Monitoring Sublethal Injury in Listeria monocytogenes During Heat Treatment of Pork Frankfurter-Type Sausages: A Single-Cell vs. Population Level Approach
Abstract
1. Introduction
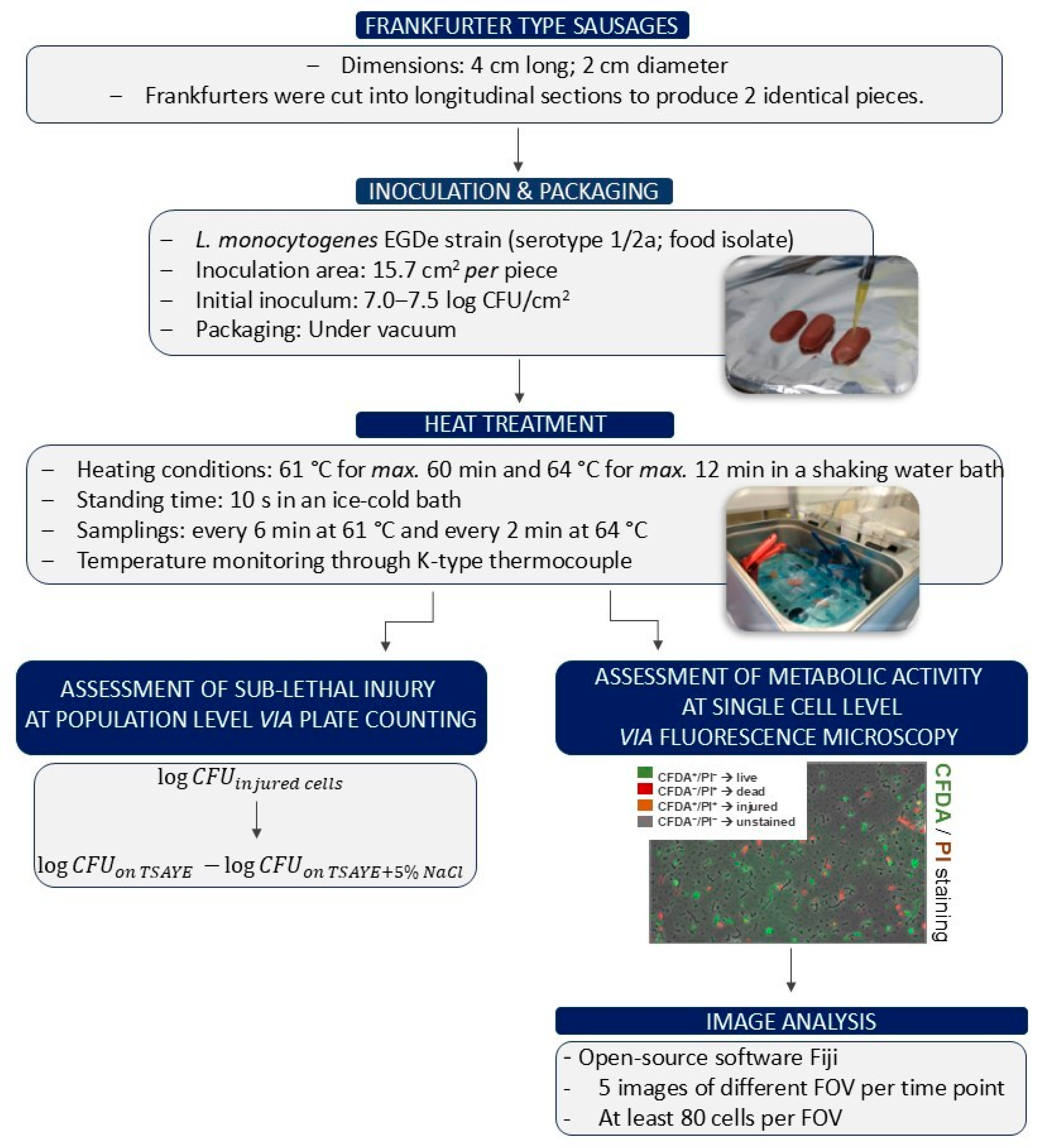
2. Materials and Methods
2.1. Microorganism and Inoculum Preparation
2.2. Experimental Procedure
2.2.1. Inoculation and Vacuum Packaging of Pork Frankfurter-Type Sausages
2.2.2. Heat Treatment of Pork Frankfurter-Type Sausages
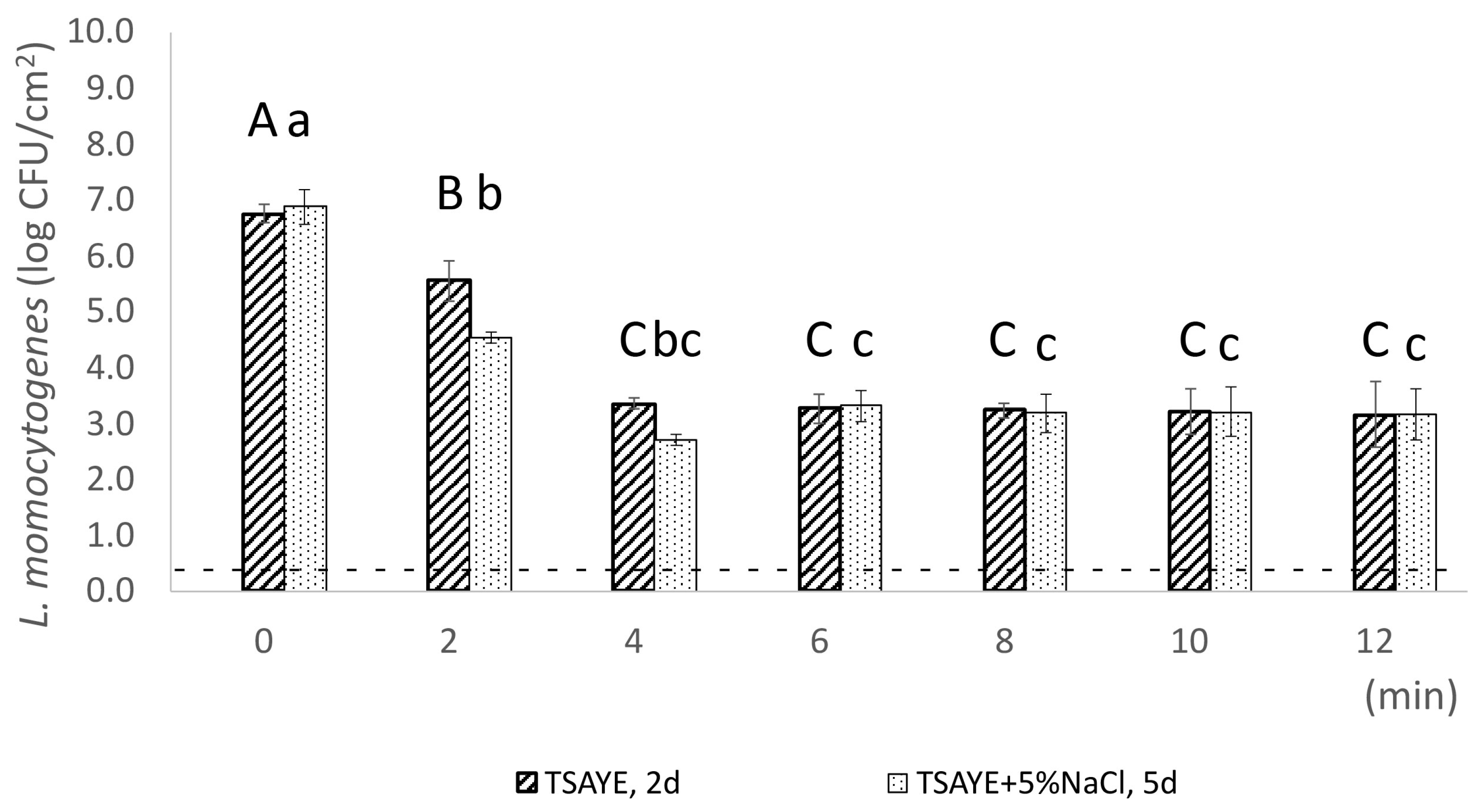
2.2.3. Assessment of Sublethal Injury at Population Level via Plate Counting
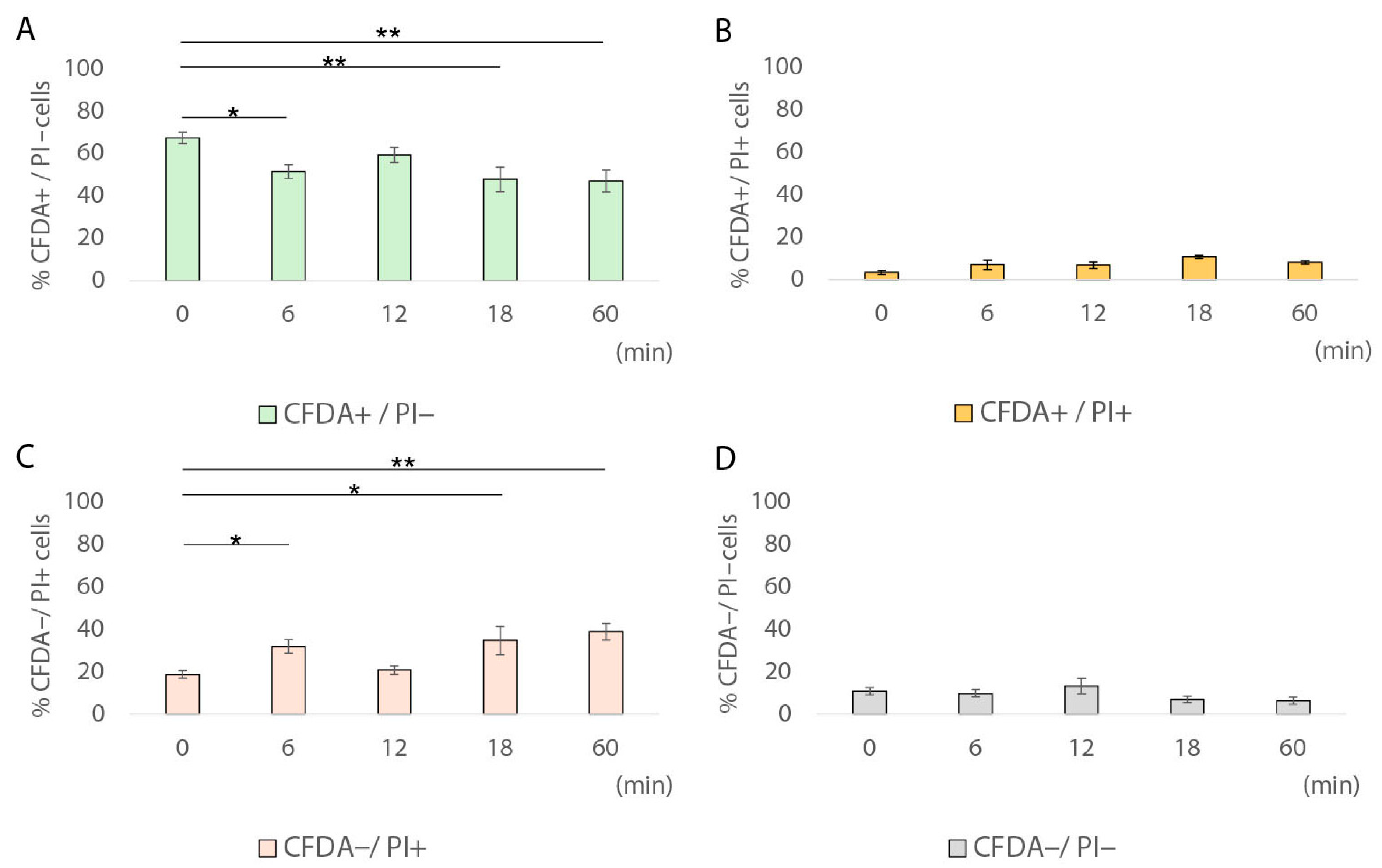
2.2.4. Assessment of Metabolic Activity at Single-Cell Level via Fluorescence Microscopy
2.3. Statistical Analysis
3. Results
3.1. Enhanced Survival of Resistant Subpopulations as Reflected by the Reported Tailing of Inactivation Curves
3.2. Induction of Sublethal Injury and VBNC State at Single-Cell Level
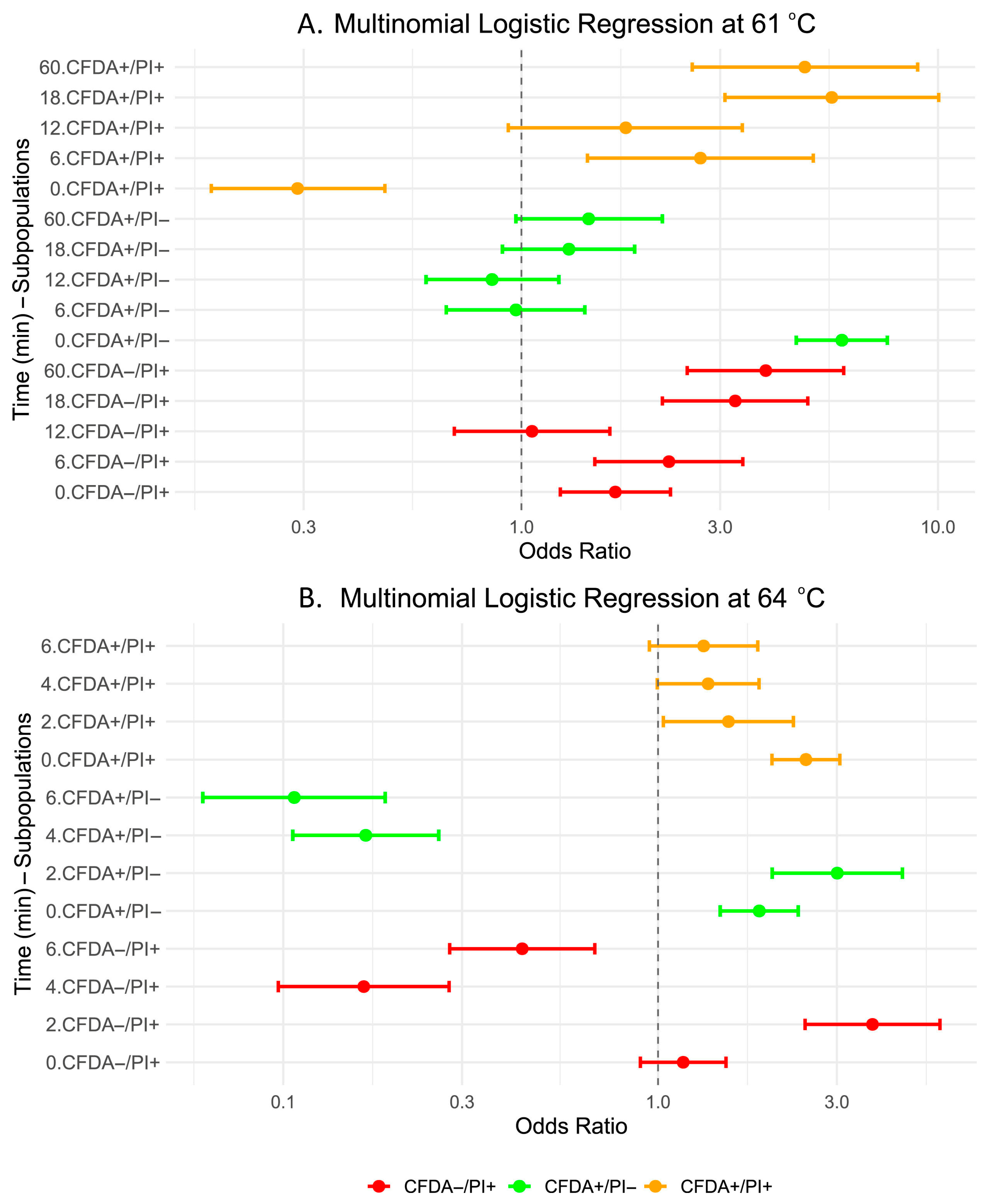
3.3. Transition of Metabolically Active Cells (CFDA+/PI−) to Sublethally Injured Cells (CFDA+/PI+) at Single-Cell Level
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Wang, S.; Chen, X.; Qu, C. Review Controlling Listeria monocytogenes in Ready-to-Eat Meat and Poultry Products: An Overview of Outbreaks, Current Legislations, Challenges, and Future Prospects. Trends Food Sci. Technol. 2021, 116, 24–35. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 15 July 2025).
- Matle, I.; Mbatha, K.R.; Madoroba, E. A Review of Listeria Monocytogenes from Meat and Meat Products: Epidemiology, Virulence Factors, Antimicrobial Resistance and Diagnosis. Onderstepoort J. Vet. Res. 2020, 87, e1–e20. [Google Scholar] [CrossRef]
- Jensen, A.K.; Björkman, J.T.; Ethelberg, S.; Kiil, K.; Kemp, M.; Nielsen, E.M. Molecular Typing and Epidemiology of Human Listeriosis Cases, Denmark, 2002–2012. Emerg. Infect. Dis. 2016, 22, 625–633. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Regan, P.; Laksanalamai, P.; Healey, S.; Hu, Z. Prevalence and Methodologies for Detection, Characterization and Subtyping of Listeria monocytogenes and L. ivanovii in Foods and Environmental Sources. Food Sci. Hum. Wellness 2017, 6, 97–120. [Google Scholar] [CrossRef]
- Bozzuto, G.; Ruggieri, P.; Molinari, A. Molecular Aspects of Tumor Cell Migration and Invasion. Ann. Ist. Super. Sanita 2010, 46, 66–80. [Google Scholar] [CrossRef]
- Smith, A.M.; Tau, N.P.; Smouse, S.L.; Allam, M.; Ismail, A.; Ramalwa, N.R.; Disenyeng, B.; Ngomane, M.; Thomas, J. Outbreak of Listeria monocytogenes in South Africa, 2017–2018: Laboratory Activities and Experiences Associated with Whole-Genome Sequencing Analysis of Isolates. Foodborne Pathog. Dis. 2019, 16, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Hot Dog and Sausages Market Report. Available online: https://introspectivemarketresearch.com/request/16649 (accessed on 17 July 2025).
- Cavalcanti, A.A.C.; Limeira, C.H.; de Siqueira, I.N.; de Lima, A.C.; de Medeiros, F.J.P.; de Souza, J.G.; de Araújo Medeiros, N.G.; de Oliveira Filho, A.A.; de Melo, M.A. The Prevalence of Listeria Monocytogenes in Meat Products in Brazil: A Systematic Literature Review and Meta-Analysis. Res. Vet. Sci. 2022, 145, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.S.; de Sá, C.V.G.C.; de Melo, C.B. Listeria monocytogenes Contamination in Industrial Sausages. Braz. J. Vet. Med. 2018, 40, e009118. [Google Scholar] [CrossRef]
- Zhang, H.; Que, F.; Xu, B.; Sun, L.; Zhu, Y.; Chen, W.; Ye, Y.; Dong, Q.; Liu, H.; Zhang, X. Identification of Listeria monocytogenes Contamination in a Ready-to-Eat Meat Processing Plant in China. Front. Microbiol. 2021, 12, 628204. [Google Scholar] [CrossRef] [PubMed]
- Romans, J.R. The Meat We Eat; Interstate Publishers: Crete, IL, USA, 2001; ISBN 9780813431758. [Google Scholar]
- Aika, L.L.; Palumbo, S.A.; Smith, J.L.; Del Corral, F.; Bhaduri, S.; Jones, C.O.; Kim, A.H. Destruction of Listeria monocytogenes during Frankfurter Processing. J. Food Prot. 1990, 53, 18–21. [Google Scholar]
- Cygnarowicz-Provost, M.; Whiting, R.C.; Craig, J.C., Jr. Steam Surface Pasteurization of Beef Frankfurters. J. Food Sci. 1994, 59, 1–5. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Food Safety and Inspection Service. Control of Listeria monocytogenes in Ready-to-Eat Meat and Poultry Products. Final Rule. Fed. Reg. 2023, 68, 34208–34254. Available online: https://www.govinfo.gov/content/pkg/FR-2003-06-06/pdf/03-14173.pdf (accessed on 4 December 2024).
- Balamurugan, S.; Inmanee, P.; De Souza, J.; Strange, P.; Pirak, T.; Barbut, S. Effects of High Pressure Processing and Hot Water Pasteurization of Cooked Sausages on Inactivation of Inoculated Listeria monocytogenes, Natural Populations of Lactic Acid Bacteria, Pseudomonas Spp., and Coliforms and Their Recovery during Storage at 4 and 10 °C. J. Food Prot. 2018, 81, 1245–1251. [Google Scholar] [CrossRef]
- Cichoski, A.J.; Rampelotto, C.; Silva, M.S.; de Moura, H.C.; Terra, N.N.; Wagner, R.; de Menezes, C.R.; Flores, E.M.M.; Barin, J.S. Ultrasound-Assisted Post-Packaging Pasteurization of Sausages. Innov. Food Sci. Emerg. Technol. 2015, 30, 132–137. [Google Scholar] [CrossRef]
- Inmanee, P.; Ratphitagsanti, W.; Kamonpatana, P.; Pirak, T. Effect of Thermosonication or Microwave Heating for Post Pasteurization on Chemical, Physical, and Sensory Characteristics of Prototype Sausage. Agric. Nat. Resour. 2020, 54, 39–47. [Google Scholar] [CrossRef][Green Version]
- Inmanee, P.; Kamonpatana, P.; Pirak, T. Ohmic Heating Effects on Listeria monocytogenes Inactivation, and Chemical, Physical, and Sensory Characteristic Alterations for Vacuum Packaged Sausage during Post Pasteurization. LWT 2019, 108, 183–189. [Google Scholar] [CrossRef]
- NSW Government Food Authority In-Pack Pasteurization for Reducing the Risk of Listeria Contamination on Ready-To-Eat (RTE) Meats. Food Safety Schemes Manual Appendix 5. Available online: https://www.foodauthority.nsw.gov.au/sites/default/files/2020-01/fssm_inpack_pasteurisation_app5.pdf (accessed on 22 July 2025).
- Yousef, A.E.; Courtney, P.D. Basics of Stress Adaptation and Implications in New-Generation Foods; CRC Press LLC: Boca Raton, FL, USA, 2002; Volume 1. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Williams, T.C.; Oliver, J.D. Bridging the Gap between Viable but Non-Culturable and Antibiotic Persistent Bacteria. Trends Microbiol. 2015, 23, 7–13. [Google Scholar] [CrossRef]
- Arvaniti, M.; Skandamis, P.N. Defining Bacterial Heterogeneity and Dormancy with the Parallel Use of Single-Cell and Population Level Approaches. Curr. Opin. Food Sci. 2022, 44, 100808. [Google Scholar] [CrossRef]
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection Method. 2nd ed. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 11290-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 2: Enumeration Method. 2nd ed. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Lee, N.; Kwon, K.Y.; Oh, S.K.; Chang, H.-J.; Chun, H.S.; Choi, S.-W. A Multiplex PCR Assay for Simultaneous Detection of Escherichia Coli O157:H7, Bacillus Cereus, Vibrio Parahaemolyticus, Salmonella spp., Listeria monocytogenes, and Staphylococcus Aureus in Korean Ready-to-Eat Food. Foodborne Pathog. Dis. 2014, 11, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, S.; Robben, C.; Alter, T.; Rossmanith, P.; Mester, P. How to Evaluate Non-Growing Cells-Current Strategies for Determining Antimicrobial Resistance of VBNC Bacteria. Antibiotics 2021, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Wideman, N.E.; Oliver, J.D.; Crandall, P.G.; Jarvis, N.A. Detection and Potential Virulence of Viable but Non-Culturable (VBNC) Listeria monocytogenes: A Review. Microorganisms 2021, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, M.; Tsakanikas, P.; Papadopoulou, V.; Giannakopoulou, A.; Skandamis, P. Listeria monocytogenes Sublethal Injury and Viable-but-Nonculturable State Induced by Acidic Conditions and Disinfectants. Microbiol. Spectr. 2021, 9, e0137721. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, M.; Tsakanikas, P.; Paramithiotis, S.; Papadopoulou, V.; Balomenos, A.; Giannakopoulou, A.; Skandamis, P. Deciphering the Induction of Listeria monocytogenes into Sublethal Injury Using Fluorescence Microscopy and RT-QPCR. Int. J. Food Microbiol. 2023, 385, 109983. [Google Scholar] [CrossRef]
- Christophe, B.; Christiane, B.; Pierre, L.; Cristel, A.; Sophie, C.; Edith, G.; Zongfu, W.; Andreas, K.; Sylvain, B.; Graciela, P.M.; et al. Comparison of Widely Used Listeria monocytogenes Strains EGD, 10403S, and EGD-e Highlights Genomic Differences Underlying Variations in Pathogenicity. MBio 2014, 5, e00969-14. [Google Scholar] [CrossRef]
- Glaser, P.; Frangeul, L.; Buchrieser, C.; Rusniok, C.; Amend, A.; Baquero, F.; Berche, P.; Bloecker, H.; Brandt, P.; Chakraborty, T.; et al. Comparative Genomics of Listeria Species. Science 2001, 294, 849–852. [Google Scholar] [CrossRef]
- Scott, V.N.; Swanson, K.M.J.; Freier, T.A.; Pruett, W.P.J.; Sveum, W.H.; Hall, P.A.; Smoot, L.A.; Brown, D.G. Guidelines for Conducting Listeria monocytogenes Challenge Testing of Foods. Food Prot. Trends 2005, 25, 818–825. [Google Scholar]
- Siderakou, D.; Zilelidou, E.; Poimenidou, S.; Tsipra, I.; Ouranou, E.; Papadimitriou, K.; Skandamis, P. Assessing the Survival and Sublethal Injury Kinetics of Listeria monocytogenes under Different Food Processing-Related Stresses. Int. J. Food Microbiol. 2021, 346, 109159. [Google Scholar] [CrossRef]
- Arvaniti, M.; Orologas-Stavrou, N.; Tsitsilonis, O.E.; Skandamis, P. Induction into Viable but Non Culturable State and Outgrowth Heterogeneity of Listeria monocytogenes Is Affected by Stress History and Type of Growth. Int. J. Food Microbiol. 2024, 421, 110786. [Google Scholar] [CrossRef]
- Arvaniti, M.; Balomenos, A.; Tsakanikas, P.; Skandamis, P. VBNC Induction and Persistence of Listeria monocytogenes Scott A as a Defence Mechanism against Free Chlorine Stress. Food Microbiol. 2025, 130, 104781. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.X. On the Criterion That a given System of Deviations from the Probable in the Case of a Correlated System of Variables Is Such That It Can Be Reasonably Supposed to Have Arisen from Random Sampling. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1900, 50, 157–175. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Monu, E.A.; Valladares, M.; D’souza, D.H.; Davidson, P.M. Determination of the Thermal Inactivation Kinetics of Listeria monocytogenes, Salmonella enterica, and Escherichia coli O157:H7 and Non-O157 in Buffer and a Spinach Homogenate. J. Food Prot. 2015, 78, 1467–1471. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D.; Niemira, B.A. Microbial Inactivation Models of Salmonella Typhimurium in Radio Frequency Treated Eggs. Food Control 2023, 148, 109634. [Google Scholar] [CrossRef]
- Noma, S.; Kajiyama, D.; Igura, N.; Shimoda, M.; Hayakawa, I. Mechanisms behind Tailing in the Pressure Inactivation Curve of a Clinical Isolate of Escherichia coli O157:H7. Int. J. Food Microbiol. 2006, 109, 103–108. [Google Scholar] [CrossRef]
- Metselaar, K.I.; den Besten, H.M.W.; Abee, T.; Moezelaar, R.; Zwietering, M.H. Isolation and Quantification of Highly Acid Resistant Variants of Listeria monocytogenes. Int. J. Food Microbiol. 2013, 166, 508–514. [Google Scholar] [CrossRef]
- Van Boeijen, I.K.H.; Moezelaar, R.; Abee, T.; Zwietering, M.H. Inactivation Kinetics of Three Listeria monocytogenes Strains under High Hydrostatic Pressure. J. Food Prot. 2008, 71, 2007–2013. [Google Scholar] [CrossRef]
- den Besten, H.M.W.; Mataragas, M.; Moezelaar, R.; Abee, T.; Zwietering, M.H. Quantification of the Effects of Salt Stress and Physiological State on Thermotolerance of Bacillus cereus ATCC 10987 and ATCC 14579. Appl. Environ. Microbiol. 2006, 72, 5884–5894. [Google Scholar] [CrossRef]
- Rajkovic, A.; Smigic, N.; Uyttendaele, M.; Medic, H.; de Zutter, L.; Devlieghere, F. Resistance of Listeria monocytogenes, Escherichia coli O157:H7 and Campylobacter jejuni after Exposure to Repetitive Cycles of Mild Bactericidal Treatments. Food Microbiol. 2009, 26, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Abee, T.; Koomen, J.; Metselaar, K.I.; Zwietering, M.H.; den Besten, H.M.W. Impact of Pathogen Population Heterogeneity and Stress-Resistant Variants on Food Safety. Annu. Rev. Food Sci. Technol. 2016, 7, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Van Boeijen, I.K.H.; Chavaroche, A.A.E.; Valderrama, W.B.; Moezelaar, R.; Zwietering, M.H.; Abee, T. Population Diversity of Listeria Monocytogenes LO28: Phenotypic and Genotypic Characterization of Variants Resistant to High Hydrostatic Pressure. Appl. Environ. Microbiol. 2010, 76, 2225–2233. [Google Scholar] [CrossRef]
- Aguirre, J.S.; Pin, C.; Rodríguez, M.R.; de Fernando, G.D.G. Analysis of the Variability in the Number of Viable Bacteria after Mild Heat Treatment of Food. Appl. Environ. Microbiol. 2009, 75, 6992–6997. [Google Scholar] [CrossRef]
- Cebrián, G.; Condón, S.; Mañas, P. Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef]
- Hurst, A. Revival of Vegetative Bacteria after Sublethal Heating. In Society for Applied Bacteriology Symposium Series; Blackwell: Oxford, UK, 1984; pp. 77–103. [Google Scholar]
- Wang, X.; Devlieghere, F.; Geeraerd, A.; Uyttendaele, M. Thermal Inactivation and Sublethal Injury Kinetics of Salmonella enterica and Listeria monocytogenes in Broth versus Agar Surface. Int. J. Food Microbiol. 2017, 243, 70–77. [Google Scholar] [CrossRef]
- Kell, D.B.; Kaprelyants, A.S.; Weichart, D.H.; Harwood, C.R.; Barer, M.R. Viability and Activity in Readily Culturable Bacteria: A Review and Discussion of the Practical Issues. Antonie Van Leeuwenhoek 1998, 73, 169–187. [Google Scholar] [CrossRef]
- McKellar, R.C.; Butler, G.; Stanich, K. Modelling the Influence of Temperature on the Recovery of Listeria monocytogenes from Heat Injury. Food Microbiol. 1997, 14, 617–625. [Google Scholar] [CrossRef]
- Ray, B. Impact of Bacterial Injury and Repair in Food Microbiology: Its Past, Present and Future. J. Food Prot. 1986, 49, 651–655. [Google Scholar] [CrossRef]
- Bhaumik, G.; Bhattacharjee, S.B. Repair of Heat Injury in Thymine Auxotrophs of Escherichia coli. Can. J. Microbiol. 1975, 21, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Bolten, S.; Mowery, J.; Luo, Y.; Gulbronson, C.; Nou, X. Susceptibility of Foodborne Pathogens to Sanitizers in Produce Rinse Water and Potential Induction of Viable but Non-Culturable State. Food Control 2020, 112, 107138. [Google Scholar] [CrossRef]
- Highmore, C.J.; Warner, J.C.; Rothwell, S.D.; Wilks, S.A.; Keevil, C.W.; Bailey, M.J. Viable-but-Nonculturable Listeria Monocytogenes and Salmonella Enterica Serovar Thompson Induced by Chlorine Stress Remain Infectious. mBio 2018, 9, e00540-18. [Google Scholar] [CrossRef]
- Noll, M.; Trunzer, K.; Vondran, A.; Vincze, S.; Dieckmann, R.; Al Dahouk, S.; Gold, C. Benzalkonium Chloride Induces a VBNC State in Listeria monocytogenes. Microorganisms 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D. Formation of Viable but Nonculturable Cells. In Starvation in Bacteria; Springer: Boston, MA, USA, 1993; ISBN 978-1-4899-2439-1. [Google Scholar]
- Besnard, V.; Federighi, M.; Declerq, E.; Jugiau, F.; Cappelier, J.-M. Environmental and Physico-Chemical Factors Induce VBNC State in Listeria monocytogenes. Vet. Res. 2002, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.W.; Ding, T. Current Perspectives on Viable but Non-Culturable State in Foodborne Pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Guillier, L.; Pardon, P.; Augustin, J.-C. Influence of Stress on Individual Lag Time Distributions of Listeria monocytogenes. Appl. Environ. Microbiol. 2005, 71, 2940–2948. [Google Scholar] [CrossRef]
- Métris, A.; George, S.M.; Mackey, B.M.; Baranyi, J. Modeling the Variability of Single-Cell Lag Times for Listeria innocua Populations after Sublethal and Lethal Heat Treatments. Appl. Environ. Microbiol. 2008, 74, 6949–6955. [Google Scholar] [CrossRef]
- Bannenberg, J.W.; Abee, T.; Zwietering, M.H.; den Besten, H.M.W. Variability in Lag Duration of Listeria monocytogenes Strains in Half Fraser Enrichment Broth after Stress Affects the Detection Efficacy Using the ISO 11290-1 Method. Int. J. Food Microbiol. 2021, 337, 108914. [Google Scholar] [CrossRef]


| Df | F Value | Pr (>F) | Sig. | |
|---|---|---|---|---|
| Time | 2 | 21.17 | <0.001 | *** |
| Temperature | 1 | 2.21 | 0.17 | |
| Time: Temperature | 2 | 1.22 | 0.34 | |
| Residuals | 9 |
| Temperature | Exposure Time (min) | FC CFDA+/PI− | FC CFDA−/PI+ | FC CFDA+/PI+ | FC CFDA−/PI− |
|---|---|---|---|---|---|
| 61 °C | 6 | 0.76 | 1.71 | 2.10 | 0.91 |
| 12 | 0.88 | 1.11 | 2.04 | 1.22 | |
| 18 | 0.71 | 1.86 | 3.22 | 0.64 | |
| 60 | 0.70 | 2.08 | 2.42 | 0.58 | |
| 64 °C | 2 | 0.53 | 1.41 | 9.37 | 0.64 |
| 4 | 0.08 | 0.24 | 20.67 | 2.03 | |
| 6 | 0.05 | 0.58 | 19.82 | 1.90 |
| Category | Time | Odds Ratio | Lower 95% CI | Upper 95% CI | p-Value | Signif. |
|---|---|---|---|---|---|---|
| CFDA+/PI− | 0 | 5.88 | 4.57 | 7.56 | <2 × 10−16 | *** |
| CFDA+/PI− | 6 | 0.97 | 0.66 | 1.42 | 0.852103 | |
| CFDA+/PI− | 12 | 0.85 | 0.59 | 1.23 | 0.398458 | |
| CFDA+/PI− | 18 | 1.30 | 0.90 | 1.87 | 0.164295 | |
| CFDA+/PI− | 60 | 1.45 | 0.97 | 2.18 | 0.070375 | |
| CFDA−/PI+ | 0 | 1.68 | 1.24 | 2.28 | 0.000746 | *** |
| CFDA−/PI+ | 6 | 2.26 | 1.50 | 3.40 | 9.82 × 10−5 | *** |
| CFDA−/PI+ | 12 | 1.06 | 0.69 | 1.63 | 0.804948 | |
| CFDA−/PI+ | 18 | 3.26 | 2.18 | 4.87 | 9.24 × 10−9 | *** |
| CFDA−/PI+ | 60 | 3.86 | 2.50 | 5.94 | 1.34 × 10−9 | *** |
| CFDA+/PI+ | 0 | 0.29 | 0.18 | 0.47 | 1.50 × 10−6 | *** |
| CFDA+/PI+ | 6 | 2.69 | 1.44 | 5.02 | 0.001892 | ** |
| CFDA+/PI+ | 12 | 1.78 | 0.93 | 3.39 | 0.081872 | |
| CFDA+/PI+ | 18 | 5.56 | 3.08 | 10.04 | 1.14 × 10−8 | *** |
| CFDA+/PI+ | 60 | 4.79 | 2.57 | 8.94 | 1.12 × 10−6 | *** |
| Category | Time | OR | Lower 95% CI | Upper 95% CI | p-Value | Signif. |
|---|---|---|---|---|---|---|
| CFDA+/PI− | 0 | 1.86 | 1.47 | 2.37 | 4.03 × 10−7 | *** |
| CFDA+/PI− | 2 | 3.01 | 2.02 | 4.49 | 5.99 × 10−8 | *** |
| CFDA+/PI− | 4 | 0.17 | 0.11 | 0.26 | 8.46 × 10−15 | *** |
| CFDA+/PI− | 6 | 0.11 | 0.06 | 0.19 | 2.45 × 10−14 | *** |
| CFDA−/PI+ | 0 | 1.17 | 0.90 | 1.52 | 0.253 | |
| CFDA−/PI+ | 2 | 3.73 | 2.47 | 5.65 | 6.68 × 10−10 | *** |
| CFDA−/PI+ | 4 | 0.16 | 0.10 | 0.28 | 3.76 × 10−11 | *** |
| CFDA−/PI+ | 6 | 0.43 | 0.28 | 0.68 | 0.000293 | *** |
| CFDA+/PI+ | 0 | 2.48 | 2.01 | 3.06 | 9.54 × 10−15 | *** |
| CFDA+/PI+ | 2 | 1.54 | 1.03 | 2.30 | 0.0344 | * |
| CFDA+/PI+ | 4 | 1.36 | 1.00 | 1.86 | 0.0545 | |
| CFDA+/PI+ | 6 | 1.32 | 0.95 | 1.85 | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvaniti, M.; Vlachou, E.; Kourteli, M.; Kapetanakou, A.E.; Skandamis, P.N. Monitoring Sublethal Injury in Listeria monocytogenes During Heat Treatment of Pork Frankfurter-Type Sausages: A Single-Cell vs. Population Level Approach. Foods 2025, 14, 3144. https://doi.org/10.3390/foods14173144
Arvaniti M, Vlachou E, Kourteli M, Kapetanakou AE, Skandamis PN. Monitoring Sublethal Injury in Listeria monocytogenes During Heat Treatment of Pork Frankfurter-Type Sausages: A Single-Cell vs. Population Level Approach. Foods. 2025; 14(17):3144. https://doi.org/10.3390/foods14173144
Chicago/Turabian StyleArvaniti, Marianna, Eleni Vlachou, Maria Kourteli, Anastasia E. Kapetanakou, and Panagiotis N. Skandamis. 2025. "Monitoring Sublethal Injury in Listeria monocytogenes During Heat Treatment of Pork Frankfurter-Type Sausages: A Single-Cell vs. Population Level Approach" Foods 14, no. 17: 3144. https://doi.org/10.3390/foods14173144
APA StyleArvaniti, M., Vlachou, E., Kourteli, M., Kapetanakou, A. E., & Skandamis, P. N. (2025). Monitoring Sublethal Injury in Listeria monocytogenes During Heat Treatment of Pork Frankfurter-Type Sausages: A Single-Cell vs. Population Level Approach. Foods, 14(17), 3144. https://doi.org/10.3390/foods14173144






