Effect of Different Disinfection Procedures on the Microbiological Quality and Germination Efficacy of Sprouted Quinoa (Chenopodium quinoa) Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Samples
2.2. Preparation of Chemical Solutions
2.3. Disinfection Procedures
2.4. Germination Process and Sprouted Quinoa Flour
2.5. Microbiological Analysis
2.6. Germination Power
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Disinfection on Mesophilic and Enterobacterial Counts
3.1.1. Chemical Treatments
3.1.2. Combined Treatments
3.2. Effect of Disinfection Procedures on Germination Power
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RH | Rosada de Huancayo quinoa variety |
| PK | Pasankalla quinoa variety |
| 200 ppm SH | 200 ppm sodium hypochlorite |
| 4% AA | 4% acetic acid |
| 70% EtOH | 70% ethanol |
| 8% H2O2 | 8% hydrogen peroxide |
| HW + H2O2 + AA | Hot water (50 °C, 10 min) followed by immersion in hydrogen peroxide (2% v/v) and acetic acid (0.1% v/v) |
| US15 | Ultrasound treatment for 15 min in water with 200 ppm sodium hypochlorite |
| US30 | Ultrasound treatment for 30 min in water with 200 ppm sodium hypochlorite |
References
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional Constituents of Pseudo Cereals and Their Potential Use in Food Systems: A Review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, Fatty Acid, Polyphenolic Profile, Techno-Functional and Antioxidant Properties of Flours Obtained from Quinoa (Chenopodium Quinoa Willd) Seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023. Urbanization, Agrifood Systems Transformation and Healthy Diets Across the Rural–Urban Continuum; FAO: Rome, Italy; IFAD: Rome, Italy; UNICEF: New York, NY, USA; WFP: Rome, Italy; WHO: Geneva, Switzerland, 2023; ISBN 978-92-5-137226-5. [Google Scholar]
- Mir, S.A.; Farooq, S.; Shah, M.A.; Sofi, S.A.; Dar, B.N.; Hamdani, A.M.; Khaneghah, A.M. An Overview of Sprouts Nutritional Properties, Pathogens and Decontamination Technologies. LWT 2021, 141, 110900. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Sun, M.; Nguyen, T.N.; Park, S.; Ayele, B.T. Molecular Mechanisms of Seed Germination. In Sprouted Grains: Nutritional Value, Production, and Applications; Elsevier: Amsterdam, The Netherland, 2018; pp. 1–24. [Google Scholar]
- Fact.MR. Sprouted Grains and Seeds Market. Available online: https://www.factmr.com/report/2246/sprouted-grains-and-seeds-market?utm_source=chatgpt.com (accessed on 28 August 2025).
- Persistence Market Research. Quinoa Seed Market Size, Share, and Growth Forecast for 2025–2032. Available online: https://www.persistencemarketresearch.com/market-research/quinoa-seed-market.asp?utm_source=chatgpt.com (accessed on 28 August 2025).
- Growth Market Reports. Sprouted Quinoa Crisp Market Research Report 2033. Available online: https://growthmarketreports.com/report/sprouted-quinoa-crisp-market?utm_source=chatgpt.com (accessed on 28 August 2025).
- Fardet, A. How Can Both the Health Potential and Sustainability of Cereal Products Be Improved? A French Perspective. J. Cereal Sci. 2014, 60, 540–548. [Google Scholar] [CrossRef]
- Hübner, F.; Arendt, E.K. Germination of Cereal Grains as a Way to Improve the Nutritional Value: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 853–861. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry: Standards for the Growing, Harvesting, Packing, and Holding of Sprouts for Human Consumption; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition: Rockville, MD, USA, 2023.
- Reale, A.; Messia, M.C.; Pulvento, C.; Lavini, A.; Nazzaro, S.; Di Renzo, T. Microbial and Qualitative Traits of Quinoa and Amaranth Seeds from Experimental Fields in Southern Italy. Foods 2023, 12, 1866. [Google Scholar] [CrossRef] [PubMed]
- Dechet, A.M.; Herman, K.M.; Chen Parker, C.; Taormina, P.; Johanson, J.; Tauxe, R.V.; Mahon, B.E. Outbreaks Caused by Sprouts, United States, 1998–2010: Lessons Learned and Solutions Needed. Foodborne Pathog. Dis. 2014, 11, 635–644. [Google Scholar] [CrossRef]
- Fang, Y.; Franke, C.; Manthei, A.; McMullen, L.; Temelli, F.; Gänzle, M.G. Effects of High-Pressure Carbon Dioxide on Microbial Quality and Germination of Cereal Grains and Beans. J. Supercrit. Fluids 2021, 175, 105272. [Google Scholar] [CrossRef]
- Shen, M.-H.; Singh, R.K. Elicitation and Disinfection during Sprout Production Using Ultraviolet Radiation and Hydrogen Peroxide: A Review. Trends Food Sci. Technol. 2024, 147, 104447. [Google Scholar] [CrossRef]
- Weissinger, W.R.; Beuchat, L.R. Comparison of Aqueous Chemical Treatments To Eliminate Salmonella on Alfalfa Seeds. J. Food Prot. 2000, 63, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Fu, T.J.; Smith, M.A. Microbial Contamination in Sprouts: How Effective Is Seed Disinfection Treatment? J. Food Sci. 2013, 78, R495–R501. [Google Scholar] [CrossRef] [PubMed]
- Trząskowska, M.; Dai, Y.; Delaquis, P.; Wang, S. Pathogen Reduction on Mung Bean Reduction of Escherichia Coli O157:H7, Salmonella Enterica and Listeria Monocytogenes on Mung Bean Using Combined Thermal and Chemical Treatments with Acetic Acid and Hydrogen Peroxide. Food Microbiol. 2018, 76, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Neetoo, H.; Chen, H. Individual and Combined Application of Dry Heat with High Hydrostatic Pressure to Inactivate Salmonella and Escherichia Coli O157:H7 on Alfalfa Seeds. Food Microbiol. 2011, 28, 119–127. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Li, W.; Zhao, Y.; Chen, Z. Comprehensive Evaluation of the Effect of Different Disinfectants on the Surface Disinfection of Quinoa Seeds and Their Germination. J. Biol. 2023, 40, 83. [Google Scholar]
- Taormina, P.J.; Beuchat, L.R. Comparison of Chemical Treatments to Eliminate Enterohemorrhagic Escherichia coli O157:H7 on Alfalfa Seeds. J. Food Prot. 1999, 62, 318–324. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Q.; Zhao, D.; Han, X.; Hao, J. Systematic Application of Slightly Acidic Electrolyzed Water (SAEW) for Natural Microbial Reduction of Buckwheat Sprouts. LWT 2019, 108, 14–20. [Google Scholar] [CrossRef]
- Tornuk, F.; Ozturk, I.; Sagdic, O.; Yetim, H. Determination and Improvement of Microbial Safety of Wheat Sprouts with Chemical Sanitizers. Foodborne Pathog. Dis. 2011, 8, 503–508. [Google Scholar] [CrossRef]
- Ngnitcho, P.F.K.; Tango, C.N.; Khan, I.; Daliri, E.B.M.; Chellian, R.; Oh, D.H. The Applicability of Weibull Model for the Kinetics Inactivation of Listeria Monocytogenes and Escherichia Coli O157: H7 on Soybean Sprouts Submitted to Chemical Sanitizers in Combination with Ultrasound at Mild Temperatures. LWT 2018, 91, 573–579. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Wakeling, C.; Bach, S.; Orban, S.; Delaquis, P. Disinfection of Alfalfa and Radish Sprouting Seed Using Oxidizing Agents and Treatments Compliant with Organic Food Production Principles. J. Food Prot. 2020, 83, 779–787. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response Surface Optimisation of Germination Conditions to Improve the Accumulation of Bioactive Compounds and the Antioxidant Activity in Quinoa. Int. J. Food Sci. Technol. 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Standard’s ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microor-ganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- Standard’s ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- Holliday, S.L.; Scouten, A.J.; Beuchat, L.R. Eff Cacy of Chemical Treatments in Eliminating Salmonella and Escherichia coli O157:H7 on Scarii Ed and Polished Alfalfa Seeds. J. Food Prot. 2001, 64, 1489–1495. [Google Scholar] [CrossRef]
- Goodburn, C.; Wallace, C.A. The Microbiological Efficacy of Decontamination Methodologies for Fresh Produce: A Review. Food Control 2013, 32, 418–427. [Google Scholar] [CrossRef]
- Wirtanen, G.; Salo, S. Disinfection in Food Processing-Efficacy Testing of Disinfectants. Rev. Environ. Sci. Bio/Technol. 2003, 2, 293–306. [Google Scholar] [CrossRef]
- Mendoza, I.C.; Luna, E.O.; Pozo, M.D.; Vásquez, M.V.; Montoya, D.C.; Moran, G.C.; Romero, L.G.; Yépez, X.; Salazar, R.; Romero-Peña, M.; et al. Conventional and Non-Conventional Disinfection Methods to Prevent Microbial Contamination in Minimally Processed Fruits and Vegetables. LWT 2022, 165, 113714. [Google Scholar] [CrossRef]
- Trinetta, V.; Vaidya, N.; Linton, R.; Morgan, M. A Comparative Study on the Effectiveness of Chlorine Dioxide Gas, Ozone Gas and e-Beam Irradiation Treatments for Inactivation of Pathogens Inoculated onto Tomato, Cantaloupe and Lettuce Seeds. Int. J. Food Microbiol. 2011, 146, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Waskow, A.; Butscher, D.; Oberbossel, G.; Klöti, D.; Rudolf von Rohr, P.; Büttner-Mainik, A.; Drissner, D.; Schuppler, M. Low-Energy Electron Beam Has Severe Impact on Seedling Development Compared to Cold Atmospheric Pressure Plasma. Sci. Rep. 2021, 11, 16373. [Google Scholar] [CrossRef]
- Luo, X.; Du, Z.; Yang, K.; Wang, J.; Zhou, J.; Liu, J.; Chen, Z. Effect of Electron Beam Irradiation on Phytochemical Composition, Lipase Activity and Fatty Acid of Quinoa. J. Cereal Sci. 2021, 98, 103161. [Google Scholar] [CrossRef]
- de Alencar, E.R.; Jojoa, W.A.; Silva, K.N.; Souza, N.O.S. Ozonation of Quinoa Seeds (Chenopodium Quinoa Willd.): Saturation and Decomposition Kinetics of Ozone and Physiological Quality of Seeds. Semin. Ciências Agrárias 2021, 42, 1019–1032. [Google Scholar] [CrossRef]
- Piletić, K.; Linšak, D.T.; Kovač, B.; Mežnarić, S.; Repustić, M.; Radmanović-Skrbić, M.; Gobin, I. Ozone Disinfection Efficiency against Airborne Microorganisms in Hospital Environment: A Case Study. Arch. Ind. Hyg. Toxicol. 2022, 73, 270–276. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of Actions of Sodium Hypochlorite in Cleaning and Disinfection Processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Government of Canada, C.F.I.A. Preventive Controls for the Hygienic Production of Sprouted Seeds. Available online: https://inspection.canada.ca/preventive-controls/fresh-fruits-or-vegetables/sprouted-seeds/eng/1524179755850/1524179758065 (accessed on 26 January 2024).
- Abdelshafy, A.M.; Hu, Q.; Luo, Z.; Ban, Z.; Li, L. Hydrogen Peroxide from Traditional Sanitizer to Promising Disinfection Agent in Food Industry. Food Rev. Int. 2023, 40, 658–690. [Google Scholar] [CrossRef]
- Li, K.; Dong, W.; Zhao, Y.; Xu, H.; Chen, J.; Xu, C. Effects of Cultivar and Ethanol Disinfection on Aseptic Germination of Loquat (Eriobotrya Japonica) Seeds. HortScience 2017, 52, 941–945. [Google Scholar] [CrossRef]
- Li, R.; Lan, Y.; Zeng, X.; Wei, J. Effects of different disinfection methods and soaking with aluminium on seed germination of Jatropha curcas. Seed 2013, 32, 5–8. [Google Scholar]
- Barampuram, S.; Allen, G.; Krasnyanski, S. Effect of Various Sterilization Procedures on the in Vitro Germination of Cotton Seeds. Plant Cell Tissue Organ Cult. 2014, 118, 179–185. [Google Scholar] [CrossRef]
- Beuchat, L. Comparison of Chemical Treatments to Kill Salmonella on Alfalfa Seeds Destined for Sprout Production. Int. J. Food Microbiol. 1997, 34, 329–333. [Google Scholar] [CrossRef]
- Bari, M.L.; Nei, D.; Enomoto, K.; Todoriki, S.; Kawamoto, A.S. Combination Treatments for Killing Escherichia Coli O157:H7 on Alfalfa, Radish, Broccoli, and Mung Bean Seeds. J. Food Prot. 2009, 72, 631–636. [Google Scholar] [CrossRef]
- Coban, H.B. Organic Acids as Antimicrobial Food Agents: Applications and Microbial Productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.; Kalantari, A.; Khalid, M.F. Eliminating Salmonella Enterica in Alfalfa and Mung Bean Sprouts by Organic Acid and Hot Water Immersions. J. Food Process. Preserv. 2008, 32, 335–342. [Google Scholar] [CrossRef]
- Singh, B.R.; Chandra, M.; Agarwal, R.; Babu, N. Curing of Salmonella Enterica, Serovar Typhimurium-Contaminated Cowpea Seeds and Sprouts with Vinegar and Chlorination. J. Food Process. Preserv. 2005, 29, 268–277. [Google Scholar] [CrossRef]
- Nei, D.; Latiful, B.M.; Enomoto, K.; Inatsu, Y.; Kawamoto, S. Disinfection of Radish and Alfalfa Seeds Inoculated with Escherichia Coli O157:H7 and Salmonella by a Gaseous Acetic Acid Treatment. Foodborne Pathog. Dis. 2011, 8, 1089–1094. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Diaz, A.; Bregoff, H.A. Seed Disinfestation Practices to Control Seed-Borne Fungi and Bacteria in Home Production of Sprouts. Foods 2023, 12, 747. [Google Scholar] [CrossRef]
- Liu, H.K.; Li, Z.H.; Zhang, X.W.; Liu, Y.P.; Hu, J.G.; Yang, C.W.; Zhao, X.Y. The Effects of Ultrasound on the Growth, Nutritional Quality and Microbiological Quality of Sprouts. Trends Food Sci. Technol. 2021, 111, 292–300. [Google Scholar] [CrossRef]
- Michalczyk, M.; Fiutak, G.; Tarko, T. Effect of Hot Water Treatment of Seeds on Quality Indicators of Alfalfa Sprouts. LWT 2019, 113, 108270. [Google Scholar] [CrossRef]
- Bari, M.L.; Enomoto, K.; Nei, D.; Kawamoto, A.S. Practical Evaluation of Mung Bean Seed Pasteurization Method in Japan. J. Food Prot. 2010, 73, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.J.; Kang, D.H. Effect of Sequential Dry Heat and Hydrogen Peroxide Treatment on Inactivation of Salmonella Typhimurium on Alfalfa Seeds and Seeds Germination. Food Microbiol. 2016, 53, 9–14. [Google Scholar] [CrossRef]
- Moragas Encuentra, M.; Valcárcel Alonso, S. Recopilación de Normas Microbiológicas de los Alimentos y Asimilados (Superficies, Aguas Diferentes de Consumo, Subproductos) y Otros Parámetros Físico-Químicos de Interés Sanitario; Gobierno Vasco, Departamento de Salud: Bilbao, España, 2021. Available online: http://www.actae.elkarteak.net/normas-microbiologicas-y-parametros-fisico-quimicos-actualizacion-enero-2021/ (accessed on 11 September 2024).
- Mileto, D.; Mancon, A.; Staurenghi, F.; Rizzo, A.; Econdi, S.; Gismondo, M.R.; Guidotti, M. Inactivation of SARS-CoV-2 in the Liquid Phase: Are Aqueous Hydrogen Peroxide and Sodium Percarbonate Efficient Decontamination Agents? ACS Chem. Health Saf. 2021, 28, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Raffellini, S.; Guerrero, S.; Alzamora, S.M. Effect of Hydrogen Peroxide Concentration and Ph on Inactivation Kinetics of Escherichia Coli. J. Food Saf. 2008, 28, 514–533. [Google Scholar] [CrossRef]
- Cords, B.; Burnett, S.; Finley, M.; Magnuson, J.; Hilgren, J. Sanitizers: Halogens, Surface-Active Agents, and Peroxides. Antimicrob. Food 2005, 145, 507–572. [Google Scholar] [CrossRef]
- Ban, G.-H.; Kim, S.-H.; Kang, D.-H.; Park, S.-H. Comparison of the Efficacy of Physical and Chemical Strategies for the Inactivation of Biofilm Cells of Foodborne Pathogens. Food Sci. Biotechnol. 2023, 32, 1679–1702. [Google Scholar] [CrossRef]
- Codes of Federal Regulations. National Organic Program. Available online: https://www.ecfr.gov/current/title-7/subtitle-B/chapter-I/subchapter-M/part-205 (accessed on 28 August 2025).
- Kitis, M. Disinfection of Wastewater with Peracetic Acid: A Review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Z.; Ji, J.; Ge, Y.; Zhong, S.; Nie, C.; Lu, S. Chlorate in Foodstuffs from South China and Its Implication for Human Exposure. Food Chem. Toxicol. 2025, 195, 115120. [Google Scholar] [CrossRef]
- Gil, M.I.; Marín, A.; Andujar, S.; Allende, A. Should Chlorate Residues Be of Concern in Fresh-Cut Salads? Food Control 2016, 60, 416–421. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The Importance of the Viable but Non-Culturable State in Human Bacterial Pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Pazos-Rojas, L.A.; Cuellar-Sánchez, A.; Romero-Cerón, A.L.; Rivera-Urbalejo, A.; Van Dillewijn, P.; Luna-Vital, D.A.; Muñoz-Rojas, J.; Morales-García, Y.E.; Bustillos-Cristales, M.d.R. The Viable but Non-Culturable (VBNC) State, a Poorly Explored Aspect of Beneficial Bacteria. Microorganisms 2023, 12, 39. [Google Scholar] [CrossRef]
- Okada, A.; Tsuchida, M.; Rahman, M.; Inoshima, Y. Two-Round Treatment With Propidium Monoazide Completely Inhibits the Detection of Dead campylobacter Spp. Cells by Quantitative PCR. Front. Microbiol. 2022, 13, 801961. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.Y.; Sung, J.M. Use of Ultrasonication to Enhance Pea Seed Germination and Microbial Quality of Pea Sprouts. Int. J. Food Sci. Technol. 2014, 49, 1699–1706. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in Pea Seed Germination. Plant Signal Behav 2012, 7, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, S.E.; Maurady, A.; Lachkar, M.; Britel, M.R.; Bakali, A.H. Effect of Temperature and Different Pre-Treatments on Seed Germination of Stachys Mouretii Batt. & Pit. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100438. [Google Scholar] [CrossRef]
- Weiss, A.; Hammes, W.P. Efficacy of Heat Treatment in the Reduction of Salmonellae and Escherichia Coli O157:H– on Alfalfa, Mung Bean and Radish Seeds Used for Sprout Production. Eur. Food Res. Technol. 2005, 221, 187–191. [Google Scholar] [CrossRef]
- Jaquette, C.B.; Beuchat, L.R.; Mahon, B.E. Efficacy of Chlorine and Heat Treatment in Killing Salmonella Stanley Inoculated onto Alfalfa Seeds and Growth and Survival of the Pathogen during Sprouting and Storage. Appl. Env. Microbiol 1996, 62, 2212–2215. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Wang, G.; Ren, P.; Wu, Y.; He, Q. Effects of Typical Antimicrobials on Growth Performance, Morphology and Antimicrobial Residues of Mung Bean Sprouts. Antibiotics 2022, 11, 807. [Google Scholar] [CrossRef]
- Kern, K.A.; Pergo, E.M.; Kagami, F.L.; Arraes, L.S.; Sert, M.A.; Ishii-Iwamoto, E.L. The Phytotoxic Effect of Exogenous Ethanol on Euphorbia heterophylla L. Plant Physiol. Biochem. 2009, 47, 1095–1101. [Google Scholar] [CrossRef]
- Masserano, G.; Moretti, B.; Bertora, C.; Vidotto, F.; Monaco, S.; Vocino, F.; Vamerali, T.; Sacco, D. Acetic Acid Disturbs Rice Germination and Post-Germination under Controlled Conditions Mimicking Green Mulching in Flooded Paddy. Ital. J. Agron. 2022, 17, 1926. [Google Scholar] [CrossRef]
- Kim, H.J.; Feng, H.; Kushad, M.M.; Fan, X. Effects of Ultrasound, Irradiation, and Acidic Electrolyzed Water on Germination of Alfalfa and Broccoli Seeds and Escherichia Coli O157:H7. J. Food Sci. 2006, 71, M168–M173. [Google Scholar] [CrossRef]
- Yang, H.; Gao, J.; Yang, A.; Chen, H. The Ultrasound-Treated Soybean Seeds Improve Edibility and Nutritional Quality of Soybean Sprouts. Food Res. Int. 2015, 77, 704–710. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, N.; Li, W.; Zhang, B.; Shi, T.; Xie, M.; Yu, M. Effect of Ultrasonic Induction on the Main Physiological and Biochemical Indicators and γ–Aminobutyric Acid Content of Maize during Germination. Foods 2022, 11, 1358. [Google Scholar] [CrossRef]
- Alfalahi, A.O.; Alobaidy, B.S.; Almarie, A.A.; Dhanoon, O.M.; Qasem, J.R.; Almehemdi, A.F.; Najda, A. Ultrasonic Treatment Enhances Germination and Affects Antioxidant Gene Expression in Soybean (Glycine max L. Merr). Agronomy 2022, 12, 2446. [Google Scholar] [CrossRef]
- Foschi, M.L.; Juan, M.; Pascual, B.; Pascual-Seva, N. Effects of High Intensity Ultrasound Stimulation on the Germination Performance of Caper Seeds. Plants 2023, 12, 2379. [Google Scholar] [CrossRef] [PubMed]
- Mylsamy, P.; Tamilmani, E.; Venugopal, R.; Murugaiyan, S.; Ranganathan, U. Cotton Seed Management: Traditional and Emerging Treatment Approaches for Enhanced Productivity. J. Cotton Res. 2025, 8, 7. [Google Scholar] [CrossRef]
- Akbari, M.; Akbari, M.; Akbari, D.; Sajedi, N.A. Influence of Sodium Hypochlorite on Seed Germination and Early Seedling Growth of Rice (Oryza sativa L.) Variety Tarum. Res. Crops 2012, 13, 11–15. [Google Scholar]
- Tao, M.; Chen, J.; Huang, K. Bio-based antimicrobial delivery systems for improving microbial safety and quality of raw or minimally processed foods. Curr. Opin. Food Sci. 2021, 41, 189–200. [Google Scholar]
- Carcione, R.; Lanzetta, L.; D’Orsi, B.; Di Sarcina, I.; Mansi, E.; Scifo, J.; Cemmi, A. Gamma Irradiation for Agrifood: Non-Destructive Approaches to Study the Secondary Effects Produced in Italian Wheat Matrices. Polysaccharides 2025, 6, 39. [Google Scholar] [CrossRef]
- Asefi, N.; Gone, S.S.J.C.; Singh, R.K. Application of Plasma-Activated Water on Lentil Seeds: Germination, Biochemical Changes, and Nutritional Quality Evaluation. Food Bioprocess Technol. 2025, 18, 5196–5217. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Asgari, A.; Bakhshi, S.; Shahriari, A.G.; Lee, C.W. Ultrasound Application for the Decontamination of Roselle (Hibiscus sabdariffa L.) Seeds: Influence on Fungal Inhibition and Seed Quality. Ultrason. Sonochemistry 2023, 95, 106404. [Google Scholar] [CrossRef]


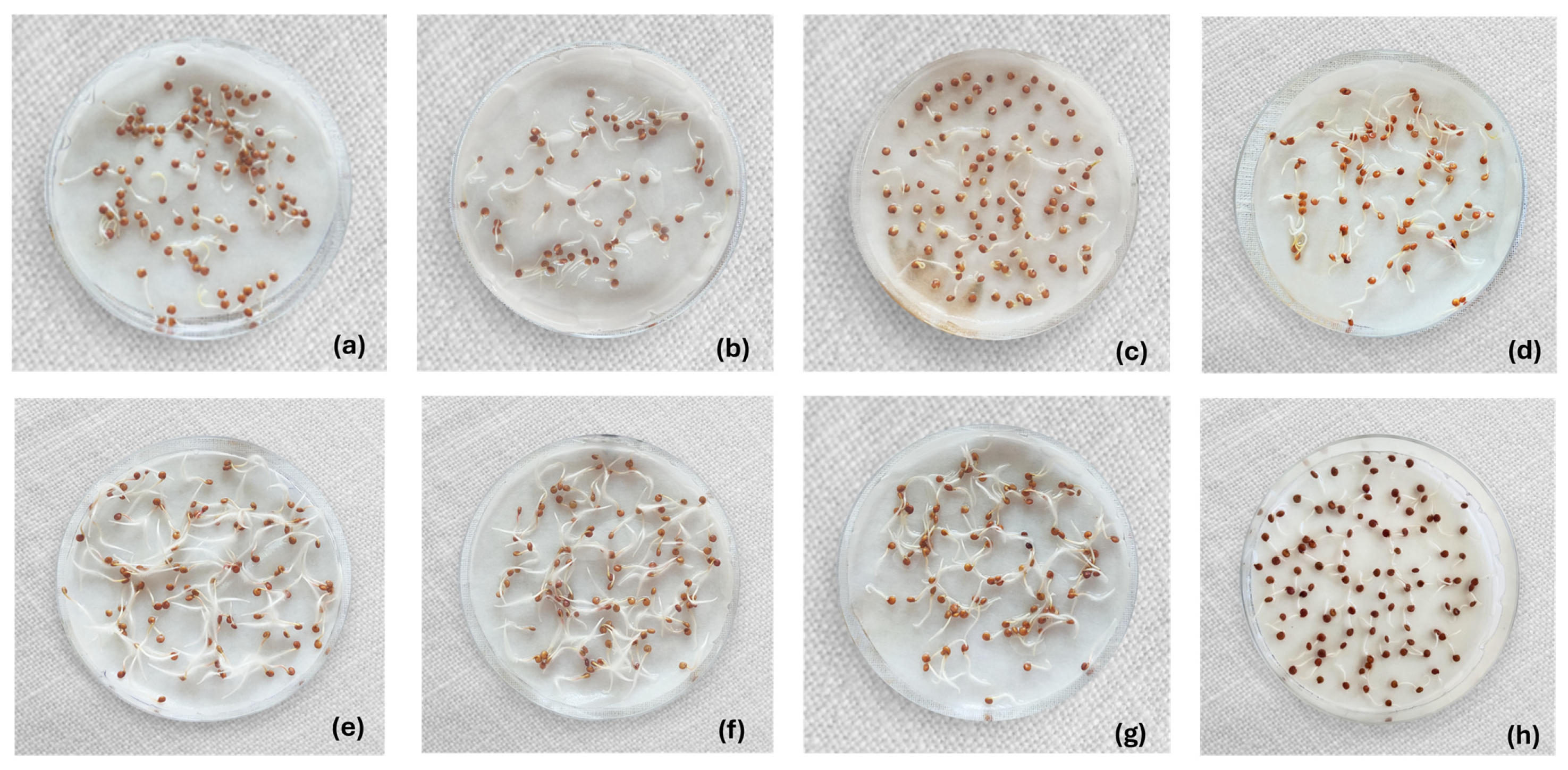
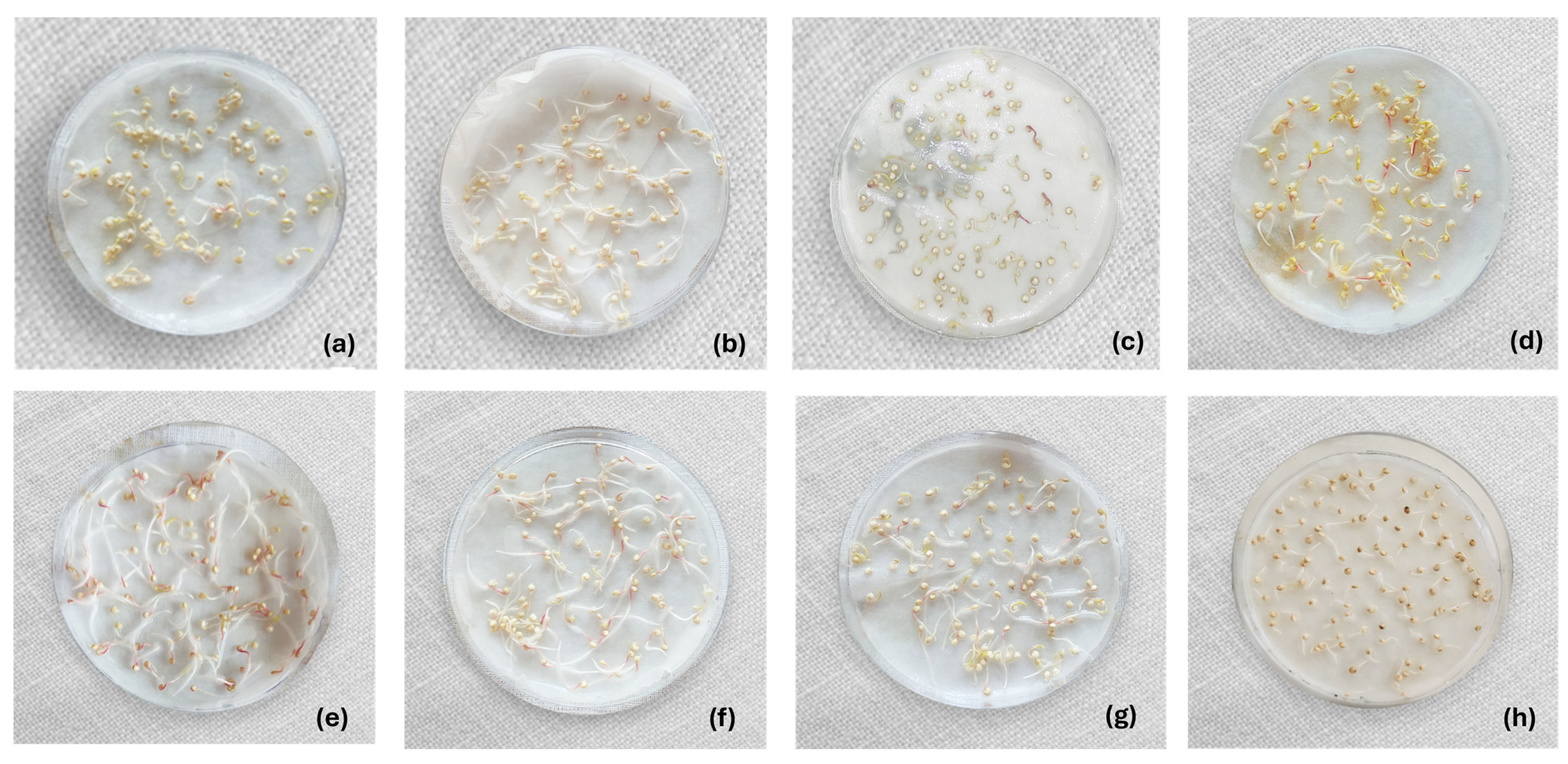
| Treatment Type | Procedure | RH (%) | PK (%) |
|---|---|---|---|
| Control | Untreated quinoa | 97.50 ± 3.02 a | 99.67 ± 0.52 A |
| Chemical | 200 ppm sodium hypochlorite | 100.00 ± 0.00 a | 100.00 ± 0.00 A |
| 8% hydrogen peroxide H2O2 | 99.00 ± 0.00 a | 100.00 ± 0.00 A | |
| 4% acetic acid | 95.67 ± 3.21 ab | 100.00 ± 0.00 A | |
| 70% alcohol | 91.00 ± 3.00 b | 89.67 ± 5.51 B | |
| Combined | Ultrasound 15 min + 200 ppm sodium hypochlorite | 100.00 ± 0.00 a | 100.00 ± 0.00 A |
| Ultrasound 30 min + 200 ppm sodium hypochlorite | 99.00 ± 1.00 a | 100.00 ± 0.00 A | |
| Water 50 °C + H2O2 2% + Acetic acid 0.1% | 98.00 ± 1.73 a | 100.00 ± 0.00 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Torres, S.M.; Teixeira, J.A.; Encina-Zelada, C.R.; Silva, C.L.M.; Gomes, A.M. Effect of Different Disinfection Procedures on the Microbiological Quality and Germination Efficacy of Sprouted Quinoa (Chenopodium quinoa) Flour. Foods 2025, 14, 3196. https://doi.org/10.3390/foods14183196
García-Torres SM, Teixeira JA, Encina-Zelada CR, Silva CLM, Gomes AM. Effect of Different Disinfection Procedures on the Microbiological Quality and Germination Efficacy of Sprouted Quinoa (Chenopodium quinoa) Flour. Foods. 2025; 14(18):3196. https://doi.org/10.3390/foods14183196
Chicago/Turabian StyleGarcía-Torres, Silvia Melissa, José António Teixeira, Christian R. Encina-Zelada, Cristina L. M. Silva, and Ana Maria Gomes. 2025. "Effect of Different Disinfection Procedures on the Microbiological Quality and Germination Efficacy of Sprouted Quinoa (Chenopodium quinoa) Flour" Foods 14, no. 18: 3196. https://doi.org/10.3390/foods14183196
APA StyleGarcía-Torres, S. M., Teixeira, J. A., Encina-Zelada, C. R., Silva, C. L. M., & Gomes, A. M. (2025). Effect of Different Disinfection Procedures on the Microbiological Quality and Germination Efficacy of Sprouted Quinoa (Chenopodium quinoa) Flour. Foods, 14(18), 3196. https://doi.org/10.3390/foods14183196











