Integration of Physiological Analysis and Untargeted Metabolomics to Explore Differences in Quality Among Four Sweet Cherry Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Materials
2.2. Reagents and Instruments
2.3. Physical Damage, Bitterness, Overall Likeability, Size, Weight, and Skin Color
2.4. Texture, Titratable Acidity (TA), Total Soluble Solids (TSS), and Solid-to-Acid Ratio
2.5. Untargeted Metabolomic Analysis of Four Sweet Cherry Fruits
2.6. Statistical Analysis
3. Results
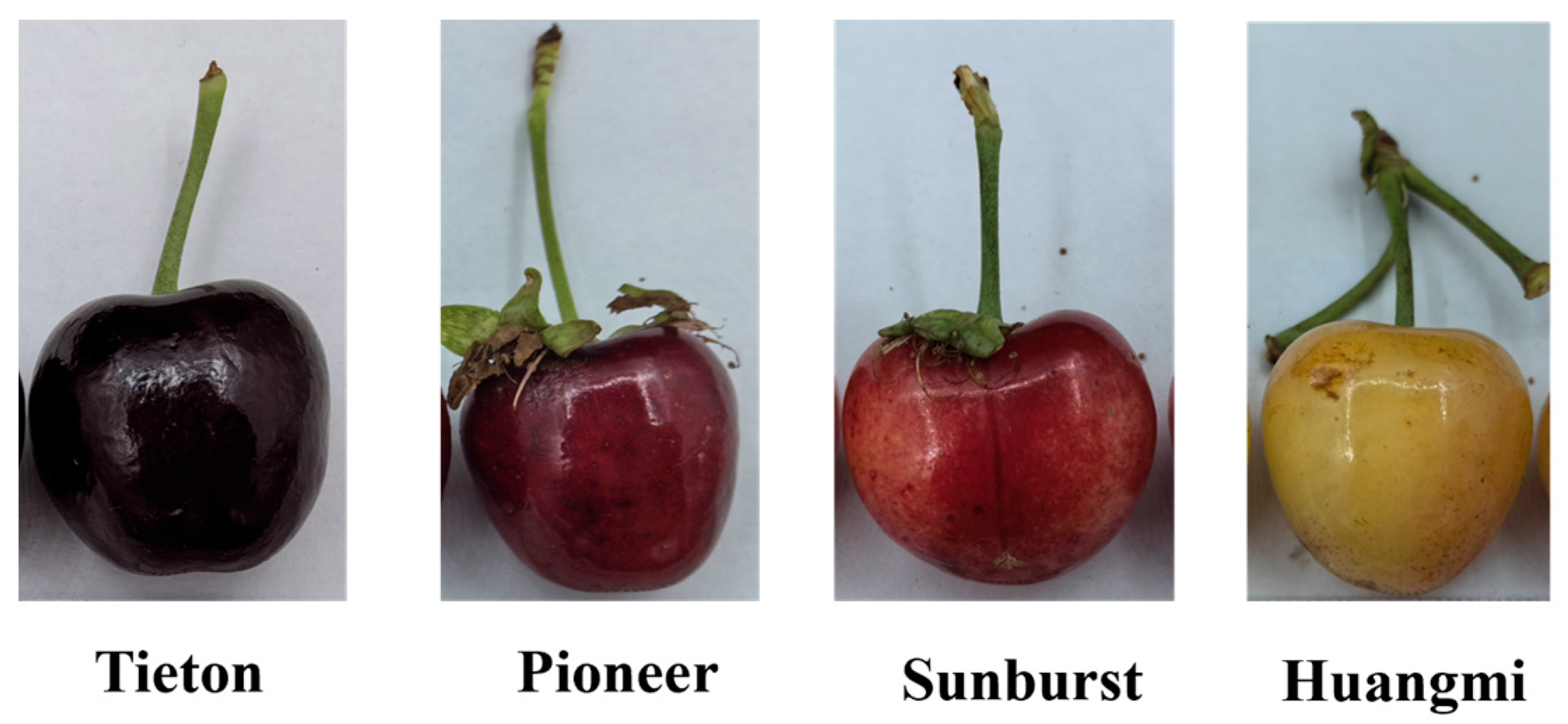
3.1. Comparison of Physical Damage, Bitterness, Overall Likeability, Size, Weight, and Skin Color in Four Sweet Cherry Fruits
3.2. Comparison of Texture, TA, TSS, and Solid-to-Acid Ratio of Four Sweet Cherry Fruits
3.3. Determination of DMEs Among Four Sweet Cherry Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.H.; D’Amato, G.; Muraglia, C.; Brunetti, G.; et al. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef] [PubMed]
- Chezanoglou, E.; Mourtzinos, I.; Goula, A.M. Sweet cherry and its by-products as sources of valuable phenolic compounds. Trends Food Sci. Technol. 2024, 145, 104367. [Google Scholar] [CrossRef]
- Xie, J.; Wu, K.; Wang, M.; Jiang, A.; Chen, C. Effects of preharvest alginate oligosaccharides treatment on water stress-induced cracking of sweet cherry fruit. Postharvest Biol. Tec. 2025, 223, 113430. [Google Scholar] [CrossRef]
- Nie, G.W.; Li, K.; Zhang, X.P.; Zhang, Y.N.; Hou, X.; Yin, K.H.; Tian, Y.Q. Current status and development suggestions for protected cultivation of cherries in Shanxi Province. J. Fruit. Res. 2025, 6, 113–115. [Google Scholar] [CrossRef]
- Li, G.Q.; Wu, J.H.; Zhu, H.M.; Du, J.J.; Erihemu; Xu, G.S.; Li, G.F. Research progress in the postharvest preservation technologies for sweet cherry fruit. Food Res. Dev. 2021, 42, 191–197. [Google Scholar]
- Han, X.; Ren, L.L.; Shang, Z.W.; Liu, B.Y.; Liu, Y.; Gong, Y.C.; Song, Y.P. Development of a full-view-type grading cup for automated sweet cherry sorters. Agronomy 2023, 13, 500. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Chen, Z.; Li, T.; Zhang, Z.; Yun, Z.; Jiang, Y.M. Metabolic variations in the pulp of four litchi cultivars during pulp breakdown. Food Res. Int. 2021, 140, 110080. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.J.; Jiang, Y.M. Insights into metabolomics in quality attributes of postharvest fruit. Curr. Opin. Food Sci. 2022, 45, 100836. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, X.P.; Mu, Q.E.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.X.; Yu, X.Y. Differences in total phenolics, antioxidant activity and metabolic characteristics in peach fruits at different stages of ripening. LWT-Food Sci. Technol. 2023, 178, 114586. [Google Scholar] [CrossRef]
- Palomino-Vasco, M.; López-Corrales, M.; Bañuls, P.; Bernalte, M.J.; Martín, A.; Serradilla, M.J. Screening of physicochemical and bioactive traits of new cherry cultivars obtained from the cross of ‘Ambrunés’ x ‘Hudson’. Agriculture 2024, 14, 1938. [Google Scholar] [CrossRef]
- Kazazic, M.; Mehic, E.; Aliman, J.; Djapo-Lavic, M. The bioactive compounds of sweet cherry fruits influenced by cultivar/rootstock combination. Hortic. Sci. 2024, 51, 23–28. [Google Scholar] [CrossRef]
- Singh, N.; Kathuria, D.; Barthwal, R.; Joshi, R. Metabolomics of chemical constituents as a tool for understanding the quality of fruits during development and processing operations. Int. J. Food Sci. Technol. 2024, 59, 4169–4184. [Google Scholar] [CrossRef]
- Kaleem, M.M.; Nawaz, M.A.; Ding, X.C.; Wen, S.Y.; Shireen, F.; Cheng, J.T.; Bie, Z.L. Comparative analysis of pumpkin rootstocks mediated impact on melon sensory fruit quality through integration of non-targeted metabolomics and sensory evaluation. Plant Physiol. Bioch. 2022, 192, 320–330. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Bonini, P.; Bontempo, L.; Camin, F.; Trevisan, M.; Lucini, L. The hierarchical contribution of organic vs. conventional farming, cultivar, and terroir on untargeted metabolomics phytochemical profile and functional traits of tomato fruits. Front. Plant Sci. 2022, 13, 856513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Li, X.; Hu, J.X.; Liu, X.; Bi, J.F. Analysis of the taste quality of specialty cold region berries based on non-targeted metabolomics. Trans. Chin. Soc. Agric. Eng. 2024, 40, 293–301. [Google Scholar]
- Brown, K.; Sims, C.; Odabasi, A.; Bartoshuk, L.; Conner, P.; Gray, D. Consumer acceptability of fresh-market muscadine grapes. J. Food Sci. 2016, 81, S2808–S2816. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Z.; Zhang, X.P.; Wang, F.; Zhao, Q.F. Effect of pressurized argon combined with controlled atmosphere on the postharvest quality and browning of sweet cherries. Postharvest Biol. Tec. 2019, 147, 59–67. [Google Scholar] [CrossRef]
- Zhang, H.M.; Tu, K.; Qiu, Z.L.; Qiao, G.; Wen, X.P. Changes in the quality of fruits of four sweet cherry cultivars grown under rain-shelter cultivation during storage at room temperature. J. Food Meas. Charact. 2022, 16, 2571–2581. [Google Scholar] [CrossRef]
- Isildak, I.; Gones, A.G. Simultaneous SIA analysis of pH and total acidity measurements in milk. J. Food Meas. Charact. 2018, 12, 403–411. [Google Scholar] [CrossRef]
- Li, G.Q.; Duan, P.R.; Wang, M.R.; Zhang, C.C.; Lv, H.Z.; Zhu, H.M.; Song, X.Q.; Zhang, S.Y.; Erihemu. Untargeted metabolomics reveals the mechanism of ultrasound combined with L-cysteine treatment inhibiting the browning of fresh-cut potatoes. Postharvest Biol. Tec. 2024, 216, 113088. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Wilson, I.D.; Kellt, D.B.; Husermet, C. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef]
- Dong, Y.X.; Qi, X.L.; Liu, C.L.; Song, L.L.; Ming, L. A sweet cherry AGAMOUS-LIKE transcription factor PavAGL15 affects fruit size by directly repressing the PavCYP78A9 expression. Sci. Hortic. 2022, 297, 110947. [Google Scholar] [CrossRef]
- Szilagyi, S.; Horvath-Kupi, T.; Desiderio, F.; Bekefi, Z. Evaluation of sweet cherry (Prunus avium L.) cultivars for fruit size by FW_G2a QTL analysis and phenotypic characterization. Sci. Hortic. 2022, 292, 110656. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Fernando, I.; Fei, J.G.; Stanley, R. Measurement and analysis of vibration and mechanical damage to bananas during long-distance interstate transport by multi-trailer road trains. Postharvest Biol. Technol. 2019, 158, 11097. [Google Scholar] [CrossRef]
- Gu, S.; Xu, D.; Zhou, F.; Feng, K.; Chen, C.; Jiang, A. Repairing ability and mechanism of methyl jasmonate and salicylic acid on mechanically damaged sweet cherries. Sci. Hortic. 2022, 292, 110567. [Google Scholar] [CrossRef]
- Tran, X.T.; Parks, S.E.; Roach, P.D.; Golding, J.B.; Nguyen, M.H. Effects of maturity on physicochemical properties of Gac fruit (Momordica cochinchinensis Spreng.). Food Sci. Nutr. 2016, 4, 305–314. [Google Scholar] [CrossRef]
- Gracia, C.; Calle, A.; Gasic, K.; Arias, E.; Wünsch, A. Genetic and QTL analyses of sugar and acid content in sweet cherry (Prunus avium L.). Hortic. Res. 2025, 12, uhae310. [Google Scholar] [CrossRef]
- Mertoglu, K. Sweet cherry fruit nutritional profile modulation and molecular docking studies of major compounds. Food Chem. 2025, 465, 142153. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.M.; Luo, T.; Han, D.M.; Zhu, D.F.; Li, Z.Y.; Wu, Z.Y.; Wu, Z.X. Multi-omics analysis revealed room temperature storage affected the quality of litchi by altering carbohydrate metabolism. Sci. Hortic. 2021, 293, 110663. [Google Scholar] [CrossRef]
- Luo, T.; Shuai, L.; Lai, T.T.; Liao, L.Y.; Li, J.; Duan, Z.H.; Xue, X.Q.; Han, D.M.; Wu, Z.X. Up-regulated glycolysis, TCA, fermentation and energy metabolism promoted the sugar receding in ‘Shixia’ longan (Dimocarpus longan Lour.) pulp. Sci. Hortic. 2021, 281, 109998. [Google Scholar] [CrossRef]
- Southan, J.; Mchugh, E.; Walker, H.; Ismail, H.M. Metabolic signature of articular cartilage following mechanical injury: An integrated transcriptomics and metabolomics analysis. Front. Mol. Biosci. 2020, 7, 592905. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yang, N.X.; Shao, Y.L.; Shen, T.; Li, W.X.; Ma, B.Q.; Wei, X.Y.; Ruan, Y.L.; Ma, F.W.; Li, M.J. An insertion in the promoter of a malate dehydrogenase gene regulates malic acid content in apple fruit. Plant Physiol. 2024, 196, 432–445. [Google Scholar] [CrossRef] [PubMed]
- He, Y.L.; Qin, H.Y.; Wen, J.L.; Wang, L.; Cao, W.Y.; Fan, S.T.; Lu, W.P.; Li, J.Q.; Li, C.Y. Characterization of amino acid composition, nutritional value, and taste of fruits from different Actinidia arguta resources. J. Food Qual. 2024, 1005194. [Google Scholar] [CrossRef]
- Xu, L.; Yu, W.; Zhu, X.; Zhang, Q.; Wu, Y.; Li, J.; Du, G.; Lv, X.; Chen, J.; Liu, L. Metabolic engineering of Escherichia coli for efficient biosynthesis of L-citrulline. Chin. J. Biotechnol. 2024, 63, 11859–11879. [Google Scholar]
- Rappu, P.; Suwal, U.; Siljamäki, E.; Heino, J. Inflammation-related citrullination of matrisome proteins in human cancer. Front. Oncol. 2022, 12, 1035188. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. 2017, 20, 92–98. [Google Scholar] [CrossRef]
- Cain, A.; Krahn, N. Overcoming challenges with biochemical studies of selenocysteine and selenoproteins. Int. J. Mol. Sci. 2024, 25, 10101. [Google Scholar] [CrossRef]
- Zhang, S.S.; He, J.L.; Peng, X.Y.; Pan, L.N.; Dong, L.; Jiang, Y.K.; Wang, J.Q.; Nie, S.P. In vitro digestion of infant formula containing partially hydrolyzed protein. J. Chin. Inst. Food Sci. Technol. 2023, 23, 86–95. [Google Scholar]
- Giacco, A.; Petito, G.; Silvestri, E.; Scopigno, N.; Vigliotti, M.; Mercurio, G.; de Lange, P.; Lombardi, A.; Moreno, M.; Goglia, F.; et al. Comparative effects of 3,5-diiodo-L-thyronine and 3,5,3’-triiodo-L-thyronine on mitochondrial damage and cGAS/STING-driven inflammation in liver of hypothyroid rats. Front. Endocrinol. 2024, 15, 1432819. [Google Scholar] [CrossRef]
- Yang, L.; Peng, J.S.; Tang, T. Transcriptional analysis of the molecular response of Arabidopsis to manganese stress and recovery. Chin. J. Biotechnol. 2024, 40, 1138–1156. [Google Scholar]
- Yang, D.D.; Zhu, H.J.; Zhao, Y.; Liu, W.G. Research progress on regulation of citrulline metabolism in vegetable crops. China Cucurbits Veg. 2023, 36, 1–10. [Google Scholar]
- Osakabe, N.; Shimizu, T.; Fujii, Y.; Fushimi, T.; Calabrese, V. Sensory nutrition and bitterness and astringency of polyphenols. Biomolecules 2024, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Wang, H.; Zhang, J.; Chen, Q.; He, W.; Zhang, Y.; Luo, Y.; Tang, H.R.; Wang, Y.; Wang, X.R. Comparative metabolomics profiling highlights unique color variation and bitter taste formation of Chinese cherry fruits. Food Chem. 2024, 439, 138072. [Google Scholar] [CrossRef]
- Jiang, C.J.; Jiang, Y.; Geng, Z.M.; Zhang, M.H.; Sun, C.; Bian, H.; Wang, D.Y.; Xu, W.M. Progress in research on hydroxyoctadecaenoic acids as oxidation products of linoleic acid. Food Sci. 2018, 39, 278–284. [Google Scholar]
- Li, Q.; Lin, H.; Lin, H.T.; Lin, M.S.; Wang, H.; Wei, W.; Chen, J.Y.; Lu, W.J.; Shao, X.F.; Fan, Z.Q. The metabolism of membrane lipid participates in the occurrence of chilling injury in cold-stored banana fruit. Food Res. Int. 2023, 173, 113415. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.D.; Tian, W.; Feng, J.R.; Zhu, S.H. Interaction between nitric oxide and storage temperature on sphingolipid metabolism of postharvest peach fruit. Plant Physiol. Bioch. 2020, 151, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kurotani, K.; Karunapema, P.; Jayaratne, K.; Sato, M.; Hayashi, T.; Kajio, H.; Fukuda, S.; Hara, H.; Okazaki, O.; Jayatilleke, A.U.; et al. Circulating odd-chain saturated fatty acids were associated with arteriosclerosis among patients with diabetes, dyslipidemia, or hypertension in Sri Lanka but not Japan. Nutr. Res. 2018, 50, 82–93. [Google Scholar] [CrossRef]
- Chen, C.; Xie, J.N.; Gang, J.; Wang, M.Y.; Wu, K.; Jiang, A.L. Metabolomic insights into the browning inhibition of fresh-cut apple by hydrogen sulfide. Food Chem. 2024, 447, 139005. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.C.; Zhou, T.B.; She, L.J.; Miao, M.S.; Bai, M. Modern biological mechanism and characteristic analysis of traditional Chinese medicine in regulation of intestinal flora for prevention and treatment of ischemic stroke. Chin. J. Exp. Tradit. Med. Formulae 2024, 30, 243–250. [Google Scholar]
- Yang, Y.Y.; Ye, Y.H.; Deng, Y.F.; Gao, L. Uridine and its role in metabolic diseases, tumors, and neurodegenerative diseases. Front. Physiol. 2024, 15, 1360891. [Google Scholar] [CrossRef]
- Magri, A.; Malorni, L.; Cozzolino, R.; Adiletta, G.; Siano, F.; Picariello, G.; Cice, D.; Capriolo, G.; Nunziata, A.; Di Matteo, M.; et al. Agronomic, physicochemical, aromatic and sensory characterization of four sweet cherry accessions of the Campania region. Plants 2023, 12, 610. [Google Scholar] [CrossRef]
- Zan, S.Y.; Wang, R.; Zhang, F.; Zhang, D.Y.; Liu, B.J.; Meng, X.H. Composition analysis of rootstock cherry (Prunus mahaleb L.), a potential source of human nutrition and dietary supplements. Eur. Food Res. Technol. 2022, 248, 1421–1435. [Google Scholar] [CrossRef]
- Liang, C.L.; Wan, T.; Xu, S.Y.; Li, B.B.; Li, X.N.; Feng, Y.; Cai, Y.L. Molecular identification and genetic analysis of cherry cultivars using capillary electrophoresis with fluorescence-labeled SSR markers. 3 Biotech. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Ropelewska, E.; Popinska, W.; Sabanci, K.; Aslan, M.F. Cultivar identification of sweet cherries based on texture parameters determined using image analysis. J. Food Process. Eng. 2021, 44, 13724. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yang, X.; Cao, Z.; Li, F.; Li, G.; Erihemu. Integration of Physiological Analysis and Untargeted Metabolomics to Explore Differences in Quality Among Four Sweet Cherry Cultivars. Foods 2025, 14, 3207. https://doi.org/10.3390/foods14183207
Li G, Yang X, Cao Z, Li F, Li G, Erihemu. Integration of Physiological Analysis and Untargeted Metabolomics to Explore Differences in Quality Among Four Sweet Cherry Cultivars. Foods. 2025; 14(18):3207. https://doi.org/10.3390/foods14183207
Chicago/Turabian StyleLi, Guoqin, Xiaosa Yang, Zhonghua Cao, Fei Li, Guifeng Li, and Erihemu. 2025. "Integration of Physiological Analysis and Untargeted Metabolomics to Explore Differences in Quality Among Four Sweet Cherry Cultivars" Foods 14, no. 18: 3207. https://doi.org/10.3390/foods14183207
APA StyleLi, G., Yang, X., Cao, Z., Li, F., Li, G., & Erihemu. (2025). Integration of Physiological Analysis and Untargeted Metabolomics to Explore Differences in Quality Among Four Sweet Cherry Cultivars. Foods, 14(18), 3207. https://doi.org/10.3390/foods14183207





