Comprehensive Evaluation of Quality and Antioxidant Capacity of Highbush Blueberries (Vaccinium corymbosum)
Abstract
1. Introduction
2. Materials and Methods
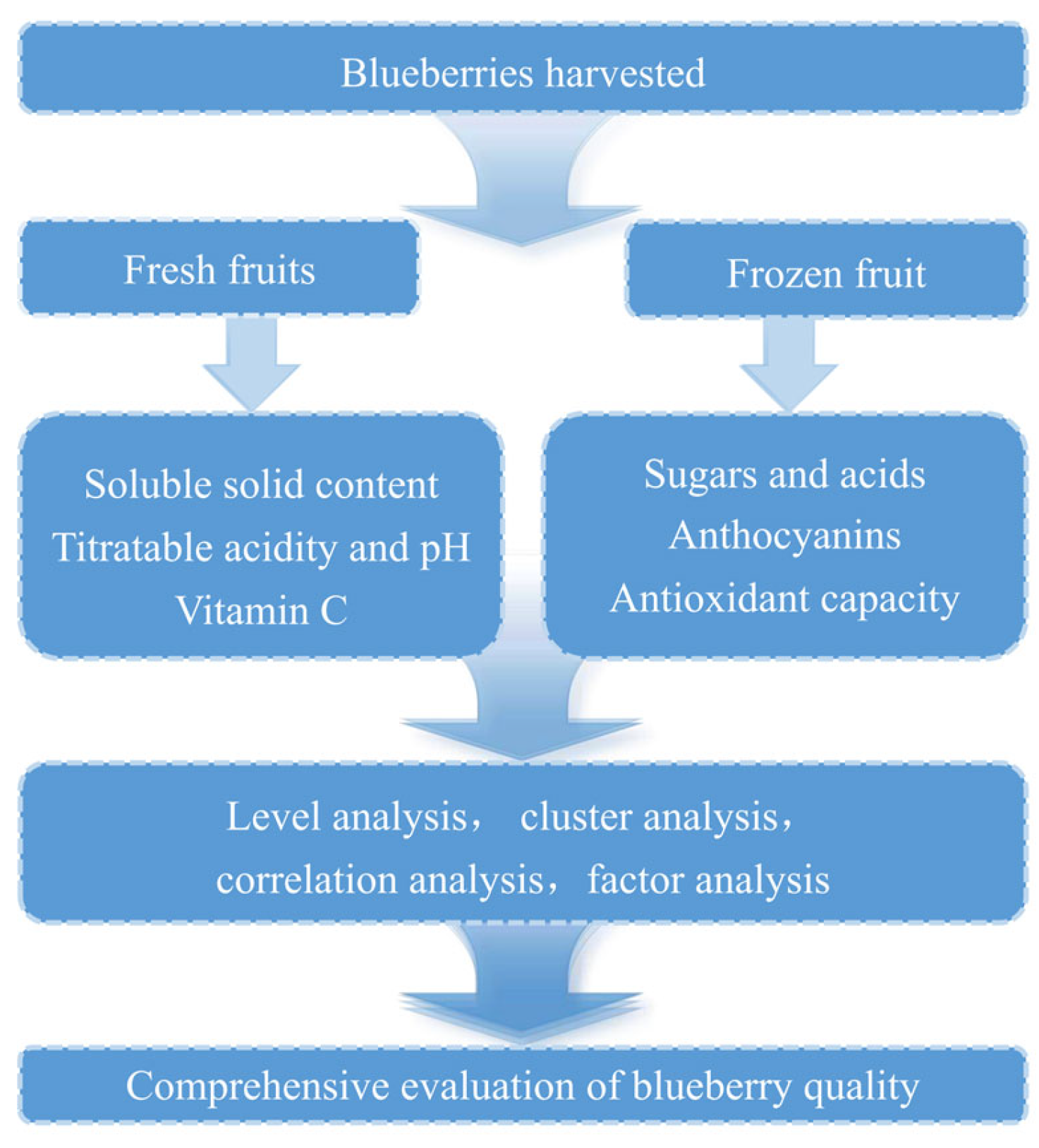
2.1. Preparation of Fruit Materials
2.2. Determination of Fruit Quality Characteristics
2.3. Determination of Major Soluble Sugars and Organic Acids
2.4. Extraction and HPLC-PDA Analysis of Anthocyanins
2.5. Antioxidant Activity Measurements
2.6. Statistical Analysis
3. Results and Discussion
3.1. Quality Characteristics of Fruit
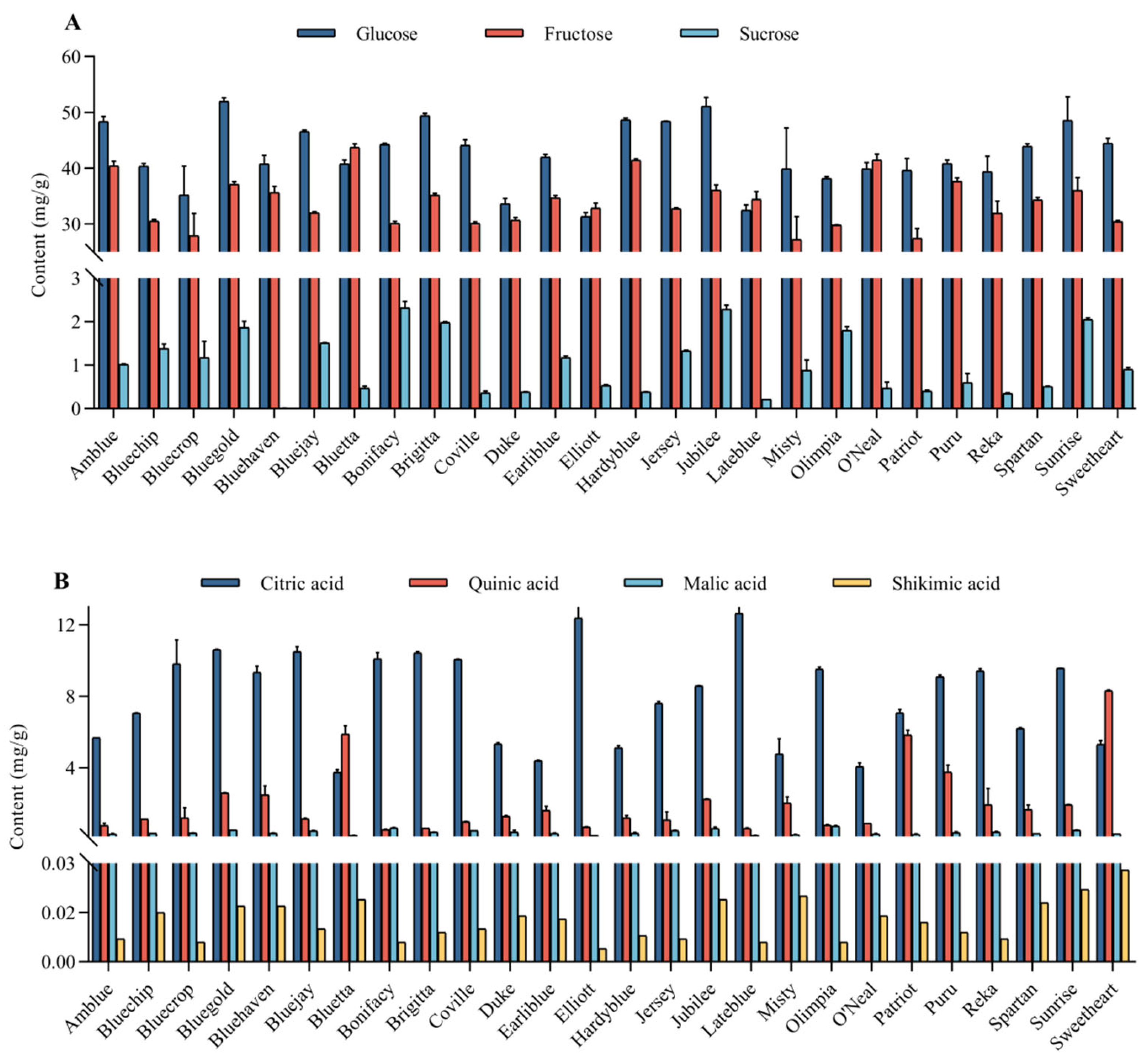
3.2. Major Soluble Sugars and Organic Acids
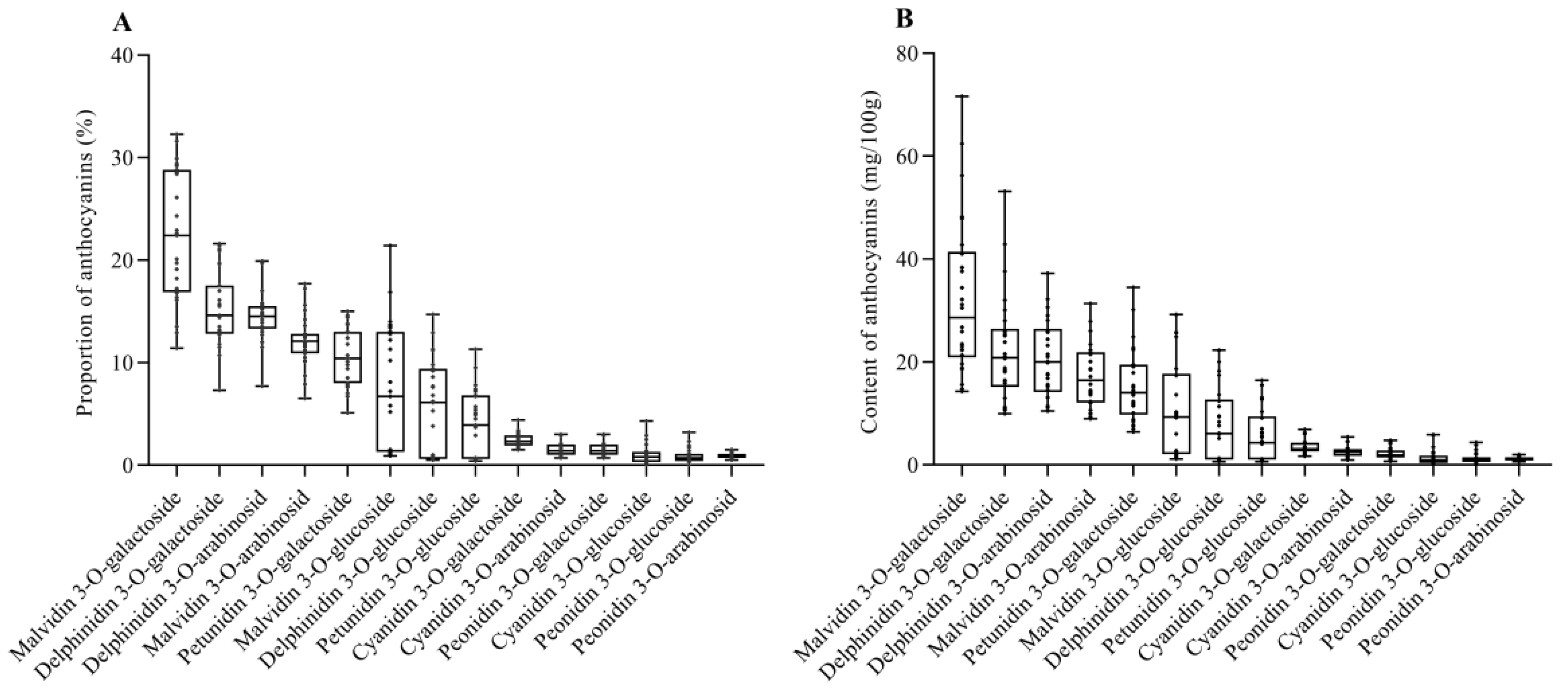
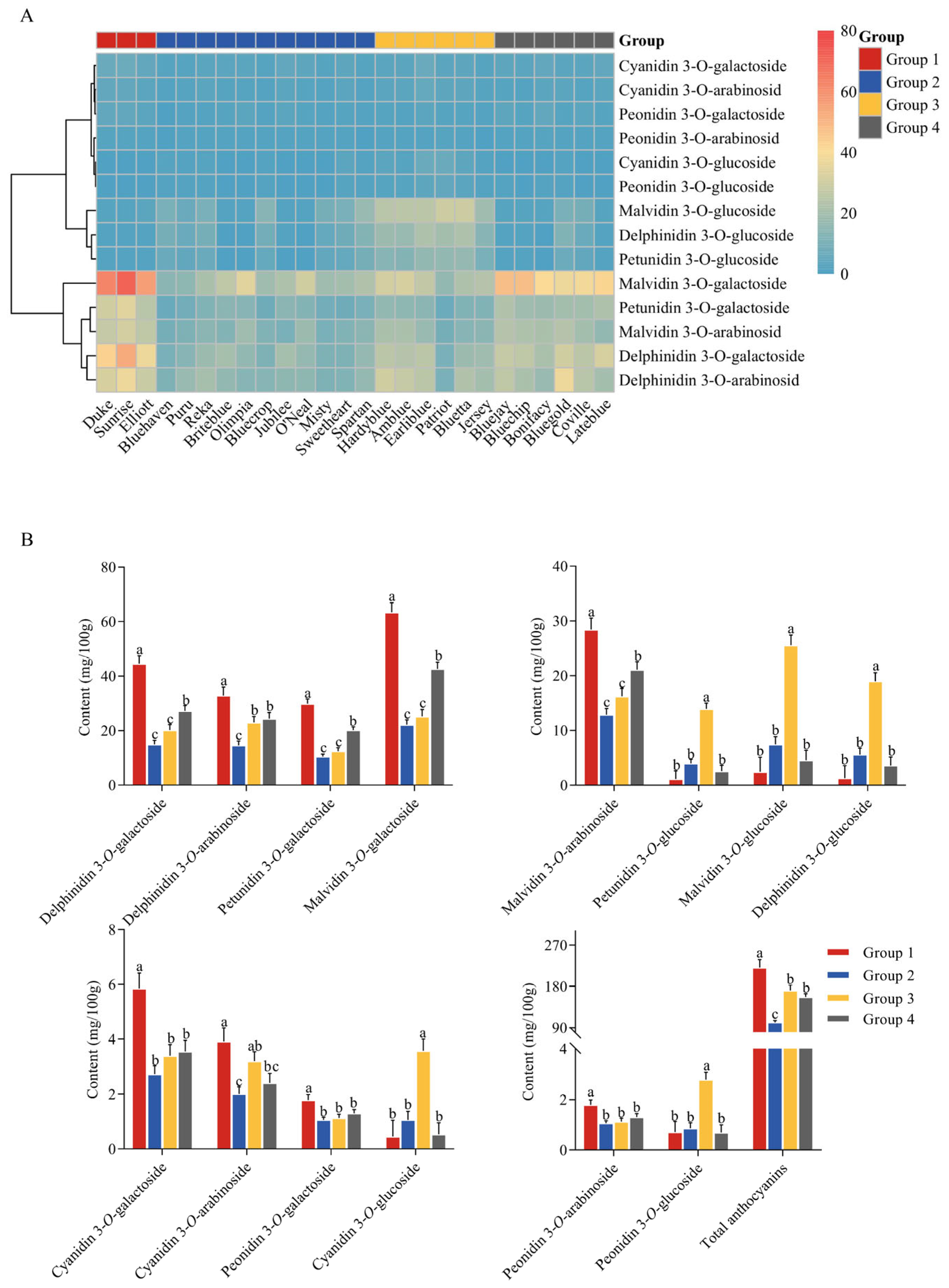
3.3. Characteristics of Anthocyanins
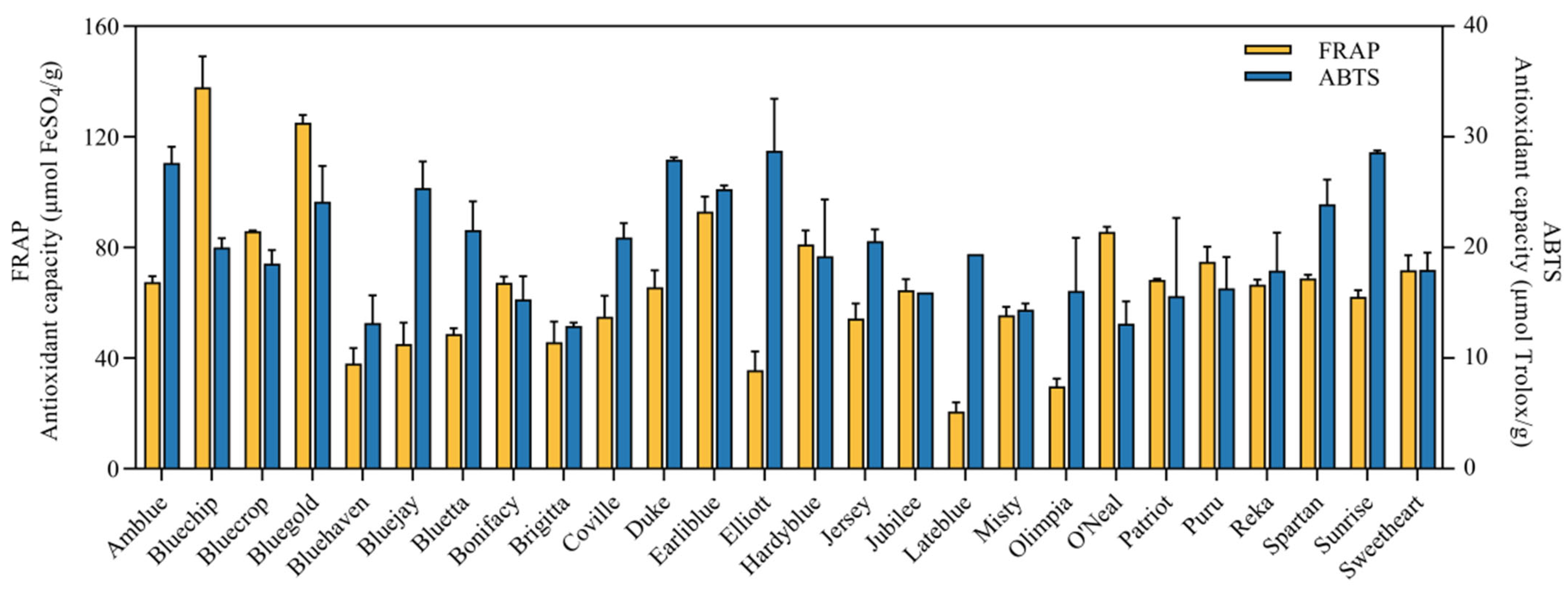
3.4. Antioxidant Capacity
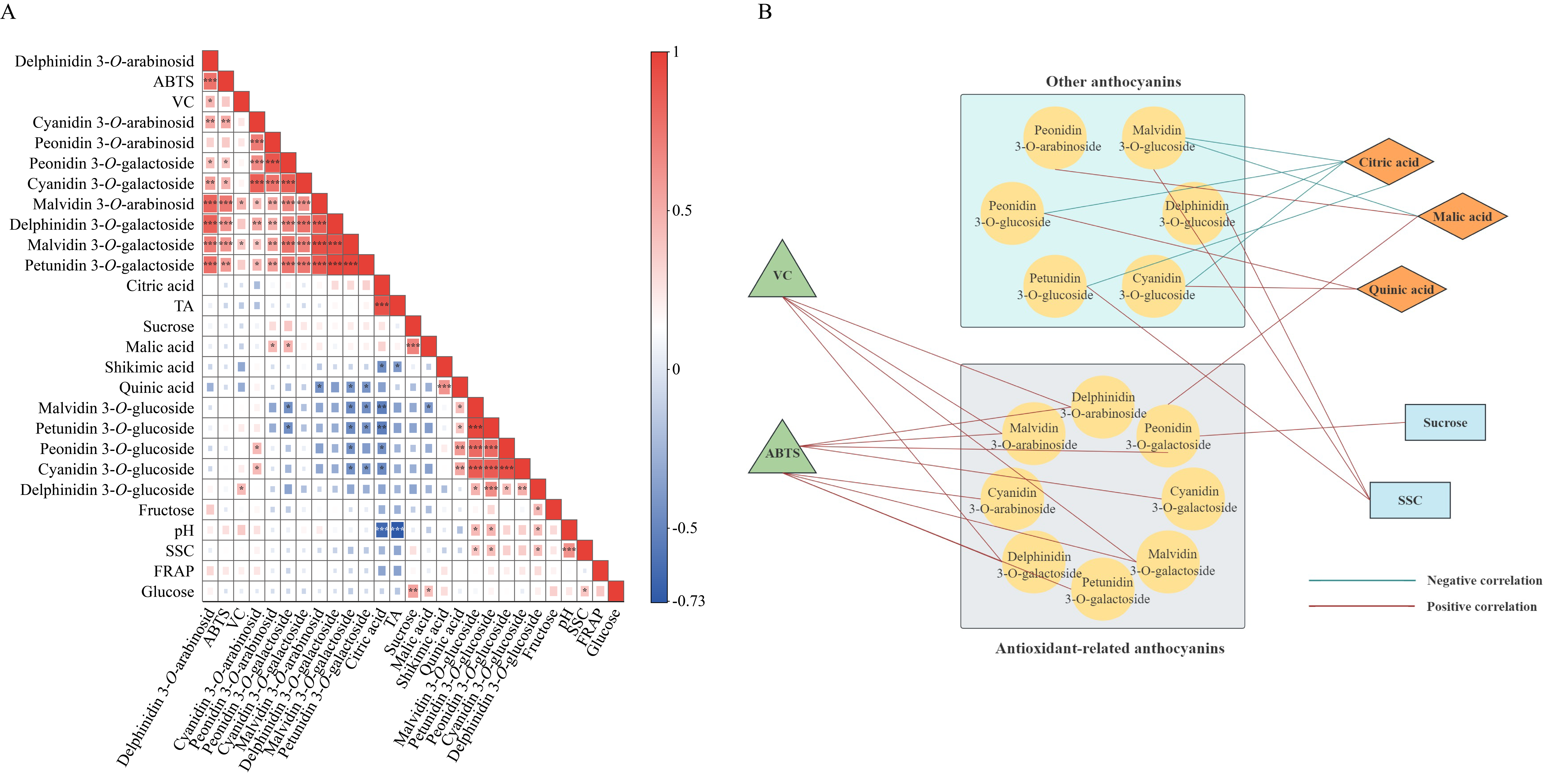
3.5. Correlation Analysis of Blueberries Indicators and Antioxidant Activity in Blueberries
3.6. Comprehensive Quality Evaluation of Blueberries Using Factor Analysis and Cultivar Ranking
3.7. Discussion on the Intake of Blueberries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FRAP | Ferric-Reducing Antioxidant Power |
| HPLC | High Performance Liquid Chromatography |
| HPLC-PDA | High-Performance Liquid Chromatography-Photodiode Array Detector |
| RP | Reversed-Phase |
| SSC | Soluble Solid Content |
| TA | Titratable Acidity |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| VC | Vitamin C |
References
- Koca, I.; Karadeniz, B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Sci. Hortic. 2009, 121, 447–450. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1–21. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W.Y. Blueberry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.X.; Nie, J.Y.; Zhang, J.Y.; Xu, G.F.; Li, J.; Cheng, Y.; Shen, Y.M.; Saqi, F. Discrimination of Geographical Origin of Blueberries from Three Major Producing Areas of China Using Mineral Element Analyses. At. Spectrosc. 2021, 42, 91–98. [Google Scholar] [CrossRef]
- Li, Y.D.; Pei, J.B.; Chen, L.; Sun, H.Y. China blueberry industry report 2020. J. Jilin Agric. Univ. 2021, 43, 1–8. [Google Scholar] [CrossRef]
- Attaribo, T.; Huang, G.Q.; Xin, X.D.; Zeng, Q.L.; Zhang, Y.Y.; Zhang, N.; Tang, L.M.; Sedjoah, R.C.A.A.; Zhang, R.; Lee, K.S.; et al. Effect of the silkworm pupa protein-glucose conjugate on the thermal stability and antioxidant activity of anthocyanins. Food Funct. 2021, 12, 4132–4141. [Google Scholar] [CrossRef]
- Pahlke, G.; Ahlberg, K.; Oertel, A.; Janson-Schaffer, T.; Grabher, S.; Mock, H.P.; Matros, A.; Marko, D. Antioxidant Effects of Elderberry Anthocyanins in Human Colon Carcinoma Cells: A Study on Structure-Activity Relationships. Mol. Nutr. Food Res. 2021, 65, 2100229. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Zheng, J.; Herrera-balandrano, D.D.; Zhang, X.; Huang, W.; Sui, Z. In vivo antioxidant activity of rabbiteye blueberry (Vaccinium ashei cv. ‘Brightwell’) anthocyanin extracts. J. Zhejiang Univ. Sci. B Biomed. Biotechnol. 2023, 24, 602–616. [Google Scholar] [CrossRef]
- Lee, S.; Jung, E.S.; Do, S.G.; Jung, G.Y.; Song, G.; Song, J.M.; Lee, C.H. Correlation between species-specific metabolite profiles and bioactivities of blueberries (Vaccinium spp.). J. Agric. Food. Chem. 2014, 62, 2126–2133. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Siddiq, M.; Dolan, K.D.; Perkins-Veazie, P.; Collins, J.K. Effect of pectinolytic and cellulytic enzymes on the physical, chemical, and antioxidant properties of blueberry (Vaccinium corymbosum L.) juice. LWT Food Sci. Technol. 2018, 92, 127–132. [Google Scholar] [CrossRef]
- Lee, S.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Contribution of anthocyanin composition to total antioxidant capacity of berries. Plant Foods Hum. Nutr. 2015, 70, 427–432. [Google Scholar] [CrossRef]
- Li, D.; Meng, X.; Li, B. Profiling of anthocyanins from blueberries produced in China using HPLC-DAD-MS and exploratory analysis by principal component analysis. J. Food Compos. Anal. 2015, 47, 1–7. [Google Scholar] [CrossRef]
- Han, T.; Wu, W.; Li, W. Transcriptome Analysis Revealed the Mechanism by Which Exogenous ABA Increases Anthocyanins in Blueberry Fruit During Veraison. Front. Plant Sci. 2021, 12, 758215. [Google Scholar] [CrossRef] [PubMed]
- Giné Bordonaba, J.; Terry, L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria × ananassa) from different cultivars using deficit irrigation. Food Chem. 2010, 122, 1020–1026. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Matos, M.; Carnide, V.; Silva, A.P.; Gonçalves, B. Variation of chemical constituents, antioxidant activity, and endogenous plant hormones throughout different ripening stages of highbush blueberry (Vaccinium corymbosum L.) cultivars produced in centre of Portugal. J. Food Biochem. 2017, 41, e12414. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, G.; Zhang, Q.; Wang, Y.; Dia, V.P.; Meng, X. Ripening affects the physicochemical properties, phytochemicals and antioxidant capacities of two blueberry cultivars. Postharvest Biol. Technol. 2020, 162, 111097. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.Y.; Li, J.; Zhang, H.; Li, Y. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Palmieri, L.; Mattivi, F. Identification and quantification of flavonol glycosides in cultivated blueberry cultivars. J. Food Compos. Anal. 2012, 25, 9–16. [Google Scholar] [CrossRef]
- Kraujalyte, V.; Venskutonis, P.R.; Pukalskas, A.; Cesoniene, L.; Daubaras, R. Antioxidant properties, phenolic composition and potentiometric sensor array evaluation of commercial and new blueberry (Vaccinium corymbosum) and bog blueberry (Vaccinium uliginosum) genotypes. Food Chem. 2015, 188, 583–590. [Google Scholar] [CrossRef]
- Okan, O.T.; Deniz, I.; Yayli, N.; Sat, I.G.; Öz, M.; Hatipoglu Serdar, G. Antioxidant Activity, Sugar Content and Phenolic Profiling of Blueberries Cultivars: A Comprehensive Comparison. Not. Bot. Horti Agrobot. 2018, 46, 639–652. [Google Scholar] [CrossRef]
- Varo, M.A.; Martín-Gómez, J.; Mérida, J.; Serratosa, M.P. Bioactive compounds and antioxidant activity of highbush blueberry (Vaccinium corymbosum) grown in southern Spain. Eur. Food Res. Technol. 2021, 247, 1199–1208. [Google Scholar] [CrossRef]
- Hellström, J.; Karhu, S.; Karhu, J.; Järvenpää, E.; Välimaa, A.L. Phenolic profiles differentiate wild bilberry and cultivated blueberry fruit. LWT Food Sci. Technol. 2024, 199, 116080. [Google Scholar] [CrossRef]
- Fan, X.G.; Zhao, H.D.; Wang, X.M.; Cao, J.K.; Jiang, W.B. Sugar and organic acid composition of apricot and their contribution to sensory quality and consumer satisfaction. Sci. Hortic. 2017, 225, 553–560. [Google Scholar] [CrossRef]
- Martínez-Valdivieso, D.; Gómez, P.; Font, R.; Alonso-Moraga, Á.; Del Río-Celestino, M. Physical and chemical characterization in fruit from 22 summer squash (Cucurbita pepo L.) cultivars. LWT Food Sci. Technol. 2015, 64, 1225–1233. [Google Scholar] [CrossRef]
- Zheng, L.J.; Nie, J.Y.; Yan, Z.; Feng, X.G.; Wang, K.; Gao, Y.; Liang, Y.M.; Amp, S. Studies on the characteristics of the composition and content of soluble sugars in apple Fruit. Acta Hortic. Sinica 2015, 42, 950–960. [Google Scholar] [CrossRef]
- Li, S.Y.; He, F.; Zhu, B.Q.; Xing, R.R.; Reeves, M.J.; Duan, C.Q. A systematic analysis strategy for accurate detection of anthocyanin pigments in red wines. Rapid Commun. Mass Spectrom. RCM 2016, 30, 1619–1626. [Google Scholar] [CrossRef]
- Liu, S.; Chang, X.; Liu, X.; Shen, Z. Effects of pretreatments on anthocyanin composition, phenolics contents and antioxidant capacities during fermentation of hawthorn (Crataegus pinnatifida) drink. Food Chem. 2016, 212, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.L.; Liu, E.H. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Sun, J.; Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef]
- Nguyen, C.T.T.; Lee, J.H.; Tran, P.T. Accumulation of sugars and associated gene expression in highbush blueberries differ by cultivar, ripening stage, and storage temperature. J. Berry Res. 2021, 11, 511–527. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, X.L.; Wang, X.F.; Xia, G.H.; Pan, Q.H.; Fan, R.C.; Wu, F.Q.; Yu, X.C.; Zhang, D.P. A shift of Phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol. 2006, 142, 220–232. [Google Scholar] [CrossRef]
- Wang, Y.; Fong, S.K.; Singh, A.P.; Vorsa, N.; Johnson-Cicalese, J. Variation of Anthocyanins, Proanthocyanidins, Flavonols, and Organic Acids in Cultivated and Wild Diploid Blueberry Species. HortScience 2019, 54, 576–585. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kadioglu, A.; Bertoft, E.; Acar, C.; Turna, I. Effect of fruit maturation on sugar and organic acid composition in two blueberries (Vaccinium Arctostaphylos and V. myrtillus) native to Turkey. N. Z. J. Crop Hortic. Sci. 2001, 29, 137–141. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Li, W.D.; He, Y.X.; Ye, L.Q.; Zhang, Z.S.; Jin, J.S. Variability in the sugar and organic acid composition of the fruit of 57 genotypes of Chinese dwarf cherry [Cerasus humilis (Bge.) Sok]. J. Hortic. Sci. Biotechnol. 2015, 90, 419–426. [Google Scholar] [CrossRef]
- Milivojevic, J.; Radivojevic, D.; Boskov, D.; Veberic, R.; Gacnik, S.; Petkovsek, M.M. Effect of a New Pruning Method On Field Performance and Fruit Quality of Highbush Blueberries Grown as a Soilless Culture—Does Cultivar Has Influence? Appl. Fruit Sci. 2025, 67, 120. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and poly-phenolics 62 during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT Food Sci. Technol. 2020, 117, 108621. [Google Scholar] [CrossRef]
- Wang, E.; Yin, Y.; Xu, C.; Liu, J. Isolation of high-purity anthocyanin mixtures and monomers from blueberries using combined chromatographic techniques. J. Chromatogr. A 2014, 1327, 39–48. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Gong, J.; Huang, J.; Xiao, G.; Chen, F.; Lee, B.; Ge, Q.; You, Y.; Liu, S.; Zhang, Y. Antioxidant capacities of fractions of Bamboo shaving extract and their antioxidant components. Molecules 2016, 21, 996. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Wu, L.; Wu, X.; Huang, Y.; Liu, B. Optimization of polysaccharides extraction from Dictyophora indusiata and determination of its antioxidant activity. Int. J. Biol. Macromol. 2017, 103, 175–181. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium Spp.) using high-performance liquid chromatography/mass spectrometry. J. Agric. Food. Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef]
- Guo, X.Q.; Fang, Z.S.; Liu, F.J.; Liao, X.J.; Hu, X.S.; Zhang, Y. Analysis of anthocyanins composition in wild blueberry varieties from northeast China and their antioxidant activities. Mod. Food Sci. Technol. 2016, 32, 320–327. [Google Scholar] [CrossRef]
- Yan, Y.; Pico, J.; Gerbrandt, E.M.; Dossett, M.; Castellarin, S.D. Comprehensive anthocyanin and flavonol profiling and fruit surface color of 20 blueberry genotypes during postharvest storage. Postharvest Biol. Technol. 2023, 199, 112274. [Google Scholar] [CrossRef]
- Holcroft, D.M.; Kader, A.A. Controlled atmosphere-induced changes in pH and organic acid metabolism may affect color of stored strawberry fruit. Postharvest Biol. Technol. 1999, 17, 19–32. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Dominguez, E. Shelf Life Potential and the Fruit Cuticle: The Unexpected Player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef]
- Cardenosa, V.; Girones-Vilaplana, A.; Muriel, J.L.; Moreno, D.A.; Moreno-Rojas, J.M. Influence of genotype, cultivation system and irrigation regime on antioxidant capacity and selected phenolics of blueberries (Vaccinium corymbosum L.). Food Chem. 2016, 202, 276–283. [Google Scholar] [CrossRef]
- Zoratti, L.; Jaakola, L.; Haggman, H.; Giongo, L. Modification of Sunlight Radiation through Colored Photo-Selective Nets Affects Anthocyanin Profile in Vaccinium spp. Berries. PLoS ONE 2015, 10, e0135935. [Google Scholar] [CrossRef]
- Rossi, G.; Woods, F.M.; Leisner, C.P. Quantification of Total Phenolic, Anthocyanin, and Flavonoid Content in a Diverse Panel of Blueberry Cultivars and Ecotypes. HortScience 2022, 57, 901–909. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Wang, J.; Lyu, L.; Wu, W.; Li, W.; Wu, Y. Fruit Quality and Metabolomic Analyses of Fresh Food Accessions Provide Insights into the Key Carbohydrate Metabolism in Blueberry. Plants 2023, 12, 3200. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Mitra, S.; Hafiz Muhammad, R.; Debnath, B.; Lu, X.; Jian, H.; Qiu, D. Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium corymbosum) at Different Developmental Stages. Molecules 2018, 23, 2209. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Zhang, H.; Wang, Y.; Wu, C.; Huang, W. Malvidin induces hepatic stellate cell apoptosis via the endoplasmic reticulum stress pathway and mitochondrial pathway. Food Sci. Nutr. 2020, 8, 5095–5106. [Google Scholar] [CrossRef] [PubMed]
- Saulite, L.; Jekabsons, K.; Klavins, M.; Muceniece, R.; Riekstina, U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine 2019, 53, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Yang, Q.; Lu, Y.; Wang, G.; Yang, H.; Dong, J.; Zhang, H. Malvidin alleviates mitochondrial dysfunction and ROS accumulation through activating AMPK-alpha/UCP2 axis, thereby resisting inflammation and apoptosis in SAE mice. Front. Pharmacol. 2022, 13, 1038802. [Google Scholar] [CrossRef]
- Gilani, S.J.; Bin-Jumah, M.N.; Al-Abbasi, F.A.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Shahid Nadeem, M.; Afzal, M.; Alzarea, S.I.; Sayyed, N.; et al. Potential of Malvidin on Aluminum Chloride Activated by the Free Radical Scavenging Property. ACS Omega 2022, 7, 24231–24240. [Google Scholar] [CrossRef]
- Xu, Y.; Ke, H.; Li, Y.; Xie, L.; Su, H.; Xie, J.; Mo, J.; Chen, W. Malvidin-3-O-Glucoside from Blueberry Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Transcription Factor EB-Mediated Lysosomal Function and Activating the Nrf2/ARE Signaling Pathway. J. Agric. Food. Chem. 2021, 69, 4663–4673. [Google Scholar] [CrossRef]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef]
- Kongthitilerd, P.; Barras, E.; Rong, W.; Thibodeaux, A.; Rigdon, M.; Yao, S.; Adisakwattana, S.; Suantawee, T.; Cheng, H. Cyanidin inhibits adipogenesis in 3T3-L1 preadipocytes by activating the PLC-IP(3) pathway. Biomed. Pharmacother. 2023, 162, 114677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Si, X.; Tan, H.; Zang, Z.; Tian, J.; Shu, C.; Wang, Y. Cyanidin-3-O-glucoside and its phenolic metabolites ameliorate intestinal diseases via modulating intestinal mucosal immune system: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2021, 63, 1629–1647. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Tamborra, P.; Bolettieri, D.; Latorraca, M.; Tamborra, M.; Paradiso, F.; Savino, M. The Shikimic Acid: An Important Metabolite for the Aglianico del Vulture Wines. Ital. J. Agron. 2014, 9, 615. [Google Scholar] [CrossRef]
- Zhu, X.-J.; Zhao, Z.; Xin, H.-H.; Wang, M.-L.; Wang, W.-D.; Chen, X.; Li, X.-H. Isolation and dynamic expression of four genes involving in shikimic acid pathway in Camellia sinensis ‘Baicha 1′ during periodic albinism. Mol. Biol. Rep. 2016, 43, 1119–1127. [Google Scholar] [CrossRef]
- Scalzo, J.; Stevenson, D.; Hedderley, D. Polyphenol compounds and other quality traits in blueberry cultivars. J. Berry Res. 2015, 5, 117–130. [Google Scholar] [CrossRef]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 227, 336–346. [Google Scholar] [CrossRef]
- Kong, X.; Xu, M.; Wang, K.; Chen, Q.; Han, L.; Li, Q.; Guo, Q.; Wan, H.; Nie, J. Development of a comprehensive evaluation system for the sensory and nutritional quality of winter jujube (Ziziphus jujuba Mill. cv. Dongzao). LWT Food Sci. Technol. 2024, 194, 115777. [Google Scholar] [CrossRef]
- Kuang, L.; Wang, Z.; Zhang, J.; Li, H.; Xu, G. Factor analysis and cluster analysis of mineral elements contents in different blueberry cultivars. J. Food Compos. Anal. 2022, 109, 104507. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A study of glycaemic effects following acute anthocyanin-rich blueberry supplementation in healthy young adults. Food Funct. 2017, 8, 3104–3110. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of Anthocyanins from Blueberries and Serum Antioxidant Status in Human Subjects. J. Agric. Food. Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef]
- Stote, K.S.; Wilson, M.M.; Hallenbeck, D.; Thomas, K.; Rourke, J.M.; Sweeney, M.I.; Gottschall-Pass, K.T.; Gosmanov, A.R. Effect of Blueberry Consumption on Cardiometabolic Health Parameters in Men with Type 2 Diabetes: An 8-Week, Double-Blind, Randomized, Placebo-Controlled Trial. Curr. Dev. Nutr. 2020, 4, nzaa030. [Google Scholar] [CrossRef] [PubMed]
| Parameter | January | February | March | April | May | June | Annual |
|---|---|---|---|---|---|---|---|
| Average rainfall (mm) | 3.1 | 5.1 | 32.2 | 54.2 | 75.9 | 99.7 | 791.5 |
| Average temperature (℃) | −3 | −1.4 | 5.8 | 11.5 | 17.2 | 21.8 | 12.2 |
| Sunshine duration (h) | 131.7 | 164.1 | 199.1 | 213.8 | 232.6 | 176.2 | 2234.6 |
| Cultivars | TA (%) | SSC (%) | VC (mg/100 g) | pH |
|---|---|---|---|---|
| Amblue | 0.34 ± 0.01 m | 20.0 ± 0.4 a | 14.5 ± 0.2 b | 4.02 ± 0.05 n |
| Bluechip | 0.32 ± 0.02 m,n | 11.2 ± 0.1 l,m | 15.6 ± 0.5 a | 3.38 ± 0.01 g,h |
| Bluecrop | 0.55 ± 0.03 k | 14.2 ± 0.1 e,f | 9.7 ± 0.1 h,i | 3.19 ± 0.06 c,d |
| Bluegold | 0.66 ± 0.01 h | 11.8 ± 0.1 j,k,l | 10.8 ± 0.8 d,e,f,g | 3.09 ± 0.03 a |
| Bluehaven | 1.09 ± 0.01 d | 12.3 ± 0.1 i,j | 11.2 ± 0.1 d,e,f | 3.10 ± 0.03 a,b |
| Bluejay | 0.56 ± 0.03 j,k | 16.2 ± 0.1 c | 11.6 ± 0.1 c,d | 3.38 ± 0.00 g,h |
| Bluetta | 0.63 ± 0.02 h,i | 14.8 ± 0.1 d,e | 10.3 ± 0.1 d,e,f,g | 3.43 ± 0.05 h,i |
| Bonifacy | 0.55 ± 0.01 k | 12.0 ± 0.2 j,k | 11.9 ± 1.6 c,d | 3.19 ± 0.01 c,d |
| Brigitta | 0.57 ± 0.01 i,j,k | 17.4 ± 0.1 b | 11.5 ± 0.2 c,d | 3.25 ± 0.01 e |
| Coville | 1.18 ± 0.04 b | 12.8 ± 0.0 h,i | 11.0 ± 0.6 d,e,f,g | 3.23 ± 0.03 d,e |
| Duke | 0.62 ± 0.01 h,i,j | 12.1 ± 0.1 j,k | 12.3 ± 0.7 c | 3.46 ± 0.01 i,j |
| Earliblue | 0.28 ± 0.01 n,o | 19.5 ± 0.2 a | 12.4 ± 0.8 c | 3.79 ± 0.03 m |
| Elliott | 1.58 ± 0.02 a | 10.8 ± 0.3 m | 12.3 ± 0.3 c | 3.16 ± 0.04 c |
| Hardyblue | 0.74 ± 0.01 g | 13.7 ± 0.1 f,g | 14.5 ± 0.6 b | 3.56 ± 0.03 k,l |
| Jersey | 0.92 ± 0.01 e | 14.5 ± 0.1 e | 10.0 ± 0.3 g,h,i | 3.56 ± 0.03 k,l |
| Jubilee | 0.48 ± 0.05 l | 13.8 ± 0.1 f,g | 9.1 ± 0.1 i,j,k | 3.31 ± 0.01 f |
| Lateblue | 1.57 ± 0.02 a | 11.5 ± 0.2 k,l,m | 10.8 ± 0.1 d,e,f,g | 3.09 ± 0.03 a |
| Misty | 0.35 ± 0.01 m | 12.0 ± 0.2 j,k | 8.6 ± 0.2 j,k | 3.47 ± 0.05 i,j |
| Olimpia | 0.56 ± 0.01 j,k | 15.3 ± 0.2 d | 10.3 ± 0.4 d,e,f,g | 3.38 ± 0.03 g |
| O’Neal | 0.26 ± 0.02 o | 11.2 ± 0.1 l,m | 9.5 ± 0.5 h,i,j | 3.56 ± 0.02 l |
| Patriot | 0.53 ± 0.01 k,l | 12.3 ± 1.4 i,j | 13.5 ± 0.1 b | 3.41 ± 0.01 g,h |
| Puru | 1.16 ± 0.01 b,c | 12.9 ± 0.1 h,i | 10.2 ± 0.6 f,g,h,i | 3.07 ± 0.01 a |
| Reka | 1.11 ± 0.02 c,d | 11.1 ± 0.1 m | 11.5 ± 0.5 c,d | 3.07 ± 0.05 a |
| Spartan | 0.84 ± 0.10 f | 14.5 ± 0.2 e | 11.3 ± 0.4 c,d,e | 3.51 ± 0.01 j,k |
| Sunrise | 0.52 ± 0.02 k,l | 12.8 ± 0.1 h,i | 12.3 ± 0.1 c | 3.51 ± 0.00 j,k,l |
| Sweetheart | 0.47 ± 0.01 l | 13.3 ± 0.1 g,h | 8.1 ± 0.4 k | 3.14 ± 0.02 b,c |
| Index | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| VC (x1) | 0.254 | 0.419 | 0.037 | −0.024 | −0.070 |
| SSC (x2) | 0.388 | 0.008 | −0.019 | 0.295 | −0.210 |
| TA (x3) | −0.170 | 0.016 | −0.031 | −0.006 | 0.951 |
| Quinic acid (x4) | 0.420 | −0.182 | 0.132 | −0.049 | 0.027 |
| Malic acid (x5) | −0.257 | 0.018 | 0.256 | 0.837 | 0.088 |
| Shikimic acid (x5) | −0.031 | −0.010 | 0.071 | 0.070 | −0.315 |
| Citric acid (x7) | −0.370 | 0.112 | −0.017 | 0.117 | 0.868 |
| Glucose (x8) | 0.126 | 0.055 | −0.172 | 0.801 | −0.048 |
| Fructose (x9) | 0.161 | 0.128 | −0.059 | 0.007 | −0.161 |
| Sucrose (x10) | −0.294 | 0.054 | 0.209 | 0.771 | 0.082 |
| FRAP (x11) | 0.164 | 0.135 | −0.023 | 0.185 | −0.219 |
| ABTS (x12) | 0.251 | 0.888 | 0.119 | −0.040 | −0.052 |
| Delphinidin 3-O-galactoside (x13) | −0.229 | 0.830 | 0.393 | −0.013 | 0.127 |
| Delphinidin 3-O-glucoside (x14) | 0.944 | −0.037 | −0.178 | −0.047 | −0.154 |
| Cyanidin 3-O-galactoside (x15) | −0.073 | 0.466 | 0.849 | −0.028 | −0.009 |
| Delphinidin 3-O-arabinosid (x16) | 0.099 | 0.900 | 0.145 | 0.089 | 0.083 |
| Cyanidin 3-O-glucoside (x17) | 0.923 | −0.184 | 0.174 | −0.124 | −0.075 |
| Petunidin 3-O-galactoside (x18) | −0.349 | 0.800 | 0.407 | 0.031 | 0.122 |
| Cyanidin 3-O-arabinosid (x19) | 0.367 | 0.369 | 0.809 | −0.021 | −0.053 |
| Petunidin 3-O-glucoside (x20) | 0.949 | −0.041 | −0.168 | −0.051 | −0.176 |
| Peonidin 3-O-galactoside (x21) | −0.281 | 0.433 | 0.801 | 0.212 | −0.012 |
| Peonidin 3-O-glucoside (x22) | 0.946 | −0.102 | 0.190 | −0.088 | −0.033 |
| Malvidin 3-O-galactoside (x23) | −0.385 | 0.802 | 0.377 | 0.001 | 0.036 |
| Peonidin 3-O-arabinosid (x24) | −0.077 | 0.283 | 0.868 | 0.172 | −0.015 |
| Malvidin 3-O-glucoside (x25) | 0.931 | −0.045 | −0.206 | −0.072 | −0.207 |
| Malvidin 3-O-arabinosid (x26) | −0.206 | 0.878 | 0.278 | 0.104 | −0.068 |
| Variance (%) | 32.6 | 22.6 | 9.8 | 9 | 7 |
| Cumulative variance (%) | 32.6 | 55.1 | 64.9 | 73.9 | 80.9 |
| Cultivars | F1 Score | F2 Score | F3 Score | F4 Score | F5 Score | Comprehensive Score | Rank |
|---|---|---|---|---|---|---|---|
| Amblue | 1.239 | −1.151 | 2.255 | −0.141 | 0.062 | 0.441 | 4 |
| Bluechip | 1.228 | −1.355 | −1.486 | 1.241 | 1.303 | 0.188 | 9 |
| Bluecrop | −0.689 | 0.041 | −0.690 | −0.442 | −0.177 | −0.414 | 23 |
| Bluegold | 0.477 | 1.766 | 0.086 | 3.044 | 0.102 | 1.042 | 2 |
| Bluehaven | −0.772 | 0.299 | −0.152 | −0.480 | 0.716 | −0.237 | 18 |
| Bluejay | 0.555 | 0.708 | 1.020 | −0.879 | −1.080 | 0.353 | 7 |
| Bluetta | −0.153 | −0.371 | 0.859 | −0.522 | −1.470 | −0.247 | 19 |
| Bonifacy | 0.134 | 0.253 | −0.359 | 0.256 | 1.747 | 0.262 | 8 |
| Briteblue | −0.392 | 0.392 | 1.943 | −0.373 | 2.483 | 0.361 | 5 |
| Coville | 0.116 | 0.563 | −0.340 | −0.258 | −0.919 | 0.054 | 11 |
| Duke | 1.381 | −1.339 | −1.561 | −0.467 | −0.946 | −0.140 | 17 |
| Earliblue | 0.864 | −1.488 | 1.122 | 0.000 | −1.324 | −0.046 | 13 |
| Elliott | 1.522 | 1.411 | −1.298 | −1.253 | −0.269 | 0.687 | 3 |
| Hardyblue | 1.026 | −0.995 | 0.453 | 1.341 | 0.248 | 0.361 | 6 |
| Jersey | −0.476 | −0.146 | 0.628 | −0.595 | −1.297 | −0.335 | 21 |
| Jubilee | −1.890 | 0.320 | 0.898 | 0.911 | −0.759 | −0.529 | 24 |
| Lateblue | −0.371 | 1.189 | −0.643 | −1.522 | 0.251 | −0.043 | 12 |
| Misty | −1.385 | −1.360 | −1.067 | −0.590 | 0.438 | −1.094 | 26 |
| Olimpia | −0.356 | 0.085 | 0.359 | −1.638 | 1.440 | −0.133 | 16 |
| O’Neal | −1.356 | −1.736 | −0.679 | 0.913 | −0.016 | −1.013 | 25 |
| Patriot | 0.430 | 0.214 | −0.904 | −0.344 | 0.456 | 0.125 | 10 |
| Puru | −0.968 | 0.824 | −0.107 | 0.910 | −0.345 | −0.102 | 15 |
| Reka | −0.196 | 0.643 | −1.093 | 0.361 | −0.662 | −0.049 | 14 |
| Spartan | −0.412 | −0.809 | 0.334 | −0.241 | 0.725 | −0.315 | 20 |
| Sunrise | 1.783 | 1.322 | 0.566 | 0.224 | 0.117 | 1.191 | 1 |
| Sweetheart | −1.338 | 0.722 | −0.144 | 0.545 | −0.824 | −0.366 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, J.; Di, Y.; Wan, H.; Wang, K.; Nie, J. Comprehensive Evaluation of Quality and Antioxidant Capacity of Highbush Blueberries (Vaccinium corymbosum). Foods 2025, 14, 3251. https://doi.org/10.3390/foods14183251
Liu X, Zhang J, Di Y, Wan H, Wang K, Nie J. Comprehensive Evaluation of Quality and Antioxidant Capacity of Highbush Blueberries (Vaccinium corymbosum). Foods. 2025; 14(18):3251. https://doi.org/10.3390/foods14183251
Chicago/Turabian StyleLiu, Xiaoli, Jia Zhang, Yindi Di, Haoliang Wan, Kunyu Wang, and Jiyun Nie. 2025. "Comprehensive Evaluation of Quality and Antioxidant Capacity of Highbush Blueberries (Vaccinium corymbosum)" Foods 14, no. 18: 3251. https://doi.org/10.3390/foods14183251
APA StyleLiu, X., Zhang, J., Di, Y., Wan, H., Wang, K., & Nie, J. (2025). Comprehensive Evaluation of Quality and Antioxidant Capacity of Highbush Blueberries (Vaccinium corymbosum). Foods, 14(18), 3251. https://doi.org/10.3390/foods14183251





