Simultaneous Presence of Mycotoxins in Feed Intended for Food-Producing Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Plan
2.2. Mycotoxin Analysis
2.2.1. Reagents and Materials
2.2.2. Standards and Internal Standards
2.2.3. Preparation of Standard Solutions and Internal Standard Solutions
2.2.4. Sample Preparation
2.2.5. LC-MS/MS Analysis
2.3. Method Validation
3. Results and Discussion
3.1. Method Validation Results
3.2. Mycotoxin Occurrence in Feed Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Comm. 2002, L140, 10–21.
- Commission Regulation (EU) No 574/2011 of 16 June 2011 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto. Off. J. Eur. Union 2011, L159, 7–24.
- Zentai, A.; Jóźwiak, Á.; Süth, M.; Farkas, Z. Carry-Over of Aflatoxin B1 from Feed to Cow Milk—A Review. Toxins 2023, 15, 195. [Google Scholar] [CrossRef]
- Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9.
- Commission Recommendation of 23 March 2013 on the presence of T-2 and HT-2 toxins in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15.
- Khan, R.; Anwar, F.; Ghazali, F.M. A comprehensive review of mycotoxins: Toxicology, detection, and effective mitigation approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2002; Volume 82.
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Risks for animal health related to the presence of ochratoxin A (OTA) in feed. EFSA J. 2023, 21, e08375. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on the risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- Annunziata, L.; Campana, G.; De Massis, M.R.; Aloia, R.; Scortichini, G.; Visciano, P. Ochratoxin A in Foods and Beverages: Dietary Exposure and Risk Assessment. Expo. Health 2024, 17, 481–494. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Chu, X.H.; Ma, R.; Wang, Y.W.; Liu, Q.; Zhang, N.Y.; Karrow, N.A.; Sun, L.H. Effects of deoxynivalenol on the porcine growth performance and intestinal microbiota and potential remediation by a modified HSCAS binder. Food Chem. Toxicol. 2020, 141, 111373. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Zhang, W.; Guang, C.; Xu, W.; Mu, W. Deoxynivalenol: Occurrence, toxicity, and degradation. Food Control 2024, 155, 110027. [Google Scholar] [CrossRef]
- Gao, X.; Sun, L.H.; Zhang, N.Y.; Li, C.; Zhang, J.; Xiao, Z.H.; Qi, D. Gestational zearalenone exposure causes reproductive and developmental toxicity in pregnant rats and female offspring. Toxins 2017, 9, 21. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, S.; Gong, Y.Y.; Zhao, Y.; Wu, Y. Human dietary and internal exposure to zearalenone based on a 24-hour duplicate diet and following morning urine study. Environ. Int. 2020, 142, 105852. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Z.; Wang, Y.; Long, M.; Wu, W.D.; Kuca, K. Fumonisin B1: Mechanisms of toxicity and biological detoxification progress in animals. Food Chem. Toxicol. 2021, 149, 111977. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2024/1038 of 9 April 2024 amending Regulation (EU) 2023/915 as regards maximum levels of T-2 and HT-2 toxins in food. Off. J. Eur. Union 2024, L1038, 1–5.
- EFSA (European Food Safety Authority). Scientific report on human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, e04972. [Google Scholar] [CrossRef]
- Kolawole, O.; Siri-Anusornsak, W.; Petchkongkaew, A.; Elliott, C. A systematic review of global occurrence of emerging mycotoxins in crops and animal feeds, and their toxicity in livestock. Emerg. Contam. 2024, 10, 100305. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, M.Y.; Xu, X.G.; Han, Y.; Zhao, X.; Li, C.H.; Zhao, Z.L. Recent advances in simultaneous detection strategies for multi-mycotoxins in foods. Crit. Rev. Food Sci. Nutr. 2022, 64, 3932–3960. [Google Scholar] [CrossRef]
- Regulation (EU) of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation). Off. J. Eur. Union 2017, L95, 1–142.
- Ministry of Health. National Plan of Official Control on Animal Feeding. PNAA 2024–2026. Rome, Italy. Available online: https://www.salute.gov.it/new/it/pubblicazione/pnaa-2024-2025-2026-piano-nazionale-di-controllo-ufficiale-sullalimentazione-degli/ (accessed on 15 July 2025).
- Nualkaw, K.; Poapolathep, S.; Zhang, Z.; Zhang, Q.; Giorgi, M.; Li, P.; Logrieco, A.F.; Poapolathep, A. Simultaneous determination of multiple mycotoxins in swine, poultry and dairy feeds using Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. Toxins 2020, 12, 253. [Google Scholar] [CrossRef]
- EURL-MP-Guidance Doc_003 (Version 1.4) Guidance Document on Performance Criteria for Methods of Analysis for Mycotoxins and Plant Toxins in Food and Feed (Draft 17 May 2024); Wageningen Food and Safety Research: Wageningen, The Netherlands, 2024.
- Wenzl, T.; Johannes, H.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; Publications Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-61768-3. [Google Scholar] [CrossRef]
- Magnusson, B.; Näykki, T.; Hovind, H.; Krysell, M.; Sahlin, E. Handbook for Calculation of Measurement Uncertainty in Environmental Laboratories; Nordtest Report (NT TR 537—Edition 4); 29 November 2017; NordTest: Taastrup, Denmark, 2017. [Google Scholar]
- Pereira, C.S.; Cunha, S.C.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Sdogati, S.; Pacini, T.; Bibi, R.; Caporali, A.; Verdini, E.; Orsini, S.; Ortenzi, R.; Pecorelli, I. Co-occurrence of aflatoxin B1, zearalenone and ochratoxin A in feed and feed materials in Central Italy from 2018 to 2022. Foods 2024, 13, 313. [Google Scholar] [CrossRef]
- Franchino, C.; Vita, V.; Iammarino, M.; De Pace, R. Monitoring of animal feed contamination by mycotoxins: Results of five years of official control by an accredited Italian laboratory. Microorganisms 2024, 12, 173. [Google Scholar] [CrossRef]
- Ferrari, L.; Fumagalli, F.; Rizzi, N.; Grandi, E.; Vallati, S.; Manoni, M.; Ottoboni, M.; Cheli, F.; Pinotti, L. An eight-year survey on aflatoxin B1 indicates high feed safety in animal feed and forages in Northern Italy. Toxins 2022, 14, 763. [Google Scholar] [CrossRef]
- Chhaya, R.S.; O’Brien, J.; Nag, R.; Cummins, E. Prevalence and concentration of mycotoxins in bovine feed and feed components: A global systematic review and meta-analysis. Sci. Total Environ. 2024, 929, 172323. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Zhang, J.; Guo, J.; Chen, L.; Qi, D.; Zhang, N. Aflatoxin B1, zearalenone and deoxynivalenol in feed ingredients and complete feed from central China. Food Addit. Contam. Part B 2016, 9, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twarużek, M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Multi-mycotoxin screening of feed and feed raw materials from Africa. World Mycotoxin J. 2018, 11, 369–383. [Google Scholar] [CrossRef]
- Birr, T.; Jensen, T.; Preußke, N.; Sönnichsen, F.D.; De Boevre, M.; De Saeger, S.; Hasler, M.; Verreet, J.-A.; Klink, H. Occurrence of Fusarium mycotoxins and their modified forms in forage maize cultivars. Toxins 2021, 13, 110. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Girgin, G.; Baydar, T.; Krska, R.; Sulyok, M. Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS. J. Sci. Food Agric. 2017, 97, 4419–4428. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, O.F.; Fon, F.N.; Nji, Q.; Adeniji, O.O.; Tangni, E.K.; Njobeh, P.B. Multiple mycotoxin contamination in livestock feed: Implications for animal Health, productivity, and food safety. Toxins 2025, 17, 365. [Google Scholar] [CrossRef]
- Dimitrakopoulou, M.E.; Manouselis, N.; Marinos, G.; Karvounis, M.; Stoitsis, G.; Thanopoulos, C.; Elliott, C. What lies behind mycotoxin presence in animal feed? A case study. J. Food Prot. 2025, 88, 100464. [Google Scholar] [CrossRef]
- Palumbo, R.; Crisci, A.; Venâncio, A.; Abrahantes, J.C.; Dorne, J.L.; Battilani, P.; Toscano, P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Sirianusornsak, W.; Kolawole, O.; Mahakarnchanakul, W.; Greer, B.; Petchkongkaew, A.; Meneely, J.; Elliott, C.; Vangnai, K. The occurrence and co-occurrence of regulated, emerging, and masked mycotoxins in rice bran and maize from Southeast Asia. Toxins 2022, 14, 567. [Google Scholar] [CrossRef]
- Scarpino, V.; Sulyok, M.; Krska, R.; Reyneri, A.; Blandino, M. The role of nitrogen fertilization on the occurrence of regulated, modified and emerging mycotoxins and fungal metabolites in maize kernels. Toxins 2022, 14, 448. [Google Scholar] [CrossRef]
- Dong, F.; Chen, X.; Lei, X.; Wu, D.; Zhang, Y.; Lee, Y.W.; Mokoena, M.P.; Olaniran, A.O.; Li, Y.; Shen, G.; et al. Effect of crop rotation on Fusarium mycotoxins and Fusarium species in cereals in Sichuan Province (China). Plant Dis. 2023, 107, 1060–1066. [Google Scholar] [CrossRef]
- Herrera, M.; Cavero, J.; Franco-Luesma, S.; Álvaro-Fuentes, J.; Ariño, A.; Lorán, S. Mycotoxins and crop yield in maize as affected by irrigation management and tillage practices. Agronomy 2023, 13, 798. [Google Scholar] [CrossRef]
- Krnjaja, V.; Mandić, V.; Stanković, S.; Obradović, A.; Vasić, T.; Lukić, M.; Bijelić, Z. Influence of plant density on toxigenic fungal and mycotoxin contamination of maize grains. Crop Prot. 2019, 116, 126–131. [Google Scholar] [CrossRef]
- Simões, D.; Carbas, B.; Soares, A.; Freitas, A.; Silva, A.S.; Brites, C.; de Andrade, E. Assessment of agricultural practices for controlling fusarium and mycotoxins contamination on maize grains: Exploratory study in maize farms. Toxins 2023, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- Chhaya, S.; Nag, R.; Cummins, E. Human health risk from co-occurring mycotoxins in dairy: A feed-to-fork approach. Food Control 2025, 168, 110954. [Google Scholar] [CrossRef]
- Akinyemi, M.O.; Braun, D.; Windisch, P.; Warth, B.; Ezekiel, C.N. Assessment of multiple mycotoxins in raw milk of three different animal species in Nigeria. Food Control 2022, 131, 108258. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Barbosa, J.; Ramos, F. Regulated and emerging mycotoxins in bulk raw milk: What is the human risk? Toxins 2023, 15, 605. [Google Scholar] [CrossRef]
- Peng, W.-X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
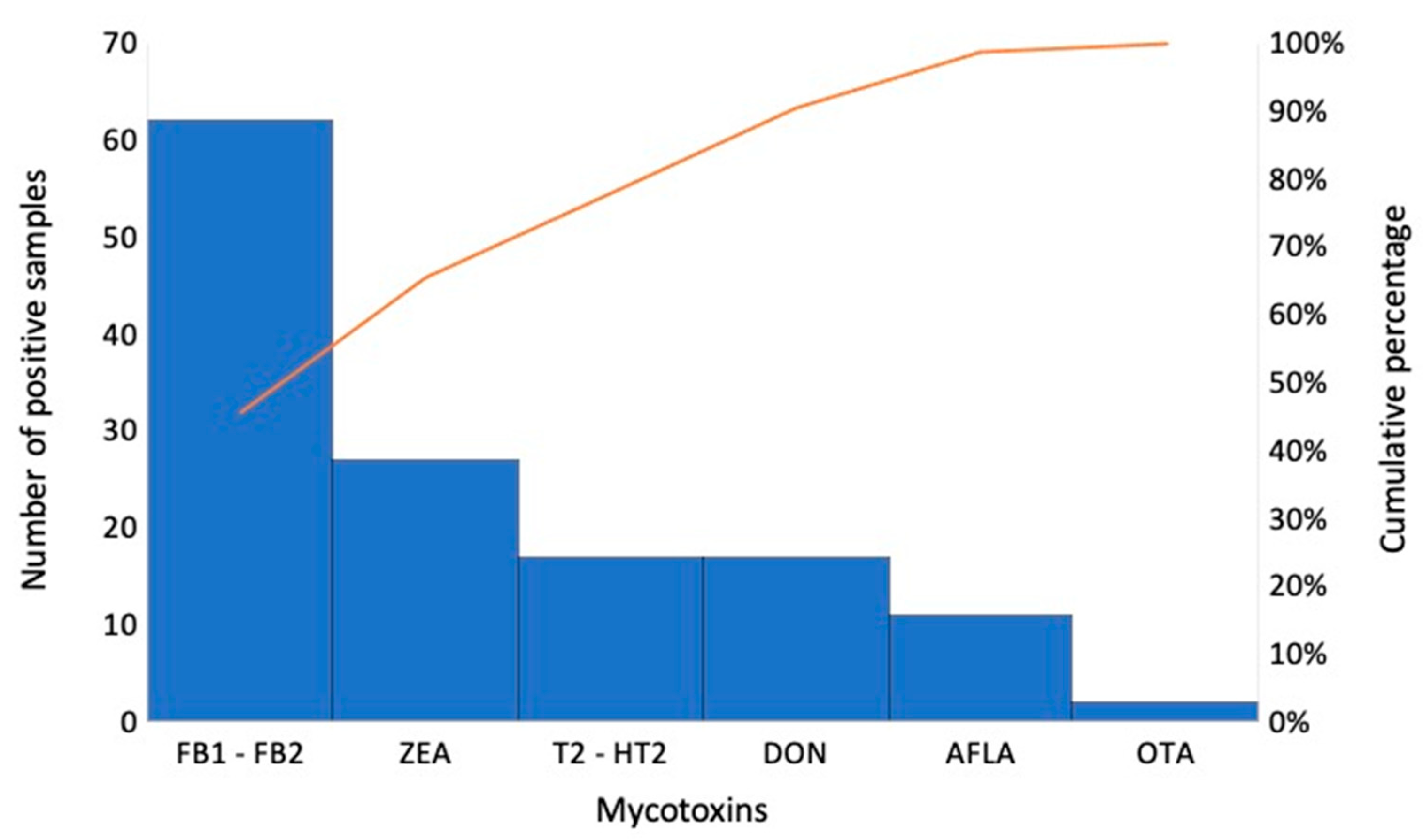
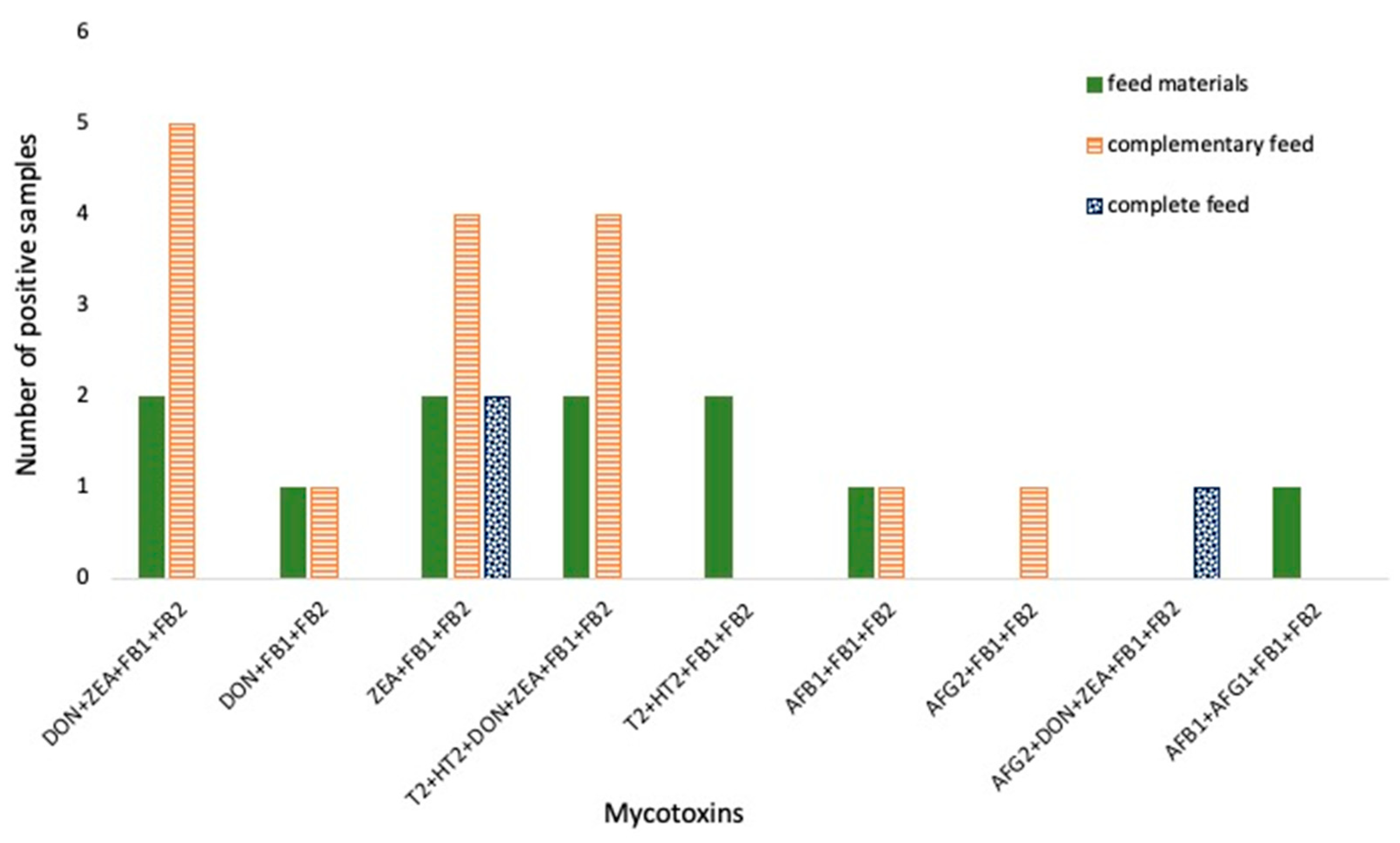
| Analyte | Rt | Precursor Ion (m/z) | Production (m/z) | DP (eV) | CE (eV) | CXP (eV) |
|---|---|---|---|---|---|---|
| AFB1 | 7.65 | 313.1 | 285.0 | 50 | 33 | 20 |
| 241.0 | 50 | 51 | 20 | |||
| IS-AFB1 | 7.65 | 330.1 | 255.1 | 70 | 50.3 | 13.5 |
| AFB2 | 7.47 | 315.0 | 287.1 | 50 | 37 | 20 |
| 259.0 | 50 | 41 | 24 | |||
| IS-AFB2 | 7.47 | 332.1 | 303.2 | 70 | 36.8 | 25 |
| AFG1 | 7.23 | 329.0 | 243.0 | 50 | 34 | 28 |
| 200.0 | 50 | 51 | 22 | |||
| IS-AFG1 | 7.23 | 346.2 | 212.1 | 70 | 54.2 | 18.1 |
| AFG2 | 7.02 | 331.1 | 313.0 | 50 | 33 | 28 |
| 285.2 | 50 | 38 | 27 | |||
| IS-AFG2 | 7.03 | 348.1 | 259.2 | 70 | 43.6 | 16.1 |
| FB1 | 8.55 | 722.0 | 352.0 | 50 | 49 | 26 |
| 334.0 | 50 | 55 | 20 | |||
| IS-FB1 | 8.54 | 756.5 | 356.4 | 83.7 | 55.9 | 17.5 |
| FB2 | 9.25 | 706.0 | 336.0 | 50 | 53 | 16 |
| 318.0 | 50 | 53 | 18 | |||
| IS-FB2 | 9.25 | 740.6 | 358.4 | 87.3 | 50.7 | 23.2 |
| T-2 | 8.61 | 484.0 | 215.0 | 69.9 | 25 | 14.8 |
| 185.0 | 69.9 | 28.9 | 16.1 | |||
| IS-T-2 | 8.61 | 508.3 | 229.2 | 77.6 | 26.7 | 16.8 |
| HT-2 | 8.16 | 442.0 | 263.0 | 66.5 | 18.0 | 24 |
| 215.0 | 66.5 | 18.6 | 24.5 | |||
| IS-HT-2 | 8.16 | 464.3 | 229.1 | 70 | 18.1 | 17.5 |
| OTA | 8.99 | 404.0 | 239.0 | 80 | 30.9 | 21 |
| 221.0 | 80 | 47.4 | 17 | |||
| IS-OTA | 8.99 | 424.2 | 250.1 | 85.1 | 32.3 | 21.4 |
| Analyte | Rt | Precursor ion (m/z) | Production (m/z) | DP (eV) | CE (eV) | CXP (eV) |
|---|---|---|---|---|---|---|
| DON | 4.17 | 355.0 | 59.0 | −58.7 | −20.37 | −17.09 |
| 295.0 | −58.7 | −13.84 | −8.34 | |||
| IS-DON | 4.17 | 370.4 | 310.1 | −57.7 | −14.37 | −9.14 |
| ZEA | 9.07 | 317.0 | 175.0 | −80 | −32.59 | −9.20 |
| 131.0 | −80 | −38 | −13 | |||
| IS-ZEA | 9.07 | 335.0 | 140.0 | −80 | −39.16 | −7.28 |
| Analyte | Matrix-Matched Standard Calibration Curves (mg/kg) |
|---|---|
| AFB1 | 0.0025–0.0050–0.010–0.020 |
| AFB2 | 0.0025–0.0050–0.010–0.020 |
| AFG1 | 0.0025–0.0050–0.010–0.020 |
| AFG2 | 0.0025–0.0050–0.010–0.020 |
| DON | 0.45–0.90–5.0–12 |
| FB1 | 0.25–0.50–2.0–6.0 |
| FB2 | 0.25–0.50–2.0–6.0 |
| OTA | 0.0050–0.010–0.10–0.25 |
| T-2 | 0.0125–0.025–0.050–0.075 |
| HT-2 | 0.0125–0.025–0.050–0.075 |
| ZEA | 0.050–0.20–0.50–3.0 |
| Analyte | Calibration Curves in Solvent (ng/mL) |
|---|---|
| AFB1 | 0.125–0.25–0.50–1.0–2.0–4.0 |
| AFB2 | 0.125–0.25–0.50–1.0–2.0–4.0 |
| AFG1 | 0.125–0.25–0.50–1.0–2.0–4.0 |
| AFG2 | 0.125–0.25–0.50–1.0–2.0–4.0 |
| DON | 22.5–45–200–500–800–1500 |
| FB1 | 12.5–50–100–200–400–800 |
| FB2 | 12.5–50–100–200–400–800 |
| OTA | 0.25–0.50–5–10–20–40 |
| T-2 | 0.625–1.25–2.5–5.0–7.5–10 |
| HT-2 | 0.625–1.25–2.5–5.0–7.5–10 |
| ZEA | 2.5–10–20–100–200–400 |
| Analyte | Matrix-Matched Standard Calibration Curves (mg/kg) |
|---|---|
| AFB1 | 0.00015–0.00030–0.00060–0.0012–0.0024 |
| AFB2 | 0.00015–0.00030–0.00060–0.0012–0.0024 |
| AFG1 | 0.00015–0.00030–0.00060–0.0012–0.0024 |
| AFG2 | 00.00015–0.00030–0.00060–0.0012–0.0024 |
| DON | 0.0125–0.025–0.050–0.10–0.15 |
| FB1 | 0.0075–0.015–0.030–0.045–0.060 |
| FB2 | 0.0075–0.015–0.030–0.045–0.060 |
| OTA | 0.00030–0.00060–0.0012–0.0024–0.0048 |
| T-2 | 0.0015–0.0030–0.0060–0.0090–0.012 |
| HT-2 | 0.0015–0.0030–0.0060–0.0090–0.012 |
| ZEA | 0.0015–0.0030–0.0060–0.012–0.015 |
| Analyte | LOQ (mg/kg) | Spiking Levels (mg/kg) | Recovery (%) (Replicates n = 6) | Within-Reproducibility (Replicates n = 6) | MU (%) |
|---|---|---|---|---|---|
| AFB1 | 0.0025 | 0.0025 | 101 | 4.1 | 13 |
| 0.0050 | 97 | 5.0 | 13 | ||
| 0.010 | 85 | 6.1 | 18 | ||
| 0.020 | 88 | 4.6 | 19 | ||
| AFB2 | 0.0025 | 0.0025 | 99 | 9.9 | 21 |
| 0.0050 | 96 | 12.1 | 21 | ||
| 0.010 | 98 | 12.6 | 26 | ||
| 0.020 | 98 | 7.3 | 26 | ||
| AFG1 | 0.0025 | 0.0025 | 92 | 12.5 | 28 |
| 0.0050 | 101 | 6.2 | 28 | ||
| 0.010 | 95 | 7.7 | 17 | ||
| 0.020 | 96 | 5.2 | 17 | ||
| AFG2 | 0.0010 | 0.0025 | 101 | 5.0 | 11 |
| 0.0050 | 105 | 5.9 | 11 | ||
| 0.010 | 93 | 1.6 | 6 | ||
| 0.020 | 100 | 2.9 | 6 | ||
| DON | 0.16 | 0.45 | 108 | 2.4 | 8 |
| 0.90 | 106 | 2.8 | 7 | ||
| 5.0 | 108 | 1.2 | 7 | ||
| 10 | 105 | 1.0 | 7 | ||
| FB1 | 0.015 | 0.25 | 99 | 6.0 | 13 |
| 0.50 | 98 | 6.7 | 13 | ||
| 2.0 | 93 | 7.7 | 16 | ||
| 6.0 | 96 | 3.6 | 17 | ||
| FB2 | 0.0075 | 0.25 | 102 | 5.8 | 12 |
| 0.50 | 106 | 2.2 | 13 | ||
| 2.0 | 102 | 3.6 | 8 | ||
| 6.0 | 104 | 3.1 | 8 | ||
| OTA | 0.0050 | 0.0050 | 105 | 1.9 | 6 |
| 0.010 | 104 | 1.1 | 6 | ||
| 0.10 | 103 | 2.5 | 6 | ||
| 0.20 | 99 | 2.8 | 6 | ||
| T-2 | 0.0060 | 0.0125 | 106 | 2.9 | 8 |
| 0.025 | 105 | 2.4 | 7 | ||
| 0.050 | 107 | 1.3 | 6 | ||
| 0.075 | 104 | 2.9 | 6 | ||
| HT-2 | 0.0080 | 0.0125 | 99 | 9.1 | 19 |
| 0.025 | 86 | 7.6 | 20 | ||
| 0.050 | 94 | 12.0 | 25 | ||
| 0.075 | 90 | 11.3 | 25 | ||
| ZEA | 0.015 | 0.050 | 102 | 1.3 | 13 |
| 0.20 | 76 | 1.1 | 10 | ||
| 0.50 | 102 | 1.0 | 4 | ||
| 3.1 | 79 | 1.1 | 4 |
| Analyte | Feed Materials | Complementary Feed | Complete Feed |
|---|---|---|---|
| AFB1 | 0.018 ± 0.021 | 0.0031 ** | 0.0030 ** |
| AFG1 | 0.0070 ± 0.0030 | - | - |
| AFG2 | 0.020 ± 0.012 | 0.0021 ** | 0.0015 ** |
| T-2 | 0.012 ** | 0.016 ± 0.0030 | 0.0083 ** |
| HT-2 | 0.031 ± 0.036 | 0.028 ± 0.019 | 0.033 ** |
| Sum T-2 and HT-2 | 0.034 ± 0.035 | 0.030 ± 0.025 | 0.041 ** |
| OTA | - | 0.038 ± 0.044 | - |
| DON | 0.57 ± 0.33 | 0.32 ± 0.21 | 0.30 ± 0.19 |
| ZEA | 0.25 ± 0.48 a | 0.032 ± 0.019 b | 0.035 ± 0.020 |
| FB1 | 2.3 ± 3.3 A | 0.19 ± 0.19 B | 0.79 ± 1.3 |
| FB2 | 0.58 ± 0.80 | 0.051 ± 0.051 | 0.45 ± 0.63 |
| Sum FB1 and FB2 | 2.6 ± 3.9 A | 0.23 ± 0.24 B | 1.1 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annunziata, L.; Campana, G.; De Massis, M.R.; Scortichini, G.; Visciano, P. Simultaneous Presence of Mycotoxins in Feed Intended for Food-Producing Animals. Foods 2025, 14, 3176. https://doi.org/10.3390/foods14183176
Annunziata L, Campana G, De Massis MR, Scortichini G, Visciano P. Simultaneous Presence of Mycotoxins in Feed Intended for Food-Producing Animals. Foods. 2025; 14(18):3176. https://doi.org/10.3390/foods14183176
Chicago/Turabian StyleAnnunziata, Loredana, Guido Campana, Maria Rosaria De Massis, Giampiero Scortichini, and Pierina Visciano. 2025. "Simultaneous Presence of Mycotoxins in Feed Intended for Food-Producing Animals" Foods 14, no. 18: 3176. https://doi.org/10.3390/foods14183176
APA StyleAnnunziata, L., Campana, G., De Massis, M. R., Scortichini, G., & Visciano, P. (2025). Simultaneous Presence of Mycotoxins in Feed Intended for Food-Producing Animals. Foods, 14(18), 3176. https://doi.org/10.3390/foods14183176






