Solid Dispersion of Hesperidin Alleviates Acetic Acid-Induced Colitis Through Modulating the Gut Microbiota Dysbiosis in Rats
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Preparation of HD Solid Dispersion by Ball Milling Method
2.3. The Characterization of HD Solid Dispersions
2.3.1. Powder X-Ray Diffraction (PXRD)
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Study on Dissolution In Vitro
2.5. Stability Analysis
2.6. In Vivo Pharmacokinetic Assay
2.7. AA-Induced Acute Colitis Rats
2.7.1. Animals and Experimental Design
2.7.2. Assessment of the DAI (Disease Activity Index) and Sample Collection
2.7.3. ELISA
2.7.4. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. The Characterization of HD-SD
3.1.1. PXRD Analysis
3.1.2. DSC Analysis
3.1.3. FT-IR Spectrum Analysis
3.2. Powder Dissolution
3.3. Stability of the Samples
3.4. Pharmacokinetic Characteristics
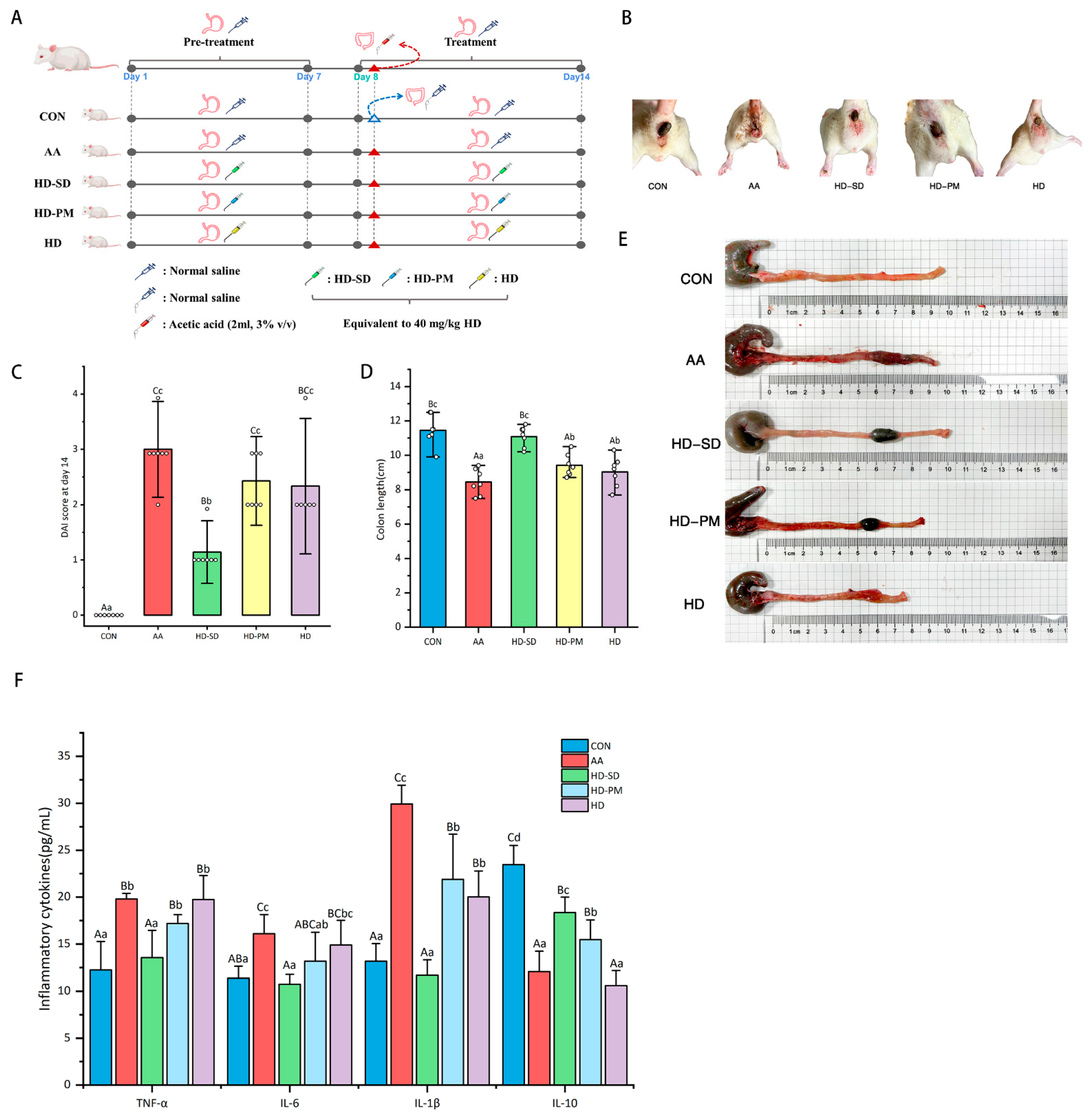
3.5. HD-SD Enhanced the Amelioration of Ameliorated AA-Induced Acute Colitis
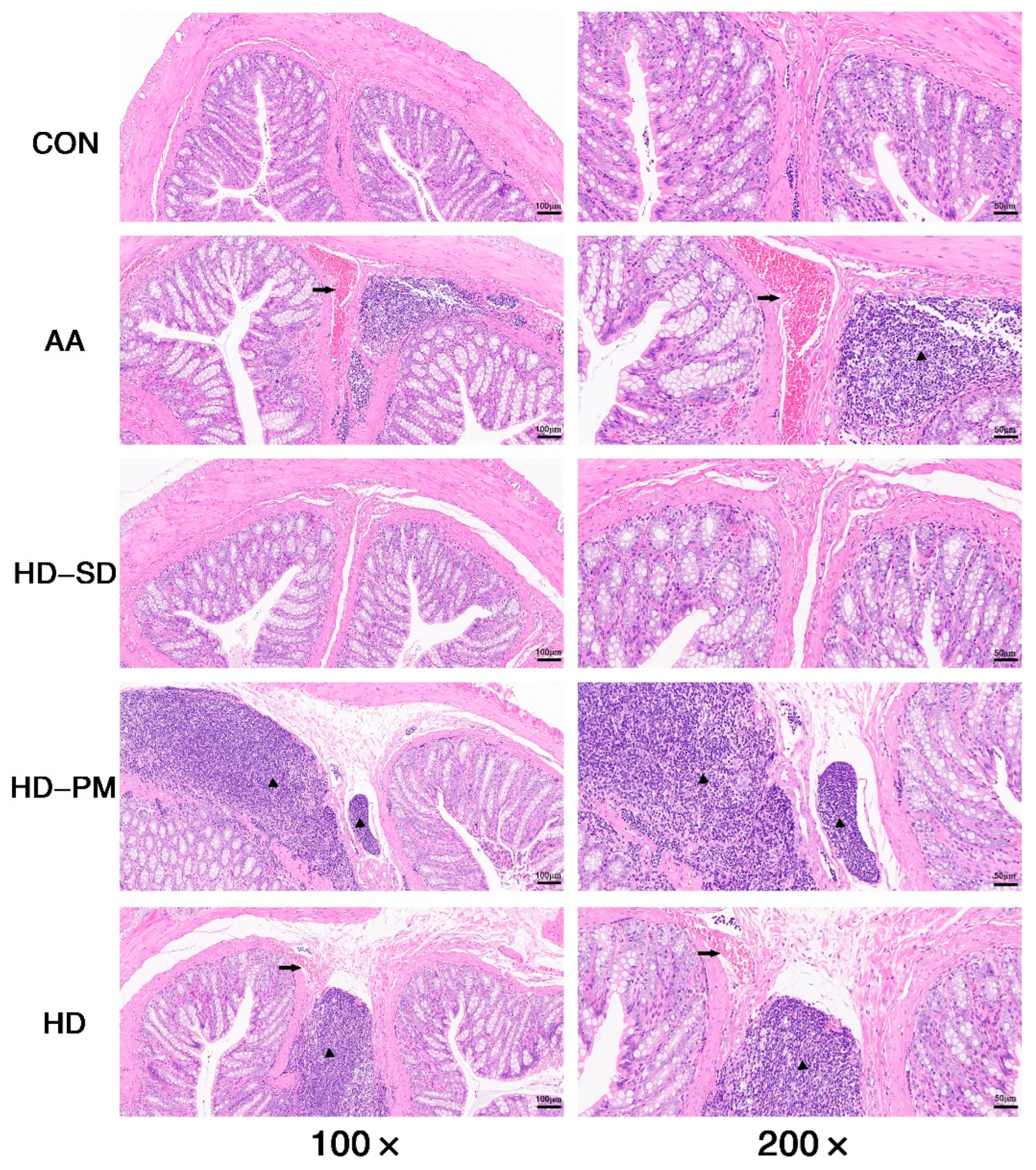
3.5.1. Assessment of DAI and Colonic Damage
3.5.2. Examination of Anti-Inflammatory Effects
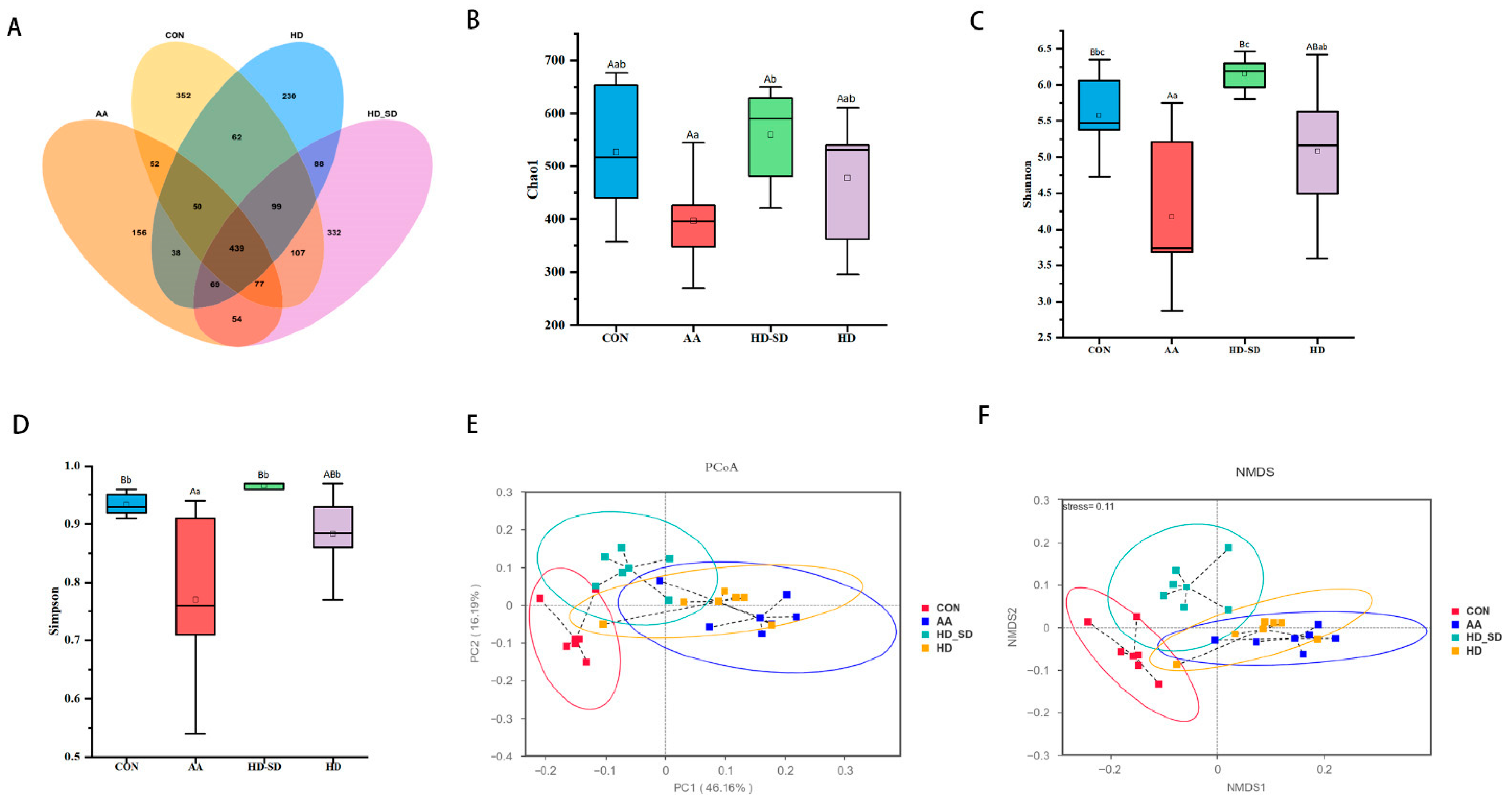
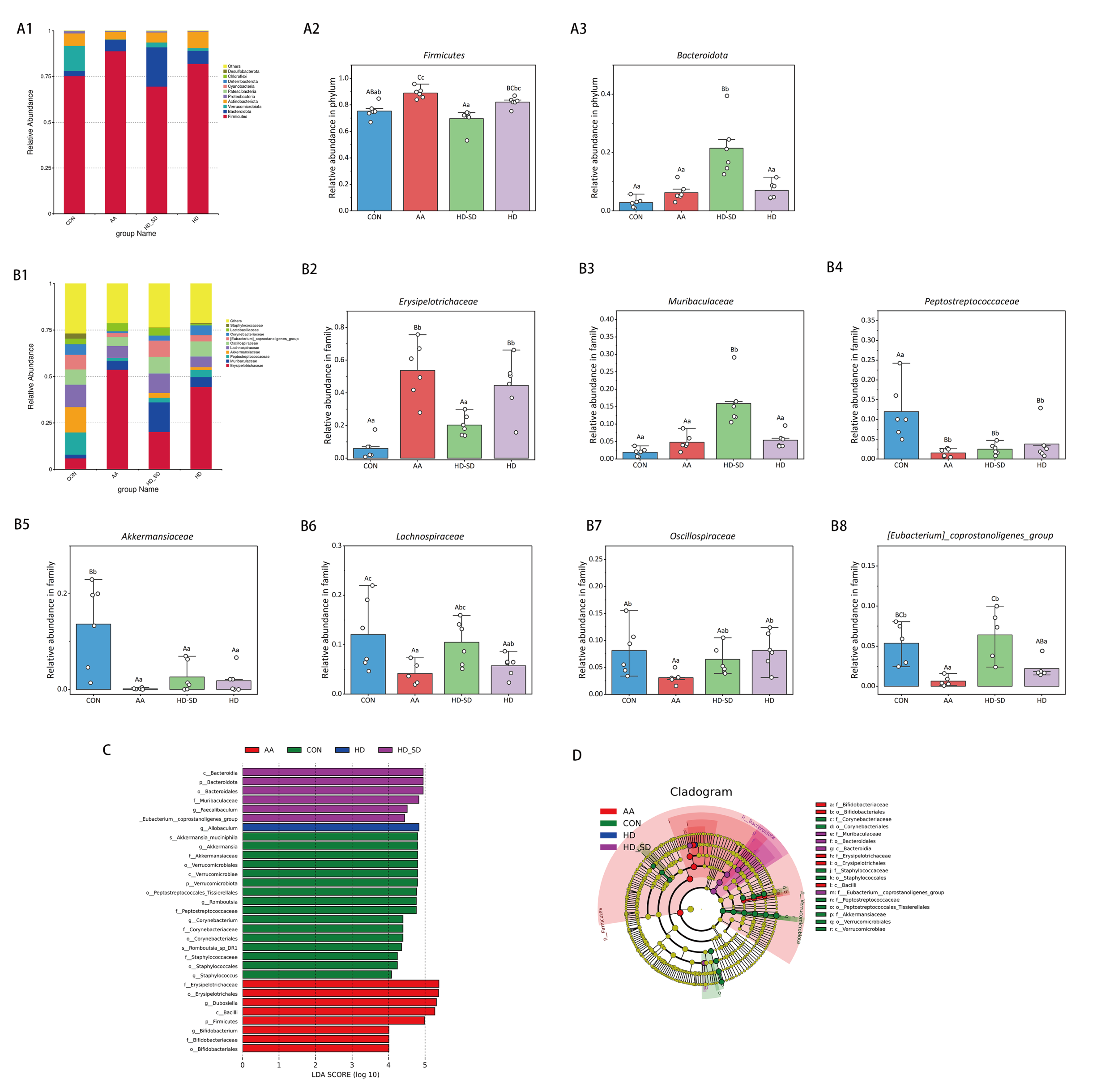
3.5.3. HD-SD Administration Modulated Gut Dysbiosis in Colitic Rat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hong, C.J.; Chen, S.Y.; Hsu, Y.H.; Yen, G.C. Protective effect of fermented okara on the regulation of inflammation, the gut microbiota, and SCFAs production in rats with TNBS-induced colitis. Food Res. Int. 2022, 157, 111390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Atractylodes macrocephala Koidz. volatile oil relieves acute ulcerative colitis via regulating gut microbiota and gut microbiota metabolism. Front. Immunol. 2023, 14, 1127785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Xu, H.M.; Liang, Y.J.; Xu, J.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Yao, J.; Wang, L.S.; Nie, Y.Q.; et al. Edible exosome-like nanoparticles from Portulaca oleracea L. mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+T cells expansion. J. Nanobiotechnol. 2023, 21, 309. [Google Scholar] [CrossRef]
- Szymon, S.; Anna, S.; Andrzej, M.; Marcin, Ż.; Judyta, C. Zein as an Effective Carrier for Hesperidin Delivery Systems with Improved Prebiotic Potential. Molecules 2023, 28, 5209. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Xia, S.; Chen, C.; Chen, X.; Zhang, Y.; Farag, M.A.; Xiao, J.; Nie, S. Celery soluble dietary fiber antagonize flavonoids ameliorative effect on dextran-sodium-sulfate-induced colitis in mice. J. Adv. Res. 2023, 52, 73–88. [Google Scholar] [CrossRef]
- Wan, L.; Qian, C.; Yang, C.; Peng, S.; Dong, G.; Cheng, P.; Zong, G.; Han, H.; Shao, M.; Gong, G.; et al. Ginseng polysaccharides ameliorate ulcerative colitis via regulating gut microbiota and tryptophan metabolism. Int. J. Biol. Macromol. 2024, 265, 130822. [Google Scholar] [CrossRef]
- Sarah, M.N.; Peddha, M.S.; Kempaiah, B.B.; Singh, N.P. Efficacy of a functional food ingredient from Ensete superbum Roxb. Cheesman peel in reducing the severity of ulcerative colitis in a murine model. Food Funct. 2022, 13, 3732–3745. [Google Scholar] [CrossRef]
- Khalilabad, S.N.; Mirzaei, A.; Askari, V.R.; Mirzaei, A.; Khademi, R.; Rahimi, V.B. How hesperidin and Hesperetin, as promising food Supplements, combat cardiovascular Diseases: A systematic review from bench to bed. J. Funct. Foods 2024, 120, 106358. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Pal, S.; Mohamed, R.; Singh, P.; Chattopadhyay, S.; China, S.P.; Porwal, K.; Sanyal, S.; Gayen, J.R.; Chattopadhyay, N. A nutraceutical composition containing diosmin and hesperidin has osteogenic and anti-resorptive effects and expands the anabolic window of teriparatide. Biomed. Pharmacother. 2019, 118, 109207. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef]
- SungSook, C.; SunHyung, L.; KyungAe, L. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, S.; Hu, H.; Hu, T.; Shi, K.; Xu, Y.; Xu, G.; Hu, H.; Pan, S. Synthesis and evaluation of novel hesperidin selenium- enriched derivatives as potential anti-inflammatory and antioxidant agents. Food Biosci. 2024, 58, 103651. [Google Scholar] [CrossRef]
- He, D.; Gao, X.; Wen, J.; Zhang, Y.; Yang, S.; Sun, X.; Cui, M.; Li, Z.; Fu, S.; Liu, J.; et al. Orally administered neohesperidin attenuates MPTP-induced neurodegeneration by inhibiting inflammatory responses and regulating intestinal flora in mice. Food Funct. 2024, 15, 1460–1475. [Google Scholar] [CrossRef]
- Li, X.; Yao, Y.; Wang, Y.; Hua, L.; Wu, M.; Chen, F.; Deng, Z.-Y.; Luo, T. Effect of Hesperidin Supplementation on Liver Metabolomics and Gut Microbiota in a High-Fat Diet-Induced NAFLD Mice Model. J. Agric. Food Chem. 2022, 70, 11224–11235. [Google Scholar] [CrossRef] [PubMed]
- Radha, G.; Raghunandhakumar, S.; Balakumar, S. Dual therapeutic 5-fluorouracil and hesperidin loaded chitosan nanocarrier system: Understanding its synergism on anti-cancer activity. J. Drug Deliv. Sci. Technol. 2023, 80, 104184. [Google Scholar] [CrossRef]
- Guo, K.; Ren, J.; Gu, G.; Wang, G.; Gong, W.; Wu, X.; Ren, H.; Hong, Z.; Li, J. Hesperidin Protects Against Intestinal Inflammation by Restoring Intestinal Barrier Function and Up-Regulating Treg Cells. Mol. Nutr. Food Res. 2020, 64, e1970058. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Massot-Cladera, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Hesperidin Effects on Gut Microbiota and Gut-Associated Lymphoid Tissue in Healthy Rats. Nutrients 2019, 11, 324. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Huang, Y.; Niu, Y.; Zhang, L.; Xiong, T.; Zhang, Y.; Zhang, R.; Zhang, H. Preparation and in vitro evaluation of hesperidin nanoparticles by antisolvent recrystallization in a double homogenate system. Food Chem. X 2023, 18, 100639. [Google Scholar] [CrossRef]
- Natalia, R.; Kamil, W.; Ewa, T.; Judyta, C. Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases. Int. J. Mol. Sci. 2022, 23, 15198. [Google Scholar] [CrossRef]
- Rajendra, J.; Osman, E.G.; Sulekha, K.; Deependra, S.; Manju, S.; Kumar, S.R.; Jiyauddin, K. Hesperidin-Loaded Lipid Polymer Hybrid Nanoparticles for Topical Delivery of Bioactive Drugs. Pharmaceuticals 2022, 15, 211. [Google Scholar] [CrossRef]
- Long, J.; Song, J.; Zhang, X.; Deng, M.; Xie, L.; Zhang, L.; Li, X. Tea saponins as natural stabilizers for the production of Hesperidin nanosuspensions. Int. J. Pharm. 2020, 583, 119406. [Google Scholar] [CrossRef] [PubMed]
- Kamil, W.; Natalia, R.; Ewa, T.; Marcin, Ż.; Anita, P.; Wojciech, P.; Judyta, C. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar]
- Li, Y.; Xu, J.; Guan, Q.; Zhang, H.; Ding, Z.; Wang, Q.; Wang, Z.; Han, J.; Liu, M.; Zhao, Y. Impact of hypromellose acetate succinate and Soluplus® on the performance of β-carotene solid dispersions with the aid of sorbitan monolaurate: In vitro-in vivo comparative assessment. Int. J. Biol. Macromol. 2023, 253, 126639. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhang, Y.; Fan, Y.; Li, S.; Gao, J.; He, X.; Zhao, X. Solid Dispersions of Genistein via Solvent Rotary Evaporation for Improving Solubility, Bioavailability, and Amelioration Effect in HFD-Induced Obesity Mice. Pharmaceutics 2024, 16, 306. [Google Scholar] [CrossRef]
- Han, J.; Tong, M.; Li, S.; Yu, X.; Hu, Z.; Zhang, Q.; Xu, R.; Wang, J. Surfactant-free amorphous solid dispersion with high dissolution for bioavailability enhancement of hydrophobic drugs: A case of quercetin. Drug Dev. Ind. Pharm. 2021, 47, 153–162. [Google Scholar] [CrossRef]
- Gao, J.; Fan, Y.; Lu, C.; Zhao, X.; He, X. The baicalein amorphous solid dispersion to enhance the dissolution and bioavailability and effects on growth performance, meat quality, antioxidant capacity and intestinal flora in Taihang chickens. Poult. Sci. 2024, 103, 103768. [Google Scholar] [CrossRef]
- Mahdy, R.N.E.; Nader, M.A.; Helal, M.G.; Abu-Risha, S.E.; Abdelmageed, M.E. Eicosapentaenoic acid mitigates ulcerative colitis-induced by acetic acid through modulation of NF-κB and TGF-β/EGFR signaling pathways. Life Sci. 2023, 327, 121820. [Google Scholar] [CrossRef]
- Tinnirello, V.; Zizzo, M.G.; Conigliaro, A.; Tabone, M.; Ganji, N.R.; Cicio, A.; Bressa, C.; Larrosa, M.; Rappa, F.; Vergilio, G.; et al. Industrial-produced lemon nanovesicles ameliorate experimental colitis-associated damages in rats via the activation of anti-inflammatory and antioxidant responses and microbiota modification. Biomed. Pharmacother. 2024, 174, 116514. [Google Scholar] [CrossRef]
- Belém, M.O.; de Andrade, G.M.M.; Carlos, T.M.; Guazelli, C.F.S.; Fattori, V.; Toginho Filho, D.O.; Dias, I.F.L.; Verri, W.A., Jr.; Araújo, E.J.A. Light-emitting diodes at 940nm attenuate colitis-induced inflammatory process in mice. J. Photochem. Photobiol. B Biol. 2016, 162, 367–373. [Google Scholar] [CrossRef]
- Guazelli, C.F.; Fattori, V.; Colombo, B.B.; Georgetti, S.R.; Vicentini, F.T.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J. Nat. Prod. 2013, 76, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Labbé, A.; Ganopolsky, J.G.; Martoni, C.J.; Prakash, S.; Jones, M.L. Bacterial bile metabolising gene abundance in Crohn’s, ulcerative colitis and type 2 diabetes metagenomes. PLoS ONE 2014, 9, e115175. [Google Scholar] [CrossRef]
- Ouyang, Q.; Li, X.; Liang, Y.; Liu, R. Sea Buckthorn Polysaccharide Ameliorates Colitis. Nutrients 2024, 16, 1280. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the development of amorphous solid dispersions: The role of polymeric carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, C.; Xiao, Z.; Huang, X.; Xu, J. Enhanced anti-hepatoma effect of a novel curcumin analog C086 via solid dispersion technology. Drug Deliv. 2020, 27, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-R.; Jia, R.-B.; Cai, X.; Luo, D.; Chen, C.; Zhao, M. Characterizations of food-derived ellagic acid-Undaria pinnatifida polysaccharides solid dispersion and its benefits on solubility, dispersity and biotransformation of ellagic acid. Food Chem. X 2023, 413, 135530. [Google Scholar] [CrossRef]
- Shahraki, O.; Shayganpour, M.; Hashemzaei, M.; Daneshmand, S. Solid lipid nanoparticles (SLNs), the potential novel vehicle for enhanced in vivo efficacy of hesperidin as an anti-inflammatory agent. Bioorg. Chem. 2023, 131, 106333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, F.; Cao, Y.; Xu, H.; Xie, Y.; Xiao, X.; Addo, K.A.; Peng, X.-F. Preparation and characterization of a solid dispersion of Hexahydrocolupulone and its application in the preservation of fresh apple juice. Food Chem. X 2023, 424, 136367. [Google Scholar] [CrossRef]
- Nandi, U.; Ajiboye, A.L.; Patel, P.; Douroumis, D.; Trivedi, V. Preparation of solid dispersions of simvastatin and soluplus using a single-step organic solvent-free supercritical fluid process for the drug solubility and dissolution rate enhancement. Pharmaceuticals 2021, 14, 846. [Google Scholar] [CrossRef] [PubMed]
- Suthar, T.; Patel, P.; Singh, P.; Datusalia, A.K.; Yadav, A.K.; Jain, K. Hesperidin microemulsion: Formulation optimization, characterization, and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2023, 80, 130–149. [Google Scholar] [CrossRef]
- Van Duong, T.; Van den Mooter, G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part II: Amorphous carriers. Expert Opin. Drug Deliv. 2016, 13, 1681–1694. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, H.; Wang, Y.; Wang, R.; Xu, J.; Zhang, C. Amorphous Solid Dispersions: Role of the Polymer and Its Importance in Physical Stability and In Vitro Performance. Pharmaceutics 2022, 14, 1747. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Dev. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef]
- Mahdy, R.N.E.; Nader, M.A.; Helal, M.G.; Risha, S.E.A.; Abdelmageed, M.E. Tiron ameliorates acetic acid-induced colitis in rats: Role of TGF-β/EGFR/PI3K/NF-κB signaling pathway. Int. Immunopharmacol. 2024, 128, 111587. [Google Scholar] [CrossRef] [PubMed]
- Niraj, P.; Kumar, B.L. Topotecan alleviates acetic acid-induced ulcerative colitis in rats via attenuation of the RORγT transcription factor. Life Sci. 2023, 328, 121915. [Google Scholar]
- Ge, H.; Cai, Z.; Chai, J.; Liu, J.; Liu, B.; Yu, Y.; Liu, J.; Zhang, T. Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 2021, 360, 129981. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Li, X.; Chen, J. Geniposidic acid attenuates DSS-induced colitis through inhibiting inflammation and regulating gut microbiota. Phytother. Res. PTR 2023, 37, 3453–3466. [Google Scholar] [CrossRef]
- Liu, J.; Cai, J.; Fan, P.; Dong, X.; Zhang, N.; Tai, J.; Cao, Y. Salidroside alleviates dextran sulfate sodium-induced colitis in mice by modulating the gut microbiota. Food Funct. 2023, 14, 7506–7519. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, C.; Wang, Z.; Guan, X.; Ma, Y.; Song, J.; Shen, S.; Song, H.; Li, M.; Liu, C. Preparation and characterisation of baicalin magnesium and its protective effect in ulcerative colitis via gut microbiota-bile acid axis modulation. Phytomedicine 2024, 126, 155416. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Y.; Bai, J.; Li, X.; Ma, L.; Man, S. Litchi Procyanidins Ameliorate DSS-Induced Colitis through Gut Microbiota-Dependent Regulation of Treg/Th17 Balance. J. Agric. Food Chem. 2024, 72, 24823–24832. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Suna, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Chen, C.; Zheng, Y.; Wu, Z.J.; Zhou, M.Q.; Liu, X.Y.; Miyashita, K.; Duan, D.L.; Du, L. Fucoxanthin Alleviates Dextran Sulfate Sodium-Induced Colitis and Gut Microbiota Dysbiosis in Mice. J. Agric. Food Chem. 2024, 72, 4142–4154. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Zhao, J.; Wang, R.; Du, J.; Zhou, C.; Gu, C.; Wang, Y.; Zhang, L.; Zhao, Y.; Lu, N. Eubacterium coprostanoligenes alleviates chemotherapy-induced intestinal mucositis by enhancing intestinal mucus barrier. Acta Pharm. Sin. B 2024, 14, 1677–1692. [Google Scholar] [CrossRef]
- Feng, W.; Liu, J.; Tan, Y.; Ao, H.; Wang, J.; Peng, C. Polysaccharides from Atractylodes macrocephala Koidz. Ameliorate ulcerative colitis via extensive modification of gut microbiota and host metabolism. Food Res. Int. 2020, 138, 109777. [Google Scholar] [CrossRef] [PubMed]

| Items | Group | |
|---|---|---|
| HD | HD-SD | |
| Tmax(0–24) (h) | 1.15 ± 0.34 | 0.96 ± 0.10 |
| Cmax(0–24) (μg/mL) | 0.61 ± 0.14 | 1.63 ± 0.17 ** |
| t1/2(0–24) (h) | 5.35 ±1.69 | 4.30 ± 1.03 |
| AUC0–24 (μg·h/mL) | 4.05 ± 0.55 | 6.06 ±0.81 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, D.; Wu, Q.; Sun, Y.; Ma, N.; He, X.; Zhao, X. Solid Dispersion of Hesperidin Alleviates Acetic Acid-Induced Colitis Through Modulating the Gut Microbiota Dysbiosis in Rats. Foods 2025, 14, 3252. https://doi.org/10.3390/foods14183252
Wang Q, Liu D, Wu Q, Sun Y, Ma N, He X, Zhao X. Solid Dispersion of Hesperidin Alleviates Acetic Acid-Induced Colitis Through Modulating the Gut Microbiota Dysbiosis in Rats. Foods. 2025; 14(18):3252. https://doi.org/10.3390/foods14183252
Chicago/Turabian StyleWang, Qiru, Dan Liu, Qi Wu, Yanling Sun, Ning Ma, Xin He, and Xinghua Zhao. 2025. "Solid Dispersion of Hesperidin Alleviates Acetic Acid-Induced Colitis Through Modulating the Gut Microbiota Dysbiosis in Rats" Foods 14, no. 18: 3252. https://doi.org/10.3390/foods14183252
APA StyleWang, Q., Liu, D., Wu, Q., Sun, Y., Ma, N., He, X., & Zhao, X. (2025). Solid Dispersion of Hesperidin Alleviates Acetic Acid-Induced Colitis Through Modulating the Gut Microbiota Dysbiosis in Rats. Foods, 14(18), 3252. https://doi.org/10.3390/foods14183252






