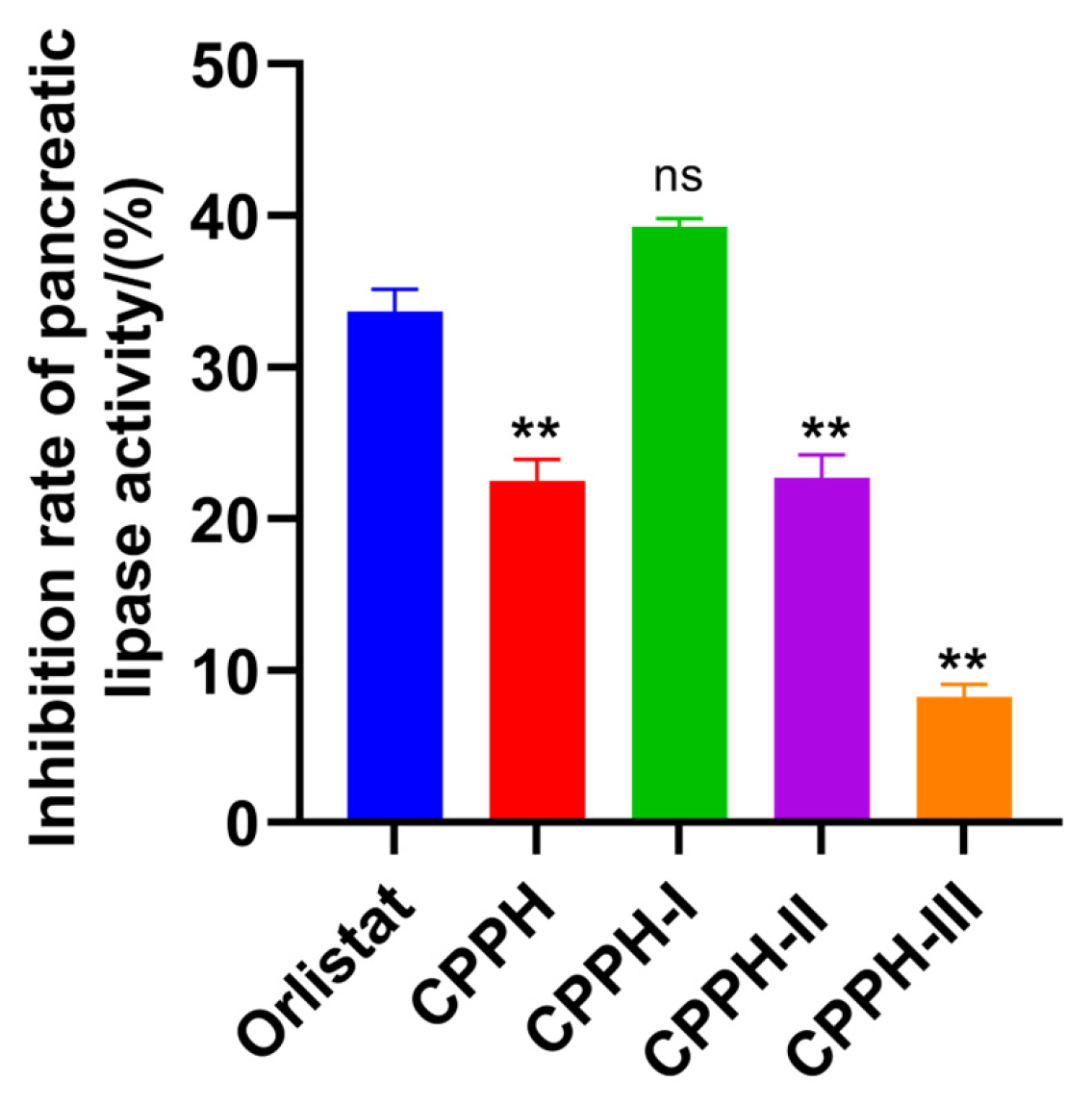
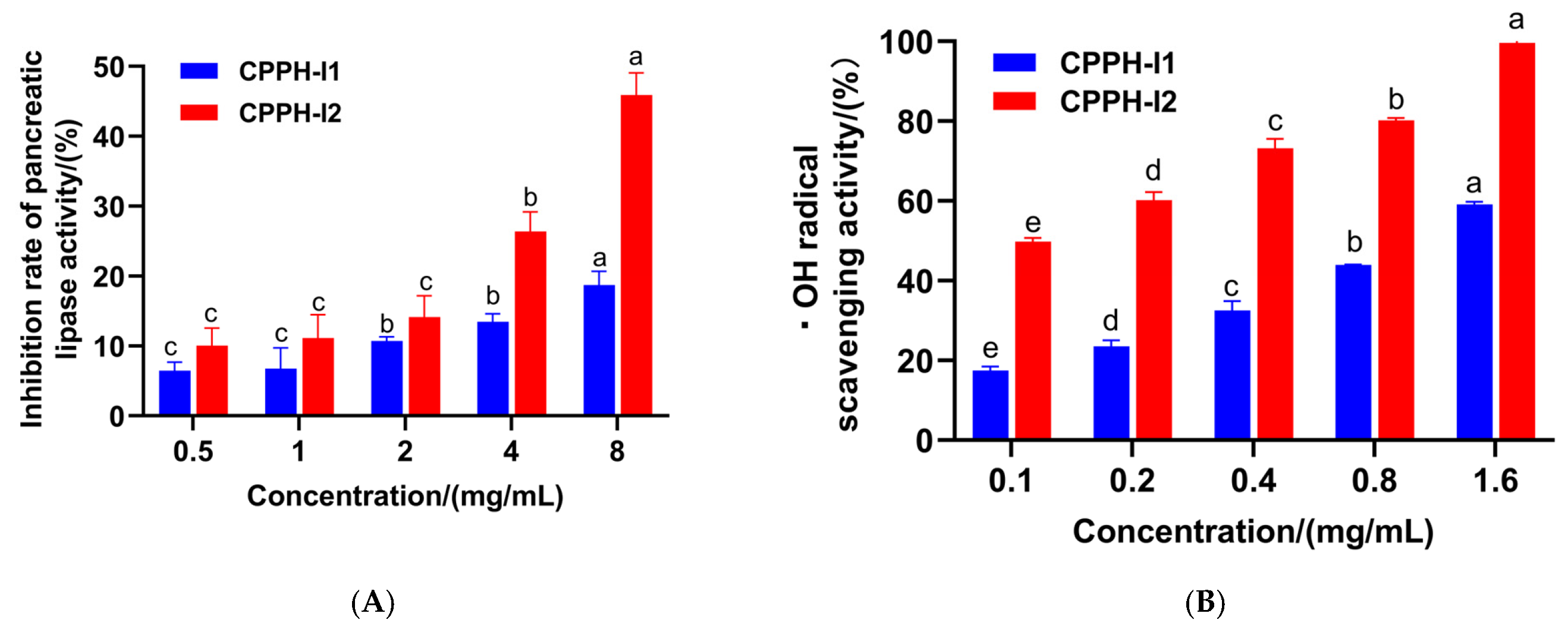
3.3. PL-I Activity and Antioxidant Activity of Purified Fractions
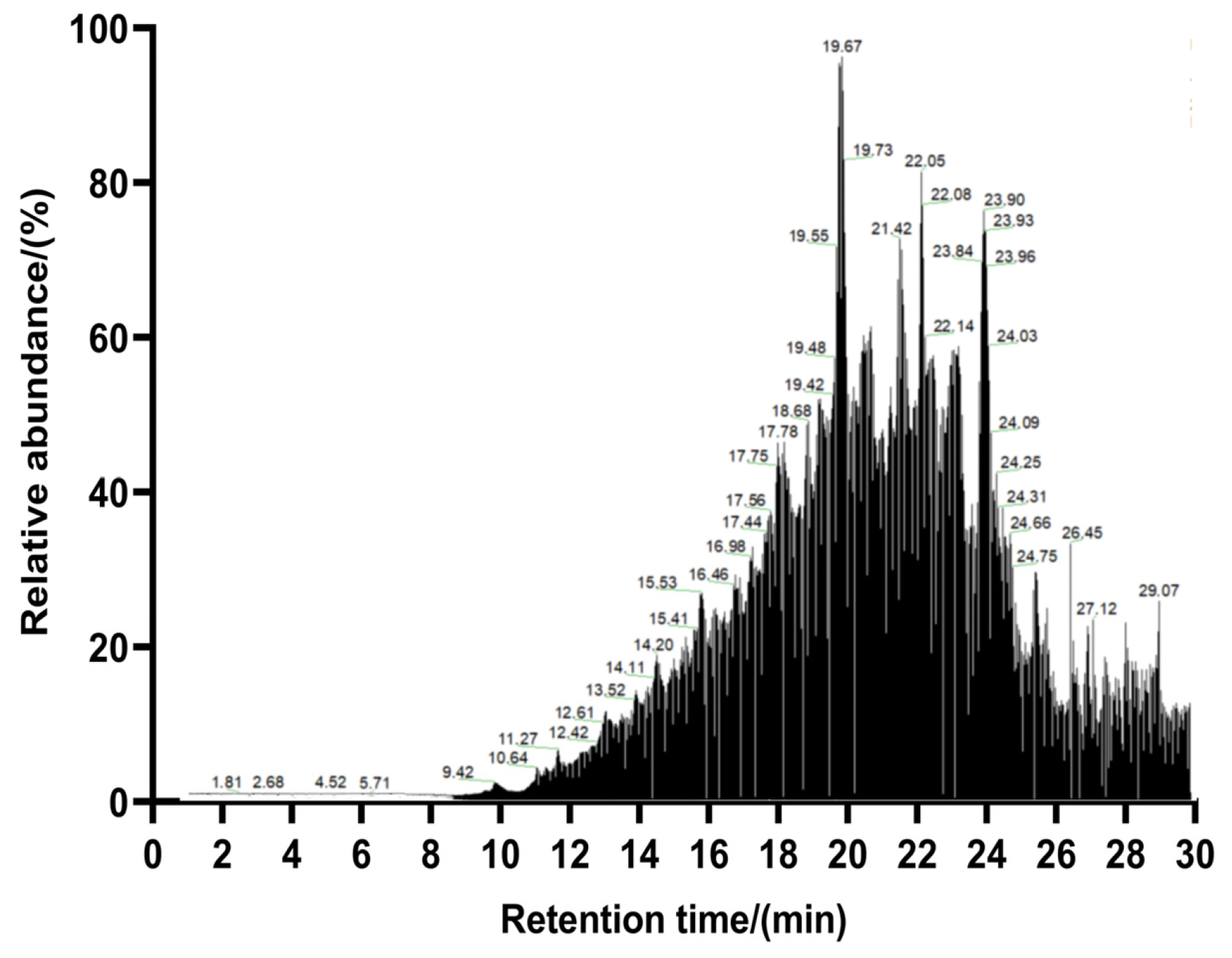
3.4. Peptide Sequence Identification and Virtual Screening
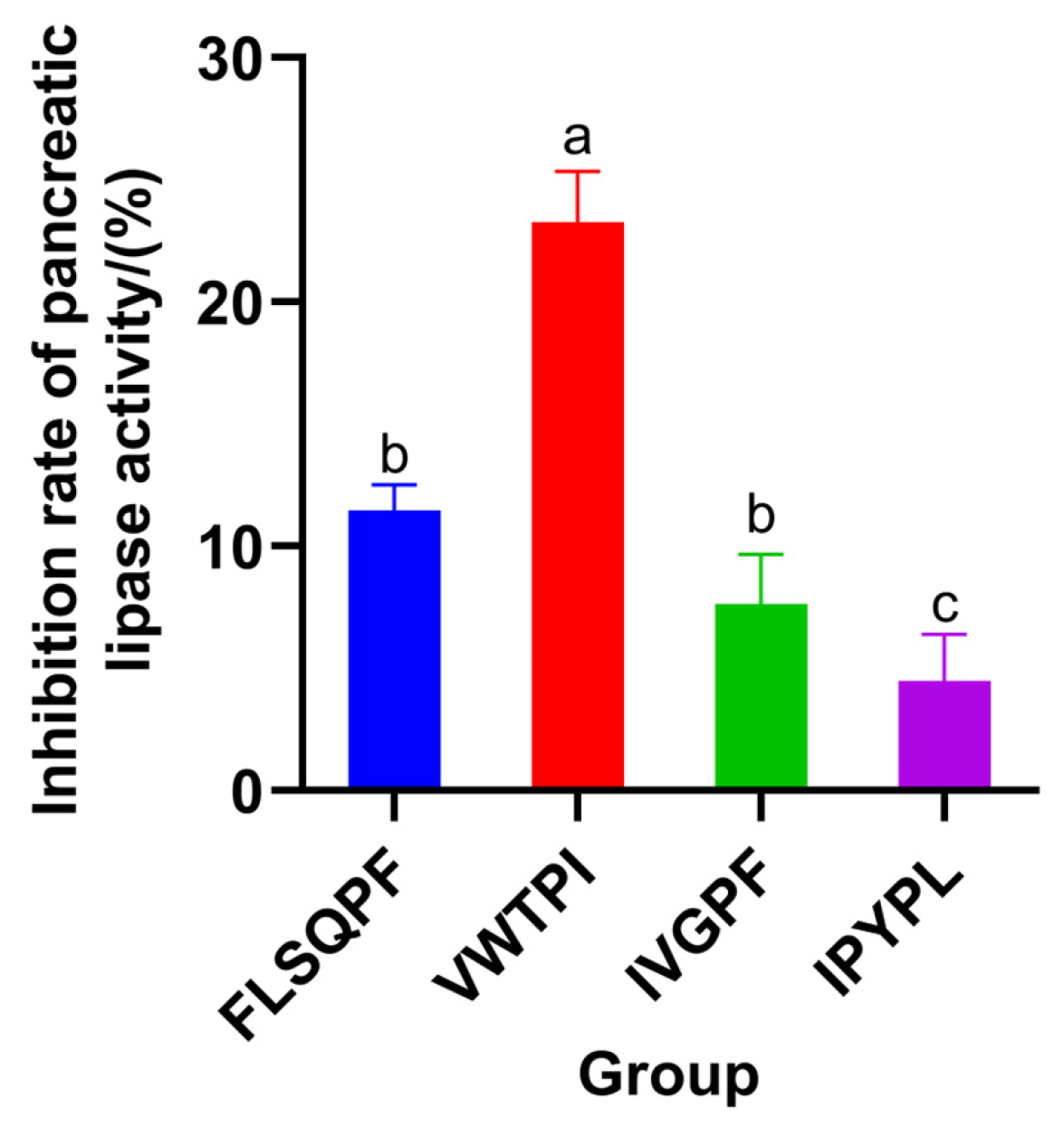
3.5. Verification of Activity of Synthetic Peptides
3.6. Characterization of the Molecular Interaction Between the Synthetic Peptide VWTPI and PL
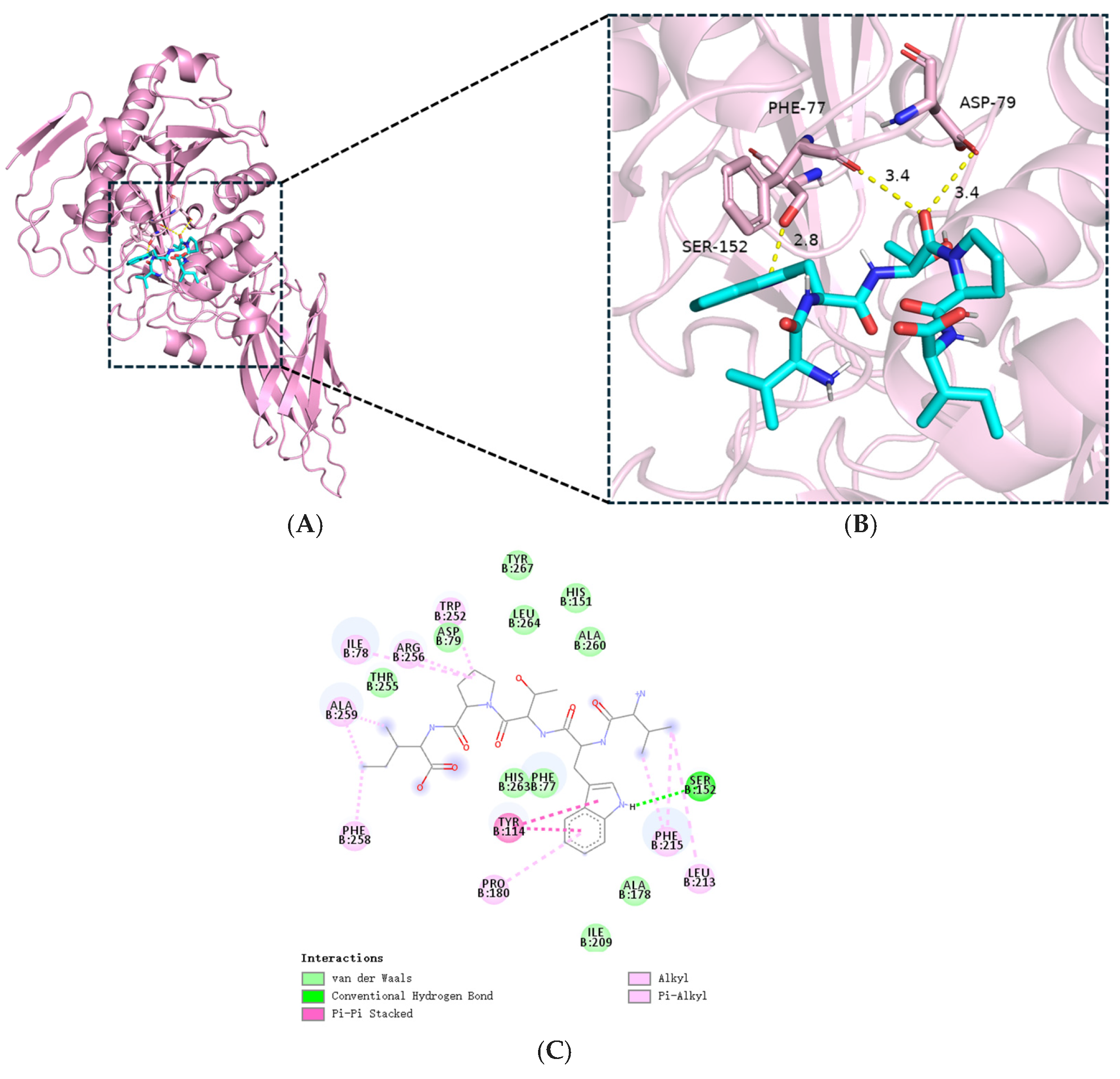
3.7. Molecular Docking Analysis of VWTPI with PL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPPH | Chlorella pyrenoidosa Protein Hydrolysate |
| PL | Pancreatic Lipase |
| PL-I | Pancreatic Lipase—Inhibitory |
References
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global Burden of Obesity in 2005 and Projections to 2030. Int. J. Obesity. 2008, 32, 431–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Yang, X.; Li, Y.; Gui, J.; Mei, Y.; Liu, H.; Guo, L.; Li, J.; Lei, Y.; et al. Obesity and Lipid Indices as Predictors of Depressive Symptoms in Middle-Aged and Elderly Chinese: Insights from a Nationwide Cohort Study. BMC Psychiatry 2024, 34, 351. [Google Scholar] [CrossRef]
- Lee, K.X.; Quek, K.F.; Ramadas, A. Dietary and Lifestyle Risk Factors of Obesity Among Young Adults: A Scoping Review of Observational Studies. Curr. Nutr. Rep. 2023, 12, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, D.; Guo, J.-J.; Tao, W.; Gong, R.-X.; Yao, L.; Zhang, X.-L.; Cao, W.-G. Active Components, Antioxidant, Inhibition on Metabolic Syndrome Related Enzymes, and Monthly Variations in Mature Leaf Hawk Tea. Molecules 2019, 24, 657. [Google Scholar] [CrossRef]
- Ren, M.; Hou, Y.; Peng, D.; Li, H.; Zhang, X.; Qiao, L.; Wang, X.; Jiang, Y.; Wu, F.; Wang, G. Ultrasonic/Compound Enzyme Extraction, Comparative Characterisation and Biological Activity of Lonicera Macranthoides Polysaccharides. Ultrason. Sonochem. 2025, 114, 107259. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Morita, S.; Yamada, H.; Koga, K.; Ota, W.; Furuta, T.; Yamatsu, A.; Kim, M. Free Linoleic Acid and Oleic Acid Reduce Fat Digestion and Absorption In Vivo as Potent Pancreatic Lipase Inhibitors Derived from Sesame Meal. Molecules 2022, 27, 4910. [Google Scholar] [CrossRef]
- Peixoto, T.C.; Moura, E.G.; de Oliveira, E.; Soares, P.N.; Guarda, D.S.; Bernardino, D.N.; Ai, X.X.; da, S.T.; Rodrigues, V.; de Souza, G.R.; et al. Cranberry (Vaccinium Macrocarpon) Extract Treatment Improves Triglyceridemia, Liver Cholesterol, Liver Steatosis, Oxidative Damage and Corticosteronemia in Rats Rendered Obese by High Fat Diet. Eur. J. Nutr. 2017, 57, 1829–1844. [Google Scholar] [CrossRef]
- Gariballa, S.; Al-Bluwi, G.S.M.; Yasin, J. Increased Fruit and Vegetable Consumption Mitigates Oxidative Damage and Associated Inflammatory Response in Obese Subjects Independent of Body Weight Change. Nutrients 2023, 15, 1638. [Google Scholar] [CrossRef]
- Björnsson, E.S. Hepatotoxicity of Statins and Other Lipid-Lowering Agents. Liver Int. 2016, 37, 173–178. [Google Scholar] [CrossRef]
- Ketprayoon, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. An in Vitro Study of Lipase Inhibitory Peptides Obtained from De-Oiled Rice Bran. RSC Adv. 2021, 11, 18915–18929. [Google Scholar] [CrossRef]
- Lafarga, T.; Álvarez, C.; Hayes, M. Bioactive Peptides Derived from Bovine and Porcine Co-Products: A Review. J. Food Biochem. 2017, 41, e12418. [Google Scholar] [CrossRef]
- Lin, H.; Li, W.; Sun, R.; Xu, C.; Zhang, C.; Gao, J.; Cao, W.; Qin, X.; Zhong, S.; Chen, Y. Purification and Characterization of a Novel Immunoregulatory Peptide from Sipunculus Nudus L. Protein. Food Sci. Nutr. 2023, 11, 7779–7790. [Google Scholar] [CrossRef]
- Mora, L.; Reig, M.; Toldrá, F. Bioactive Peptides Generated from Meat Industry By-Products. Food Res. Int. 2014, 65, 344–349. [Google Scholar] [CrossRef]
- Esfandi, R.; Seidu, I.; Willmore, W.; Tsopmo, A. Antioxidant, Pancreatic Lipase, and α-Amylase Inhibitory Properties of Oat Bran Hydrolyzed Proteins and Peptides. J. Food Biochem. 2022, 46, e13762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ge, J.; Wei, L.; Shi, J.; Ji, Y.; Xing, X.; Shi, Y.; Dong, Y. Isolation and Identification of Novel Hemp Seed Protein-Derived Pancreatic Lipase Inhibitory Peptides. Food Biosci. 2025, 63, 105834. [Google Scholar] [CrossRef]
- Fisayo Ajayi, F.; Mudgil, P.; Gan, C.-Y.; Maqsood, S. Identification and Characterization of Cholesterol Esterase and Lipase Inhibitory Peptides from Amaranth Protein Hydrolysates. Food Chem. X 2021, 12, 100165. [Google Scholar] [CrossRef]
- Sergi, D.; Melloni, M.; Passaro, A.; Neri, L.M. Influence of Type 2 Diabetes and Adipose Tissue Dysfunction on Breast Cancer and Potential Benefits from Nutraceuticals Inducible in Microalgae. Nutrients 2024, 16, 3243. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, H.J.; Lee, H.-J.; Kang, M.-H.; Park, Y.K. Six-Week Supplementation with Chlorella Has Favorable Impact on Antioxidant Status in Korean Male Smokers. Nutrition 2010, 26, 175–183. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Separation, Antitumor Activities, and Encapsulation of Polypeptide from Chlorella Pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, M.; Zhang, Z.; Huang, P.; Xu, B.; Chen, C.; Li, P. N-Nitrosodimethylamine Reduction by Lactobacillus Pentosus R3 in Fermented Cooked Sausages. Food Control 2021, 124, 107869. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Q.; Wei, X.; Qin, X. Pancreatic Lipase and Cholesterol Esterase Inhibitory Effect of Camellia Nitidissima Chi Flower Extracts in Vitro and in Vivo. Food Biosci. 2020, 37, 100682. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. In Vitro Antioxidant Activity and in Vivo Anti-Fatigue Effect of Loach (Misgurnus Anguillicaudatus) Peptides Prepared by Papain Digestion. Food Chem. 2010, 124, 188–194. [Google Scholar] [CrossRef]
- Huang, X.; Tu, Z.; Xiao, H.; Li, Z.; Zhang, Q.; Wang, H.; Hu, Y.; Zhang, L. Dynamic High Pressure Microfluidization-Assisted Extraction and Antioxidant Activities of Sweet Potato (Ipomoea Batatas L.) Leaves Flavonoid. Food Bioprod. Process. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and Free Radical-Scavenging Activities of Chickpea Protein Hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Handa, B.K.; Keech, E. FMOC Solid Phase Synthesis of an Endothelin Converting Enzyme Substrate. Int. J. Pept. Protein Res. 1992, 40, 66–71. [Google Scholar] [CrossRef]
- Zhao, L.; Ai, X.; Pan, F.; Zhou, N.; Zhao, L.; Cai, S.; Tang, X. Novel Peptides with Xanthine Oxidase Inhibitory Activity Identified from Macadamia Nuts: Integrated in Silico and in Vitro Analysis. Eur. Food Res. Technol. 2022, 248, 2031–2042. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, F.; He, Z.; Fang, X.; Liu, X. Optimization and Molecular Mechanism of Novel α-Glucosidase Inhibitory Peptides Derived from Camellia Seed Cake through Enzymatic Hydrolysis. Foods 2023, 12, 393. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; Gonzalez de Mejia, E.; Amaya-Llano, S.L. Hard-to-Cook Bean (Phaseolus Vulgaris L.) Proteins Hydrolyzed by Alcalase and Bromelain Produced Bioactive Peptide Fractions That Inhibit Targets of Type-2 Diabetes and Oxidative Stress. Food Res. Int. 2015, 76, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shi, L.; Ren, Z.; Weng, W. Antioxidant Characteristics of Hydrolysate from Low-Value Sea Cucumber: In Vitro and in Vivo Activities of Caenorhabditis Elegans. Food Chem. X 2023, 19, 100836. [Google Scholar] [CrossRef]
- Tawalbeh, D.; Al-U’datt, M.H.; Wan Ahmad, W.A.N.; Ahmad, F.; Sarbon, N.M. Recent Advances in In Vitro and In Vivo Studies of Antioxidant, ACE-Inhibitory and Anti-Inflammatory Peptides from Legume Protein Hydrolysates. Molecules 2023, 28, 2423. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of Bioactive Peptides with Antidiabetic, Antihypertensive, and Antioxidant Activities and Identification of α-Glucosidase Inhibitory Peptides from Soy Protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; Liceaga, A.M.; Reddivari, L.; Kim, K.-H.; Anderson, J.M. Enzyme Kinetics, Molecular Docking, and in Silico Characterization of Canary Seed (Phalaris Canariensis L.) Peptides with ACE and Pancreatic Lipase Inhibitory Activity. J. Funct. Foods 2022, 88, 104892. [Google Scholar] [CrossRef]
- Yin, S.; Siahaan, E.A.; Niu, L.; Shibata, M.; Liu, Y.; Hagiwara, T. Real Time Monitoring and Evaluation of the Inhibition Effect of Fucoxanthin against α-Amylase Activity by Using QCM-A. Front. Nutr. 2023, 9, 1110615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Fan, Y.; Zhao, L.; Zhu, Y.; Jiang, Y.; Gu, J.; Xue, Y.; Hao, Z.; Shen, Q. Identification and Molecular Binding Mechanism of Novel Pancreatic Lipase and Cholesterol Esterase Inhibitory Peptides from Heat-Treated Adzuki Bean Protein Hydrolysates. Food Chem. 2023, 439, 138129. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Pérez-Pérez, A.; Vilariño-García, T.; Jiménez-Cortegana, C.; Muriana, F.J.G.; Millán-Linares, M.C.; Sánchez-Margalet, V. Nutritional Modulation of Leptin Expression and Leptin Action in Obesity and Obesity-Associated Complications. J. Nutr. Biochem. 2020, 86, 108561. [Google Scholar] [CrossRef]
- Wang, X.; Ai, X.; Zhu, Z.; Zhang, M.; Pan, F.; Yang, Z.; Wang, O.; Zhao, L.; Zhao, L. Pancreatic Lipase Inhibitory Effects of Peptides Derived from Sesame Proteins: In Silico and in Vitro Analyses. Int. J. Biol. Macromol. 2022, 222, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Stefanucci, A.; Dimmito, M.P.; Zengin, G.; Luisi, G.; Mirzaie, S.; Novellino, E.; Mollica, A. Discovery of Novel Amide Tripeptides as Pancreatic Lipase Inhibitors by Virtual Screening. New J. Chem. 2019, 43, 3208–3217. [Google Scholar] [CrossRef]
- Du, X.; Bai, M.; Huang, Y.; Jiang, Z.; Chen, F.; Ni, H.; Li, Q. Inhibitory Effect of Astaxanthin on Pancreatic Lipase with Inhibition Kinetics Integrating Molecular Docking Simulation. J. Funct. Foods 2018, 48, 551–557. [Google Scholar] [CrossRef]
- Li, S.; Hu, X.; Pan, J.; Gong, D.; Zhang, G. Mechanistic Insights into the Inhibition of Pancreatic Lipase by Apigenin: Inhibitory Interaction, Conformational Change and Molecular Docking Studies. J. Mol. Liq. 2021, 335, 116505. [Google Scholar] [CrossRef]
- Mudgil, P.; Baba, W.N.; Kamal, H.; FitzGerald, R.J.; Hassan, H.M.; Ayoub, M.A.; Gan, C.-Y.; Maqsood, S. A Comparative Investigation into Novel Cholesterol Esterase and Pancreatic Lipase Inhibitory Peptides from Cow and Camel Casein Hydrolysates Generated upon Enzymatic Hydrolysis and In-Vitro Digestion. Food Chem. 2022, 367, 130661. [Google Scholar] [CrossRef] [PubMed]


| Code | Peptide | RT | Mass | m/z | Area | −10lgP | Relative Content (%) |
|---|---|---|---|---|---|---|---|
| 1 | VWTPI | 21.68 | 614.3428 | 615.3505 | 1.40 × 109 | 29.73 | 3.36 |
| 2 | IAYPL | 19.37 | 575.3318 | 576.3395 | 1.27 × 109 | 29.16 | 3.05 |
| 3 | IGIML | 23.88 | 545.3247 | 546.3293 | 1.17 × 109 | 26.79 | 2.81 |
| 4 | VLPLF | 24.16 | 587.3683 | 588.3754 | 9.37 × 108 | 24.47 | 2.25 |
| 5 | FLSQPF | 22.21 | 737.3748 | 738.3818 | 9.22 × 108 | 44.04 | 2.21 |
| 6 | GLAPF | 19.93 | 503.2744 | 504.2817 | 7.76 × 108 | 31.03 | 2.16 |
| 7 | IVGPF | 20.77 | 531.3057 | 532.3133 | 5.92 × 108 | 33.36 | 1.86 |
| 8 | IPYPL | 22.16 | 601.3475 | 602.3566 | 5.53 × 108 | 25.55 | 1.42 |
| 9 | YLPPL | 21.46 | 601.3475 | 602.3548 | 5.37 × 108 | 23.73 | 1.33 |
| 10 | SYLPPL | 22.02 | 688.3795 | 689.387 | 5.01 × 108 | 44.30 | 1.29 |
| Code | Peptide | Affinity (kcal/mol) | PeptideRanker Score | Solubility | Toxicity |
|---|---|---|---|---|---|
| 1 | FLSQPF | −9.94 | 0.527 | Good | Non-Toxic |
| 2 | VWTPI | −9.76 | 0.636 | Good | Non-Toxic |
| 3 | IVGPF | −9.69 | 0.702 | Good | Non-Toxic |
| 4 | IPYPL | −9.63 | 0.816 | Good | Non-Toxic |
| 5 | SYLPPL | −9.50 | 0.853 | Good | Non-Toxic |
| 6 | YLPPL | −9.28 | 0.908 | Good | Non-Toxic |
| 7 | VLPLF | −9.03 | 0.793 | Good | Non-Toxic |
| 8 | GLAPF | −9.03 | 0.850 | Good | Non-Toxic |
| 9 | IGIML | −8.82 | 0.870 | Good | Non-Toxic |
| 10 | IAYPL | −8.70 | 0.853 | Good | Non-Toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lin, L.; Liang, P.; Liu, W.; Ma, W.; Wang, Y.; Chen, J. Exploring the Novel Pancreatic Lipase-Inhibitory Peptides in Chlorella pyrenoidosa: Preparation, Purification, Identification, and Molecular Docking. Foods 2025, 14, 3277. https://doi.org/10.3390/foods14183277
Wang D, Lin L, Liang P, Liu W, Ma W, Wang Y, Chen J. Exploring the Novel Pancreatic Lipase-Inhibitory Peptides in Chlorella pyrenoidosa: Preparation, Purification, Identification, and Molecular Docking. Foods. 2025; 14(18):3277. https://doi.org/10.3390/foods14183277
Chicago/Turabian StyleWang, Dengmi, Luan Lin, Peng Liang, Wenjun Liu, Wenrui Ma, Ying Wang, and Jicheng Chen. 2025. "Exploring the Novel Pancreatic Lipase-Inhibitory Peptides in Chlorella pyrenoidosa: Preparation, Purification, Identification, and Molecular Docking" Foods 14, no. 18: 3277. https://doi.org/10.3390/foods14183277
APA StyleWang, D., Lin, L., Liang, P., Liu, W., Ma, W., Wang, Y., & Chen, J. (2025). Exploring the Novel Pancreatic Lipase-Inhibitory Peptides in Chlorella pyrenoidosa: Preparation, Purification, Identification, and Molecular Docking. Foods, 14(18), 3277. https://doi.org/10.3390/foods14183277






