Evaluating the Antimicrobial Efficacy of Citral Nano-Emulsion Against Vibrio parahaemolyticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Strain and Culture Conditions
2.3. Preparation of Citral Nano-Emulsion
2.4. Experiment Design
2.5. Characterization of Citral Nano-Emulsion
2.6. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.7. Determination of AKP Content
2.8. MDA Content Assay
2.9. Extracellular Glucose Content Assay
2.10. Field Emission Scanning Electron Microscope (FESEM) Observation
2.11. Motility Assay
2.12. Biofilm Formation Assay
2.13. Extracellular Polysaccharide (EPS) Assay
2.14. Determination of Extracellular Protease
2.15. Determination of Preservation Effect of Citral Nano-Emulsion on Salmon
2.16. Data Analysis
3. Results and Discussion
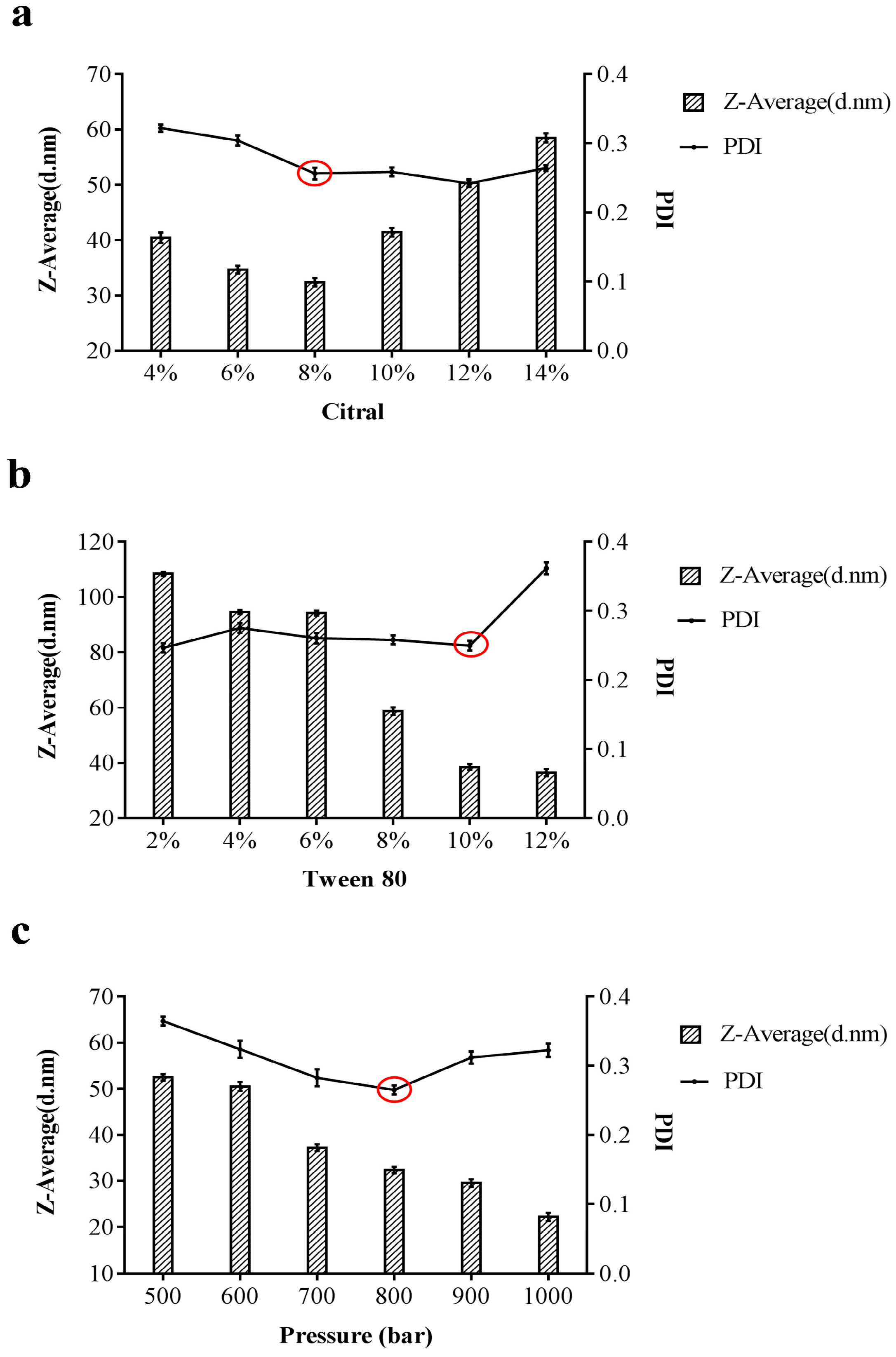
3.1. Optimization of Synthesis Conditions of Citral Nano-Emulsion
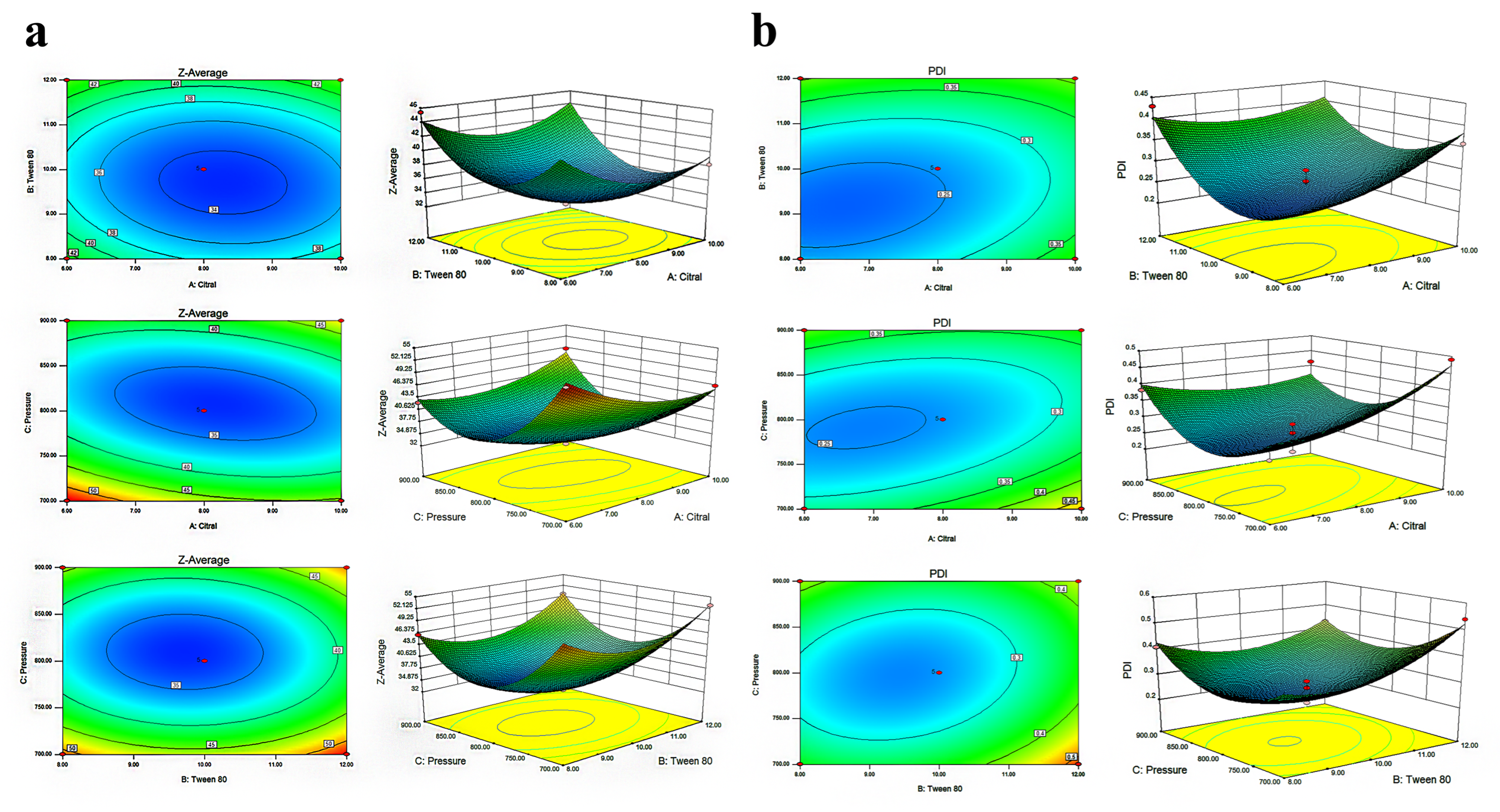
3.2. Response Surface Methodology Analysis
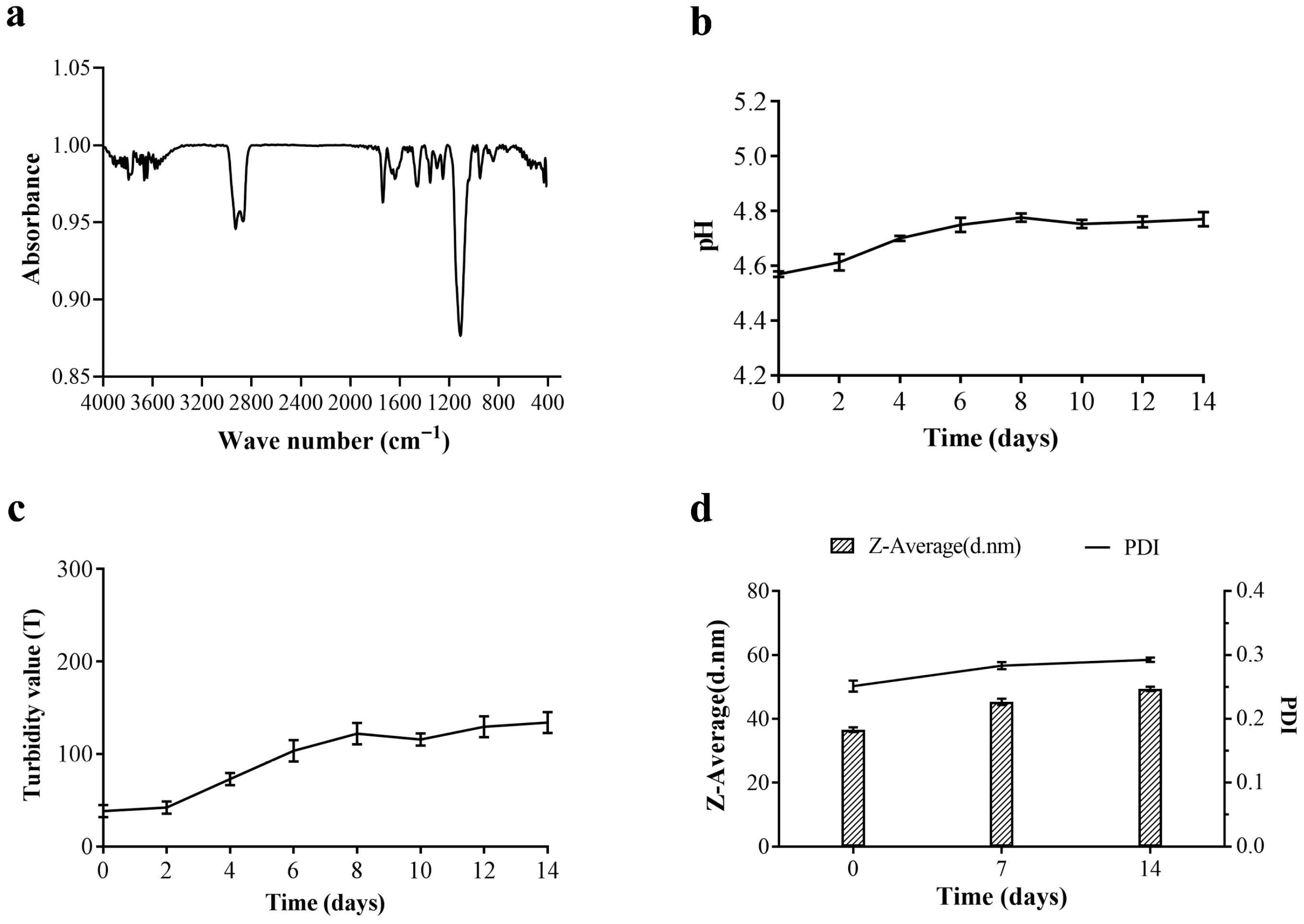
3.3. Physicochemical Properties of Citral Nano-Emulsion
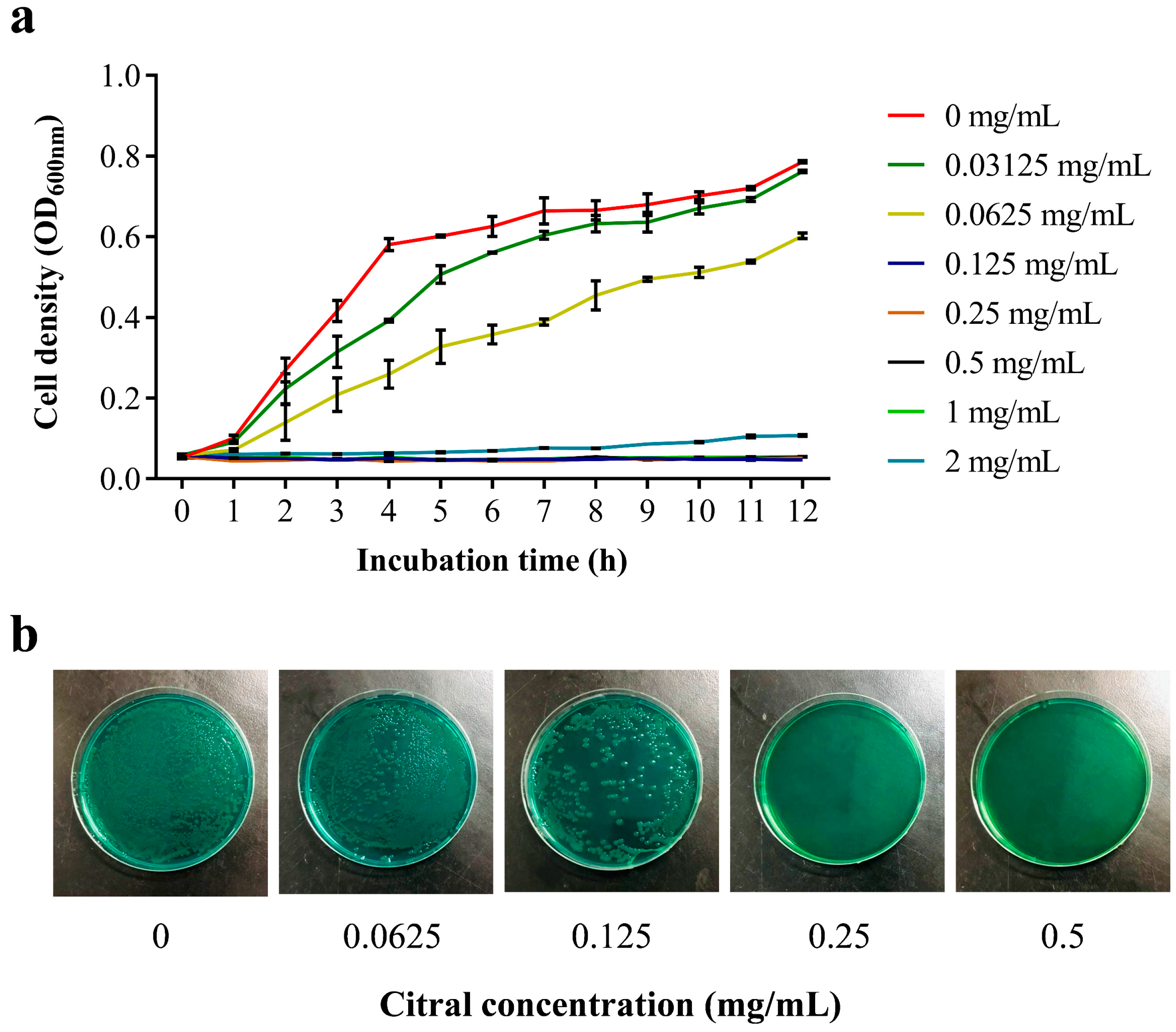
3.4. Antibacterial Activity of Citral Nano-Emulsion Against V. parahaemolyticus
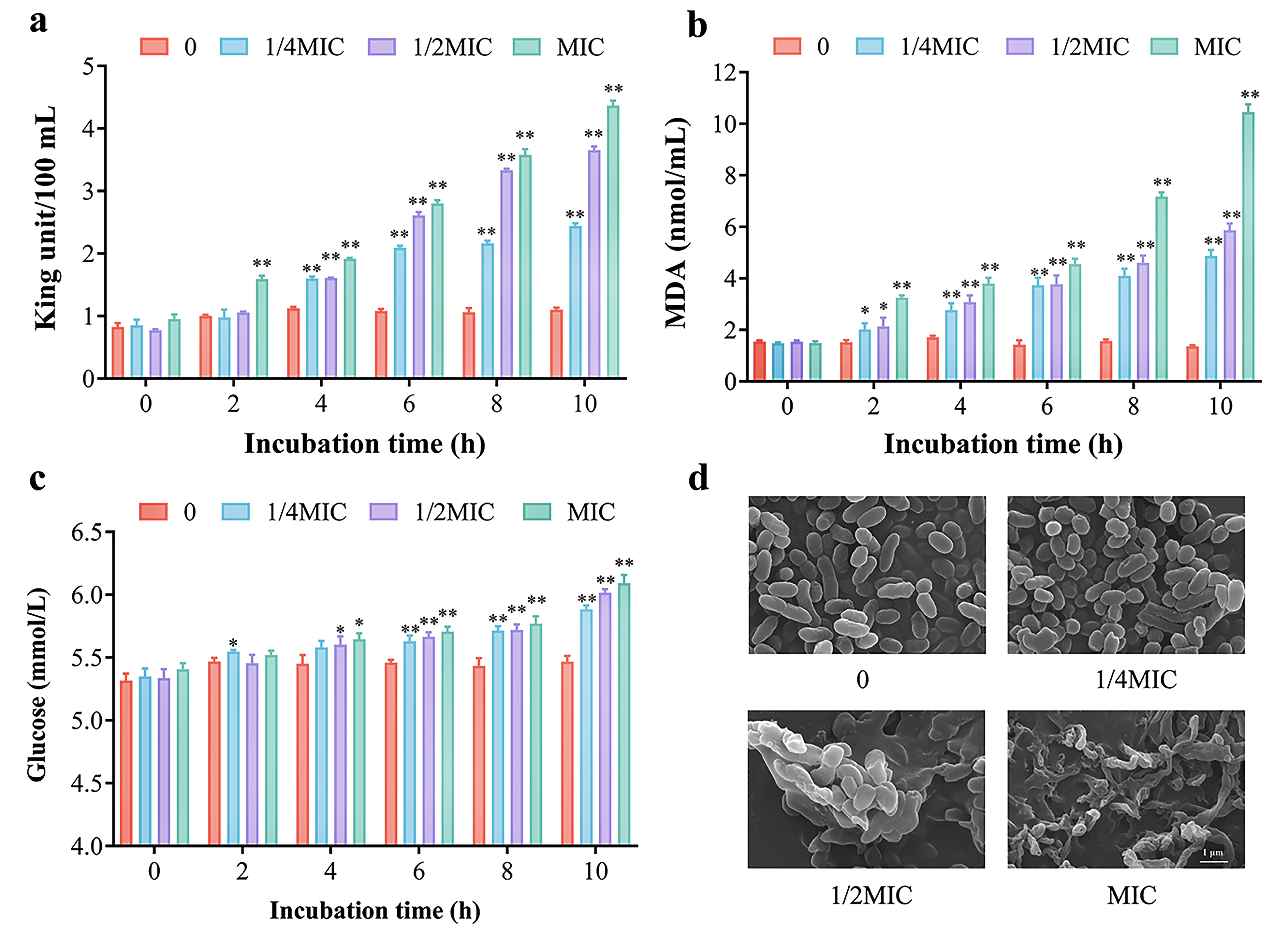
3.5. Antibacterial Activity and Mechanism Analysis of Citral Nano-Emulsion Against V. parahaemolyticus
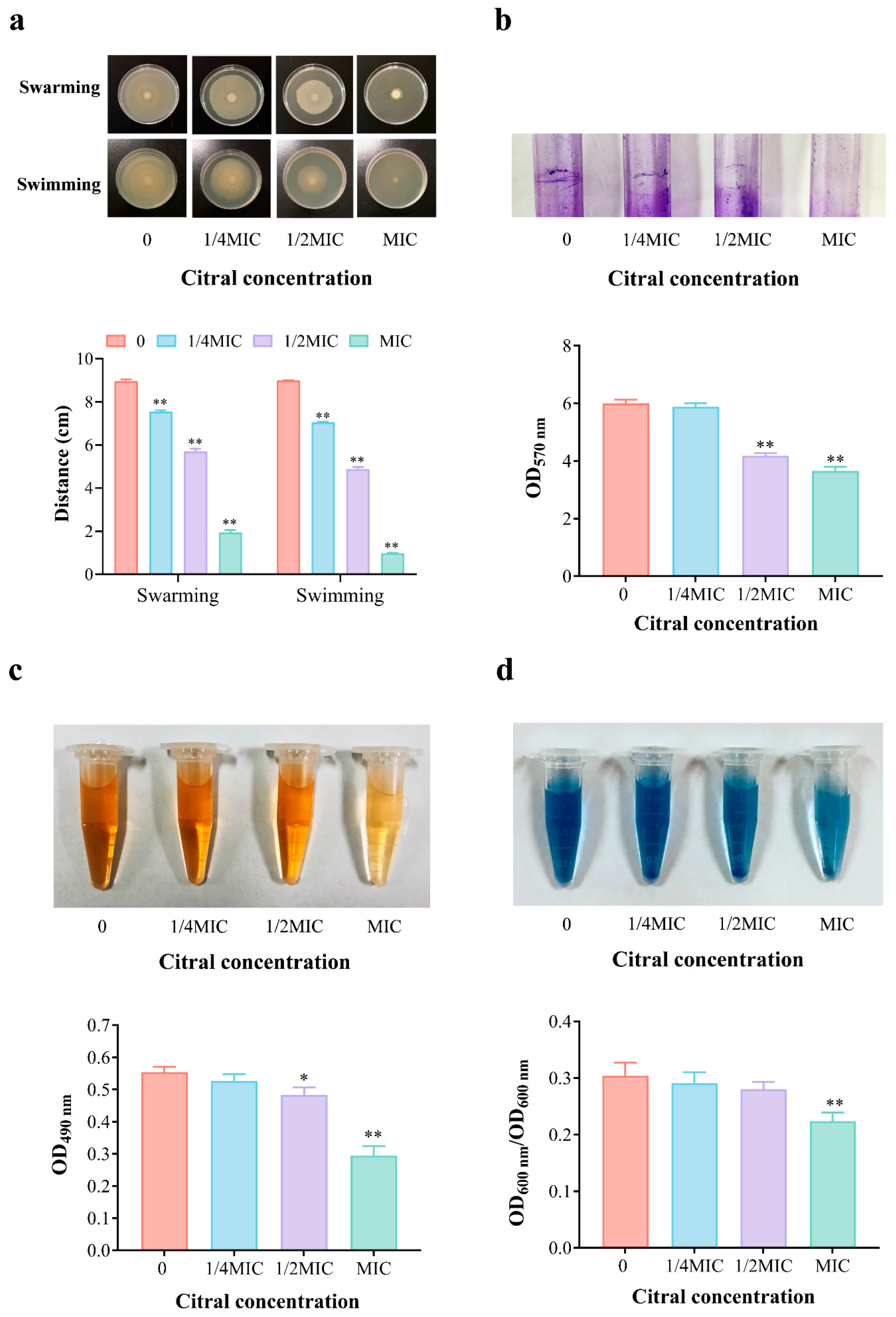
3.6. Effect of Citral Nano-Emulsion on Virulence of V. parahaemolyticus
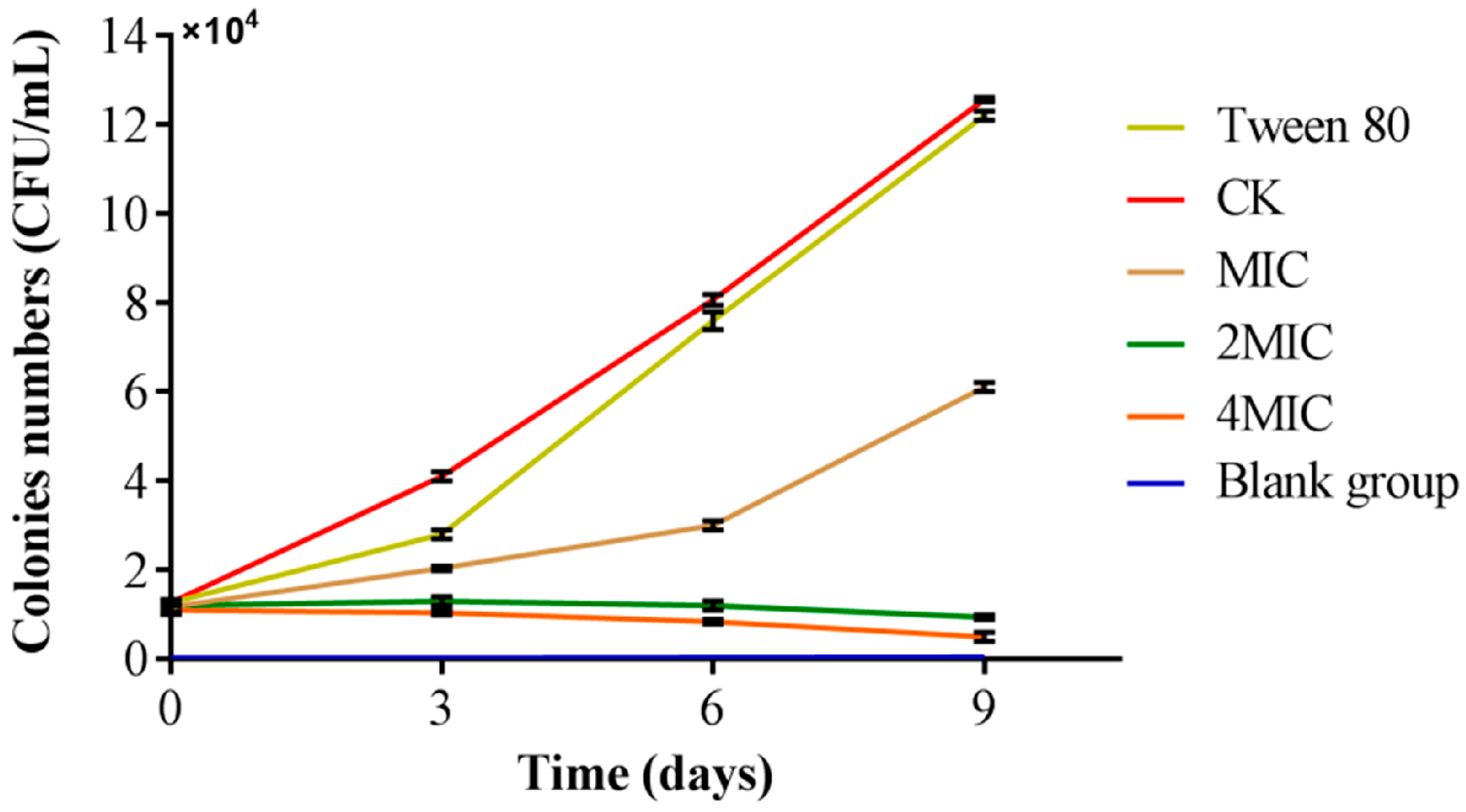
3.7. Analysis of the Preservation Effect of Citral Nano-Emulsion on Salmon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Wang, Z.; Huang, X.; Hu, X.; Li, Y.; Zhou, Y.; Wang, X.; Zhang, R.; Wei, X.; Zhai, X.; et al. H-bond modulation mechanism for moisture-driven bacteriostat evolved from phytochemical formulation. Adv. Funct. Mater. 2023, 34, 2312053. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, Y.; Li, F.; Chen, L.; Huang, X. Analysis of the antibacterial properties of compound essential oil and the main antibacterial components of unilateral essential oils. Molecules 2023, 28, 6304. [Google Scholar] [CrossRef]
- Manju, S.; Malaikozhundan, B.; Withyachumnarnkul, B.; Vaseeharan, B. Essential oils of Nigella sativa protects Artemia from the pathogenic effect of Vibrio parahaemolyticus Dahv2. J. Invertebr. Pathol. 2016, 136, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Hu, M.; Zhang, Y.; Niu, C.; Yue, T.; Yuan, Y.; Wang, Z. Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT-Food Sci. Technol. 2019, 106, 50–56. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, M.M.; Torres-Moreno, H.; Flores-Lopez, M.L.; Velázquez Guadarrama, N.; Ayala-Zavala, J.F.; Ortega-Ramírez, L.A.; López-Romero, J.C. Mechanisms and applications of citral’s antimicrobial properties in food preservation and pharmaceuticals formulations. Antibiotics 2023, 12, 1608. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Cao, J.; Jiang, H.; Yao, J.; Gong, G.; Chen, X.; Xu, W.; He, X. Antimicrobial activity and virulence attenuation of citral against the fish pathogen Vibrio alginolyticus. Aquaculture 2020, 515, 734578. [Google Scholar] [CrossRef]
- Ju, J.; Lei, Y.; Guo, Y.; Yu, H.; Cheng, Y.; Yao, W. Eugenol and citral kills Aspergillus niger through the tricarboxylic acid cycle and its application in food preservation. LWT-Food Sci. Technol. 2023, 173, 114226. [Google Scholar] [CrossRef]
- López-Romero, J.C.; García-Dávila, J.; Peña-Ramos, E.A.; González-Ríos, H.; Valenzuela-Melendres, M.; Osoria, M.; Juneja, V.K. Effect of citral on the thermal inactivation of Escherichia coli O104:H4 in ground beef. J. Food Prot. 2022, 85, 1635–1639. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Zhang, R.; Yao, W. Synergistic inhibition effect of citral and eugenol against Aspergillus niger and their application in bread preservation. Food Chem. 2020, 310, 125974. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Edible coatings enriched with essential oils for extending the shelf-life of ‘Bravo de Esmolfe’ fresh-cut apples. Int. J. Food Sci. Technol. 2015, 51, 87–95. [Google Scholar] [CrossRef]
- Chien, S.-Y.; Sheen, S.; Sommers, C.; Sheen, L.-Y. Modeling the inactivation of Escherichia coli O157:H7 and Uropathogenic E. coli in ground beef by high pressure processing and citral. Food Control 2017, 73, 672–680. [Google Scholar] [CrossRef]
- Kim, J.M.; Marshall, M.; Cornell, J.A.; Preston-iii, J.F.; Wei, C.I. Antibacterial activity of carvacrol, citral, and geraniol against Salmonella Typhimurium in culture medium and on fish cubes. J. Food Sci. 1995, 60, 1364–1368. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Xing, Z.; Liu, X.; Bai, X.; Yang, Y.; Guo, D.; Xia, X.; Zhang, C.; Shi, C. Antibacterial effect of citral on yersinia enterocolitica and its mechanism. Food Control 2022, 135, 108775. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B 2014, 114, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Pey, C.M.; Maestro, A.; Solé, I.; González, C.; Solans, C.; Gutiérrez, J.M. Optimization of nano-emulsions prepared by low-energy emulsification methods at constant temperature using a factorial design study. Colloids Surf. A 2006, 288, 144–150. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent insights into nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Özogul, Y.; Durmus, M.; Ucar, Y.; Özogul, F.; Regenstein, J.M. Comparative study of nanoemulsions based on commercial oils (sunflower, canola, corn, olive, soybean, and hazelnut oils): Effect on microbial, sensory, and chemical qualities of refrigerated farmed sea bass. Innov. Food Sci. Emerg. Technol. 2016, 33, 422–430. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef]
- Ambrosone, L.; Mosca, M.; Ceglie, A. Impact of edible surfactants on the oxidation of olive oil in water-in-oil emulsions. Food Hydrocoll. 2007, 21, 1163–1171. [Google Scholar] [CrossRef]
- Katsouli, M.; Polychniatou, V.; Tzia, C. Influence of surface-active phenolic acids and aqueous phase ratio on w/o nano-emulsions properties; Model fitting and prediction of nano-emulsions oxidation stability. J. Food Eng. 2017, 214, 40–46. [Google Scholar] [CrossRef]
- Suwannasang, S.; Thumthanaruk, B.; Zhong, Q.; Uttapap, D.; Puttanlek, C.; Vatanyoopaisarn, S.; Rungsardthong, V. The improved properties of zein encapsulating and stabilizing sacha inchi oil by surfactant combination of lecithin and Tween 80. Food Bioprocess Technol. 2021, 14, 2078–2090. [Google Scholar] [CrossRef]
- Riquelme, N.; Zúñiga, R.N.; Arancibia, C. Physical stability of nanoemulsions with emulsifier mixtures: Replacement of tween 80 with quillaja saponin. LWT-Food Sci. Technol. 2019, 111, 760–766. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Braschi, G.; Lanciotti, R. Combined use of natural antimicrobial based nanoemulsions and ultra high pressure homogenization to increase safety and shelf-life of apple juice. Food Control 2020, 111, 107051. [Google Scholar] [CrossRef]
- Chong, W.-T.; Tan, C.-P.; Cheah, Y.-K.; Lajis, A.F.B.; Habi Mat Dian, N.L.; Kanagaratnam, S.; Lai, O.-M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS ONE 2018, 13, e0202771. [Google Scholar] [CrossRef]
- Zhang, X.; Song, R.; Liu, X.; Xu, Y.; Wei, R. Fabrication of vitamin D3 nanoemulsions stabilized by Tween 80 and Span 80 as a composite surface-active surfactant: Characterization and stability. Colloids Surf. A 2022, 645, 128873. [Google Scholar] [CrossRef]
- Hou, K.; Xu, Y.; Cen, K.; Gao, C.; Feng, X.; Tang, X. Nanoemulsion of cinnamon essential oil Co-emulsified with hydroxypropyl-β-cyclodextrin and Tween-80: Antibacterial activity, stability and slow release performance. Food Biosci. 2021, 43, 101232. [Google Scholar] [CrossRef]
- Cao, J.; Liu, H.; Wang, Y.; He, X.; Jiang, H.; Yao, J.; Xia, F.; Zhao, Y.; Chen, X. Antimicrobial and antivirulence efficacies of citral against foodborne pathogen Vibrio parahaemolyticus RIMD2210633. Food Control 2021, 120, 107507. [Google Scholar] [CrossRef]
- Nouri, M.; Baghaee-Ravari, S.; Emadzadeh, B. Nano-emulsified savory and thyme formulation show limited efficacy to suppress Pectobacterium carotovorum subsp. carotovorum compared with pure oil. Ind. Crop. Prod. 2021, 161, 113216. [Google Scholar] [CrossRef]
- Pinto, L.d.A.; Machado, F.P.; Esteves, R.; Farias, V.M.; Köptcke, F.B.N.; Ricci-Junior, E.; Rocha, L.; Keller, L.A.M. Characterization and inhibitory effects of essential oil and nanoemulsion from Ocotea indecora (Shott) Mez in Aspergillus species. Molecules 2023, 28, 3437. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yan, P.; Yin, X.; Shu, Z.; Mintah, B.K.; He, R.; Ma, H. Surfactin and its antibacterial mechanism on Staphylococcus aureus and application in pork preservation. Food Bioprocess Technol. 2024, 18, 1311–1324. [Google Scholar] [CrossRef]
- Yang, H.; Zhan, X.; Song, L.; Cheng, S.; Su, R.; Zhang, Y.; Guo, D.; Lü, X.; Xia, X.; Shi, C. Synergistic antibacterial and anti-biofilm mechanisms of ultrasound combined with citral nanoemulsion against Staphylococcus aureus 29213. Int. J. Food Microbiol. 2023, 391-393, 110150. [Google Scholar] [CrossRef]
- Sugumar, S.; Ghosh, V.; Nirmala, M.J.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: Antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. Sonochem. 2014, 21, 1044–1049. [Google Scholar] [CrossRef]
- Zhong, W.; Tang, P.; Liu, T.; Zhao, T.; Guo, J.; Gao, Z. Linalool nanoemulsion preparation, characterization and antimicrobial activity against Aeromonas hydrophila. Int. J. Mol. Sci. 2021, 22, 11003. [Google Scholar] [CrossRef]
- Moghimi, R.; Aliahmadi, A.; McClements, D.J.; Rafati, H. Investigations of the effectiveness of nanoemulsions from sage oil as antibacterial agents on some food borne pathogens. LWT-Food Sci. Technol. 2016, 71, 69–76. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, S.; Liu, Z.; Zhou, G.; Zhang, X.; Kang, S.; Hua, Q.; Wu, Y.; Liu, Z. Utilization of nanoemulsion to enhance antibacterial capacity of Zanthoxylum bungeanum pericarp essential oils against foodborne pathogenic bacteria. LWT-Food Sci. Technol. 2024, 207, 116657. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Shemesh, M. The bacillary postbiotics, including 2-Undecanone, suppress the virulence of pathogenic microorganisms. Pharmaceutics 2022, 14, 962. [Google Scholar] [CrossRef]
- Lou, Z.; Letsididi, K.S.; Yu, F.; Pei, Z.; Wang, H.; Letsididi, R. Inhibitive effect of eugenol and its nanoemulsion on quorum sensing–mediated virulence factors and biofilm formation by Pseudomonas aeruginosa. J. Food Prot. 2019, 82, 379–389. [Google Scholar] [CrossRef]
- da Silva, L.B.; Menezes, M.C.; Kitano, E.S.; Oliveira, A.K.; Abreu, A.G.; Souza, G.O.; Heinemann, M.B.; Isaac, L.; Fraga, T.R.; Serrano, S.M.T.; et al. Leptospira interrogans secreted proteases degrade extracellular matrix and plasma proteins from the host. Front. Cell. Infect. Microbiol. 2018, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kang, X.; Zhou, W.; Lee, J.; Wang, S.; Cui, Z.; Zhang, H.; Mo, H.; Hu, L. Ferrous sulfate efficiently kills Vibrio parahaemolyticus and protects salmon sashimi from its contamination. Int. J. Food Microbiol. 2022, 382, 109929. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Peng, S.; Wei, S.; Lei, Y.; Zhang, S.; Hu, Y.; Lv, Y. Antimicrobial and biofilm inhibition effects of p-anisaldehyde against Vibrio parahaemolyticus. Food Control 2023, 154, 110021. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Bai, B. Nanoemulsion-based edible coatings loaded with fennel essential oil/cinnamaldehyde: Characterization, antimicrobial property and advantages in pork meat patties application. Food Control 2021, 127, 108151. [Google Scholar] [CrossRef]
| Experiment Number | A Citral Content/(%) | B Tween 80 Content/(%) | C Pressure/(bar) | Mean Particle Size/(d.nm) | PDI |
|---|---|---|---|---|---|
| 1 | 8.00 | 10.00 | 800 | 33.57 | 0.263 |
| 2 | 8.00 | 8.00 | 700 | 51.66 | 0.374 |
| 3 | 8.00 | 12.00 | 900 | 49.98 | 0.431 |
| 4 | 6.00 | 10.00 | 900 | 42.35 | 0.383 |
| 5 | 10.00 | 10.00 | 700 | 46.35 | 0.49 |
| 6 | 8.00 | 10.00 | 800 | 32.51 | 0.203 |
| 7 | 8.00 | 10.00 | 800 | 33.95 | 0.251 |
| 8 | 6.00 | 12.00 | 800 | 45.35 | 0.429 |
| 9 | 10.00 | 10.00 | 900 | 49.11 | 0.415 |
| 10 | 6.00 | 10.00 | 700 | 52.99 | 0.28 |
| 11 | 10.00 | 8.00 | 800 | 38.31 | 0.359 |
| 12 | 8.00 | 8.00 | 900 | 46.03 | 0.408 |
| 13 | 8.00 | 12.00 | 700 | 53.01 | 0.539 |
| 14 | 6.00 | 8.00 | 800 | 43.33 | 0.293 |
| 15 | 8.00 | 10.00 | 800 | 33.92 | 0.261 |
| 16 | 10.00 | 12.00 | 800 | 43.59 | 0.376 |
| 17 | 8.00 | 10.00 | 800 | 33.26 | 0.289 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 859.92 | 9 | 95.55 | 84.32 | <0.0001 | Significant |
| A-Citral | 5.54 | 1 | 5.54 | 4.89 | 0.0626 | - |
| B-Tween 80 | 19.85 | 1 | 19.85 | 17.51 | 0.0041 | - |
| C-Pressure | 34.20 | 1 | 34.20 | 30.18 | 0.0009 | - |
| AB | 2.66 | 1 | 2.66 | 2.34 | 0.1696 | - |
| AC | 44.89 | 1 | 44.89 | 39.62 | 0.0004 | - |
| BC | 1.69 | 1 | 1.69 | 1.49 | 0.2615 | - |
| A2 | 47.72 | 1 | 47.72 | 42.11 | 0.0003 | - |
| B2 | 143.43 | 1 | 143.43 | 126.58 | <0.0001 | - |
| C2 | 499.47 | 1 | 499.47 | 440.79 | <0.0001 | - |
| Residual | 7.93 | 7 | 1.13 | - | - | - |
| Lack of fit | 6.53 | 3 | 2.18 | 6.20 | 0.0552 | Not significant |
| Pure error | 1.40 | 4 | 0.35 | - | - | - |
| Cor total | 867.86 | 16 | - | - | - | - |
| R2 = 0.9909 R2adj = 0.9791 | ||||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.12 | 9 | 0.014 | 8.08 | 0.0058 | Significant |
| A-Citral | 8.128 × 10−3 | 1 | 8.128 × 10−3 | 4.75 | 0.0656 | - |
| B-Tween 80 | 0.015 | 1 | 0.015 | 8.50 | 0.0225 | - |
| C-Pressure | 2.645 × 10−4 | 1 | 2.645 × 10−4 | 0.15 | 0.7058 | - |
| AB | 3.540 × 10−3 | 1 | 3.540 × 10−3 | 2.07 | 0.1934 | - |
| AC | 7.921 × 10−3 | 1 | 7.921 × 10−3 | 4.63 | 0.0684 | - |
| BC | 5.041 × 10−3 | 1 | 5.041 × 10−3 | 2.95 | 0.1297 | - |
| A2 | 4.427 × 10−3 | 1 | 4.427 × 10−3 | 2.59 | 0.1517 | - |
| B2 | 0.026 | 1 | 0.026 | 15.14 | 0.0060 | - |
| C2 | 0.047 | 1 | 0.047 | 27.75 | 0.0012 | - |
| Residual | 0.012 | 7 | 1.710 × 10−3 | - | - | - |
| Lack of fit | 8.009 × 10−3 | 3 | 2.670 × 10−3 | 2.69 | 0.1812 | Not significant |
| Pure error | 3.963 × 10−3 | 4 | 9.908 × 10−4 | - | - | - |
| Cor total | 0.14 | 16 | - | - | - | - |
| R2 = 0.9122 R2adj = 0.8741 | ||||||
| Experiment Times | Particle Size/(nm) * | PDI |
|---|---|---|
| 1 | 35.53 ± 1.48 | 0.238 ± 0.112 |
| 2 | 32.64 ± 2.36 | 0.259 ± 0.045 |
| 3 | 37.47 ± 1.61 | 0.264 ± 0.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zhang, X.; Qi, Z.; Liu, H. Evaluating the Antimicrobial Efficacy of Citral Nano-Emulsion Against Vibrio parahaemolyticus. Foods 2025, 14, 3272. https://doi.org/10.3390/foods14183272
Cao J, Zhang X, Qi Z, Liu H. Evaluating the Antimicrobial Efficacy of Citral Nano-Emulsion Against Vibrio parahaemolyticus. Foods. 2025; 14(18):3272. https://doi.org/10.3390/foods14183272
Chicago/Turabian StyleCao, Juanjuan, Xiaoxu Zhang, Zihe Qi, and Huan Liu. 2025. "Evaluating the Antimicrobial Efficacy of Citral Nano-Emulsion Against Vibrio parahaemolyticus" Foods 14, no. 18: 3272. https://doi.org/10.3390/foods14183272
APA StyleCao, J., Zhang, X., Qi, Z., & Liu, H. (2025). Evaluating the Antimicrobial Efficacy of Citral Nano-Emulsion Against Vibrio parahaemolyticus. Foods, 14(18), 3272. https://doi.org/10.3390/foods14183272





