3.1. Phenolic Compounds Profile of Tinctures
In all analyzed tinctures from blackthorn fruits, four groups of polyphenols were identified: anthocyanins < flavan-3-ols < flavonols < phenolic acids. During the initial stage of maceration (measurement on day 28), the tinctures exhibited the same profile of polyphenolic compounds. A significant change occurred in the flavonols profile in samples from days 56 and 84 of maceration, as quercetin-3-O-rutinoside was not detected (
Table 2). The dominant compound in blackthorn tinctures throughout the maceration period was neochlorogenic acid.
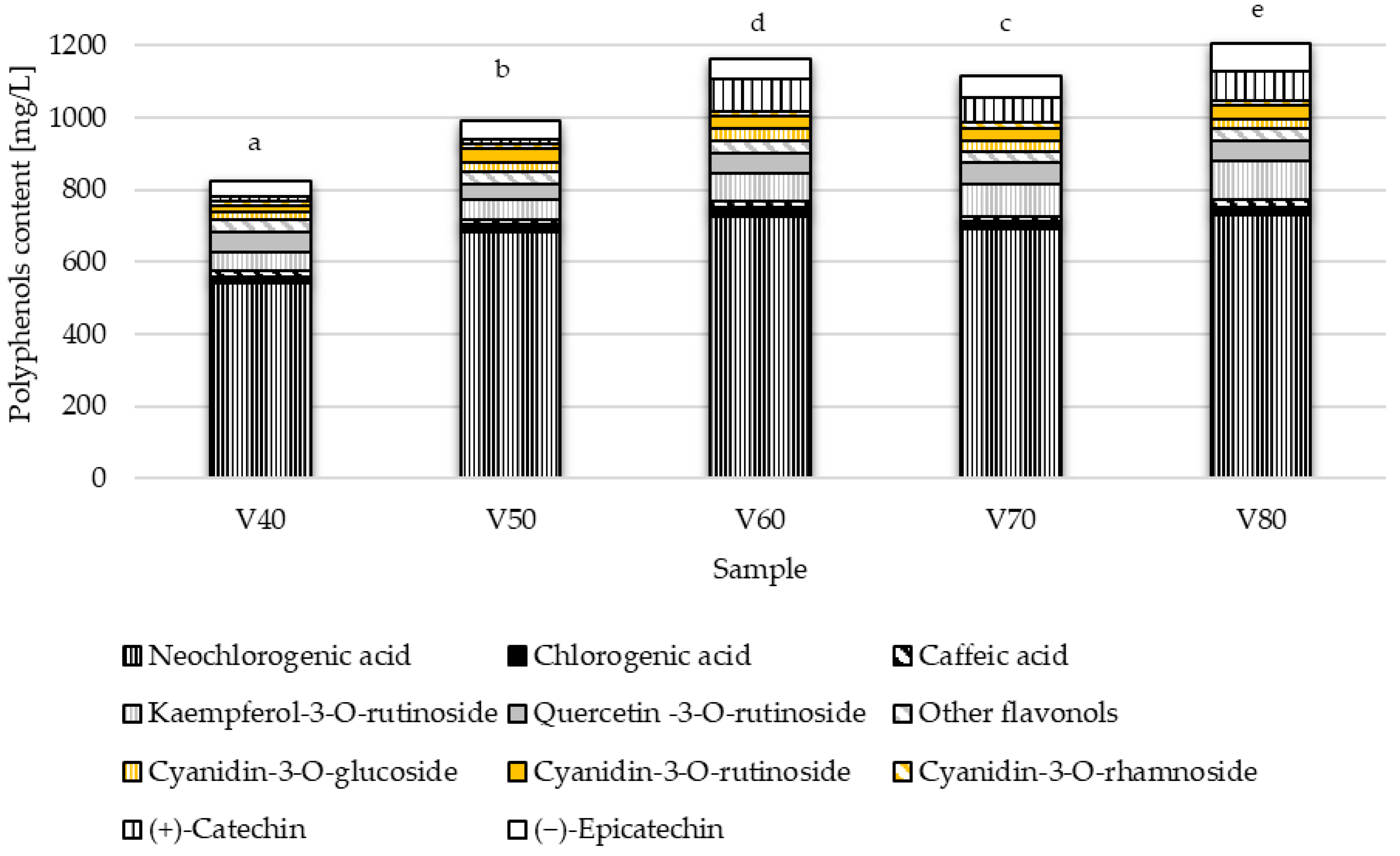
The solubility of phenolic compounds depends on the polarity of the solvent, the degree of depolymerization of phenols, the interactions of polyphenols with other components of the plant material, and the formation of insoluble complexes. In studies using fixed extraction conditions, as in the production of tinctures, it is necessary to select the concentration and type of extractant suitable for the specific raw material. The effect of ethanol concentration on polyphenols extraction was tested in five variants of the blackthorn tincture. The results are shown in
Figure 1.
Based on the conducted research, it was found that tinctures prepared with ethanol concentrations of 40% and 50% are characterized by the lowest total phenolic compound content (
p < 0.05). The highest content, amounting to 1204.32 mg/L, was recorded in the tincture with an ethanol concentration of 80% (
v/
v) during the first stage of the study. However, if polyphenol groups are considered separately, it should be noted that anthocyanins are an exception, for which it is best to use 60–70% ethanol for extraction. In the group of phenolic acids, similar results were achieved with 60% and 80% ethanol (766.60 mg/L and 770.41 mg/L, respectively). The most significant impact of alcohol concentration on the amount of extracted compounds was observed in the flavan-3-ols group, where the concentration of these compounds in the V40 sample was 57.01 mg/L. In contrast, in the V80 sample, it was 155.11 mg/L. In the flavonols group, the extraction efficiency increased with the rising concentration of the applied alcohol. The obtained results can be explained by the fact that ethanol concentration influences solvent polarity, which in turn affects the solubility of different classes of phenolic compounds. Higher ethanol content can enhance the extraction of less polar compounds, such as hydroxycinnamic acids. The extraction of bioactive compounds from plant materials using organic solvents occurs through mass transfer, including convective diffusion at the particle surface and molecular diffusion within the particles [
22]. Effective penetration of the solvent into the plant matrix is essential to intensify this process. Solvent viscosity plays a key role in the rate and extent of compound release. Low viscosity is advantageous because it minimizes molecular resistance and facilitates compound transfer, leading to higher extraction efficiency [
23]. The results of the present study confirm that increasing ethanol content in the solvent enhances the concentration of extracted bioactive compounds, likely due to a reduction in solvent viscosity with higher ethanol levels [
24].
The effectiveness of high-concentration alcoholic solutions for extracting polyphenols from blackthorn fruit has been confirmed in previous studies. Kotsou et al. [
7] obtained the highest total polyphenol yield from
P. spinosa fruit using a 75% ethanol solution combined with pulsed electric field and ultrasound pre-treatment. Similarly, Magiera et al. [
5] used 75% methanol to recover bioactive compounds from
P. spinosa. However, extraction conditions should be tailored to the specifics of the intended technological process. For instance, Kotsou et al. [
7] focused on rapid, technologically assisted extraction of powdered fruit under laboratory conditions at elevated temperature. In contrast, the tincture production process simulated in the present study—featuring prolonged maceration time and sugar addition—required a higher optimal ethanol concentration. Given that the primary aim of the first stage was to identify the ethanol concentration yielding the highest total polyphenolic content, 80% ethanol (V80) was selected for the subsequent experimental stages.
In the second stage of the research, it was checked which method of preparing the raw material would allow for obtaining the most considerable amount of bioactive compounds in the tincture, what effect the maceration time has, and, additionally, whether the timing of sugar addition makes a difference. The quantitative and qualitative characteristics of the extracted polyphenolic compounds in different tincture variants are presented in
Table 2. As shown by ANOVA analysis (
Table 3), the processing method of blackthorn fruits (variant) and maceration time, as well as their interactions, significantly (
p < 0.05) influenced the extraction of total polyphenols in the tested tinctures. Additionally, it can be seen that the time of sugar addition is significant (V1 and V2). The greatest extractability of polyphenols was observed in all variants during the first maceration period, over the first 28 days. In the following weeks, the extraction rate was slower. Although the individual increments between days 56 and 84 of maceration differed significantly (
p < 0.05) in variants V2, V3, and V4, they were only 1.09%, 2.97%, and 6.92%, respectively. Similar observations were made by Narwojsz et al. [
14] during the maceration of quince in ethanol, where 81.9% of polyphenols were extracted into the tincture in the first week of the process. The highest total amounts of polyphenols were obtained in the V2 variant, where the fruit was only washed, but sugar was added at the beginning of the process. At the same time, on the 28 day of maceration, the total amount of polyphenols for V2 was the lowest of all variants (up to 12.38%). This can be explained by the “dilution” of the V2 sample due to the 20% (
w/
v) sugar addition at the beginning of the process. Similar conclusions were drawn by Sokół-Łętowska et al. [
13] when determining the total polyphenols in tincture samples based on cherry juice, for which the difference due to the added sugar averaged 24%.
The dominant group of polyphenolic compounds identified in tinctures at both stages of research was phenolic acids, specifically hydroxycinnamic acids. The extraction of phenolic acids using 40% ethanol was found to be the least effective, averaging 23% (
Figure 1). Generally, the longer the maceration lasted, the higher the content of phenolic acids increased (
Table 2). The predominant compound among all identified polyphenols extracted from blackthorn fruits was neochlorogenic acid, which accounted for about 60% of the total polyphenolic compounds, which is consistent with other studies [
4,
7,
25]. The presence of chlorogenic and caffeic acids was also noted in the studied tinctures. Research on extracting blackthorn fruits for food was also conducted by Koraqi et al. [
26]. Using a solvent system of lactic acid, maltose, and water (66:16:16
v/
v/
v), they obtained extracts containing phenolic acids (vanillic acid, chlorogenic acid, caffeic acid, and gallic acid) and anthocyanins (cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, peonidin-3-O-glucoside, and delphinidin-3-O-β-O-glucoside) as the most prevalent bioactive compounds. Stoenescu et al. [
27] confirmed the presence of gallic, neochlorogenic, and caffeic acids in dry blackthorn fruit samples extracted with methanol combined with ultrasonic bathing. The differences in the results obtained in our studies can be attributed to the additional identification of chlorogenic acid, but without gallic acid.
On the other hand, Magiera et al. [
5] identified neochlorogenic, chlorogenic, and cryptochlorogenic acids in a methanol–water (75:25,
v/
v) extract of fresh fruits. Furthermore, protocatechuic, p-hydroxybenzoic, vanillic, and p-coumaric acids were recorded after an additional sequential liquid–liquid extraction using organic solvents. The reasons for the differences in the results of our studies and those of other authors can be sought in the different extractants, the place of fruit collection, the form of the fruit (powdered or fresh), the ratio of fruit to solvent, as well as the conditions and time of extraction.
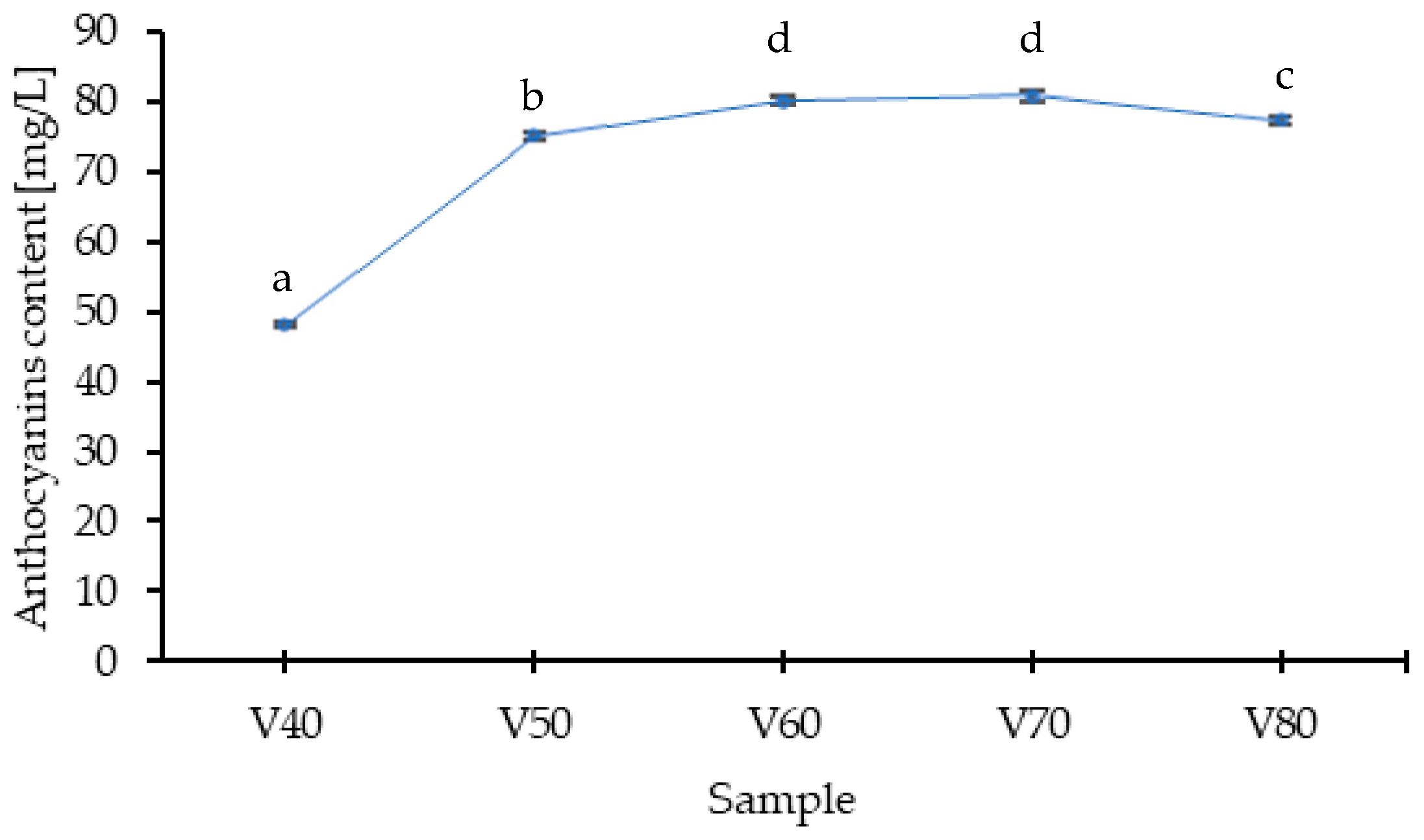
Considering the diversity of the polyphenolic profile of the analyzed tinctures, most compounds identified belonged to the flavonoid class. In the anthocyanin group, three compounds were identified, namely cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside as the dominant ones, and cyanidin-3-O-rhamnoside. The dominant anthocyanins in tinctures are also found in the highest concentration in blackthorn fruits [
28]. The content of anthocyanins in the extract increased within the ethanol concentration range from 40% to 70% (
Figure 2), with no statistically significant difference in the V60 and V70 samples. Due to their molecular structure (numerous hydroxyl and glycosyl groups), anthocyanins are compounds that dissolve well in polar solvents, so it can be assumed that in solvents containing relatively high amounts of water, extraction will be high. The obtained results indicate that in 40% ethanol, the recovery of anthocyanins is the lowest; this may stem from the specifics of the fruit form used, namely that whole, undamaged blackthorn fruits were subjected to extraction, and in this case, the ethanol concentration was insufficient for effective penetration of the cellular structures. Kotsou et al. [
7] studied the key factors influencing the extraction process of bioactive compounds from powdered fruits of
P. spinosa L. The parameters studied, in addition to temperature and duration of extraction, included the solvent composition (water, ethanol, and their mixtures at 25, 50, and 75%
v/
v). These authors proved that lower concentrations of solvent resulted in insufficient extraction, and the optimal conditions for the extraction of anthocyanins were considered to be the use of a 50% ethanol solution at a temperature of 65 °C for 30 min. It should be noted, however, that to achieve optimal extraction, they applied a preliminary ultrasound treatment. This method allows for the rupture of cell membranes, facilitating the extraction of desired compounds.
The anthocyanins are sensitive to many factors, such as pH, storage temperature, copigments, light, sugars and their degradation products, oxygen, enzymes, proteins, and metallic ions [
29]. They are therefore unstable compounds, a fact confirmed by the present study. The highest content of these compounds was recorded on the 28 day of the process. Gironés-Vilaplana et al. [
16] showed that anthocyanins transfer from fruits to liqueurs within the first 30–60 days of maceration, with maximum anthocyanin levels observed on the 60th day for the liqueur based on
Prunus spinosa L., after which degradation occurred in the following days. In the presented studies, the concentration of anthocyanins decreased over time during maceration, but starting from the 28 day, which is related to the concentration of the applied solvent (80%
v/
v). It has been demonstrated that the anthocyanins’ degradation rate increases with ethanol concentration. The main causes of accelerated anthocyanin degradation in the presence of higher ethanol concentrations include reduced self-association of anthocyanin molecules, which exposes more reactive and unstable forms, and a decrease in the energy of the lowest unoccupied molecular orbital (LUMO) of the flavylium cation. This decrease in LUMO energy facilitates nucleophilic attack by water, leading to hydrolysis of the O-glycosidic bonds at the C-3 position of the flavylium molecule. Additionally, higher ethanol content can shift the equilibrium toward the colorless hemiketal and chalcone forms, further promoting degradation. The combination of these factors—glycosidic bond cleavage, structural rearrangements, and increased susceptibility to hydration—explains the reduced stability of anthocyanins in ethanol-rich solutions [
30,
31]. The smallest loss of these compounds, at 11.9%, occurred in sample V1-unprocessed fruits, while the largest, at 26.4%, was in V3, where the fruits were blanched.
Among flavonols, the presence of quercetin-3-O-rutinoside and kaempferol-3-O-rutinoside was confirmed, as well as a particular group of unidentified compounds referred to as ‘other flavonols’ (
Figure 1,
Table 2). The most significant impact of ethanol concentration on flavonol extraction was observed in the case of kaempferol-3-O-rutinoside, which had a concentration of 51.94 mg/L in the tincture produced with 40% ethanol; however, when 80% ethanol was used, it increased to 103.56 mg/L. The increase in quercetin-3-O-rutinoside in these same samples was 11.2%. Interestingly, in the second stage of the study, on days 56 and 84 of the process, the presence of this compound was not recorded, or its quantity was below the detection level of the device. Such changes in the concentration of quercetin-3-O-rutinoside may have been related to polymerization reactions occurring during maceration [
15]. The concentration of kaempferol-3-O-rutinoside decreased during maceration by 2–12%, indicating its greater stability. It should also be added that the concentration of quercetin-3-O-rutinoside was, on average, 3.5 times lower than that of kaempferol-3-O-rutinoside (on the 28 day of maceration). Other authors in studies on the extraction of bioactive compounds from
P. spinosa fruits report the presence of different compounds from this group of flavonoids in the extracts, such as rutin, quercetin 3-O-galactoside, quercetin 3-β-D-glucoside, kaempferol 3-O-β-rutinoside, kaempferol 3-glucoside, avicularin, hyperoside, and isoquercitrin [
5,
7].
In the analyzed tinctures, monomers of flavan-3-ols in the form of (+)-catechin and (−)-epicatechin were identified. For both compounds, an increase in ethanol concentration improved the effectiveness of extraction, especially at an alcohol concentration ≥ 60%. During the maceration of blackthorn fruits in 80% ethanol, the lowest concentration of (+)-catechin was recorded in sample V1, while the highest was in V2. Still, the maximum increases in the content of this compound were only at the level of 5%. For (−)-epicatechin in samples V2, V3, and V4, a maximum concentration was observed on the 56 day of maceration, with a difference of about 20 mg/L (35%) compared to the 28 day. Interestingly, in tincture V2, to which sugar was added at the beginning of the process, the concentration of flavan-3-ols was the highest (155.65 mg/L), which may indicate the protective effect of sucrose on these compounds. In addition, in sample V1, in which, as in V2, no raw material treatment was applied, but sugar was added at day 56 of maceration, the concentration of flavan-3-ols was the lowest (107.94 mg/L) and at a similar level throughout the maceration period. Shpigelman et al. [
32] investigated the protective mechanisms of sugars on epigallocatechin-3-gallate against degradation. They confirmed that sugars (fructose and sucrose) protect the tested compound through multiple mechanisms, including reducing oxygen solubility, chelating transition metal ions, and scavenging reactive oxygen species. Based on these findings, the higher concentration of flavan-3-ols observed in V2 in our study, where sugar was added at the beginning of maceration, may result from the effect of sucrose, which likely reduced oxygen solubility in the solvent and thereby limited oxidative degradation of flavan-3-ols. Additionally, potential chelation of trace metal ions and scavenging of reactive oxygen species by sugar could have contributed to the preservation of these compounds.
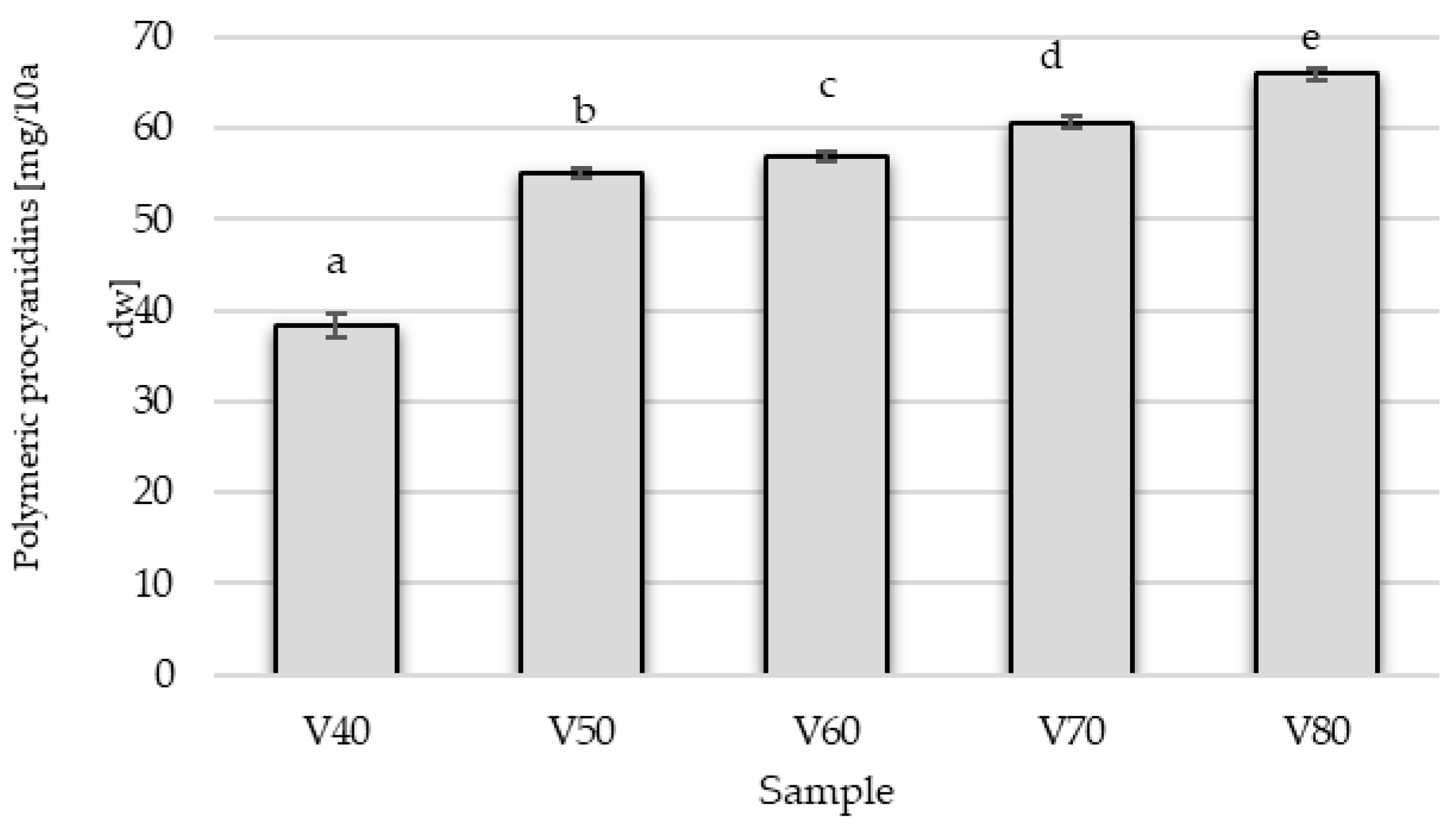
The tinctures were characterized by a low content of polymeric procyanidins (PPCs) with degrees of polymerization (DP) ranging from 1.07 to 1.23, whose concentration was higher when the more concentrated ethanol solution was used for extraction (
Figure 3).
Research on fruit juice production has shown that PPCs are bound mainly to insoluble cell wall polysaccharides and do not pass into the juice but remain in the pomace [
33]. In tinctures V1 and V2, the fruit has not undergone any treatment, and the extraction of these compounds is lowest (
Table 4). The highest concentration of polymeric procyanidins was in the tincture in which the fruit peel was mechanically damaged before maceration (V4). The optimal maceration time for samples in which sugar was added on the 56 day of the process—regardless of the raw material pre-treatment method—was 56 days. In contrast, the sample in which sugar was added at the beginning of the process exhibited the lowest content of polymeric procyanidins. The influence of the tested factors is supported by the statistical analysis (
Table 5). It is important to emphasize that the differences between the variants are pronounced and statistically significant, as indicated by a very high F value and a
p-value below 0.05 (in fact, <0.001). Although Mikulic-Petkovsek et al. [
28] reported that blackthorn fruit contains not only (+)-catechins and (−)-epicatechins but also procyanidin dimers and some trimers in the highest amounts, our study demonstrates that primarily flavan-3-ol monomers are extracted into the tincture.
Ethanol, methanol, and water are the most commonly used solvents when extracting polyphenols from a plant matrix for use in food or cosmetic products. Adding water to the extraction mixture increases the polarity of the environment, which enhances the desorption of polyphenols, especially the glycosidic forms, by breaking their hydrogen bonds [
34]. Pinacho et al. [
12] spoiled blackthorn extracts based on dichloromethane, ethyl acetate, ethanol, and water. They reported the best results regarding total polyphenol content in the ethanol extract. In their study, a solution of ethanol in water was used as a non-toxic substance and approved for tincture production [
3].
3.2. Antioxidant Capacity of Blackthorn Tinctures
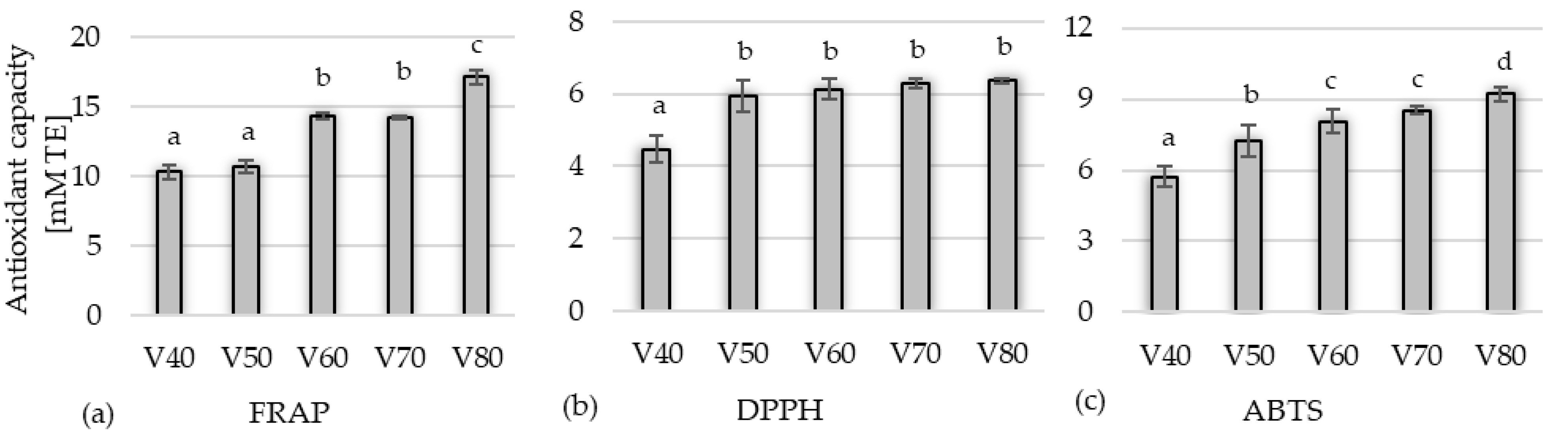
Between the FRAP, DPPH, and ABTS tests, a significantly strong correlation (Pearson correlation (r)) was found in both the first (0.7369 ≤ r ≤ 0.9335) and the second (0.7840 ≤ r ≤ 0.9056) stages of the research.
The antioxidant activity of the tinctures in the first stage of the study (
Figure 4), assessed using the FRAP test, ranged from 10.31 to 17.10 mM TE; for the DPPH test, it ranged from 4.47 to 6.36 mM TE, while for the ABTS test, it ranged from 5.73 to 9.23 mM TE. Generally, the higher the alcohol concentration used to obtain the tincture, the greater activity was observed against Fe
3+ ions and ABTS•+ cation radicals. Such a correlation was not observed for DPPH, for which the activity did not differ significantly (
p < 0.05) in the samples from V50 to V80. The antioxidant capacity measured by all methods was strongly correlated with the content of polyphenolic compounds. This study confirms the other reports, e.g., Popović et al. [
35] showed that the polyphenols of blackthorn contribute to the overall antioxidant capacity. Pinacho et al. [
12] attempted to establish a relationship between antioxidant activity and the nature of active compounds in branches, leaves, and fruits of
P. spinosa L. The authors concluded that the activity might be mainly attributed to phenolic compounds.
The correlations between the results of the second research stage were considered separately for each variant of the tincture. In the ABTS test, the highest activity was recorded for sample V1 on the 56 day of maceration. This result may have been influenced by the highest anthocyanin content among the other samples, positively correlated with ABTS (r = 0.5648 and 0.7870 for cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside, respectively). Interestingly, despite the highest concentration of polyphenols (
Table 2) in sample V2 (sugar was added at the beginning of the process), the antioxidant activity measured by FRAP and ABTS tests was the lowest (
Table 6). Based on this, one can infer a strong influence of polymeric procyanidins (r = 0.9786 and 0.8844 for FRAP and ABTS, respectively), whose concentration in this sample was at the lowest level (
Table 4). On the other hand, in sample V4, the highest extraction of polymeric procyanidins correlated with the highest activity measured by FRAP and DPPH tests (r = 0.9999 and 0.9729, respectively). In the studied tinctures, an unidentified group of flavonols (‘other flavonols’), caffeic acid, and PPC were significantly positively correlated.
At the same time, quercetin-3-O-rutinoside was significantly negatively correlated with antioxidant activity, regardless of the method of raw material processing. Drăghici-Popa et al. [
9] in their research on optimizing extraction conditions of polyphenols from Romanian blackthorn fruits also reported a strongly directed correlation between phenolic acids (caffeic and protocatechuic acids) and antioxidant capacity. In the current study, a strong positive effect of all identified phenolic acids (0.9188 ≤ r ≤ 0.9999), as well as (−)-epicatechin (0.8066 ≤ r ≤ 0.9773), on the formation of antioxidant capacity in tincture sample V2 was observed. In the same sample, contrary to some studies [
11,
36], correlations between antioxidant determinations and anthocyanins (cyanidin-3-O-glucoside and cyanidin-3-O-rhamnoside), and between ABTS and cyanidin-3-O-rutinoside were inversely correlated (−0.9985 ≤ r ≤ −0.9449). Wojdyło et al. [
37], in their studies on 33 different sour cherry cultivars, also suggest that antioxidant activity is not related to the presence of anthocyanins but may be attributed to polymeric procyanidins.
The antioxidant capacity of blackthorn tinctures was significantly affected by both the variant type (raw material pre-treatment and sugar addition timing) and maceration time, as confirmed by two-way ANOVA (
p < 0.001 for all three assays: FRAP, DPPH, and ABTS) (
Table 7). The interaction between these two factors was also statistically significant (
p < 0.001), indicating that the effect of maceration duration depends on the specific variant.