Identification of Key Aroma-Active Compounds in Commercial Coffee Using GC-O/AEDA and OAV Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Samples
2.2. Chemicals
2.3. Color Measurements
2.4. Determination of Volatile Substances
2.4.1. Isolation of Volatiles by Solvent-Assisted Flavor Evaporation (SAFE)
2.4.2. GC-MS Analysis
2.5. Determination of Non-Volatile Substances
2.5.1. Organic Acid Analysis
2.5.2. Caffeine and Chlorogenic Acids Analysis
2.6. Gas Chromatography–Olfactometry (GC-O) Analysis and Aroma Extraction Dilution Analysis (AEDA)
2.7. Identification and Quantitation of Volatile Compounds
2.8. Calculation of Odor Activity Values (OAVs)
2.9. Recombination and Omission Experiments
2.10. Aroma Profile Evaluation
2.11. Statistical Analysis
3. Results
3.1. Color Value of Four Kinds of Coffee
3.2. Sensory Analysis of Four Kinds of Coffee
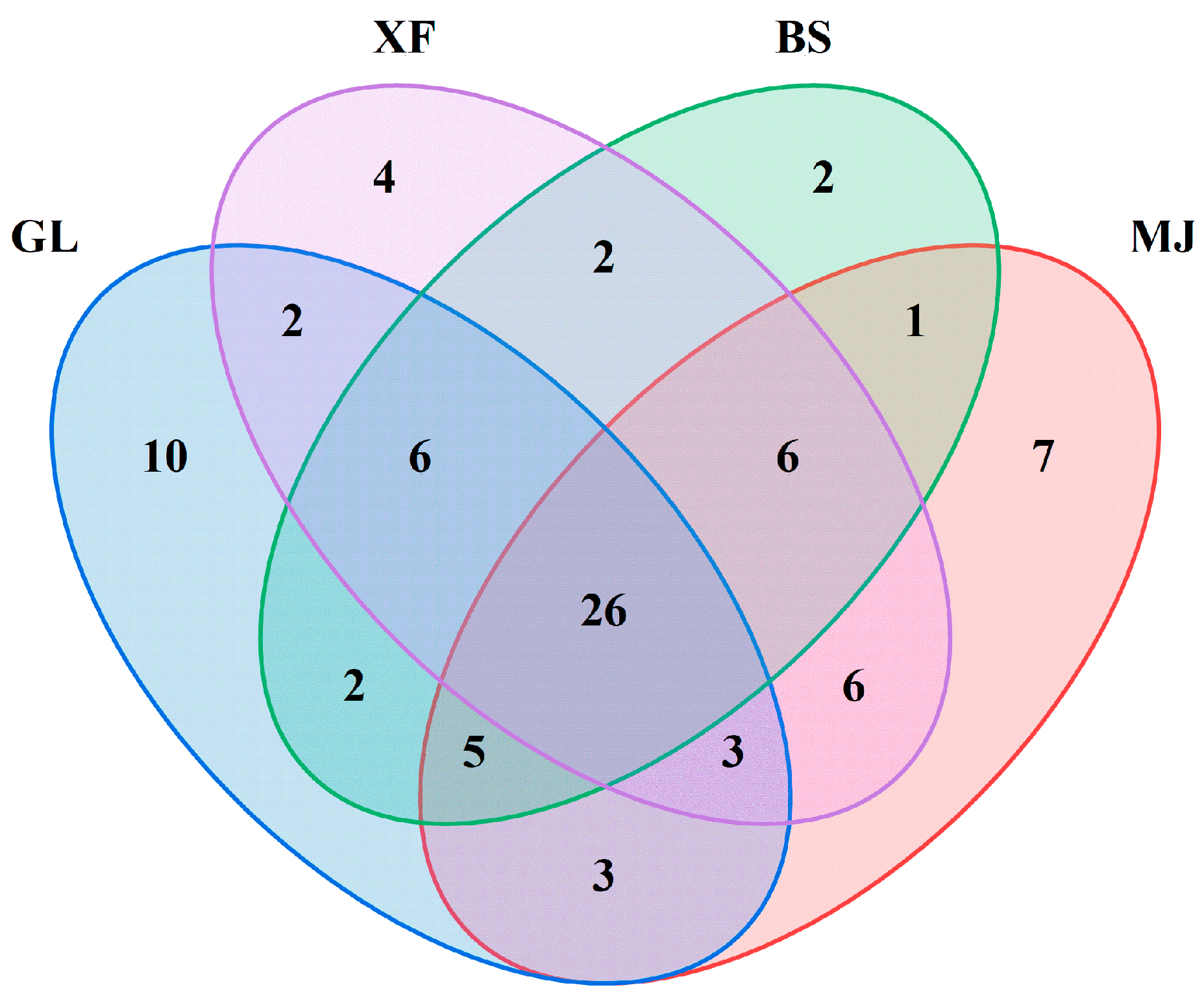
3.3. Volatiles in Four Kinds of Coffee
3.4. Non-Volatiles in Four Kinds of Coffee
3.5. Aroma-Active Compounds in Coffee
3.6. Quantitation of the Aroma-Active Compounds and OAVs
3.7. Aroma Recombination
3.8. Omission Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAFE | Solvent-assisted flavor evaporation |
| GC-MS | Gas chromatography–mass spectrometry |
| GC-O | Gas chromatography–olfactometry |
| AEDA | Aroma extract dilution analysis |
| OAV | Odor activity value |
| LC-MS | Liquid chromatography–mass spectrometry |
| HPLC | High-performance liquid chromatography |
| QDA | Quantitative descriptive analysis |
References
- Martins, V.d.C.; Godoy, R.L.d.O.; Gouvêa, A.C.M.S.; Santiago, M.C.P.d.A.; Borguini, R.G.; Braga, E.C.d.O.; Pacheco, S.; Nascimento, L.d.S.d.M.D. Fraud investigation in commercial coffee by chromatography. Food Qual. Saf. 2018, 2, 121–133. [Google Scholar] [CrossRef]
- Ongo, E.A.; Montevecchi, G.; Antonelli, A.; Sberveglieri, V.; Iii, F.S. Metabolomics fingerprint of Philippine coffee by SPME-GC-MS for geographical and varietal classification. Food Res. Int. 2020, 134, 109227. [Google Scholar] [CrossRef] [PubMed]
- Knysak, D. Volatile compounds profiles in unroasted Coffea arabica and Coffea canephora beans from different countries. Food Sci. Technol. 2017, 37, 444–448. [Google Scholar] [CrossRef]
- Cui, D.D.; Liu, Y.; Chen, Y.P.; Feng, X.; Lu, Y.; Yu, B. Application of SPME-GC-TOFMS, E-nose, and sensory evaluation to investigate the flavor characteristics of Chinese Yunnan coffee at three different conditions (beans, ground powder, and brewed coffee). Flavour Fragr. J. 2020, 35, 541–560. [Google Scholar] [CrossRef]
- Toledo, P.R.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship Between the Different Aspects Related to Coffee Quality and Their Volatile Compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef]
- Amalia, F.; Aditiawati, P.; Yusianto; Putri, S.P.; Fukusaki, E. Gas chromatography/mass spectrometry-based metabolite profiling of coffee beans obtained from different altitudes and origins with various postharvest processing. Metabolomics 2021, 17, 69. [Google Scholar] [CrossRef]
- Bichlmaier, C.; Fröhlich, S.M.; Brychcy, V.; Graßl, A.; Behrens, M.; Lang, R. Contribution of mozambioside roasting products to coffee’s bitter taste. Food Chem. 2024, 469, 142547. [Google Scholar] [CrossRef]
- Rune, C.J.; Giacalone, D.; Steen, I.; Duelund, L.; Münchow, M.; Clausen, M.P. Acids in brewed coffees: Chemical composition and sensory threshold. Curr. Res. Food Sci. 2023, 6, 100485. [Google Scholar] [CrossRef]
- Yeager, S.E.; Batali, M.E.; Guinard, J.-X.; Ristenpart, W.D. Acids in coffee: A review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2023, 63, 1010–1036. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Huang, D.; Chen, S.; Zhu, S. Characterization and discrimination of volatile compounds in roasted Arabica coffee beans from different origins by combining GC-TOFMS, GC-IMS, and GC-E-Nose. Food Chem. 2025, 481, 144079. [Google Scholar] [CrossRef] [PubMed]
- Portela, C.d.S.; de Almeida, I.F.; Mori, A.L.B.; Yamashita, F.; Benassi, M.d.T. Brewing conditions impact on the composition and characteristics of cold brew Arabica and Robusta coffee beverages. LWT 2021, 143, 111090. [Google Scholar] [CrossRef]
- Marek, G.; Dobrzański, B.; Oniszczuk, T.; Combrzyński, M.; Ćwikła, D.; Rusinek, R. Detection and differentiation of volatile compound profiles in roasted coffee arabica beans from different countries using an electronic nose and GC-MS. Sensors 2020, 20, 2124. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Yoon, S.; Jo, S.M.; Hong, S.J.; Kim, Y.J.; Kim, J.K.; Shin, E. Chemical sensory investigation in green and roasted beans Coffea arabica L. (cv. Yellow bourbon) by various brewing methods using electronic sensors. J. Food Sci. 2023, 88, 1033–1047. [Google Scholar] [CrossRef]
- Pua, A.; Lau, H.; Liu, S.Q.; Tan, L.P.; Goh, R.M.V.; Lassabliere, B.; Leong, K.-C.; Sun, J.; Cornuz, M.; Yu, B. Improved detection of key odourants in Arabica coffee using gas chromatography-olfactometry in combination with low energy electron ionisation gas chromatography-quadrupole time-of-flight mass spectrometry. Food Chem. 2020, 302, 125370. [Google Scholar] [CrossRef]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. Characterization of fatty acid, amino acid and volatile compound compositions and bioactive components of seven coffee (Coffea robusta) cultivars grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef]
- Freitas, A.M.C.; Mosca, A.I. Coffee geographic origin—An aid to coffee differentiation. Food Res. Int. 1999, 32, 565–573. [Google Scholar]
- Nie, R.; Zhang, C.; Liu, H.; Wei, X.; Gao, R.; Shi, H.; Zhang, D.; Wang, Z. Characterization of key aroma compounds in roasted chicken using SPME, SAFE, GC-O, GC–MS, AEDA, OAV, recombination-omission tests, and sensory evaluation. Food Chem. X 2024, 21, 101167. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Wen, B.; Tian, J.; Cheng, Z.; Tang, S.; Nie, Y.; Wu, X.; Guo, X.; Li, B. Characterization and metabolism pathway of volatile compounds in blueberries of different varieties and origins analyzed via HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2025, 480, 143813. [Google Scholar] [CrossRef]
- Lau, H.; Liu, S.Q.; Xu, Y.Q.; Lassabliere, B.; Sun, J.; Yu, B. Characterising volatiles in tea (Camellia sinensis). Part I: Comparison of headspace-solid phase microextraction and solvent assisted flavour evaporation. LWT 2018, 94, 178–189. [Google Scholar] [CrossRef]
- Zhai, H.; Dong, W.; Tang, Y.; Hu, R.; Yu, X.; Chen, X. Characterization of the volatile flavour compounds in Yunnan Arabica coffee prepared by different primary processing methods using HS-SPME/GC-MS and HS-GC-IMS. LWT 2024, 192, 115717. [Google Scholar] [CrossRef]
- Yu, M.; Li, T.; Song, H. Characterization of key aroma-active compounds in four commercial oyster sauce by SGC/GC × GC–O–MS, AEDA, and OAV. J. Food Compos. Anal. 2022, 107, 104368. [Google Scholar] [CrossRef]
- Pang, X.; Yin, H.; Li, J.; Shi, Y.; Yang, Z. Molecular insights into the contribution of oak barrel aging to the aroma of beer with high alcohol content using SAFE-GC-O/AEDA and OAV calculation. Food Chem. 2025, 491, 145329. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Mayer, F.; Grosch, W. Sensory study on the character impact odorants of roasted arabica coffee. J. Agric. Food Chem. 1999, 47, 695–699. [Google Scholar] [CrossRef]
- Moon, S.A.; Wongsakul, S.; Kitazawa, H.; Saengrayap, R. Impact of Roasting and Storage Conditions on the Shelf Stability of Thai Arabica Coffee. J. Agric. Food Res. 2025, 22, 102060. [Google Scholar] [CrossRef]
- Tsai, C.F.; Jioe, I.P.J. The analysis of chlorogenic acid and caffeine content and its correlation with coffee bean color under different roasting degree and sources of coffee (Coffea arabica typica). Processes 2021, 9, 2040. [Google Scholar] [CrossRef]
- Alcantara, G.M.; Martins, L.C.; Gomes, W.P.; Dresch, D.; Rocha, F.R.; Melchert, W.R. Effect of roasting on chemical composition of coffee. Food Chem. 2025, 477, 143169. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Alessandroni, L.; Fioretti, L.; Sagratini, G.; Vittori, S.; Maggi, F.; Caprioli, G. Discrimination of Filter Coffee Extraction Methods of a Medium Roasted Specialty Coffee Based on Volatile Profiles and Sensorial Traits. Foods 2023, 12, 3199. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Borompichaichartkul, C.; Suppavorasatit, I.; Cadwallader, K.R. Impact of drying process on chemical composition and key aroma components of Arabica coffee. Food Chem. 2019, 291, 49–58. [Google Scholar] [CrossRef]
- Angeloni, S.; Mustafa, A.M.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F.K.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E.; et al. Characterization of the Aroma Profile and Main Key Odorants of Espresso Coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef]
- Seninde, D.R.; Chambers, E. Coffee flavor: A review. Beverages 2020, 6, 44. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Hu, F.; Miyakawa, T.; Tanokura, M. Two-dimensional 1H–13C nuclear magnetic resonance (NMR)-based comprehensive analysis of roasted coffee bean extract. J. Agric. Food Chem. 2011, 59, 9065–9073. [Google Scholar] [CrossRef] [PubMed]
- Santanatoglia, A.; Angeloni, S.; Caprioli, G.; Fioretti, L.; Ricciutelli, M.; Vittori, S.; Alessandroni, L. Comprehensive investigation of coffee acidity on eight different brewing methods through chemical analyses, sensory evaluation and statistical elaboration. Food Chem. 2024, 454, 139717. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Wang, M.-Q.; Ma, W.-J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.-P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]





| CIELAB Color Space | GL | MJ | XF | BS |
|---|---|---|---|---|
| L* | 25.56 ± 0.21 | 27.92 ± 0.42 | 30.98 ± 0.6 | 28.34 ± 0.05 |
| a* | 9.13 ± 0.13 | 11.86 ± 0.08 | 12.14 ± 0.07 | 11.68 ± 0.07 |
| b* | 10.6 ± 0.13 | 14.45 ± 0.17 | 16.09 ± 0.29 | 14.74 ± 0.11 |
| Compounds | Content (mg/g) | |||
|---|---|---|---|---|
| GL | MJ | XF | BS | |
| Lactic acid | 1.22 ± 0.06 ab | 1.18 ± 0.04 a | 1.18 ± 0.07 a | 1.31 ± 0.04 b |
| Malic acid | 1.24 ± 0.03 a | 2.44 ± 0.04 b | 2.84 ± 0.03 c | 2.83 ± 0.03 c |
| Citric acid | 3.43 ± 0.16 a | 4.89 ± 0.07 b | 5.94 ± 0.11 c | 7.51 ± 0.18 d |
| Quinic acid | 8.34 ± 0.15 a | 6.77 ± 0.2 b | 6.76 ± 0.13 b | 7.51 ± 0.23 c |
| Succinic acid | ND e | ND | ND | ND |
| Tartaric acid | ND | ND | ND | ND |
| Caffeine | 12.21 ± 0.13 a | 12.05 ± 0.11 a | 12.08 ± 0.11 a | 11.99 ± 0.17 a |
| Chlorogenic acid | 4.27 ± 0.06 a | 21.26 ± 0.45 b | 22.33 ± 0.2 c | 15.77 ± 0.76 d |
| Compounds | Odor Description a | RI | FD d | Identification e | ||||
|---|---|---|---|---|---|---|---|---|
| TG-Wax b | Literature c | GL | MJ | XF | BS | |||
| Pyridine | Smoky | 1165 | 1179 | 1 | 3 | - | 1 | MS, RI, Std, O |
| Limonene | Lemon | 1180 | 1185 | 1 | - | - | - | MS, RI, Std, O |
| 2,4,5-trimethyloxazole | Nuts | 1186 | 1190 | 1 | 3 | 1 | - | MS, RI, Std, O |
| Pyrazine | Roasted potatoes | 1193 | 1210 | 1 | - | - | - | MS, RI, Std, O |
| Methyl furfuryl ether | Coffee | 1224 | 1243 | 3 | 3 | 9 | 3 | MS, RI, Std, O |
| 3-methyl-3-butene-1-ol | Fruity | 1234 | 1236 | 3 | - | - | - | MS, RI, Std, O |
| Styrene | Floral | 1236 | 1254 | 1 | - | - | 3 | MS, RI, Std, O |
| 2-methyltetrahydrofurano-3-one | Nuts | 1244 | 1246 | 1 | - | 1 | - | MS, RI, Std, O |
| 2-methylpyrazine | Nuts, roasted | 1246 | 1261 | 1 | - | - | 1 | MS, RI, Std, O |
| 3-hydroxy-2-butanone | Butter | 1263 | 1286 | 9 | - | 9 | 9 | MS, RI, Std, O |
| Hydroxyacetone | Caramel | 1278 | 1275 | 9 | 27 | 9 | 27 | MS, RI, Std, O |
| 2,5-dimethylpyrazine | Roasted potatoes | 1300 | 1303 | 3 | 9 | 9 | 3 | MS, RI, Std, O |
| 2,6-dimethylpyrazine | Nuts, roasted meat | 1306 | 1328 | 9 | 9 | 3 | 3 | MS, RI, Std, O |
| 2-ethylpyrazine | Peanut, woody | 1309 | 1292 | 1 | - | 1 | 1 | MS, RI, Std, O |
| 2-hydroxy-3-pentanone | Earthy | 1336 | 1361 | 1 | - | 1 | 1 | MS, RI, Std, O |
| Methylcyclopentenolone | Nuts | 1347 | 1366 | 3 | - | - | - | MS, RI, Std, O |
| 2-ethyl-6-methylpyrazine | Roasted, nuts | 1368 | 1386 | 3 | 27 | 9 | 27 | MS, RI, Std, O |
| 2-ethyl-5-methylpyrazine | Coffee | 1374 | 1383 | 81 | 3 | 3 | 9 | MS, RI, Std, O |
| 2,3,5-trimethylpyrazine | Coffee and cocoa | 1389 | 1405 | 81 | - | 27 | 27 | MS, RI, Std, O |
| 2-propyrazine | Vegetables, nuts | 1396 | - | 9 | - | - | - | MS, Std, O |
| Allyl butyrate | Fruity | 1414 | - | 3 | - | - | - | MS,O |
| Acetic acid | Vinegar | 1429 | 1465 | 3 | 3 | 3 | 1 | MS, RI, Std, O |
| Furfural | Almonds, nuts | 1445 | 1466 | 2187 | 2187 | 2187 | 2187 | MS, RI, Std, O |
| Acetylacetone peroxide | Nuts | 1457 | 1469 | 3 | - | 9 | 3 | MS, RI, Std, O |
| Furyl methyl sulfide | Onion, spicy | 1460 | 1503 | 81 | - | - | - | MS, RI, Std, O |
| Tetrahydrofurfuryl alcohol | Caramel | 1472 | 1481 | 243 | - | - | - | MS, RI, Std, O |
| 2-acetylfuran | Cocoa, caramel, Coffee | 1483 | 1479 | 729 | 3 | 729 | 1 | MS, RI, Std, O |
| Pyrrole | Mold | 1487 | 1505 | 243 | 3 | - | 27 | MS, RI, Std, O |
| Furyl acetate | Sweety | 1519 | 1521 | 729 | 3 | 243 | 1 | MS, RI, Std, O |
| Linalool | Floral, lemon, rose | 1538 | 1537 | 3 | 27 | 81 | 9 | MS, RI, Std, O |
| 5-methylfurfural | Caramel | 1550 | 1558 | 243 | 3 | 243 | 3 | MS, RI, Std, O |
| 2-acetylpyridine | Barbecue | 1570 | 1590 | 81 | 243 | - | - | MS, RI, Std, O |
| Methyl 2-furan propionate | Fruity | 1578 | 1599 | 3 | - | 81 | - | MS, RI, Std, O |
| 5-methyl-6, 7-dihydro-5H-cyclopentanopyrazine | Nuts, barbecue | 1581 | 1616 | 1 | 1 | - | 9 | MS, RI, Std, O |
| 2-(furan-2-ylmethyl)furan | Savory | 1584 | 1628 | 81 | - | - | - | MS, RI, Std, O |
| γ -butyrolactone | Caramel, roasted nuts | 1590 | 1601 | 243 | 81 | 81 | 9 | MS,RI,O |
| 2-acetylpyrazine | Popcorn | 1594 | 1604 | 243 | 81 | - | 81 | MS, RI, Std, O |
| Butyric acid | Butter, cheese, sour | 1610 | 1628 | 9 | 1 | 1 | 3 | MS, RI, Std, O |
| 2-acetyl-1-methylpyrrole | Soil | 1624 | 1609 | 1 | 1 | - | - | MS, RI, Std, O |
| Furfuryl alcohol | Bread | 1643 | 1660 | 9 | 9 | 9 | 9 | MS, RI, Std, O |
| 2(5H)-furanone | Butter | 1718 | 1712 | 3 | - | 9 | 9 | MS, RI, Std, O |
| Methyl salicylate | Mint | 1741 | 1735 | 1 | - | - | - | MS, RI, Std, O |
| 2-hydroxy-2-cyclopentene-1-ketone | Caramel | 1747 | - | 3 | 27 | - | - | MS, Std, O |
| 3,3-dimethacrylic acid | Dairy | 1777 | 1776 | 3 | 1 | - | 1 | MS, RI, Std, O |
| Isovaleric acid | Sour, stinky | 1649 | 1655 | 243 | 243 | 729 | 243 | MS, RI, Std, O |
| 1-(2-furanyl methyl)-1H-pyrrole | Vegetables | 1795 | 1820 | 729 | 9 | 3 | 3 | MS, RI, Std, O |
| Guaiacol | Woody | 1830 | 1836 | 2187 | 2187 | 2187 | 2187 | MS, RI, Std, O |
| Ethyl cyclopentenolone | Caramel | 1866 | 1845 | 9 | 9 | 1 | 1 | MS, RI, Std, O |
| Maltol | Caramel | 1935 | 1943 | 27 | 27 | 9 | 9 | MS, RI, Std, O |
| 2-acetylpyrrole | Barbecue | 1941 | 1949 | 9 | 3 | 3 | 3 | MS, RI, Std, O |
| Difuryl ether | Coffee | 1960 | 1977 | 1 | 9 | 3 | - | MS, RI, Std, O |
| Phenol | Sweet medicine | 1979 | 1992 | 729 | 3 | 1 | - | MS, RI, Std, O |
| 2-pyrrolidine formaldehyde | Mold, coffee | 1986 | 2030 | 81 | 1 | 1 | 1 | MS, RI, Std, O |
| 4-ethyl-2-methoxyphenol | Smokey | 1996 | 2014 | 729 | 27 | 1 | 27 | MS, RI, Std, O |
| Furaneol | Marshmallows, caramels | 2008 | 2037 | 2187 | 2187 | 2187 | 2187 | MS, RI, Std, O |
| 4-vinyl-2-methoxyphenol | Smoky | 2168 | 2156 | 243 | 729 | 243 | 2187 | MS, RI, Std, O |
| 5-hydroxymethylfurfural | Mold | 2483 | 2509 | 3 | 3 | 1 | 3 | MS, RI, Std, O |
| Lauryl | Woody | 1154 | 1145 | - | 1 | 1 | - | MS, RI, Std, O |
| (+)-limonene | Orange | 1178 | - | - | 1 | - | - | MS, Std, O |
| (E)-3,7-dimethylocta-1,3,6-triene | Sweet herb | 1218 | 1242 | - | 1 | - | - | MS,RI,O |
| 1-hydroxy-2-butanone | Sweet | 1351 | 1375 | - | 9 | 3 | 3 | MS, RI, Std, O |
| 2-ethyl-3-methylpyrazine | Nuts | 1387 | 1402 | - | 1 | 3 | - | MS, RI, Std, O |
| 2-vinylpyrazine | Nuts | 1417 | 1438 | - | 27 | 243 | 9 | MS, RI, Std, O |
| 3-ethyl-2, 5-dimethylpirazine | Peanuts | 1427 | 1438 | - | 27 | - | 9 | MS, RI, Std, O |
| 2-methyl-6-vinyl pyrazine | Roasted | 1470 | 1485 | - | 3 | - | - | MS, RI, Std, O |
| Furyl formate | Sweet | 1480 | 1504 | - | 3 | - | - | MS,RI,O |
| Dihydro-2-methyl-3 (2H)-thiophenone | Sulfur | 1493 | 1506 | - | 3 | 3 | 27 | MS, RI, Std, O |
| Benzaldehyde | Almond | 1496 | 1508 | - | 729 | 729 | 729 | MS, RI, Std, O |
| N-methyl-2-pyrrolidine formaldehyde | Roasted nuts | 1586 | 1610 | - | 3 | 3 | - | MS, RI, Std, O |
| 1-(6-methyl-2-pyrazinyl) acetone | Coffee, cocoa | 1671 | 1676 | - | 1 | 3 | 3 | MS,RI,O |
| Benzyl alcohol | Floral | 1847 | 1877 | - | 1 | 3 | - | MS, RI, Std, O |
| Phenylethanol | Rose | 1878 | 1905 | - | 3 | 1 | - | MS, RI, Std, O |
| α -ethylene-phenylacetaldehyde | Floral, honey, cocoa | 1893 | 1906 | - | 1 | 1 | - | MS, RI, Std, O |
| 2-thiophene methanol | Coffee | 1911 | 1890 | - | 9 | 27 | 1 | MS, RI, Std, O |
| 3,4-dimethoxystyrene | Fruity, oranges | 2019 | 2014 | - | 3 | - | - | MS, RI, Std, O |
| Ethyl palmitate | Fruity, creamy | 2230 | 2250 | - | 3 | - | - | MS, RI, Std, O |
| Indole | Fruity, floral | 2403 | 2412 | - | 1 | - | - | MS, RI, Std, O |
| 2-n-pentylfuran | Fruity | 1215 | 1229 | - | - | 1 | - | MS, RI, Std, O |
| 2,3-dimethylpyrazine | Nuts | 1332 | 1342 | - | - | 3 | 3 | MS, RI, Std, O |
| Hexyl formate | Fruity | 1340 | 1382 | - | - | 1 | - | MS, RI, Std, O |
| 2-ethyl-3,5-dimethylpyrazine | Almond | 1413 | 1437 | - | - | 27 | - | MS, RI, Std, O |
| 3-thiol-3-methyl-1-butanol | Roasted vegetables | 1631 | 1658 | - | - | 3 | - | MS, RI, Std, O |
| 3-methylcyclopentane-1,2-dione | Sweet, woody | 1800 | 1781 | - | - | 3 | 3 | MS, RI, Std, O |
| 2,3-butanediol | Butter | 1528 | 1542 | - | - | - | 243 | MS, RI, Std, O |
| 2, 3-dihydro-3, 5-dihydroxy-6-methyl-4 (H)-pyran-4-one | Smokey | 2235 | 2225 | - | - | - | 1 | MS, RI, Std, O |
| Compounds | Standard Curves | Concentration (μg/g) a | Odor Threshold (mg/kg) b | OAV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GL | MJ | XF | BS | GL | MJ | XF | BS | |||
| Furyl acetate | y = 0.6798x + 0.196 | 41.53 ± 7.15 | - | 4.39 ± 0.55 | - | - c | - | - | - | - |
| Furfuryl alcohol | y = 1.7166x + 0.0086 | 58.95 ± 7.53 | 49.25 ± 1.76 | 31.91 ± 2.72 | 48.03 ± 3.59 | 1.9 | 31 | 26 | 17 | 25 |
| Guaiacol | y = 2.1769x + 0.0223 | 1.14 ± 0.44 | 0.07 ± 0.03 | 0.07 ± 0.04 | 0 | 0.02 | 63 | 12 | 9 | 8 |
| 2-ethyl-5-methylpyrazine | y = 0.7862x + 0.0037 | 2.1 ± 0.45 | - | - | 3.05 ± 0.19 | 0.04 | 52 | - | - | 76 |
| Furyl methyl sulfide | y = 2.1424x − 0.0023 | 0.44 ± 0.03 | - | - | - | - | - | - | - | - |
| Butyric acid | y = 0.5846x + 0.0008 | 1.54 ± 0.25 | - | - | - | 0.204 | 8 | - | - | - |
| 2-acetylpyrazine | y = 0.711x + 0.0018 | 0.55 ± 0.09 | 0.59 ± 0.06 | - | 0.7 ± 0.06 | 0.06 | 9 | 10 | - | 12 |
| Ethyl cyclopentenolone | y = 0.7003x + 0.0034 | 2.1 ± 0.94 | 0.63 ± 0.11 | - | - | - | - | - | - | - |
| Furaneol | y = 0.7x + 0.0003 | 1.76 ± 0.16 | 1.75 ± 0.19 | 1.96 ± 0.45 | 2.5 ± 0.16 | 0.01 | 176 | 175 | 196 | 250 |
| 3-hydroxy-2-butanone | y = 0.5683x + 0.0331 | 5.19 ± 0.16 | - | 5.94 ± 0.26 | 7.53 ± 0.62 | 0.055 | 94 | - | 108 | 137 |
| 2, 6-dimethylpyrazine | y = 1.2255x + 0.0071 | 3.16 ± 0.31 | 3.01 ± 0.3 | - | - | 0.4 | 8 | 8 | - | - |
| 2,3, 5-trimethylpyrazine | y = 1.2279x + 0.0161 | 1.76 ± 0.38 | - | 0 | 1.99 ± 0.09 | 0.022 | 77 | - | 1 | 90 |
| 2-(furan-2-methyl-furan) furan | y = 0.739x + 0.0105 | 3.08 ± 0.37 | - | - | - | - | - | - | - | - |
| Isovaleric acid | y = 0.8538x + 0.0062 | 5.01 ± 0.8 | 13.99 ± 1 | 10.88 ± 0.1 | 7.16 ± 0.53 | 0.07 | 72 | 200 | 155 | 102 |
| 1-(2-furanyl methyl)-1H-pyrrole | y = 2.4452x + 0.0041 | 0.92 ± 0.23 | 0.34 ± 0.02 | - | - | 0.1 | 9 | 3 | - | - |
| 2-acetylpyrrole | y = 1.6549x + 0.0117 | 2.76 ± 0.98 | - | - | - | 58.58 | <1 | - | - | - |
| Hydroxyacetone | y = 0.7044x − 0.0126 | 16.9 ± 0.93 | 15.12 ± 4.07 | 31.9 ± 9.1 | 29.97 ± 8.43 | 10 | 2 | 2 | 3 | 3 |
| Furfural | y = 1.2762x + 0.0097 | 4.32 ± 0.26 | 15.12 ± 4.08 | 16.72 ± 3.4 | 6.26 ± 0.95 | 0.282 | 15 | 62 | 59 | 22 |
| 2-acetylfuran | y = 1.3788x + 0.0354 | 3.1 ± 0.4 | - | 1.65 ± 0.2 | - | 10 | <1 | - | <1 | - |
| 5-methylfurfural | y = 0.5462x + 0.1844 | 11.37 ± 2.61 | - | 23.82 ± 2.13 | - | 0.5 | 23 | - | 48 | - |
| γ -butyrolactone | y = 0.3166x − 0.0365 | 49.86 ± 4.77 | 27.45 ± 0.68 | 16.99 ± 1.14 | 23.39 ± 1.5 | 16 | 3 | 2 | 1 | 1 |
| Maltol | y = 0.8127x − 0.0059 | 6.1 ± 2.3 | 2.09 ± 0.29 | 1.7 ± 0.43 | 3.49 ± 0.23 | 0.21 | 29 | 10 | 8 | 17 |
| Phenol | y = 1.1515x + 0.0375 | 1.14 ± 0.6 | - | - | - | 0.5 | 2 | - | - | - |
| 4-vinyl-2-methoxyphenol | y = 1.2822x + 0.0307 | 2.28 ± 1.17 | 1.02 ± 0.08 | 1.59 ± 0.46 | 1.47 ± 0.1 | 0.003 | 761 | 340 | 530 | 491 |
| 2-propyrazine | y = 0.9629x − 0.0003 | 0.28 ± 0.04 | - | - | - | 0.3 | <1 | - | - | - |
| Tetrahydrofurfuryl alcohol | y = 0.7516x − 0.0024 | 0.83 ± 0.08 | - | - | - | - | - | - | - | - |
| Pyrrole | y = 0.8933x − 0.0063 | 1.86 ± 0.07 | - | - | 0.8 ± 0.04 | 10 | <1 | - | - | <1 |
| 2-acetylpyridine | y = 1.0978x − 0.0047 | 0.78 ± 0.13 | 0.58 ± 0.03 | - | - | 0.019 | 41 | 31 | - | - |
| 2-pyrrolidine formaldehyde | y = 1.0864x − 0.0021 | 2.19 ± 0.51 | - | - | - | 65 | <1 | - | - | - |
| 4-ethyl-2-methoxyphenol | y = 1.4011x − 0.0003 | 1.26 ± 0.4 | 0.19 ± 0.01 | - | 0.31 ± 0.04 | 0.016 | 79 | 12 | - | 19 |
| 2, 5-dimethylpyrazine | y = 1.3423x + 0.0011 | - | 3.52 ± 0.35 | 2.3 ± 0.19 | - | 0.08 | - | 44 | 29 | - |
| 2-ethyl-6-methylpyrazine | y = 1.532x − 0.0158 | - | 2.29 ± 0.05 | 1.82 ± 0.06 | 2.88 ± 0.07 | 0.04 | - | 57 | 45 | 72 |
| 2-vinylpyrazine | y = 0.6536 x − 0.0000 | - | 0.28 ± 0.09 | 0.29 ± 0.02 | 0.12 ± 0.01 | 0.7 | - | <1 | <1 | <1 |
| 3-ethyl-2, 5-methylpyrazine | y = 1.3365x − 0.0001 | - | 0.19 ± 0.09 | - | 0.04 ± 0.04 | 0.005 | - | 38 | - | 9 |
| Benzaldehyde | y = 0.9385 x − 0.0001 | - | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.3 | - | <1 | <1 | <1 |
| Linalool | y = 0.8852x − 0.0047 | - | 1.33 ± 0.03 | 1.12 ± 0.12 | 0.43 ± 0.02 | 0.006 | - | 222 | 187 | 72 |
| 2-hydroxy-2-cyclopentene-1-ketone | y = 0.3923x − 0.0001 | - | 0.45 ± 0.05 | - | - | - | - | - | - | - |
| 2-thiophene methanol | y = 0.657x − 0.0003 | - | 0.61 ± 0.02 | 0.5 ± 0.05 | - | 15 | - | <1 | <1 | - |
| Difuryl ether | y = 1.1231x − 0.0011 | - | 0.23 ± 0.04 | - | - | - | - | - | - | - |
| Methyl furfuryl ether | y = 0.8407x − 0.0004 | - | - | 0.47 ± 0.02 | - | - | - | - | - | - |
| 2-ethyl-3, 5-dimethylpyrazine | y = 0.5048x − 0.0001 | - | - | 0.17 ± 0.01 | - | 0.001 | - | - | 170 | - |
| Acetylacetone peroxide | y = 0.5575x + 0.0645 | - | - | 9.8 ± 0.01 | - | - | - | - | - | - |
| Methyl 2-furan propionate | y = 0.8096x − 0.0004 | - | - | 0.54 ± 0.01 | - | - | - | - | - | - |
| 2(5H)-furanone | y = 0.2708x − 0.0002 | - | - | 2.76 ± 0.23 | 3.69 ± 0.27 | - | - | - | - | - |
| Dihydro-2-methyl-3 (2H)-thiophenone | y = 0.8033x − 0.0029 | - | - | - | 0.9 ± 0.06 | - | - | - | - | - |
| 5-methyl-6, 7-dihydro-5H-cyclopentanopyrazine | y = 1.0406x − 0.0014 | - | - | - | 0.52 ± 0.01 | - | - | - | - | - |
| No. | Compound Omitted | Significance a | |||
|---|---|---|---|---|---|
| GL | MJ | XF | BS | ||
| 1 | Furfuryl alcohol | NS | NS | NS | NS |
| 2 | γ -butyrolactone | * | * | * | * |
| 3 | Hydroxyacetone | * | * | * | ** |
| 4 | 5-methylfurfural | NS | - | NS | - |
| 5 | 3-hydroxy-2-butanone | * | - | * | NS |
| 6 | Isovaleric acid | ** | * | * | * |
| 7 | Furfural | NS | NS | NS | * |
| 8 | 2, 6-dimethylpyrazine | NS | NS | - | - |
| 9 | 4-vinyl-2-methoxyphenol | ** | ** | * | * |
| 10 | 2-ethyl-5-methylpyrazine | ** | - | - | ** |
| 11 | Furaneol | * | * | * | *** |
| 12 | 2,3, 5-trimethylpyrazine | *** | - | * | * |
| 13 | Butyric acid | NS | - | - | - |
| 14 | 4-ethyl-2-methoxyphenol | * | * | - | * |
| 15 | Guaiacol | * | * | * | *** |
| 16 | Phenol | NS | - | - | - |
| 17 | 1-(2-furanyl methyl)-1H-pyrrole | * | * | - | - |
| 18 | 2-acetylpyridine | * | * | - | - |
| 19 | 2-acetylpyrazine | NS | NS | - | NS |
| 20 | 2, 5-dimethylpyrazine | - | * | * | - |
| 21 | 2-ethyl-6-methylpyrazine | - | *** | * | * |
| 22 | Maltol | * | * | * | NS |
| 23 | Linalool | - | ** | * | * |
| 24 | 3-ethyl-2,5-dimethylpyrazine | - | * | - | * |
| 25 | 2-ethyl-3, 5-dimethylpyrazine | - | - | *** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wu, P.; Wang, S.; Sun, J.; Chen, H. Identification of Key Aroma-Active Compounds in Commercial Coffee Using GC-O/AEDA and OAV Analysis. Foods 2025, 14, 3192. https://doi.org/10.3390/foods14183192
Chen X, Wu P, Wang S, Sun J, Chen H. Identification of Key Aroma-Active Compounds in Commercial Coffee Using GC-O/AEDA and OAV Analysis. Foods. 2025; 14(18):3192. https://doi.org/10.3390/foods14183192
Chicago/Turabian StyleChen, Xiaomei, Panpan Wu, Shuwei Wang, Jie Sun, and Haitao Chen. 2025. "Identification of Key Aroma-Active Compounds in Commercial Coffee Using GC-O/AEDA and OAV Analysis" Foods 14, no. 18: 3192. https://doi.org/10.3390/foods14183192
APA StyleChen, X., Wu, P., Wang, S., Sun, J., & Chen, H. (2025). Identification of Key Aroma-Active Compounds in Commercial Coffee Using GC-O/AEDA and OAV Analysis. Foods, 14(18), 3192. https://doi.org/10.3390/foods14183192





