Sourdough Breads Made with Selected Lactobacillus Strains and Spelt Flour Contain Peptides That Positively Impact Intestinal Barrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms Growth Conditions and Enumeration
2.2. Sourdoughs and Breads Preparation
2.3. Extraction, Characterization, and Fractionation of Water-Soluble Extracts
2.3.1. Free Amino Acid Determination in Water–Salt-Soluble Extracts
2.3.2. Isolation of Low Molecular Weight Peptides
2.3.3. Mass Spectrometry Analysis
2.4. Biological Activity of Peptides on RAW 264.7 Cells
2.4.1. Cell Viability
2.4.2. Reactive Oxygen Species (ROS) Assay
2.5. Biological Activity of Peptides on Caco-2 Monolayer
2.5.1. Intestinal Epithelium Differentiation and Cell Viability
2.5.2. Intestinal Barrier Permeability
2.5.3. Monolayer Immunofluorescence Staining
2.6. Inflammatory and Immune Activation Markers in Mouse Jejunum Organoids
2.7. Statistical Analysis
3. Results
3.1. Dough Analysis
3.2. Extraction and Characterization of Water-Soluble Extracts
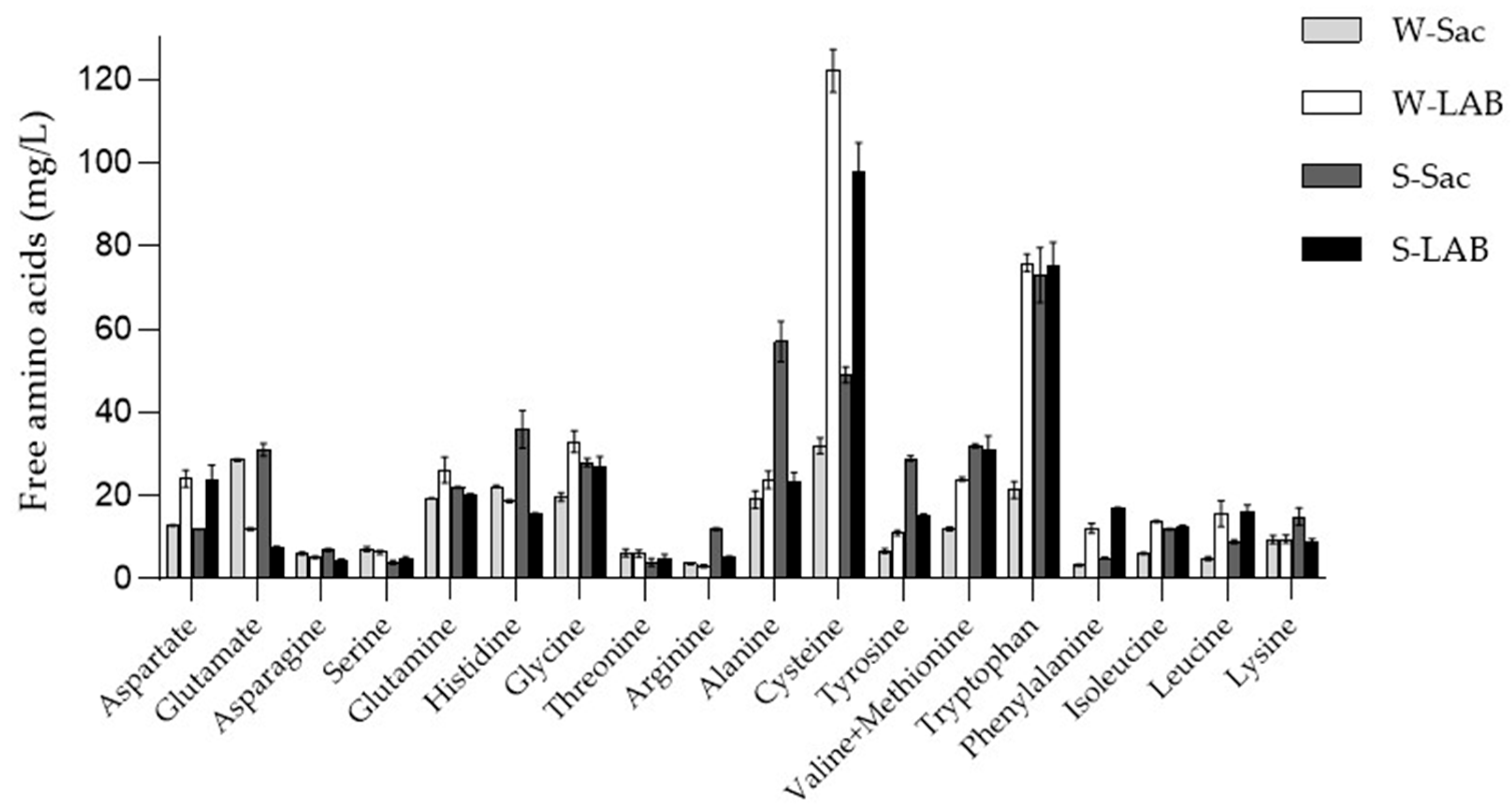
3.2.1. Free Amino Acid Content in the WSE Extracts
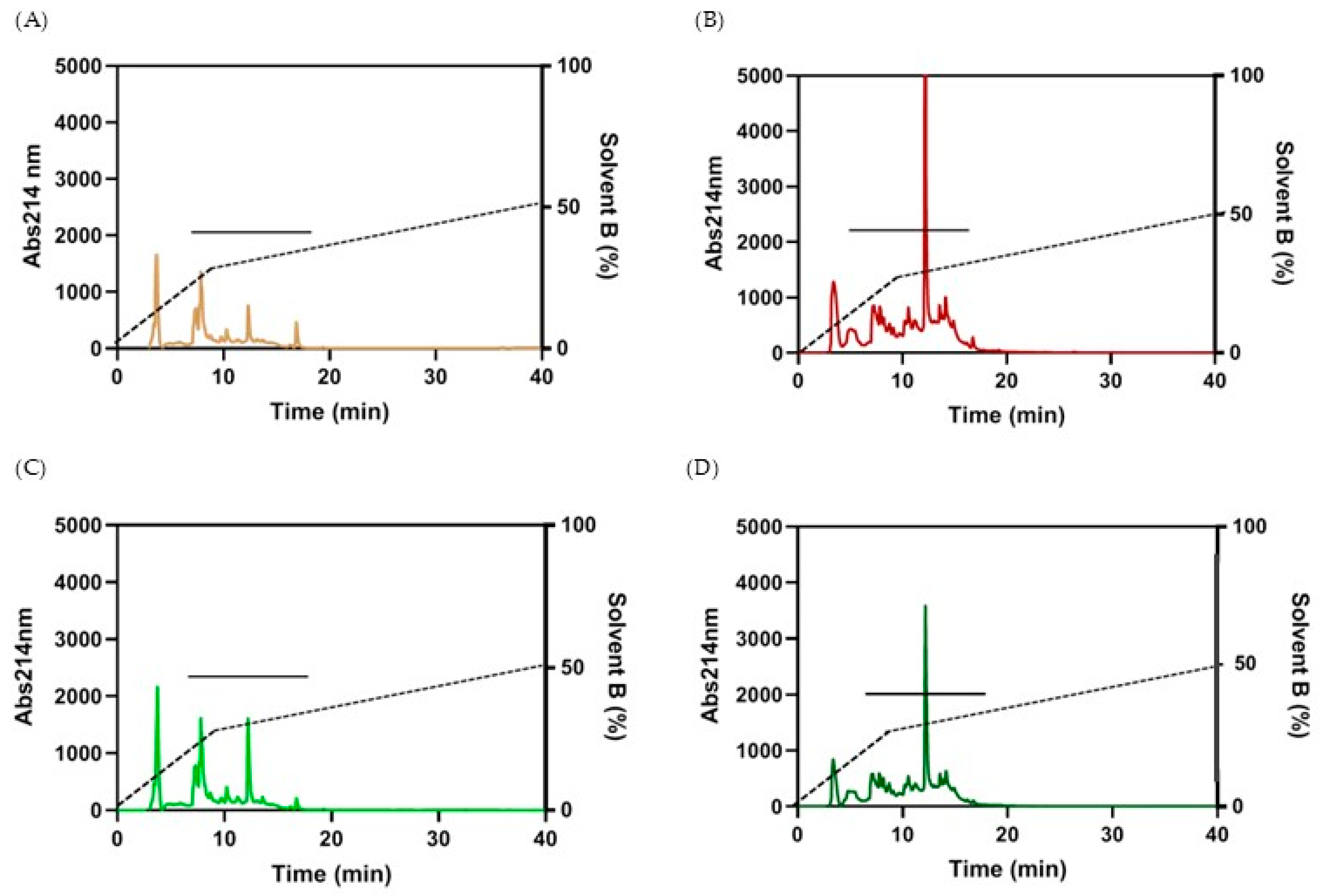
3.2.2. Fractionation of the Peptides
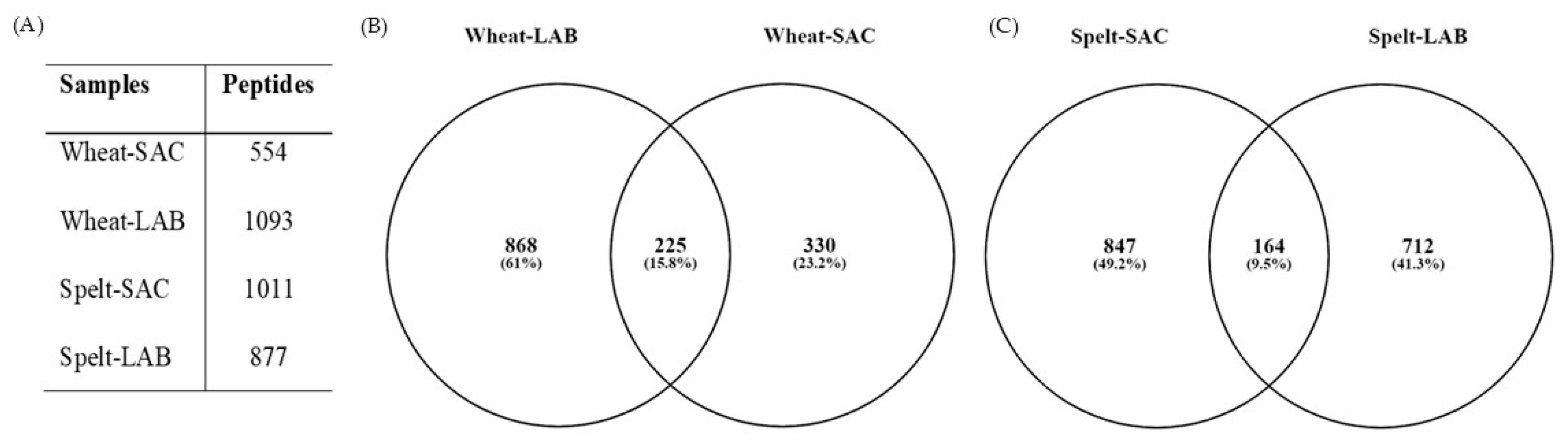
3.2.3. Peptide Content Analysis by Mass Spectrometry
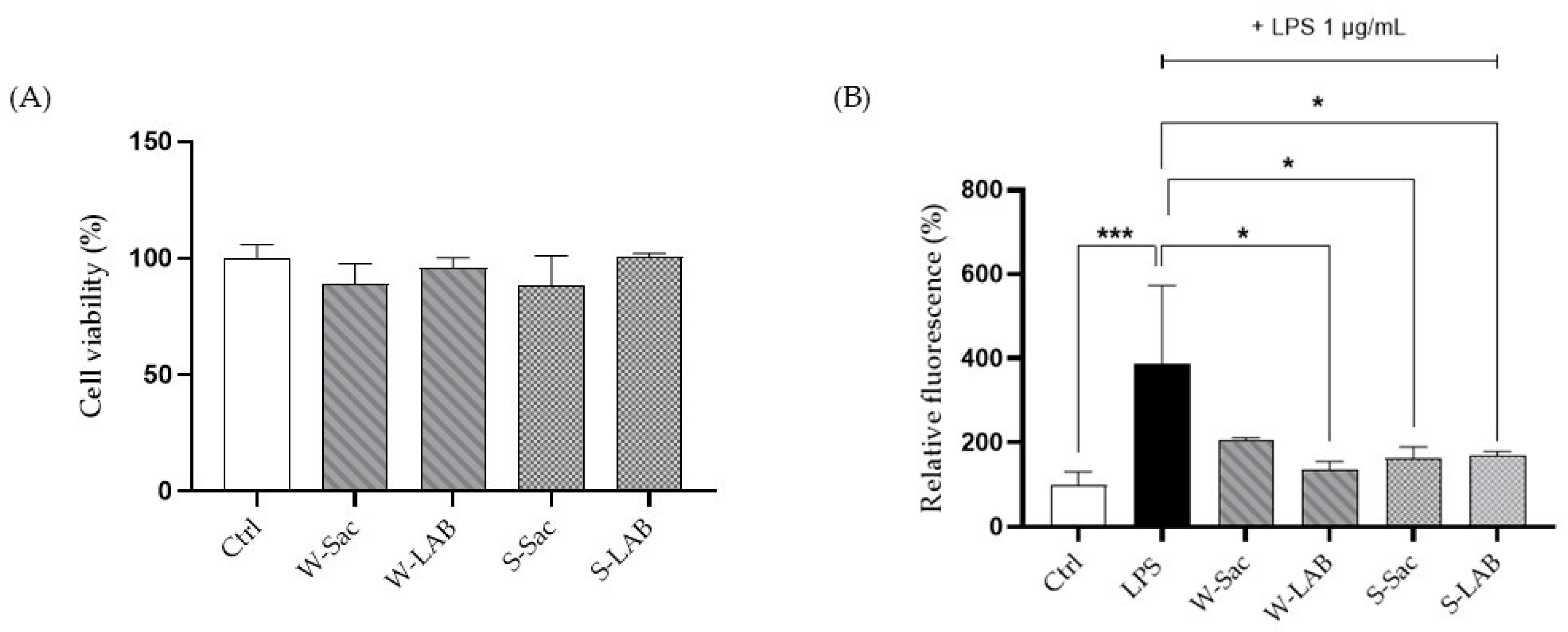
3.3. Effect of Peptides on RAW 264.7 Cell Viability and ROS Production
3.4. Impact of Peptides on Intestinal Permeability and Barrier Integrity
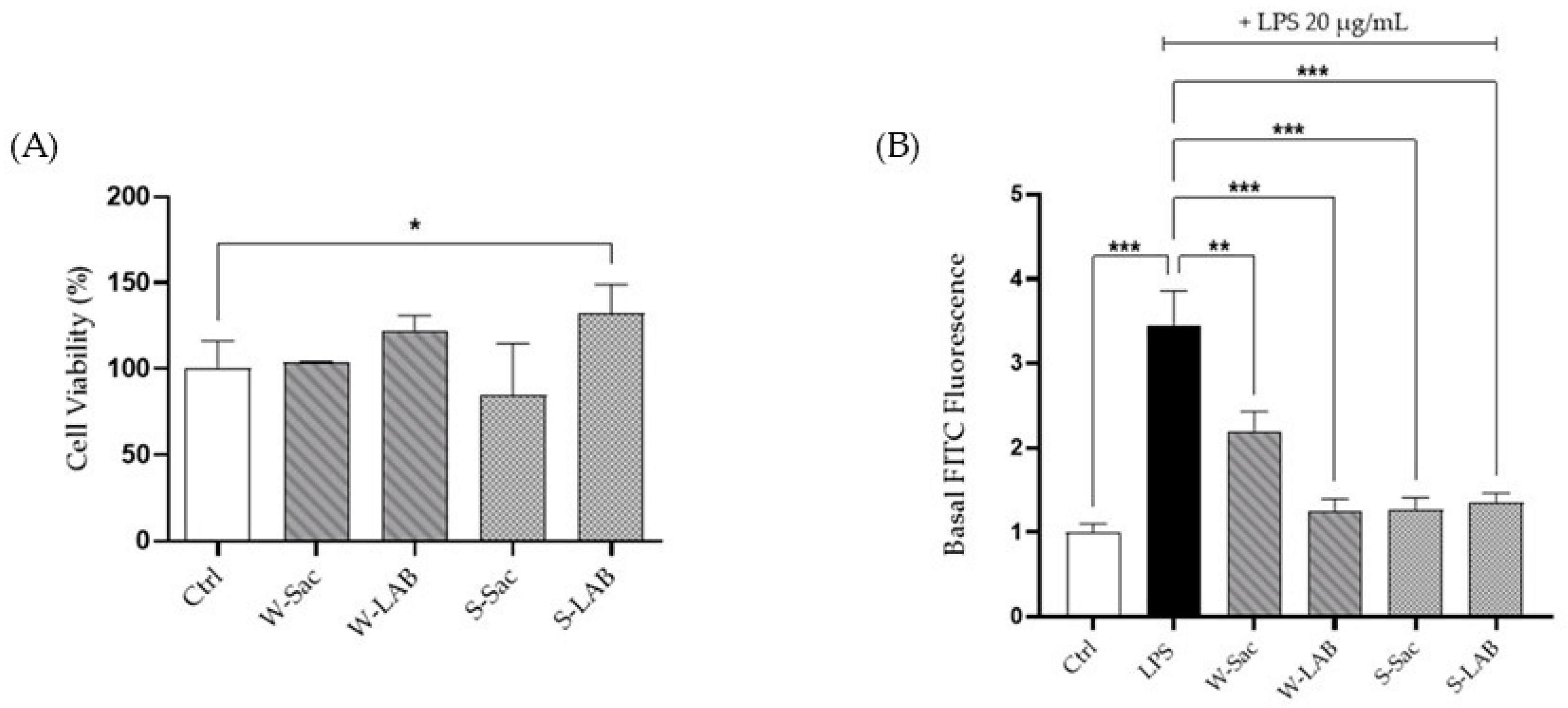
3.4.1. Cell Viability and Intestinal Barrier Permeability
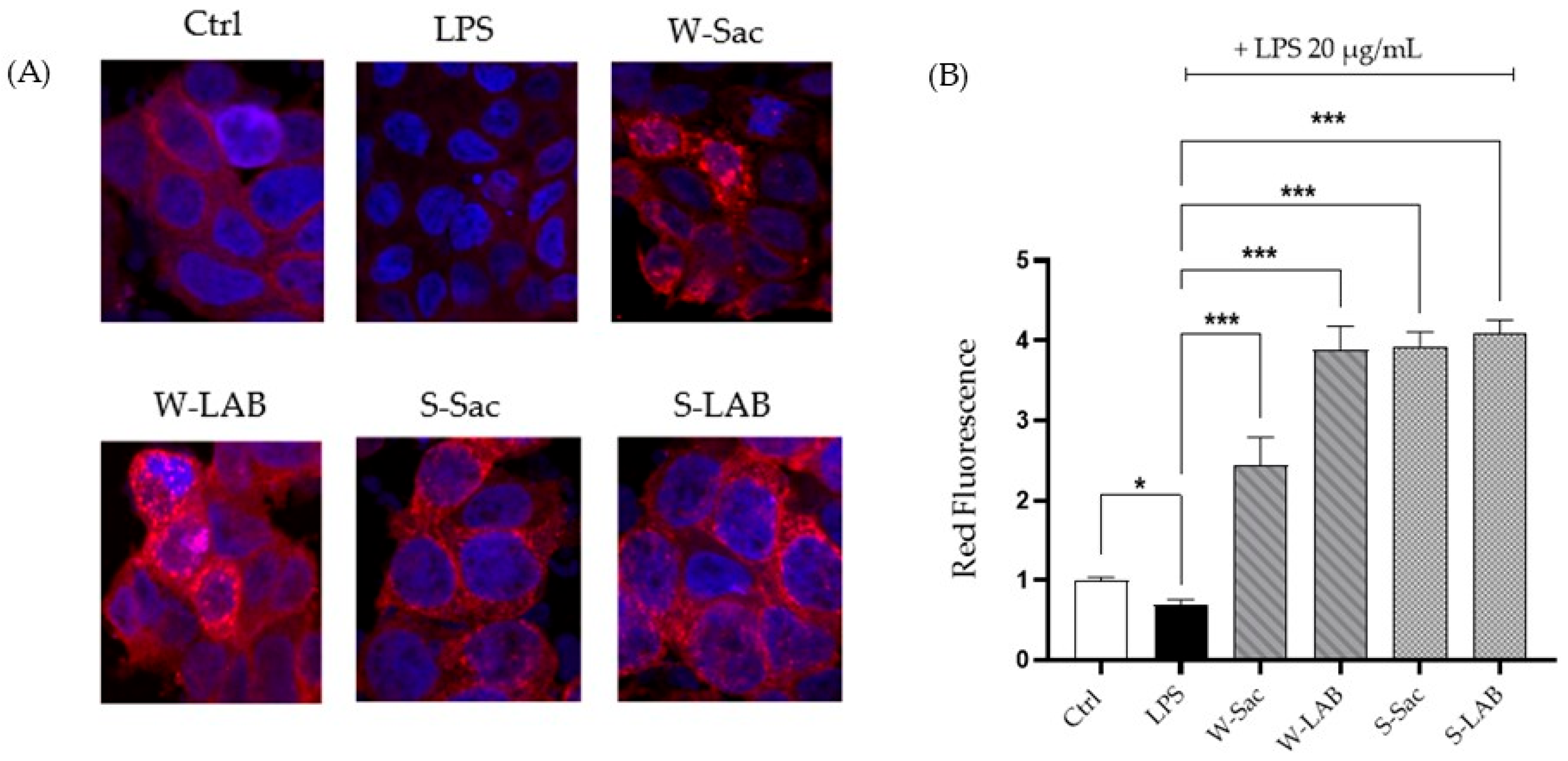
3.4.2. Effects of Peptides on Gap-Junction Protein Expression
3.5. Inflammatory and Immune Activation Markers in Mouse Jejunum Organoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPs | Bioactive peptides |
| LAB | Lactobacilli |
| WSE | Water-soluble extracts |
| W-Sac | Dough with wheat flour fermented by baker’s yeasts |
| W-LAB | Dough with wheat flour fermented by a sourdough |
| S-Sac | Dough with spelt flour fermented by baker’s yeasts |
| S-LAB | Dough with spelt flour fermented by a sourdough |
References
- Poole, N.; Donovan, J.; Erenstein, O. Continuing Cereals Research for Sustainable Health and Well-Being. Int. J. Agric. Sustain. 2022, 20, 693–704. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The Contribution of Wheat to Human Diet and Health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; Nosworthy, M.G.; Shoveller, A.-K. Cereal Proteins in the Human Diet: Reflecting on Their Contributions to Daily Protein Intake. J. Cereal Sci. 2024, 117, 103908. [Google Scholar] [CrossRef]
- Pequeno, D.N.L.; Ferreira, T.B.; Fernandes, J.M.C.; Singh, P.K.; Pavan, W.; Sonder, K.; Robertson, R.; Krupnik, T.J.; Erenstein, O.; Asseng, S. Production Vulnerability to Wheat Blast Disease Under Climate Change. Nat. Clim. Change 2024, 14, 178–183. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; DePauw, R.; Jin, Z.; Garvin, D.; Yue, X.; Anderson, W.; Li, T.; Dong, X.; Zhang, T.; et al. Climate Change May Outpace Current Wheat Breeding Yield Improvements in North America. Nat. Commun. 2022, 13, 5591. [Google Scholar] [CrossRef]
- Ortiz, A.M.D.; Outhwaite, C.L.; Dalin, C.; Newbold, T. A Review of the Interactions between Biodiversity, Agriculture, Climate Change, and International Trade: Research and Policy Priorities. One Earth 2021, 4, 88–101. [Google Scholar] [CrossRef]
- Sugár, E.; Fodor, N.; Sándor, R.; Bónis, P.; Vida, G.; Árendás, T. Spelt Wheat: An Alternative for Sustainable Plant Production at Low N-Levels. Sustainability 2019, 11, 6726. [Google Scholar] [CrossRef]
- Dumalasová, V.; Grausgruber, H.; Zelba, O.; Hanzalová, A.; Buerstmayr, H.; Weyermann, V.; dell’Avo, F.; Cuendet, C.; Koppel, R.; Sooväli, P.; et al. Spelt Wheat Resistance to Rusts, Powdery Mildew, Leaf Blotch and Common Bunt. Cereal Res. Commun. 2025, 53, 451–467. [Google Scholar] [CrossRef]
- Ruibal-Mendieta, N.L.; Delacroix, D.L.; Mignolet, E.; Pycke, J.-M.; Marques, C.; Rozenberg, R.; Petitjean, G.; Habib-Jiwan, J.-L.; Meurens, M.; Quetin-Leclercq, J.; et al. Spelt (Triticum aestivum ssp. spelta) as a Source of Breadmaking Flours and Bran Naturally Enriched in Oleic Acid and Minerals but Not Phytic Acid. J. Agric. Food Chem. 2005, 53, 2751–2759. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Topakas, E.; Tzia, C. Chemical Characterization and Breadmaking Potential of Spelt Versus Wheat Flour. J. Cereal Sci. 2018, 79, 50–56. [Google Scholar] [CrossRef]
- Geisslitz, S.; Wieser, H.; Scherf, K.A.; Koehler, P. Gluten Protein Composition and Aggregation Properties as Predictors for Bread Volume of Common Wheat, Spelt, Durum Wheat, Emmer and Einkorn. J. Cereal Sci. 2018, 83, 204–212. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef]
- Gabriele, M.; Arouna, N.; Árvay, J.; Longo, V.; Pucci, L. Sourdough Fermentation Improves the Antioxidant, Antihypertensive, and Anti-Inflammatory Properties of Triticum Dicoccum. Int. J. Mol. Sci. 2023, 24, 6283. [Google Scholar] [CrossRef]
- Coda, R.; Cagno, R.D.; Gobbetti, M.; Rizzello, C.G. Sourdough Lactic Acid Bacteria: Exploration of Non-Wheat Cereal-Based Fermentation. Food Microbiol. 2014, 37, 51–58. [Google Scholar] [CrossRef]
- Alkay, Z.; Falah, F.; Cankurt, H.; Dertli, E. Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential. Foods 2024, 13, 1732. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in Sourdough Fermentations: Mechanisms and Potential for Improved Bread Quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Lu, S.; An, M.; Sun, R.; Luo, H.; Wen, Y.; Li, H.; Wang, J.; Sun, B. Organic Acid Profiles of Traditional Sourdough Microbiota Dominates the Fermentation Effect on Whole Wheat Bread Texture. J. Cereal Sci. 2025, 124, 104229. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Dai, Y.; Yang, R.; Zhao, R.; Sun, G.; Zhou, W.-W.; Feng, S.; Feng, Y.; Li, N.; et al. Effect of Probiotic Fermentation on the Extraction Rate and Bioactivity of Plant-Based Polysaccharides: A Review. Innov. Food Sci. Emerg. Technol. 2024, 98, 103863. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the Sourdough May Affect the Functional Features of Leavened Baked Goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hui, Y.; Gao, T.; Shu, G.; Chen, H. Function and Characterization of Novel Antioxidant Peptides by Fermentation with a Wild Lactobacillus Plantarum 60. LWT 2021, 135, 110162. [Google Scholar] [CrossRef]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Ferro, S.; Arrigoni, G.; Bindoli, A.; Feller, E.; et al. Identification of New Peptides from Fermented Milk Showing Antioxidant Properties: Mechanism of Action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Ye, H.; Tao, X.; Zhang, W.; Chen, Y.; Yu, Q.; Xie, J. Food-Derived Bioactive Peptides: Production, Biological Activities, Opportunities and Challenges. J. Future Foods 2022, 2, 294–306. [Google Scholar] [CrossRef]
- Galli, V.; Mazzoli, L.; Luti, S.; Venturi, M.; Guerrini, S.; Paoli, P.; Vincenzini, M.; Granchi, L.; Pazzagli, L. Effect of Selected Strains of Lactobacilli on the Antioxidant and Anti-Inflammatory Properties of Sourdough. Int. J. Food Microbiol. 2018, 286, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhao, J.; Xie, Y.; Tang, J.; Wang, Q.; Zhao, J.; Xu, M.; Liu, P. Corrigendum to “Identification and Molecular Mechanisms of Novel Antioxidant Peptides from Fermented Broad Bean Paste: A Combined In Silico and In Vitro Study” [Food Chemistry 450 (2024) 139297/FOCH_FOODCHEM-D-23-08808]. Food Chem. 2024, 451, 139578. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Chelliah, R.; Daliri, E.B.-M.; Sultan, G.; Madar, I.H.; Kim, N.-H.; Shabbir, U.; Oh, D.-H. Antioxidant Activities of Novel Peptides from Limosilactobacillus Reuteri Fermented Brown Rice: A Combined In Vitro and In Silico Study. Food Chem. 2023, 404, 134747. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Pan, D.; Liu, Z.; Xiao, C.; Xiong, Y.; Du, L.; Cai, Z.; Lu, W.; Dang, Y.; et al. Identification and Virtual Screening of Novel Anti-Inflammatory Peptides from Broccoli Fermented by Lactobacillus Strains. Front. Nutr. 2023, 9, 1118900. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Da, P.; Chao, Y.; Hui, X.; Ligang, Y.; Shaokang, W.; Guiju, S. Wheat Oligopeptides Enhance the Intestinal Mucosal Barrier and Alleviate Inflammation via the TLR4/Myd88/MAPK Signaling Pathway in Aged Mice. Food Nutr. Res. 2022, 66, 5690. [Google Scholar] [CrossRef]
- Cicchi, C.; Paoli, P.; Modesti, A.; Mannelli, F.; Scicutella, F.; Buccioni, A.; Fontanarosa, C.; Luti, S.; Pazzagli, L. Effect of Bovine Milk Peptides on Cell Inflammation, Proliferation and Differentiation: Milk Potential Benefits Are Preserved in an Unconventional Cow Feeding Strategy. Biology 2023, 12, 1162. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Silván, J.M.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Peptides Derived from in Vitro Gastrointestinal Digestion of Germinated Soybean Proteins Inhibit Human Colon Cancer Cells Proliferation and Inflammation. Food Chem. 2018, 242, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, X.; Wang, J.; Zheng, X. Corn Protein Hydrolysate with Glutamine-Rich Peptides Protects Intestinal Barrier in Caco-2 Cells: Insights into Structural Characteristics of Identified Glutamine Peptides. J. Funct. Foods 2024, 117, 106232. [Google Scholar] [CrossRef]
- Liang, Q.; Ren, X.; Chalamaiah, M.; Ma, H. Simulated Gastrointestinal Digests of Corn Protein Hydrolysate Alleviate Inflammation in Caco-2 Cells and a Mouse Model of Colitis. J. Food Sci. Technol. 2020, 57, 2079–2088. [Google Scholar] [CrossRef]
- Ji, Z.-H.; Xie, W.-Y.; Zhao, P.-S.; Wu, H.-Y.; Ren, W.-Z.; Hu, J.-P.; Gao, W.; Yuan, B. Oat Peptides Alleviate Dextran Sulfate Sodium Salt-Induced Colitis by Maintaining the Intestinal Barrier and Modulating the Keap1-Nrf2 Axis. Nutrients 2023, 15, 5055. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, X.; Wang, J.; Ma, Y.; Zheng, X. Production of Corn Protein Hydrolysate with Glutamine-Rich Peptides and Its Antagonistic Function in Ulcerative Colitis In Vivo. Foods 2022, 11, 3359. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Zhao, C.; Zhang, Y.; Lv, H.; Ji, X.; Wang, J.; Pang, W.; Wang, X.; Wang, S. Protective Effects of Bioactive Peptides in Foxtail Millet Protein Hydrolysates against Experimental Colitis in Mice. Food Funct. 2022, 13, 2594–2605. [Google Scholar] [CrossRef]
- Luti, S.; Mazzoli, L.; Ramazzotti, M.; Galli, V.; Venturi, M.; Marino, G.; Lehmann, M.; Guerrini, S.; Granchi, L.; Paoli, P.; et al. Antioxidant and Anti-Inflammatory Properties of Sourdoughs Containing Selected Lactobacilli Strains Are Retained in Breads. Food Chem. 2020, 322, 126710. [Google Scholar] [CrossRef] [PubMed]
- Graça, C.; Lima, A.; Raymundo, A.; Sousa, I. Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products. Fermentation 2021, 7, 246. [Google Scholar] [CrossRef]
- D’Amico, V.; Gänzle, M.; Call, L.; Zwirzitz, B.; Grausgruber, H.; D’Amico, S.; Brouns, F. Does Sourdough Bread Provide Clinically Relevant Health Benefits? Front. Nutr. 2023, 10, 1230043. [Google Scholar] [CrossRef]
- Kelly, M.T.; Blaise, A.; Larroque, M. Rapid Automated High Performance Liquid Chromatography Method for Simultaneous Determination of Amino Acids and Biogenic Amines in Wine, Fruit and Honey. J. Chromatogr. A 2010, 1217, 7385–7392. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of Phenotypic and Functional Stability of RAW 264.7 Cell Line through Serial Passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- More, G.K.; Makola, R.T. In-Vitro Analysis of Free Radical Scavenging Activities and Suppression of LPS-Induced ROS Production in Macrophage Cells by Solanum Sisymbriifolium Extracts. Sci. Rep. 2020, 10, 6493. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 Cell Models in Food-Intestine Interaction Study: Current Applications and Future Trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Panse, N.; Gerk, P.M. The Caco-2 Model: Modifications and Enhancements to Improve Efficiency and Predictive Performance. Int. J. Pharm. 2022, 624, 122004. [Google Scholar] [CrossRef]
- Córdova, S.; Tena-Garitaonaindia, M.; Álvarez-Mercado, A.I.; Gámez-Belmonte, R.; Gómez-Llorente, M.A.; de Medina, F.S.; Martínez-Cañavate, A.; Martínez-Augustin, O.; Gómez-Llorente, C. Differential Modulation of Mouse Intestinal Organoids with Fecal Luminal Factors from Obese, Allergic, Asthmatic Children. Int. J. Mol. Sci. 2024, 25, 866. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Lattanzi, A.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Influence of Artisan Bakery- or Laboratory-Propagated Sourdoughs on the Diversity of Lactic Acid Bacterium and Yeast Microbiotas. Appl. Environ. Microbiol. 2012, 78, 5328–5340. [Google Scholar] [CrossRef]
- Oliveros, J.C. (2007–2015) Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 10 June 2025).
- Chatonidi, G.; Pradal, I.; De Vuyst, L.; Courtin, C.M.; Verbeke, K. Effect of Lactic Acid-Rich Sourdough Bread on Appetite Regulation: A Randomized, Double-Blind Controlled Trial. Curr. Res. Food Sci. 2025, 10, 100956. [Google Scholar] [CrossRef]
- Pérez-Vega, K.A.; Sanllorente, A.; Zomeño, M.-D.; Quindós, A.; Muñoz-Martínez, J.; Malcampo, M.; Aldea-Perona, A.; Hernáez, Á.; Lluansí, A.; Llirós, M.; et al. Sourdough Bread with Different Fermentation Times: A Randomized Clinical Trial in Subjects with Metabolic Syndrome. Nutrients 2024, 16, 2380. [Google Scholar] [CrossRef]
- Geisslitz, S.; Scherf, K.A. Rediscovering Ancient Wheats. Cereal Foods World 2020, 65, 1–10. [Google Scholar] [CrossRef]
- Shewry, P. Wheat Grain Proteins: Past, Present, and Future. Cereal Chem. 2023, 100, 9–22. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Gioulatos, S.; Tsakalidou, E.; Kalantzopoulos, G. Interactions between Saccharomyces Cerevisiae and Lactic Acid Bacteria in Sourdough. Process Biochem. 2006, 41, 2429–2433. [Google Scholar] [CrossRef]
- Tóth, V.; Láng, L.; Vida, G.; Mikó, P.; Rakszegi, M. Characterization of the Protein and Carbohydrate Related Quality Traits of a Large Set of Spelt Wheat Genotypes. Foods 2022, 11, 2061. [Google Scholar] [CrossRef]
- Diana, M.; Rafecas, M.; Quílez, J. Free Amino Acids, Acrylamide and Biogenic Amines in Gamma-Aminobutyric Acid Enriched Sourdough and Commercial Breads. J. Cereal Sci. 2014, 60, 639–644. [Google Scholar] [CrossRef]
- Dogan, C.E.; Cebi, N.; Develioglu, A.; Olgun, E.O.; Sagdic, O. Detection of Cystine and Cysteine in Wheat Flour Using a Robust LC-MS/MS Method. J. Cereal Sci. 2018, 84, 49–54. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef]
- Martínez-Augustin, O.; Rivero-Gutiérrez, B.; Mascaraque, C.; de Medina, F.S. Food Derived Bioactive Peptides and Intestinal Barrier Function. Int. J. Mol. Sci. 2014, 15, 22857–22873. [Google Scholar] [CrossRef]
- Korbecki, J.; Maruszewska, A.; Bosiacki, M.; Chlubek, D.; Baranowska-Bosiacka, I. The Potential Importance of CXCL1 in the Physiological State and in Noncancer Diseases of the Cardiovascular System, Respiratory System and Skin. Int. J. Mol. Sci. 2022, 24, 205. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Sultana, M.F.; Suzuki, M.; Yamasaki, F.; Kubota, W.; Takahashi, K.; Abo, H.; Kawashima, H. Identification of Crucial Amino Acid Residues for Antimicrobial Activity of Angiogenin 4 and Its Modulation of Gut Microbiota in Mice. Front. Microbiol. 2022, 13, 900948. [Google Scholar] [CrossRef]
- Wijatniko, B.D.; Yamamoto, Y.; Hirayama, M.; Suzuki, T. Identification and Molecular Mechanism of Anti-Inflammatory Peptides Isolated from Jack Bean Protein Hydrolysates: In Vitro Studies with Human Intestinal Caco-2BBe Cells. Plant Foods Hum. Nutr. 2024, 79, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhong, Y.; Liu, H. Characterization and Relationship Analysis of Antioxidant and Anti-Inflammatory Peptides in Pomelo Fruitlet Albumin. Food Chem. 2024, 446, 138798. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Majumder, K. Structural-Features of Food-Derived Bioactive Peptides with Anti-Inflammatory Activity: A Brief Review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef] [PubMed]

| Breads | ||||
|---|---|---|---|---|
| Ingredients | W-Sac | W-LAB | S-Sac | S-LAB |
| Wheat flour type 00 (g) | 525 | 525 | -- | -- |
| Spelt flour (g) | -- | -- | 525 | 525 |
| Water (g) | 225 | 225 | 225 | 225 |
| Sourdough (g) | -- | 250 | -- | 250 |
| Baker’s yeast (g) | 10 | -- | 10 | -- |
| Dough yield (DY) | 153 | 153 | 153 | 153 |
| Sample | Final pH | Final TTA (mL) | ΔV/V × 100 | LAB (CFU/g) | Yeasts (CFU/g) |
|---|---|---|---|---|---|
| W-Sac | 5.76 ± 0.46 b | 3.64 ± 0.29 ab | 144.4 ± 10.1 a | -- | (1.35 ± 0.20) × 108 b |
| W-LAB | 4.21 ± 0.46 a | 5.34 ± 0.43 c | 138.9 ± 11.1 a | (7.55 ± 0.20) × 108 a | (3.11 ± 0.30) × 107 a |
| S-Sac | 5.85 ± 0.64 b | 2.77 ± 0.19 a | 144.4 ± 11.6 a | -- | (1.40 ± 0.88) × 108 b |
| S-LAB | 4.22 ± 0.34 a | 4.40 ± 0.48 b | 133.3 ± 9.3 a | (7.99 ± 0.16) × 108 a | (1.07 ± 0.07) × 107 a |
| Sample | Protein content (mg/mL) |
|---|---|
| W-Sac | 0.89 ± 0.19 a |
| W-LAB | 1.77 ± 0.08 b |
| S-Sac | 1.15 ± 0.05 a |
| S-LAB | 1.78 ± 0.24 b |
| Sample | FAA content (mg/L) |
|---|---|
| W-Sac | 241.1 ± 10.3 a |
| W-LAB | 443.1 ± 20.1 b |
| S-Sac | 444.5 ± 15.4 b |
| S-LAB | 411.1 ± 12.6 b |
| Amino Acid | W-Sac | W-LAB | S-Sac | S-LAB |
|---|---|---|---|---|
| Ala (A) | 5.5% | 8.8% | 4.2% | 4.9% |
| Arg (R) | 2.9% | 3.1% | 3.2% | 3.7% |
| Asn (N) | 2.7% | 2.8% | 2.8% | 2.9% |
| Asp (D) | 1.6% | 2.5% | 1.2% | 1.7% |
| Cys (C) | 0.1% | 0.2% | 0.7% | 1.2% |
| Gln (Q) | 19.3% | 10.8% | 25.4% | 21.7% |
| Glu (E) | 3.2% | 4.3% | 3.0% | 3.3.% |
| Gly (G) | 9.1% | 11.5% | 6.0% | 6.2% |
| His (H) | 1.2% | 1.6% | 1.2% | 1.5% |
| Ile (I) | 2.8% | 4.6% | 3.4% | 4.5% |
| Leu (L) | 6.4% | 8.0% | 7.9% | 9.2% |
| Lys (K) | 1.9% | 3.9% | 1.9% | 2.8% |
| Met (M) | 0.9% | 1.6% | 1.8% | 2.0% |
| Phe (F) | 2.8% | 2.8% | 2.8% | 2.5% |
| Pro (P) | 13.8% | 9.9% | 14.4.% | 12.3% |
| Ser (S) | 6.4% | 6.9% | 6.0% | 5.5% |
| Thr (T) | 5.8% | 5.0% | 2.8% | 3.1% |
| Trp (W) | 0.3% | 0.4% | 0.4% | 0.6% |
| Tyr (Y) | 1.4% | 1.4% | 2.2% | 2.1% |
| Val (V) | 11.7% | 10.1% | 8.6% | 8.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicchi, C.; Leri, M.; Bucciantini, M.; Galli, V.; Guerrini, S.; Jiménez-Ortas, Á.; Ceacero-Heras, D.; Martínez-Augustín, O.; Pazzagli, L.; Luti, S. Sourdough Breads Made with Selected Lactobacillus Strains and Spelt Flour Contain Peptides That Positively Impact Intestinal Barrier. Foods 2025, 14, 3184. https://doi.org/10.3390/foods14183184
Cicchi C, Leri M, Bucciantini M, Galli V, Guerrini S, Jiménez-Ortas Á, Ceacero-Heras D, Martínez-Augustín O, Pazzagli L, Luti S. Sourdough Breads Made with Selected Lactobacillus Strains and Spelt Flour Contain Peptides That Positively Impact Intestinal Barrier. Foods. 2025; 14(18):3184. https://doi.org/10.3390/foods14183184
Chicago/Turabian StyleCicchi, Costanza, Manuela Leri, Monica Bucciantini, Viola Galli, Simona Guerrini, Ángela Jiménez-Ortas, Diego Ceacero-Heras, Olga Martínez-Augustín, Luigia Pazzagli, and Simone Luti. 2025. "Sourdough Breads Made with Selected Lactobacillus Strains and Spelt Flour Contain Peptides That Positively Impact Intestinal Barrier" Foods 14, no. 18: 3184. https://doi.org/10.3390/foods14183184
APA StyleCicchi, C., Leri, M., Bucciantini, M., Galli, V., Guerrini, S., Jiménez-Ortas, Á., Ceacero-Heras, D., Martínez-Augustín, O., Pazzagli, L., & Luti, S. (2025). Sourdough Breads Made with Selected Lactobacillus Strains and Spelt Flour Contain Peptides That Positively Impact Intestinal Barrier. Foods, 14(18), 3184. https://doi.org/10.3390/foods14183184








