Sustainable Bioethanol Production and Phenolic Compounds from Avocado Stone Biomass Based on Microwave Pretreatment
Abstract
1. Introduction
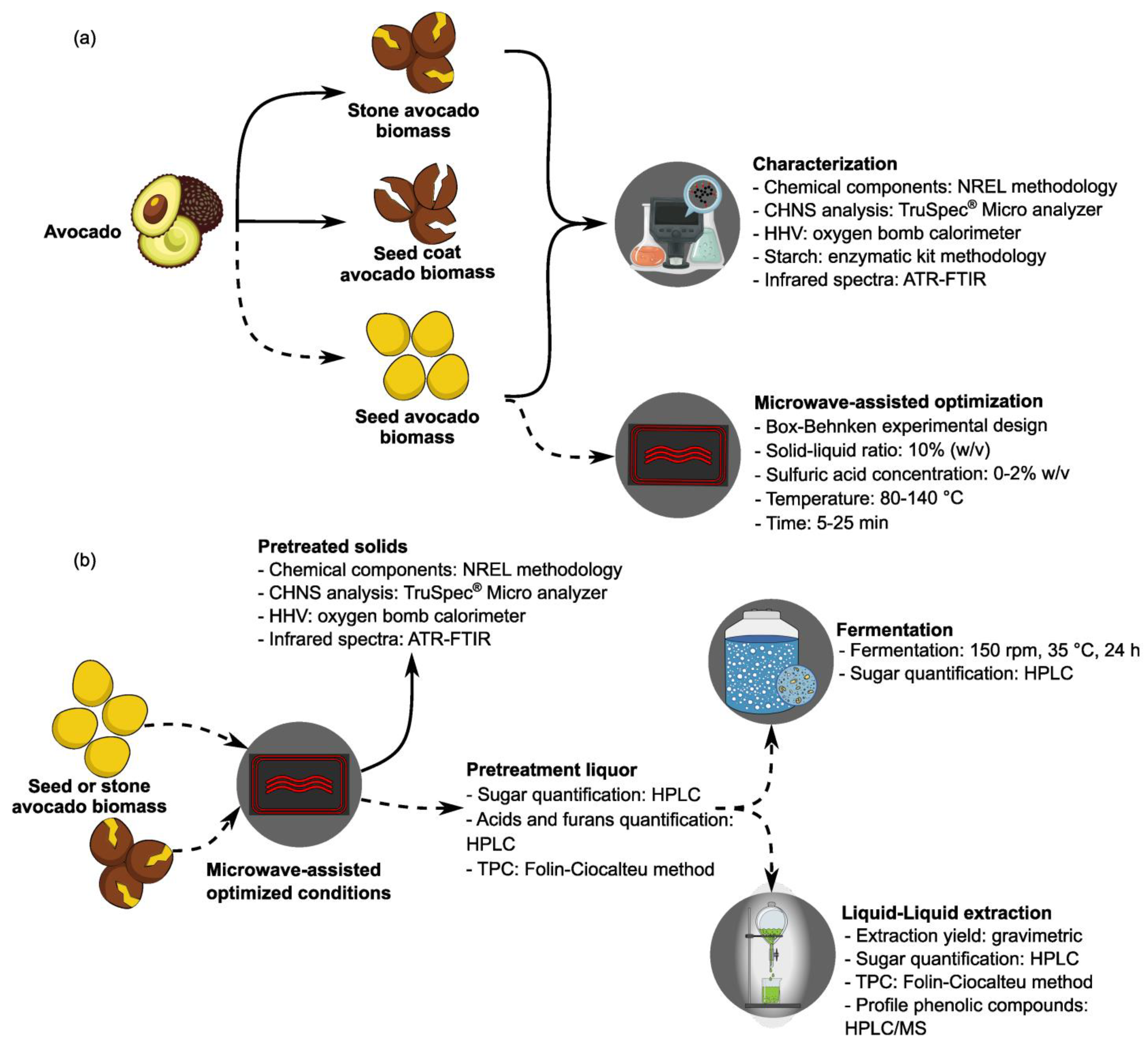
2. Materials and Methods
2.1. Raw Biomass Conditioning
2.2. Reagents
2.3. Chemical Characterization of the Raw Biomass and Pretreated Solids
2.3.1. Chemical Composition
2.3.2. CHNS and Energetic Content Determination
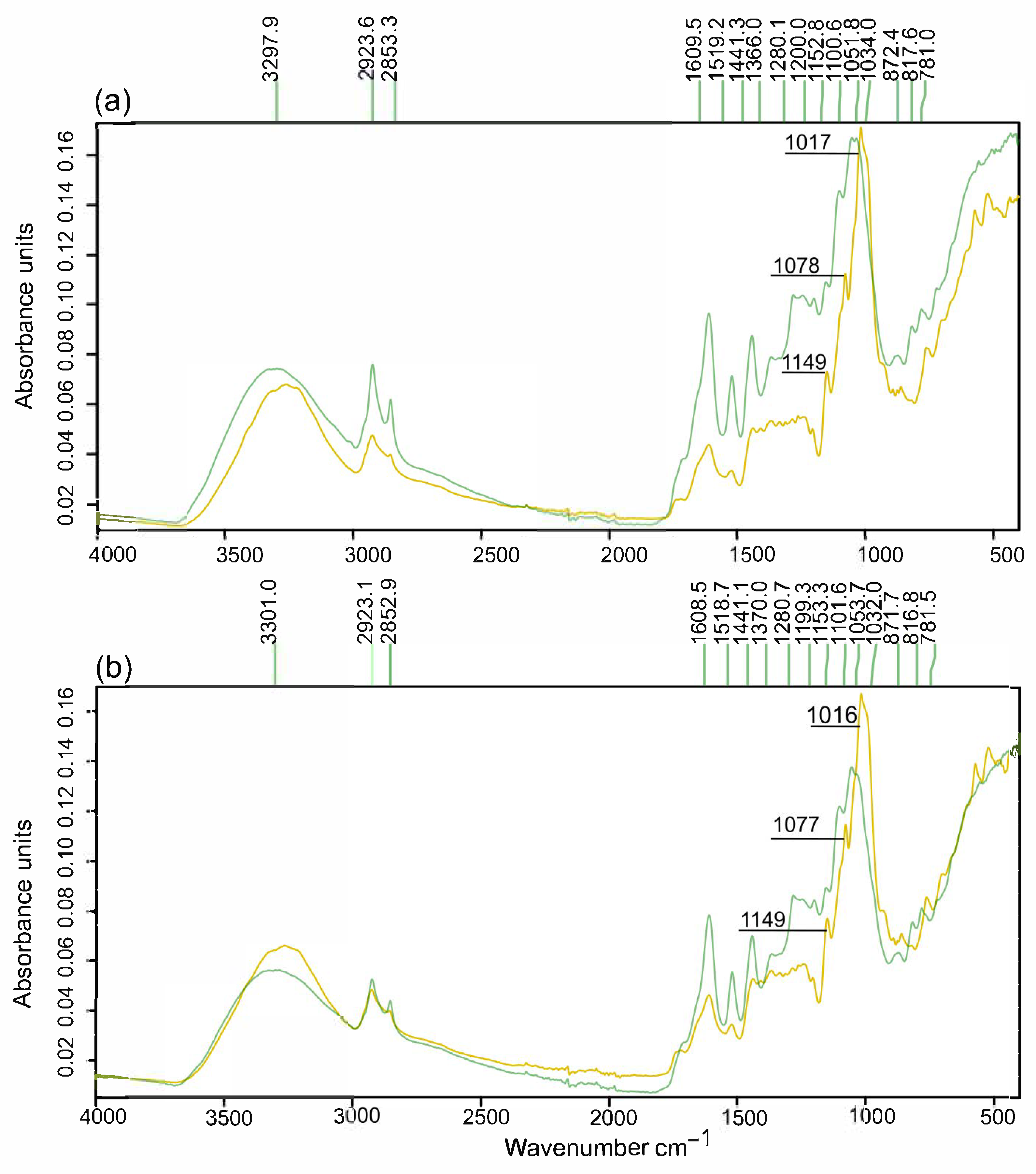
2.3.3. Infrared Spectroscopy
2.4. Microwave-Assisted Diluted Acid Pretreatment and Design
2.5. Determination of Sugars and Inhibitors in the Pretreatment Liquors
2.6. Fermentation
2.7. Liquid–Liquid Extraction of Pretreated Liquors
2.8. Characterization of Phenolic Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Raw Biomass Characterization
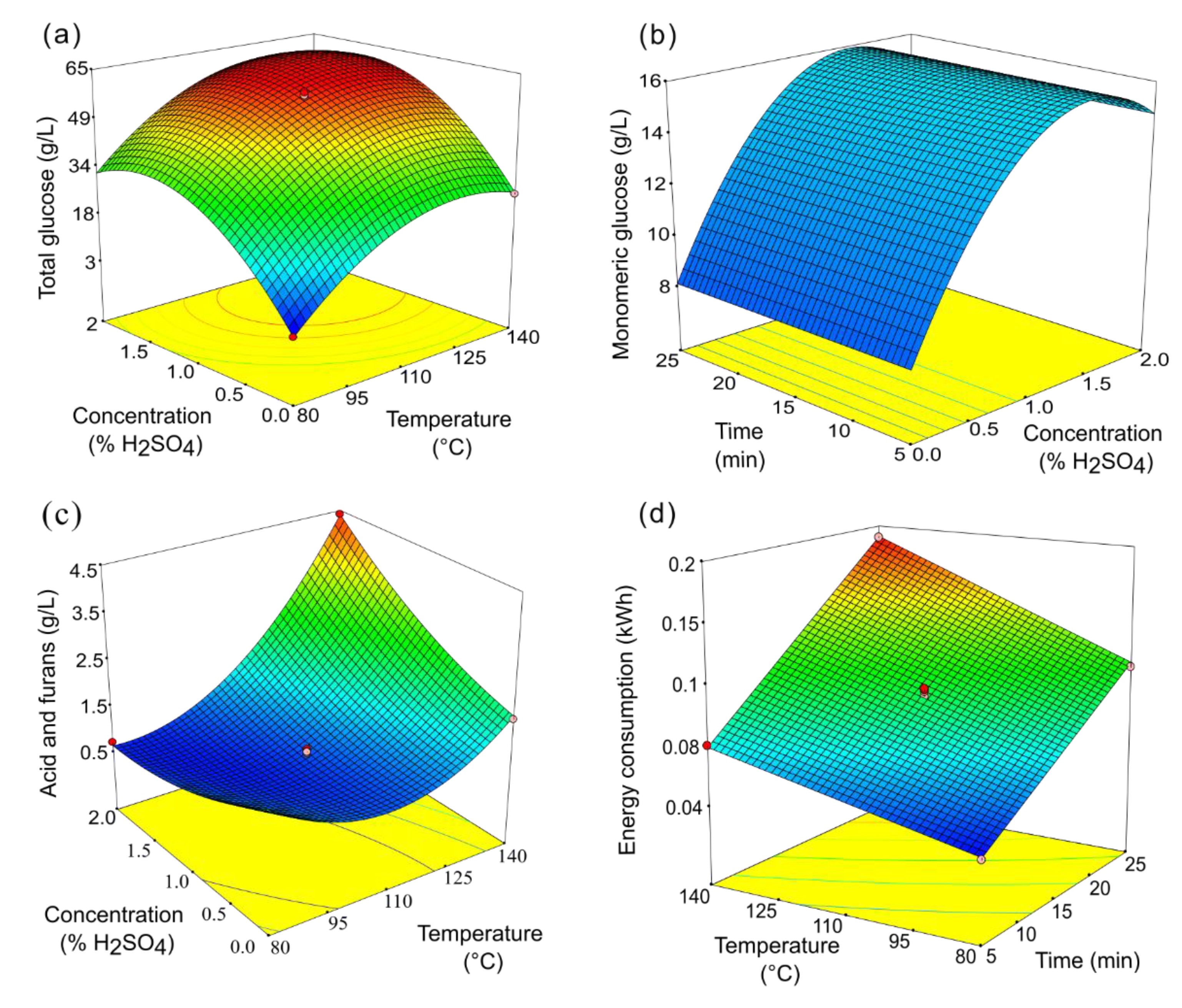
3.2. BBD of the Pretreatment: Analysis of the Pretreatment Liquors
3.3. Effect of Microwave-Assisted Diluted Acid Parameters on the Studied Responses
3.4. Multiple Optimization
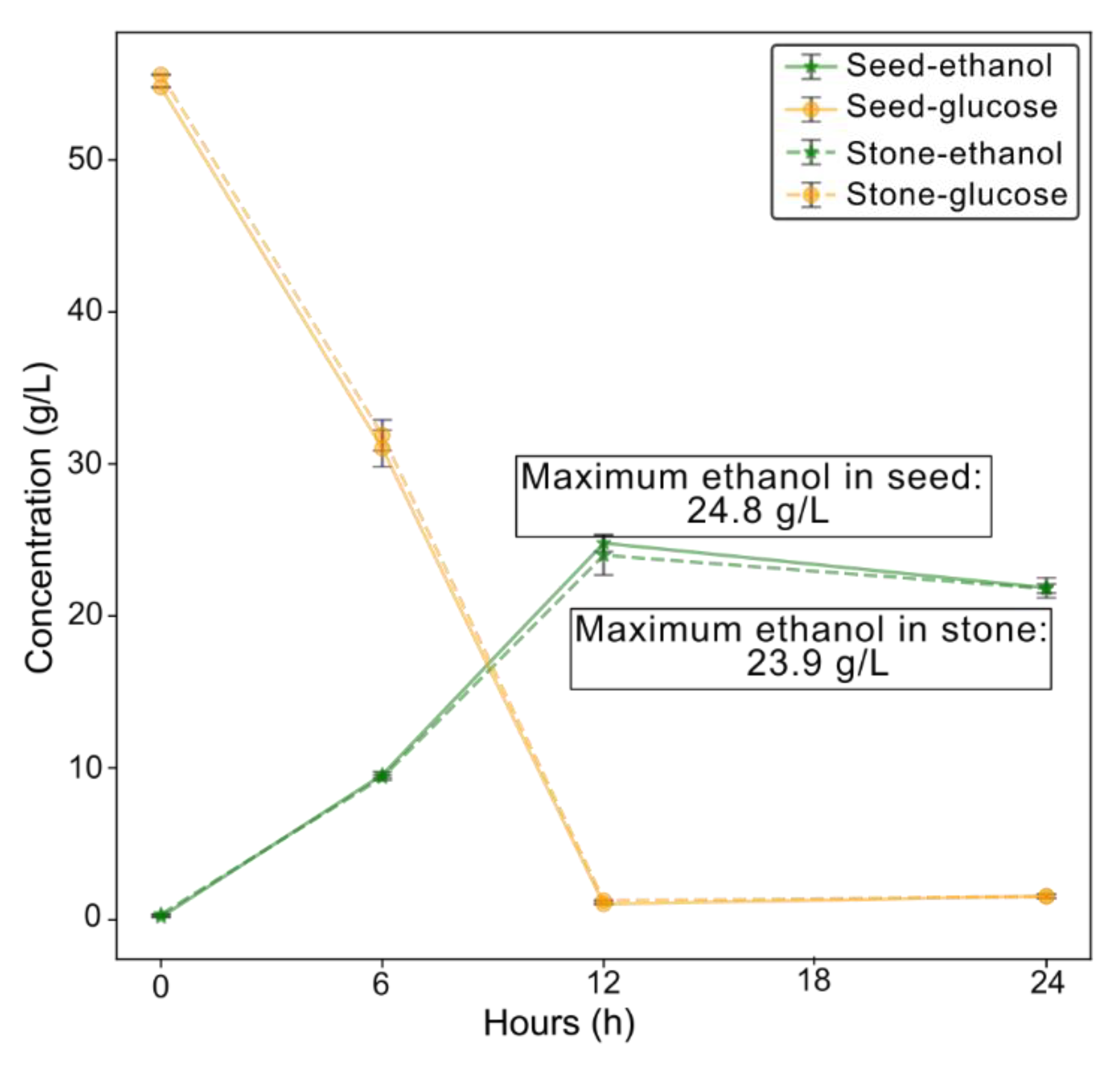
3.5. Production of Bioethanol
3.6. Recovery of Phenolic Compounds and Characterization
3.7. Characterization of the Pretreated Solids
3.8. Perspectives of an Avocado Stone Biorefinery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Attenuated total reflectance |
| BBD | Box–Behnken design |
| CSF | Combined severity factor |
| FTIR | Fourier transform infrared |
| GAE | Gallic acid equivalents |
| HHV | High heating value |
| HMF | Hydroxymethylfurfural |
| HPLC | High-performance liquid chromatography |
| MS | Mass spectrometry |
| RSM | Response surface methodology |
| RT | Retention time |
| TPC | Total phenolic content |
References
- Byun, J.; Kwon, O.; Kim, J.; Han, J. Carbon-Negative Food Waste-Derived Bioethanol: A Hybrid Model of Life Cycle Assessment and Optimization. ACS Sustain. Chem. Eng. 2022, 10, 4512–4521. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, Q.; Hou, J.; Mohsin, A.; Zhang, M.; Guo, M.; Zhu, Y.; Bao, J.; Wang, J.; Xiao, W.; et al. In-Depth Two-Stage Transcriptional Reprogramming and Evolutionary Engineering of Saccharomyces cerevisiae for Efficient Bioethanol Production from Xylose with Acetate. J. Agric. Food Chem. 2019, 67, 12002–12012. [Google Scholar] [CrossRef] [PubMed]
- de C., L. e Penalva Santos, D.; Correa, C.; Amaral Alves, Y.; Gomes Souza, C.; A. Mancebo Boloy, R. Brazil and the World Market in the Development of Technologies for the Production of Second-Generation Ethanol. Alex. Eng. J. 2023, 67, 153–170. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Arantes, V. A Review on Commercial-Scale High-Value Products That Can Be Produced alongside Cellulosic Ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef]
- Chaipornchalerm, P.; Prasertsab, A.; Prasanseang, W.; Wattanakit, C. Perspectives on Recent Advances in Hierarchical Zeolites for Bioethanol Conversion to Chemicals, Jet Fuels, and Carbon Nanotubes. Energy Fuels 2024, 38, 13612–13636. [Google Scholar] [CrossRef]
- Hans, M.; Lugani, Y.; Chandel, A.K.; Rai, R.; Kumar, S. Production of First- and Second-Generation Ethanol for Use in Alcohol-Based Hand Sanitizers and Disinfectants in India. Biomass Convers. Biorefinery 2023, 13, 7423–7440. [Google Scholar] [CrossRef]
- López-Linares, J.C.; García-Cubero, M.T.; Lucas, S.; Coca, M. Integral Valorization of Cellulosic and Hemicellulosic Sugars for Biobutanol Production: ABE Fermentation of the Whole Slurry from Microwave Pretreated Brewer’s Spent Grain. Biomass Bioenergy 2020, 135, 105524. [Google Scholar] [CrossRef]
- Zhang, B.; Khushik, F.A.; Zhan, B.; Bao, J. Transformation of Lignocellulose to Starch-like Carbohydrates by Organic Acid-Catalyzed Pretreatment and Biological Detoxification. Biotechnol. Bioeng. 2021, 118, 4105–4118. [Google Scholar] [CrossRef]
- Singh, P.; Gangwar, P.; Kumar, N.; Ghosh, S. Continuous Bioethanol Production from Glucose-Rich Hydrolysate Derived from Wheat Straw Using a Unique Fed-Batch Cultivation Method in a Bioreactor. Process. Saf. Environ. Prot. 2025, 193, 74–86. [Google Scholar] [CrossRef]
- Del-Castillo-Llamosas, A.; Eibes, G.; Ferreira-Santos, P.; Pérez-Pérez, A.; Del-Río, P.G.; Gullón, B. Microwave-Assisted Autohydrolysis of Avocado Seed for the Recovery of Antioxidant Phenolics and Glucose. Bioresour. Technol. 2023, 385, 129432. [Google Scholar] [CrossRef]
- Cardoza, D.; Contreras, M.d.M.; Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martín, J.M.; Romero, I.; Castro, E. Sustainable Vine Shoots-to-Ethanol Valorisation by a Sequential Acid/Organosolv Pretreatment. Process Saf. Environ. Prot. 2024, 183, 1059–1070. [Google Scholar] [CrossRef]
- Di Lena, G.; del Pulgar, J.S.; Boccia, G.L.; Casini, I.; Nicoli, S.F. Corn Bioethanol Side Streams: A Potential Sustainable Source of Fat-Soluble Bioactive Molecules for High-Value Applications. Foods 2020, 9, 1788. [Google Scholar] [CrossRef] [PubMed]
- García-Vargas, M.C.; Contreras, M.d.M.; Castro, E. Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Appl. Sci. 2020, 10, 8195. [Google Scholar] [CrossRef]
- Rubí-Arriaga, M.; Franco-Malvaíz, A.L.; Rebollar-Rebollar, S.; Bobadilla-Soto, E.E.; Martínez-De La Cruz, I.; Siles-Hernández, Y. Situación Actual del Cultivo del Aguacate (Persea americana Mill.) en el Estado de México, México. Trop. Subtrop. Agroecosyst. 2013, 16, 93–101. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Romaní, A.; Eibes, G.; Garrote, G.; Gullón, B.; del Río, P.G. Potential and Prospects for Utilization of Avocado By-Products in Integrated Biorefineries. Bioresour. Technol. 2022, 364, 128034. [Google Scholar] [CrossRef]
- Tesfaye, T.; Ayele, M.; Gibril, M.; Ferede, E.; Limeneh, D.Y.; Kong, F. Beneficiation of Avocado Processing Industry By-Product: A Review on Future Prospect. Curr. Res. Green Sustain. Chem. 2022, 5, 100253. [Google Scholar] [CrossRef]
- Caballero-Sanchez, L.; Lázaro-Mixteco, P.E.; Vargas-Tah, A.; Castro-Montoya, A.J. Pilot-Scale Bioethanol Production from the Starch of Avocado Seeds Using a Combination of Dilute Acid-Based Hydrolysis and Alcoholic Fermentation by Saccharomyces cerevisiae. Microb. Cell Factories 2023, 22, 119. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass (NREL/TP-510-42618). Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 30 January 2024).
- García-Vargas, M.C.; Contreras, M.d.M.; Gómez-Cruz, I.; Romero-García, J.M.; Castro, E. Avocado-Derived Biomass: Chemical Composition and Antioxidant Potential. Proceedings 2021, 70, 100. [Google Scholar]
- Gómez-Cruz, I.; Contreras, M.d.M.; Romero, I.; Castro, E. Lower Energy-Demanding Extraction of Bioactive Triterpene Acids by Microwave as the First Step towards Biorefining Residual Olive Skin. Antioxidants 2024, 13, 1212. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Denkin, A.I.; Tikhova, V.D.; Lomovsky, O.I. Prediction of Higher Heating Values of Plant Biomass from Ultimate Analysis Data. J. Therm. Anal. Calorim. 2017, 130, 1399–1405. [Google Scholar] [CrossRef]
- MacAskill, J.J.; Suckling, I.D.; Lloyd, J.A.; Manley-Harris, M. Unravelling the Effect of Pretreatment Severity on the Balance of Cellulose Accessibility and Substrate Composition on Enzymatic Digestibility of Steam-Pretreated Softwood. Biomass Bioenergy 2018, 109, 284–290. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Susmozas, A.; Padilla-Rascón, C.; Manzanares, P.; Castro, E.; Oliva, J.M.; Romero, I. Ethanol Production from Olive Stones Using Different Process Strategies. Renew. Energy 2022, 194, 1174–1183. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Dávila, I.; Labidi, J.; Gullón, P. Antioxidant and Antimicrobial Activities of Extracts Obtained from the Refining of Autohydrolysis Liquors of Vine Shoots. Ind. Crop Prod. 2017, 107, 105–113. [Google Scholar] [CrossRef]
- Medfai, W.; Contreras, M.D.M.; Lama-Muñoz, A.; Mhamdi, R.; Oueslati, I.; Castro, E. How Cultivar and Extraction Conditions Affect Antioxidants Type and Extractability for Olive Leaves Valorization. ACS Sustain. Chem. Eng. 2020, 8, 5107–5118. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive Characterization of Phenolic and Other Polar Compounds in the Seed and Seed Coat of Avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as Valuable Tools for the Determination of Phenolic and Other Polar Compounds in the Edible Part and by-Products of Avocado. LWT 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Beiro-Valenzuela, M.G.; Serrano-García, I.; Monasterio, R.P.; Moreno-Tovar, M.V.; Hurtado-Fernández, E.; González-Fernández, J.J.; Hormaza, J.I.; Pedreschi, R.; Olmo-García, L.; Carrasco-Pancorbo, A. Characterization of the Polar Profile of Bacon and Fuerte Avocado Fruits by Hydrophilic Interaction Liquid Chromatography–Mass Spectrometry: Distribution of Non-Structural Carbohydrates, Quinic Acid, and Chlorogenic Acid between Seed, Mesocarp, and Exocarp at Different Ripening Stages. J. Agric. Food Chem. 2023, 71, 5674–5685. [Google Scholar] [CrossRef]
- European MassBank. Available online: https://massbank.eu/ (accessed on 25 October 2024).
- King-Loeza, Y.; Ciprián-Macías, D.A.; Cardador-Martínez, A.; Martín-del-Campo, S.T.; Castañeda-Saucedo, M.C.; Ramírez-Anaya, J.d.P. Functional Composition of Avocado (Persea americana Mill. Var Hass) Pulp, Extra Virgin Oil, and Residues Is Affected by Fruit Commercial Classification. J. Agric. Food Res. 2023, 12, 100573. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Ortiz-Sanchez, M.; Restrepo-Serna, D.L.; Peroza Piñeres, P.; Pérez Cordero, A.; Cardona Alzate, C.A. Influence of Products Portfolio and Process Contextualization on the Economic Performance of Small- and Large-Scale Avocado Biorefineries. Bioresour. Technol. 2021, 342, 126060. [Google Scholar] [CrossRef]
- Kringel, D.H.; Dias, A.R.G.; Zavareze, E.d.R.; Gandra, E.A. Fruit Wastes as Promising Sources of Starch: Extraction, Properties, and Applications. Starch-Stärke 2020, 72, 1900200. [Google Scholar] [CrossRef]
- Tesfaye, T.; Gibril, M.; Sithole, B.; Ramjugernath, D.; Chavan, R.; Chunilall, V.; Gounden, N. Valorisation of Avocado Seeds: Extraction and Characterisation of Starch for Textile Applications. Clean Technol. Environ. Policy 2018, 20, 2135–2154. [Google Scholar] [CrossRef]
- Basak, B.; Patil, S.; Kumar, R.; Ha, G.-S.; Park, Y.-K.; Ali Khan, M.; Kumar Yadav, K.; Fallatah, A.M.; Jeon, B.-H. Integrated Hydrothermal and Deep Eutectic Solvent-Mediated Fractionation of Lignocellulosic Biocomponents for Enhanced Accessibility and Efficient Conversion in Anaerobic Digestion. Bioresour. Technol. 2022, 351, 127034. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Huisjes, E.; de Hulster, E.; van Dam, J.; Pronk, J.; Van Maris, A.J. Galacturonic Acid Inhibits the Growth of Saccharomyces cerevisiae on Galactose, Xylose, and Arabinose. Appl. Environ. Microbiol. 2012, 78, 5052–5059. [Google Scholar] [CrossRef]
- Singh, A.; Palma Toloza, C.; Singhania, R.R.; Carvajal Guevara, A. Hydrothermal Pretreatment with Microwaves: A New Strategy for Ensuring the Sustainability of Biorefineries. Energy Environ. 2025, 36, 1038–1062. [Google Scholar] [CrossRef]
- González-Mendoza, M.; Martínez-Bustos, F.; Castaño-Tostado, E.; Amaya-Llano, S.L. Effect of Microwave Irradiation on Acid Hydrolysis of Faba Bean Starch: Physicochemical Changes of the Starch Granules. Molecules 2022, 27, 3528. [Google Scholar] [CrossRef]
- Vaez, S.; Karimi, K.; Mirmohamadsadeghi, S.; Jeihanipour, A. An Optimal Biorefinery Development for Pectin and Biofuels Production from Orange Wastes without Enzyme Consumption. Process Saf. Environ. Prot. 2021, 152, 513–526. [Google Scholar] [CrossRef]
- Benvenutti, L.; Moura, F.M.; Zanghelini, G.; Barrera, C.; Seguí, L.; Zielinski, A.A.F. An Upcycling Approach from Fruit Processing By-Products: Flour for Use in Food Products. Foods 2025, 14, 153. [Google Scholar] [CrossRef]
- Meijnen, J.-P.; Randazzo, P.; Foulquié-Moreno, M.R.; van den Brink, J.; Vandecruys, P.; Stojiljkovic, M.; Dumortier, F.; Zalar, P.; Boekhout, T.; Gunde-Cimerman, N.; et al. Polygenic Analysis and Targeted Improvement of the Complex Trait of High Acetic Acid Tolerance in the Yeast Saccharomyces cerevisiae. Biotechnol. Biofuels 2016, 9, 5. [Google Scholar] [CrossRef]
- Del Castillo-Llamosas, A.; Rodríguez-Martínez, B.; del Río, P.G.; Eibes, G.; Garrote, G.; Gullón, B. Hydrothermal Treatment of Avocado Peel Waste for the Simultaneous Recovery of Oligosaccharides and Antioxidant Phenolics. Bioresour. Technol. 2021, 342, 125981. [Google Scholar] [CrossRef]
- Flourat, A.L.; Combes, J.; Bailly-Maitre-Grand, C.; Magnien, K.; Haudrechy, A.; Renault, J.-H.; Allais, F. Accessing P-Hydroxycinnamic Acids: Chemical Synthesis, Biomass Recovery, or Engineered Microbial Production? ChemSusChem 2021, 14, 118–129. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G.A. Phenolic Compound Profiles and Antioxidant Capacity of Persea americana Mill. Peels and Seeds of Two Varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Razola-Díaz, M.d.C.; De Montijo-Prieto, S.; Aznar-Ramos, M.J.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Effect of Lactic Acid Bacteria Fermentation on the Polar Compounds Content with Antioxidant and Antidiabetic Activity of Avocado Seed Extracts. Fermentation 2023, 9, 420. [Google Scholar] [CrossRef]
- Ohashi, H.; Koma, D.; Ohmoto, T.; Ohashi, T.; Satoh, Y.; Misaki, R.; Yamanaka, H. Enhancing Hydroxytyrosol Stability via Site-Specific Glucosylation. Carbohydr. Res. 2025, 556, 109618. [Google Scholar] [CrossRef] [PubMed]
- Chatzikonstantinou, A.V.; Giannakopoulou, A.; Spyrou, S.; Simos, Y.V.; Kontogianni, V.G.; Peschos, D.; Katapodis, P.; Polydera, A.C.; Stamatis, H. Production of Hydroxytyrosol Rich Extract from Olea europaea Leaf with Enhanced Biological Activity Using Immobilized Enzyme Reactors. Environ. Sci. Pollut. Res. 2022, 29, 29624–29637. [Google Scholar] [CrossRef]
- Villasante, J.; Vilas-Franquesa, A.; Fogliano, V. Solid-State Fermentation of Avocado Seed by Aspergillus oryzae and Aspergillus awamori: Effects on Nutritional Composition, Antioxidant Activity, and Volatile Compounds. Food Chem. 2025, 489, 144990. [Google Scholar] [CrossRef]
- Valanciene, E.; Malys, N. Advances in Production of Hydroxycinnamoyl-Quinic Acids: From Natural Sources to Biotechnology. Antioxidants 2022, 11, 2427. [Google Scholar] [CrossRef]
- Izu, G.O.; Mfotie Njoya, E.; Tabakam, G.T.; Nambooze, J.; Otukile, K.P.; Tsoeu, S.E.; Fasiku, V.O.; Adegoke, A.M.; Erukainure, O.L.; Mashele, S.S.; et al. Unravelling the Influence of Chlorogenic Acid on the Antioxidant Phytochemistry of Avocado (Persea americana Mill.) Fruit Peel. Antioxidants 2024, 13, 456. [Google Scholar] [CrossRef]
- Kızılyıldırım, S.; Kandemir, T.; Kendir, G.; Muhammed, M.T.; Köroğlu, A.; Ozogul, F. Antibacterial Activity of Avocado Extract (Persea americana Mill.) against Aminoglycoside-Resistant Klebsiella Pneumoniae Strains. Food Biosci. 2024, 60, 104523. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Contreras, M.d.M.; Romero, I.; Castro, E. Towards the Integral Valorization of Olive Pomace-Derived Biomasses through Biorefinery Strategies. ChemBioEng Rev. 2024, 11, 253–277. [Google Scholar] [CrossRef]
- Wang, E.; Jiang, Y.; Zhao, C. Hydroxytyrosol Isolation, Comparison of Synthetic Routes and Potential Biological Activities. Food Sci. Nutr. 2024, 12, 6899–6912. [Google Scholar] [CrossRef]
- Sambyal, K.; Singh, R.V. Production of Salicylic Acid; a Potent Pharmaceutically Active Agent and Its Future Prospects. Crit. Rev. Biotechnol. 2021, 41, 394–405. [Google Scholar] [CrossRef]
- Permal, R.; Chia, T.; Arena, G.; Fleming, C.; Chen, J.; Chen, T.; Chang, W.L.; Seale, B.; Hamid, N.; Kam, R. Converting Avocado Seeds into a Ready to Eat Snack and Analysing for Persin and Amygdalin. Food Chem. 2023, 399, 134011. [Google Scholar] [CrossRef]
- Jaszczak-Wilke, E.; Polkowska, Ż.; Koprowski, M.; Owsianik, K.; Mitchell, A.E.; Bałczewski, P. Amygdalin: Toxicity, Anticancer Activity and Analytical for Its Determination in Plant Seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef]
- Freitas, M.S.; Pereira, A.H.B.; Pereira, G.O.; Menezes, I.S.; Lucena, A.R.; Almeida, C.R.F.; Pereira, E.G.; Santos, L.A.; Tozin, L.R.S.; Alves, F.M.; et al. Acetogenin-Induced Fibrotic Heart Disease from Avocado (Persea americana, Lauraceae) Poisoning in Horses. Toxicon 2022, 219, 106921. [Google Scholar] [CrossRef]
- Nazimudheen, G.; Sekhar, N.C.; Sunny, A.; Kallingal, A.B.H. Physiochemical Characterization and Thermal Kinetics of Lignin Recovered from Sustainable Agrowaste for Bioenergy Applications. Int. J. Hydrogen Energy 2021, 46, 4798–4807. [Google Scholar] [CrossRef]
- Maksimuk, Y.; Antonava, Z.; Krouk, V.; Korsakova, A.; Kursevich, V. Prediction of Higher Heating Value Based on Elemental Composition for Lignin and Other Fuels. Fuel 2020, 263, 116727. [Google Scholar] [CrossRef]
- Lin, H.; Bean, S.R.; Tilley, M.; Peiris, K.H.S.; Brabec, D. Qualitative and Quantitative Analysis of Sorghum Grain Composition Including Protein and Tannins Using ATR-FTIR Spectroscopy. Food Anal. Methods 2021, 14, 268–279. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Sillero, L.; Prado, R.; Labidi, J. Simultaneous Microwave-Ultrasound Assisted Extraction of Bioactive Compounds from Bark. Chem. Eng. Process. Process Intensif. 2020, 156, 108100. [Google Scholar] [CrossRef]
- Reis Kemita, L.; França Lopes da Silva, L.; Pratto, B. Optimizing Dilute Acid Pretreatment for Enhanced Recovery and Co-Fermentation of Hexose and Pentose Sugars for Ethanol and Butanol Production. Fuel 2024, 372, 132187. [Google Scholar] [CrossRef]
- Torabi, S.; Satari, B.; Hassan-Beygi, S.R. Process Optimization for Dilute Acid and Enzymatic Hydrolysis of Waste Wheat Bread and Its Effect on Aflatoxin Fate and Ethanol Production. Biomass Convers. Biorefinery 2021, 11, 2617–2625. [Google Scholar] [CrossRef]
- Martins, R.P.; Schmatz, A.A.; de Freita, L.A.; Mutton, M.J.R.; Brienzo, M. Solubilization of Hemicellulose and Fermentable Sugars from Bagasse, Stalks, and Leaves of Sweet Sorghum. Ind. Crop. Prod. 2021, 170, 113813. [Google Scholar] [CrossRef]
- Yildirim, O.; Tunay, D.; Ozkaya, B. Optimization of Enzymatic Hydrolysis Conditions of Chemical Pretreated Cotton Stalk Using Response Surface Methodology for Enhanced Bioethanol Production Yield. Biomass Convers. Biorefinery 2023, 13, 6623–6634. [Google Scholar] [CrossRef]
- Salazar-Irrazabal, M.D.; Ramirez-Tixe, E.E.; Velasquez-Barreto, F.F.; Bello-Pérez, L.A. Avocado Seed Starch: Effect of the Variety on Molecular, Physicochemical, and Digestibility Characteristics. Int. J. Biol. Macromol. 2023, 247, 125746. [Google Scholar] [CrossRef]
- Martins, S.H.F.; Pontes, K.V.; Fialho, R.L.; Fakhouri, F.M. Extraction and Characterization of the Starch Present in the Avocado Seed (Persea americana Mill) for Future Applications. J. Agric. Food Res. 2022, 8, 100303. [Google Scholar] [CrossRef]
- Statista Exports of Guacamole from Mexico from 2013 to 2018. Available online: https://www.statista.com/statistics/977525/guacamole-exports-volume-mexico/ (accessed on 24 July 2025).
- de Abreu, Í.B.S.; Silva, R.K.; Siqueira, J.G.W.; da Silva, P.K.N.; Sonego, J.L.S.; de Souza, R.B.; Antonino, A.C.D.; Menezes, R.S.C.; Dutra, E.D. Brazilian Food Waste as a Substrate for Bioethanol Production. Foods 2024, 13, 4032. [Google Scholar] [CrossRef]
| Component | Seed Coat | Seed | Stone |
|---|---|---|---|
| Compositional analysis | |||
| Moisture (%) | 48.1 ± 2.9 | 61.5 ± 2.5 | 60.6 ± 2.3 |
| Aqueous extractives (%) | 28.73 ± 3.45 | 17.17 ± 3.29 | 17.49 ± 0.90 |
| Ethanolic extractives (%) | 4.64 ± 0.83 | 11.33 ± 1.96 | 10.15 ± 0.18 |
| Hexanic extractives (%) | 9.09 ± 1.09 | 4.73 ± 0.09 | 4.64 ± 0.23 |
| Glucans 1 (%) | 11.60 ± 3.24 | 52.17 ± 5.29 | 53.30 ± 0.54 |
| Starch (%) | 0.48 ± 0.06 | 42.78 ± 0.29 | 41.66 ± 2.09 |
| Hemicellulose (%) 2 | 3.97 ± 1.02 | 1.02 ± 0.28 | 1.27 ± 0.10 |
| Xylose (%) | 1.35 ± 0.44 | n.d. | n.d. |
| Galactose (%) | 1.21 ± 0.15 | 0.99 ± 0.25 | 1.14 ± 0.08 |
| Arabinose (%) | 1.83 ± 0.52 | 0.13 ± 0.06 | 0.26 ± 0.03 |
| Mannose (%) | 0.06 ± 0.04 | n.d. | n.d. |
| Galacturonic acid (%) | 0.57 ± 0.06 | 0.40 ± 0.02 | 0.58 ± 0.03 |
| Total lignin (%) 3 | 28.33 ± 1.50 | 13.08 ± 1.17 | 14.93 ± 2.75 |
| Acid-soluble lignin (%) | 0.83 ± 0.24 | 0.77 ± 0.07 | 0.675 ± 0.001 |
| Acid insoluble lignin (%) | 27.50 ± 1.26 | 12.32 ± 1.10 | 14.25 ± 2.75 |
| Protein 4 (%) | 6.15 ± 0.12 | 3.28 ± 0.12 | 2.93 ± 0.08 |
| Ash (%) | 4.34 ± 0.36 | 2.63 ± 0.01 | 2.62 ± 0.01 |
| Acetyl group (%) | 0.32 ± 0.04 | n.d. | n.d. |
| Composition of extractives | |||
| Glucose 5 (%) | 1.14 ± 0.02 | 3.08 ± 0.55 | 3.24 ± 0.34 |
| Xylose 5 (%) | 0.68 ± 0.06 | 0.06 ± 0.03 | 0.13 ± 0.01 |
| Galactose 5 (%) | 0.97 ± 0.13 | 0.22 ± 0.03 | 0.21 ± 0.03 |
| Arabinose 5 (%) | 2.12 ± 0.08 | 0.35 ± 0.08 | 0.30 ± 0.05 |
| Manose 5 (%) | 0.26 ± 0.05 | 0.079 ± 0.002 | 0.08 ± 0.01 |
| Galacturonic acid 5 (%) | 0.92 ± 0.04 | 0.38 ± 0.06 | 0.33 ± 0.08 |
| Acetic acid (%) | 1.56 ± 0.31 | 0.31 ± 0.03 | 0.31 ± 0.04 |
| TPC (%) | 7.42 ± 0.29 | 2.00 ± 0.02 | 1.99 ± 0.32 |
| Ultimate analysis | |||
| C (%) | 46.00 ± 0.36 | 43.81 ± 0.19 | 44.73 ± 0.45 |
| H (%) | 5.46 ± 0.16 | 6.03 ± 0.13 | 6.14 ± 0.12 |
| N (%) | 0.98 ± 0.02 | 0.53 ± 0.02 | 0.47 ± 0.01 |
| S (%) | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.08 ± 0.01 |
| HHV 6 (kJ/g) | 18.44 | 17.49 | 17.89 |
| HHV 7 (kJ/g) | ND | 16.90 ± 0.09 | 16.53 ± 0.29 |
| Run | Temperature (°C) | Time (min) | H2SO4 (% w/v) | Total Gluc. 1 (g/L) | Gluc. (g/L) | Other Sugars 2 (g/L) | Gluc. Rec. 3 (%) | Energy (kWh) 4 | CSF 5 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 110 (0) | 15 (0) | 1 (0) | 58.75 | 12.46 | 13.07 | 96.0 | 0.106 | 0.64 |
| 2 | 110 (0) | 15 (0) | 1 (0) | 57.70 | 12.96 | 12.93 | 94.4 | 0.104 | 0.64 |
| 3 | 110 (0) | 25 (1) | 2 (1) | 56.66 | 48.58 | 13.24 | 92.9 | 0.155 | 1.01 |
| 4 | 110 (0) | 5 (−1) | 2 (1) | 58.93 | 13.99 | 13.08 | 96.4 | 0.060 | 0.31 |
| 5 | 140 (1) | 5 (−1) | 1 (0) | 59.48 | 59.48 | 14.40 | 97.5 | 0.078 | 1.05 |
| 6 | 80 (−1) | 15 (0) | 2 (1) | 42.76 | 3.34 | 10.77 | 69.9 | 0.081 | −0.09 |
| 7 | 110 (0) | 15 (0) | 1 (0) | 57.46 | 12.04 | 12.62 | 94.1 | 0.105 | 0.64 |
| 8 | 80 (−1) | 15 (0) | 0 (−1) | 3.26 | 3.10 | 10.52 | 5.3 | 0.078 | ND |
| 9 | 140 (1) | 15 (0) | 0 (−1) | 27.07 | 3.19 | 14.17 | 44.3 | 0.125 | ND |
| 10 | 80 (−1) | 15 (0) | 1 (0) | 38.14 | 3.07 | 11.48 | 62.4 | 0.113 | −0.02 |
| 11 | 110 (0) | 15 (0) | 1 (0) | 58.30 | 12.81 | 12.88 | 95.5 | 0.106 | 0.64 |
| 12 | 140 (1) | 15 (0) | 2 (1) | 57.06 | 57.06 | 12.89 | 93.3 | 0.132 | 1.67 |
| 13 | 140 (1) | 25 (1) | 1 (0) | 58.48 | 58.48 | 13.36 | 95.7 | 0.182 | 1.75 |
| 14 | 110 (0) | 15 (0) | 1 (0) | 57.29 | 12.78 | 13.19 | 93.7 | 0.101 | 0.64 |
| 15 | 80 (−1) | 5 (−1) | 1 (0) | 30.95 | 3.07 | 12.74 | 50.6 | 0.040 | −0.72 |
| 16 | 110 (0) | 25 (1) | 0 (−1) | 34.50 | 3.03 | 15.19 | 56.5 | 0.149 | ND |
| 17 | 110 (0) | 5 (−1) | 0 (−1) | 40.16 | 3.13 | 15.32 | 65.8 | 0.060 | ND |
| Parameter 1 | Total Glucose 2 (g/L) | Glucose (g/L) | Other Sugars (g/L) | Acids and Furans 3 (g/L) | TPC (g/L) | Energy Consumption (kW h) |
|---|---|---|---|---|---|---|
| Equation terms | ||||||
| 57.90 | 12.09 | 12.94 | 0.86 | 1.39 | 0.104 | |
| A | 1.79 | - | −0.28 | 0.10 | −0.01 | 0.045 |
| B | 12.19 | 26.79 | 0.90 | 0.99 | 0.08 | 0.026 |
| C | 14.49 | 3.13 | −0.91 | 0.32 | −0.13 | 0.002 |
| AB | −2.05 | - | - | 0.84 | −0.05 | 0.008 |
| AC | −3.17 | - | - | 0.10 | −0.25 | - |
| BC | - | - | - | 0.89 | −0.60 | - |
| A 2 | 1.26 | - | 0.83 | −0.35 | −0.32 | - |
| B 2 | −12.40 | 19.58 | −0.77 | 1.04 | −0.08 | - |
| C 2 | −15.61 | −3.48 | 0.45 | 0.31 | 0.13 | - |
| Statistics | ||||||
| Model (p-value) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Lack-of-fit | 0.30 | 0.05 | 0.10 | 0.05 | 0.50 | 0.40 |
| CV (%) | 1.4 | 12.6 | 2.67 | 6.3 | 3.6 | 2.2 |
| R2 | 0.999 | 0.992 | 0.946 | 0.998 | 0.994 | 0.997 |
| Adjusted R2 | 0.998 | 0.989 | 0.909 | 0.994 | 0.986 | 0.996 |
| Component | Predicted Value | Experimental Value | Relative Error (%) 4 | Experimental Value |
|---|---|---|---|---|
| Seed | Stone | |||
| Total glucose (g/L) 1 | 58.67 | 56.80 ± 0.01 | 3.2 | 55.95 ± 0.08 |
| Glucose (g/L) | 58.36 | 56.60 ± 0.33 | 3.0 | 55.46 ± 0.08 |
| Other carbohydrates (g/L) | 14.21 | 13.92 ± 0.04 | 2.0 | 15.05 ± 0.59 |
| Acids and furans (g/L) 2 | 1.56 | 1.53 ± 0.04 | 1.6 | 1.813 ± 0.009 |
| Galacturonic acid (g/L) | - | 0.43 ± 0.15 | - | 0.493 ± 0.003 |
| Formic acid (g/L) | - | 0.25 ± 0.01 | - | 0.219 ±0.003 |
| Acetic acid (g/L) | - | 0.48 ± 0.01 | - | 0.75 ± 0.02 |
| Levulinic acid (g/L) | - | 0.032 ± 0.003 | - | 0.034 ± 0.003 |
| Furfural (g/L) | - | n.d. | - | n.d. |
| HMF (g/L) | - | 0.34 ± 0.02 | - | 0.30 ± 0.02 |
| TPC (g/L) | - | 1.56 ± 0.07 | - | 1.69 ± 0.08 |
| TPC (g/L) in extracts 3 | - | 0.24 ± 0.03 | - | 0.25 ± 0.02 |
| Energy consumption (kWh) | 0.077 | 0.079 ± 0.000 | 2.5 | 0.080 ± 0.000 |
| RT (min) | Exp. m/z | Molecular Formula | Score | Error (ppm) | Main MS/MS Fragments | Proposed Compound |
|---|---|---|---|---|---|---|
| Negative ionization mode | ||||||
| 1.22 | 167.04 | C8H8O4 | 98 | 0.03 | 123.04, 108.02, 109.03, 93.03 | Dihydroxyphenylacetic acid |
| 1.37 | 153.06 | C8H10O3 | 99 | −0.49 | 123.04 | Hydroxytyrosol |
| 1.57 | 153.02 | C7H6O4 | 99 | 0.08 | 109.03, 108.02, 91.02 | Dihydroxybenzoic acid |
| 2.32 | 137.02 | C7H6O3 | 99 | −0.91 | 119.01, 108.02, 92.03 | Hydroxybenzoic acid |
| 2.37 | 353.09 | C16H18O9 | 96 | −1.37 | 191.06, 179.03, 135.04 | Caffeoylquinic acid |
| 2.73 | 177.02 | C9H6O4 | 99 | 0.21 | 149.02, 121.03, 93.04 | Unknown |
| 3.30 | 337.09 | C16H18O8 | 99 | −1.80 | 191.06, 163.04, 119.05 | Coumaroylquinic acid |
| 3.93 | 337.09 | C16H18O8 | 97 | −0.45 | 191.05, 163.04, 119.05 | Coumaroylquinic acid is. 1 |
| 5.29 | 353.09 | C16H18O9 | 99 | −0.75 | 191.06, 179.06, 135.04 | Caffeoylquinic acid is. 1 |
| 5.44 | 337.09 | C16H18O8 | 99 | −0.53 | 173.05 | Coumaroylquinic acid is. 2 |
| 5.02 | 367.10 | C17H20O9 | 98 | 0.46 | 193.05, 134.04 | Ferulolylquinic acid |
| 5.82 | 367.10 | C17H20O9 | 98 | 0.01 | 161.02 | Caffeoyl-methylquinic acid |
| 6.53 | 337.09 | C16H18O8 | 98 | 1.09 | 173.05 | Coumaroylquinic acid is. 3 |
| 7.31 | 367.10 | C17H20O9 | 95 | 1.90 | 193.05, 173.04 | Ferulolylquinic acid is. 1 |
| 7.31 | 367.10 | C17H20O9 | 96 | −0.22 | - | Ferulolylquinic acid is. 2 |
| 15.66 | 387.14 | C21H24O7 | 100 | −0.14 | 187.11 | Unknown |
| Positive ionization mode | ||||||
| 0.69 | 139.04 | C7H6O3 | 99 | −0.70 | 110.04, 81.03, 55.06, 53.04 | Dihydroxybenzaldehyde |
| 0.97 | 127.04 | C6H6O3 | 99 | 1.79 | 109.03, 81.03, 55.06, 53.04 | Pyrogallol |
| 2.30 | 139.04 | C7H6O3 | 99 | 1.07 | 110.04, 93.03, 65.04 | Hydroxybenzoic acid |
| 4.88 | 169.05 | C8H8O4 | 99 | −1.35 | 109.03, 81.03, 53.04 | Dihydroxy-methoxy benzaldehyde |
| 5.94 | 139.04 | C7H6O3 | 97 | 3.10 | 55.06, 53.04 | Dihydroxybenzaldehyde is. 1 |
| Component | Seed | Stone |
|---|---|---|
| Compositional analysis | ||
| Glucans 1 (%) | 10.96 ± 1.47 | 12.98 ± 0.29 |
| Glucose (%) | 12.06 ± 0.51 | 14.29 ± 0.32 |
| Hemicellulose 2 (%) | n.d. | n.d. |
| Galacturonic acid (%) | 0.23 ± 0.02 | 0.27 ± 0.01 |
| Total lignin 3 (%) | 67.02 ± 2.39 | 74.18 ± 0.49 |
| Acid-soluble lignin (%) | 1.51 ± 0.16 | 1.66 ± 0.10 |
| Acid-insoluble lignin (%) | 65.51 ± 2.24 | 72.51 ± 0.39 |
| Protein 4 (%) | 10.56 ± 0.02 | 8.73 ± 0.03 |
| Ash (%) | 0.315 ± 0.004 | 0.269 ± 0.007 |
| Ultimate analysis | ||
| C (%) | 55.76 ± 0.54 | 56.36 ± 0.07 |
| H (%) | 6.64 ± 0.11 | 6.41 ± 0.08 |
| N (%) | 1.69 ± 0.01 | 1.40 ± 0.01 |
| S (%) | 0.16 ± 0.02 | 0.15 ± 0.03 |
| HHV 5 (kJ/g) | 22.71 | 22.97 |
| HHV 6 (kJ/g) | 22.84 ± 0.03 | 22.35 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morán-Alarcón, L.C.; Contreras, M.d.M.; Romero-García, J.M.; Galán-Martín, Á.; Castro, E. Sustainable Bioethanol Production and Phenolic Compounds from Avocado Stone Biomass Based on Microwave Pretreatment. Foods 2025, 14, 3160. https://doi.org/10.3390/foods14183160
Morán-Alarcón LC, Contreras MdM, Romero-García JM, Galán-Martín Á, Castro E. Sustainable Bioethanol Production and Phenolic Compounds from Avocado Stone Biomass Based on Microwave Pretreatment. Foods. 2025; 14(18):3160. https://doi.org/10.3390/foods14183160
Chicago/Turabian StyleMorán-Alarcón, Luis Carlos, María del Mar Contreras, Juan Miguel Romero-García, Ángel Galán-Martín, and Eulogio Castro. 2025. "Sustainable Bioethanol Production and Phenolic Compounds from Avocado Stone Biomass Based on Microwave Pretreatment" Foods 14, no. 18: 3160. https://doi.org/10.3390/foods14183160
APA StyleMorán-Alarcón, L. C., Contreras, M. d. M., Romero-García, J. M., Galán-Martín, Á., & Castro, E. (2025). Sustainable Bioethanol Production and Phenolic Compounds from Avocado Stone Biomass Based on Microwave Pretreatment. Foods, 14(18), 3160. https://doi.org/10.3390/foods14183160









