Design and Evaluation of a Cinnamomum burmannii Essential Oil-Loaded Preservative Film for Enhancing the Quality and Shelf Life of Squaliobarbus curriculus Filets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Instrument
2.3. Fabrication of Active Preservative Films
2.3.1. Preparation of Essential Oil Nanoemulsion
2.3.2. Preparation of Preservative Films
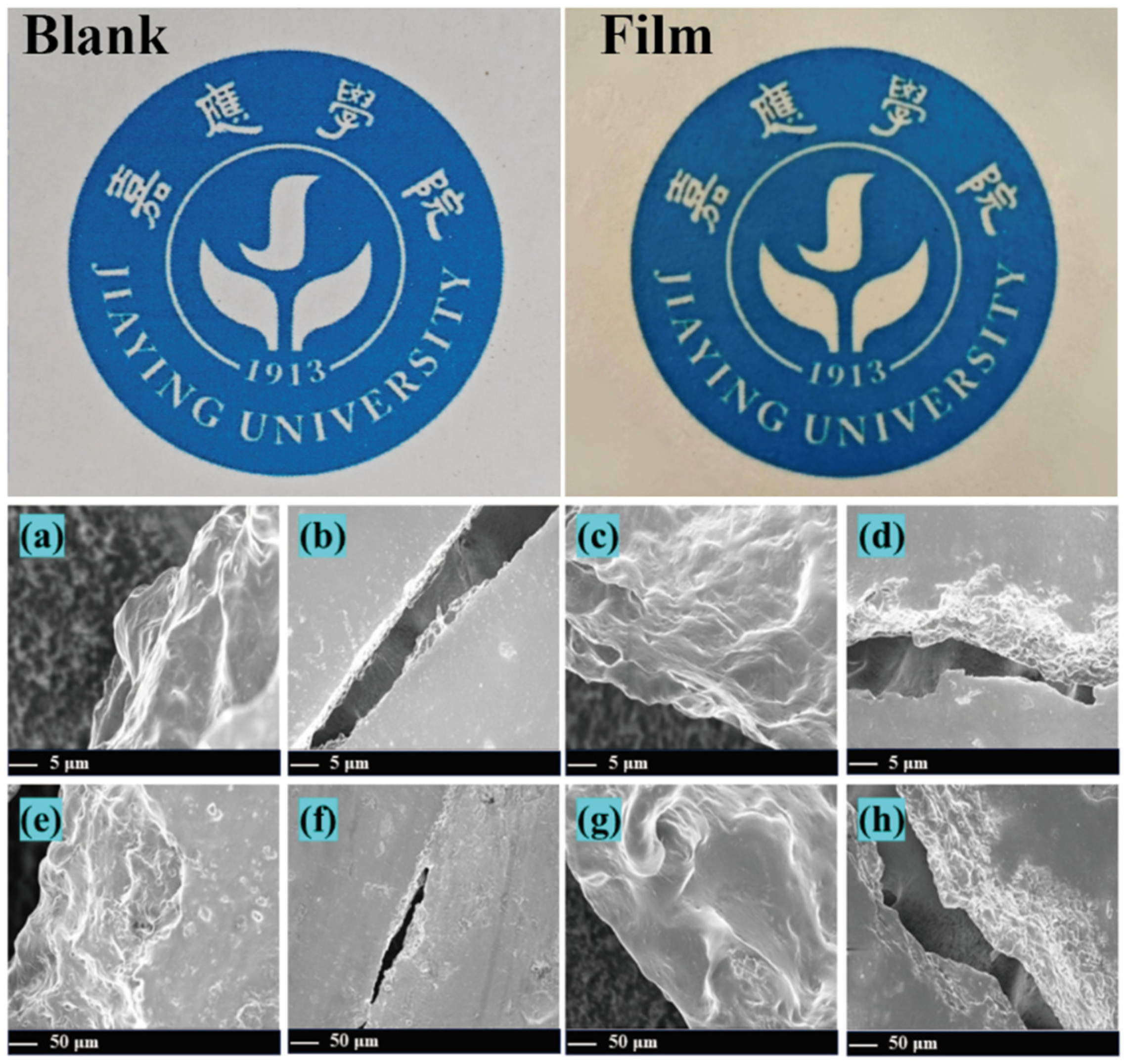
2.4. Scanning Electron Microscopy Observation of Preservative Films
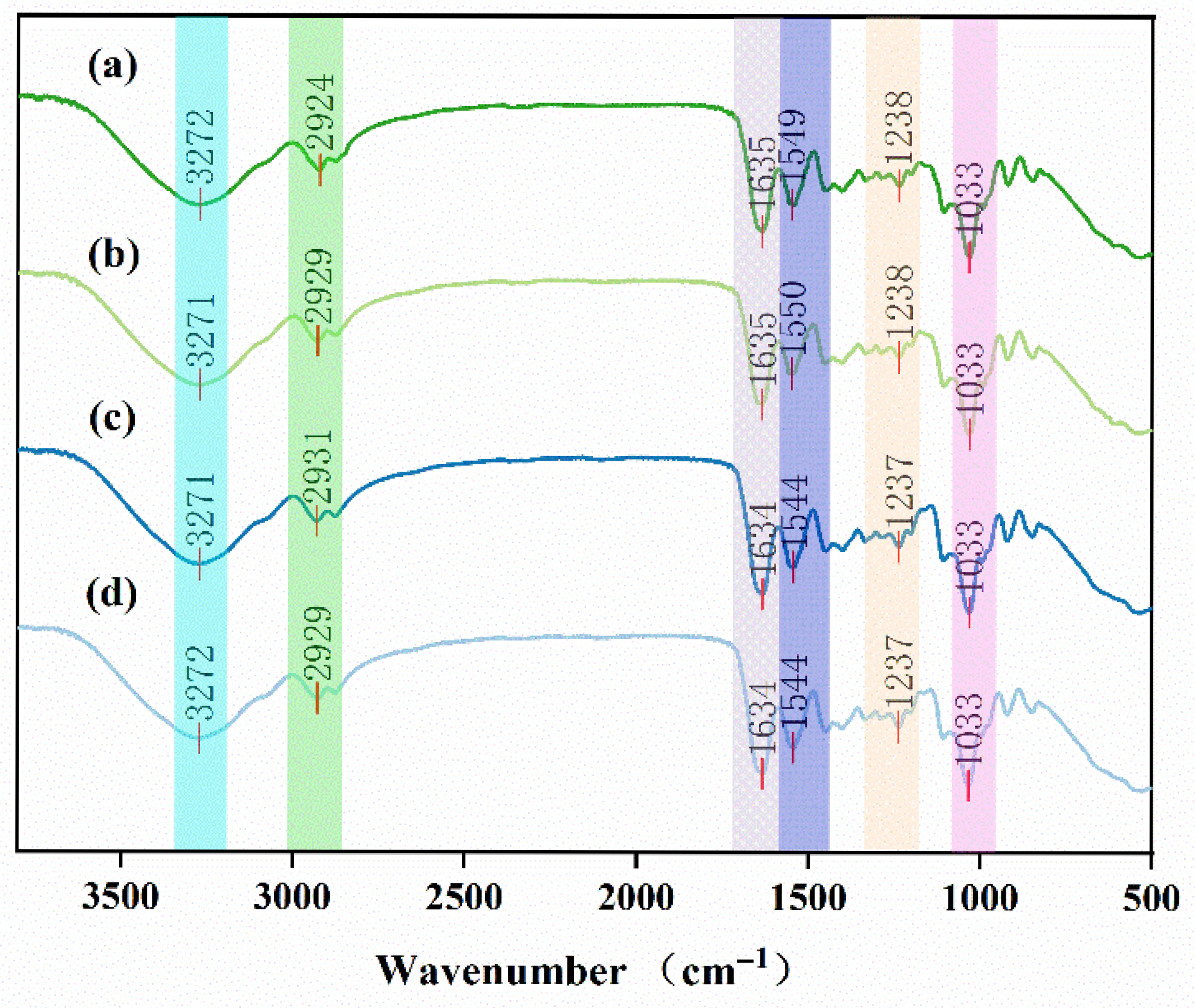
2.5. Fourier Transform Infrared Spectroscopy Observations of Preservative Films
2.6. Measurement of the Light Transmittance of Preservative Films via a UV-Vis Spectrophotometer
2.7. Analysis of Volatile Odor Compounds via Electronic Nose
2.8. Detection of Volatile Compounds in Samples by Gas Chromatography–Mass Spectrometry
2.9. Physical Properties of the Preservative Film
2.10. Inhibitory Activity Experiments of Escherichia coli and Staphylococcus aureus
2.11. Changes in the Physicochemical Quality and Color Attributes of Squaliobarbus curriculus Filets Treated with Preservative Film and Microbiological Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Microscopic Morphology of the Preservative Films
3.2. Chemical Structure Analysis of Preservative Films via Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Physical Properties of the Preservative Films
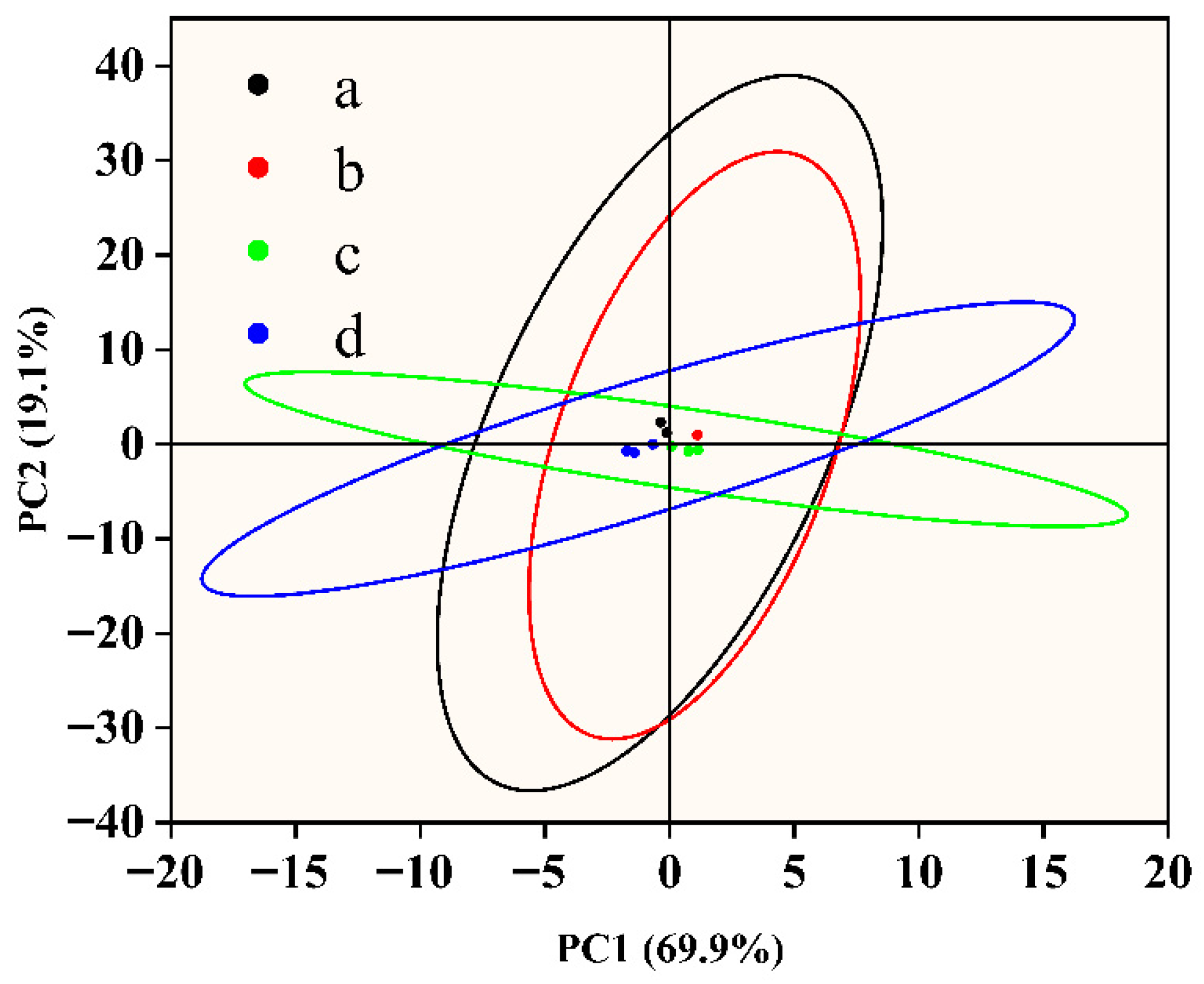
3.4. The Electronic Nose Determines the Odor of a Sample
3.4.1. Odor Characteristics of Fish Flesh
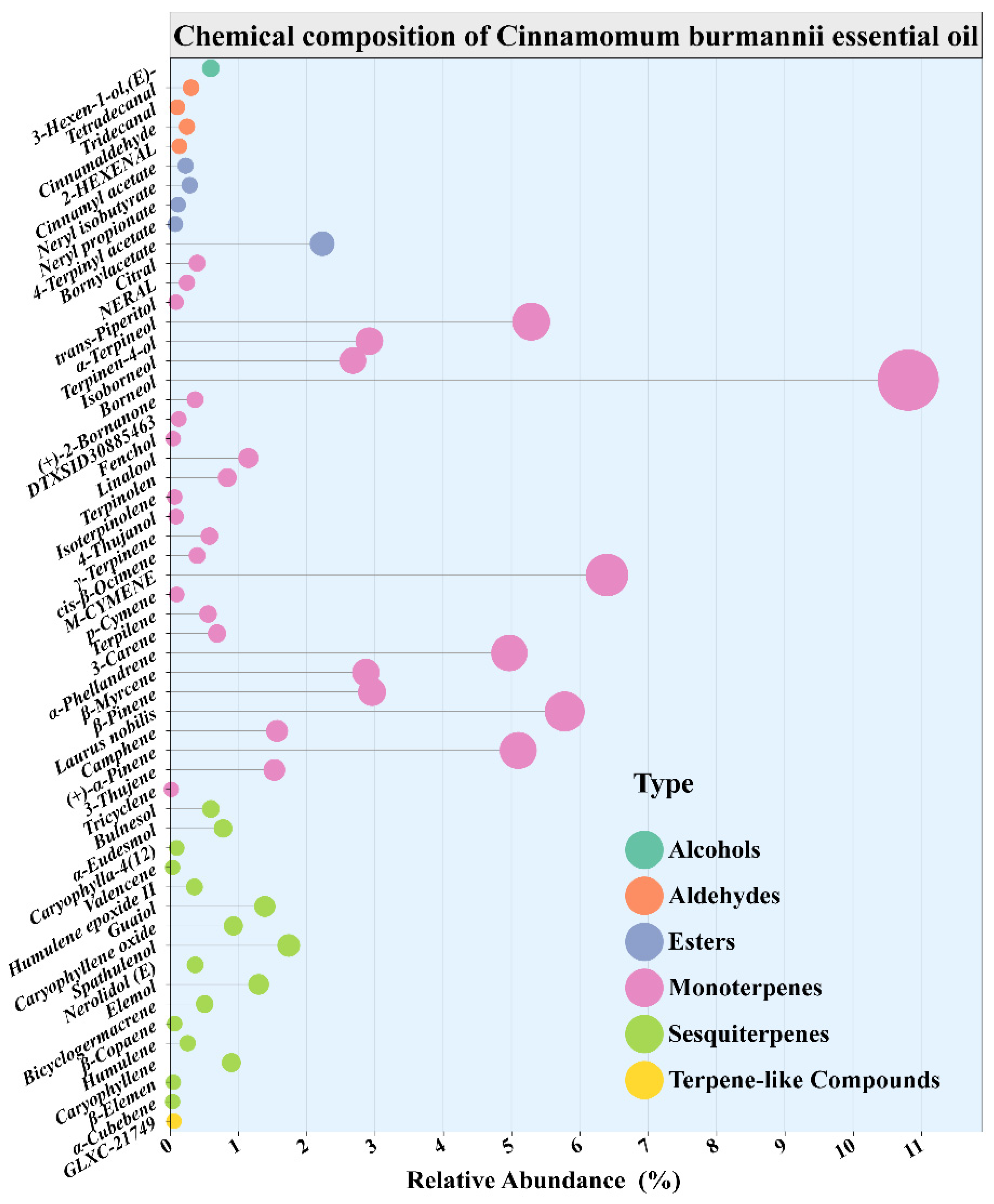
3.4.2. Detection of Essential Oil Compounds by Gas Chromatography-Mass Spectrometry
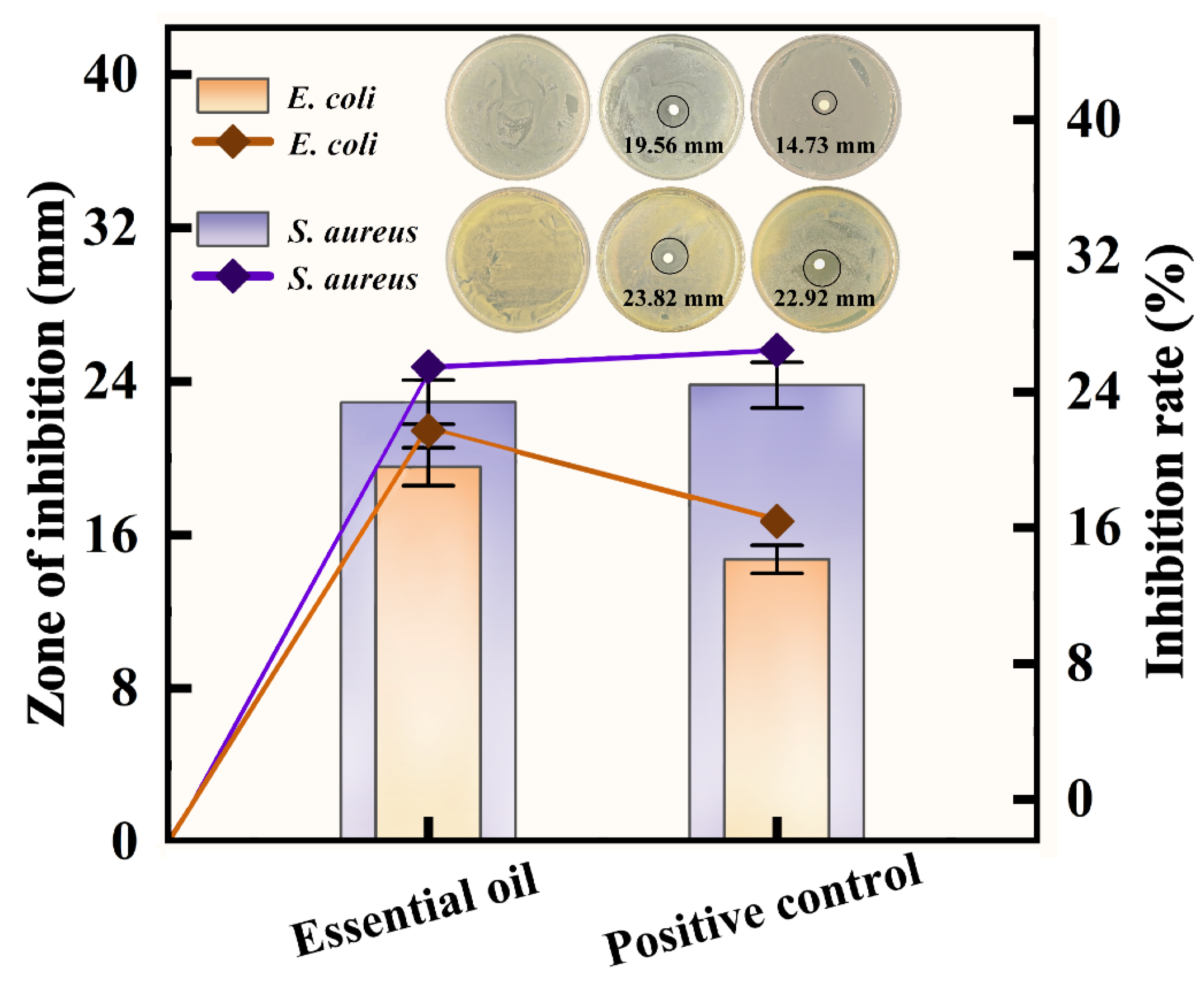
3.5. Inhibitory Activity of the Essential Oil Against Escherichia coli and Staphylococcus aureus
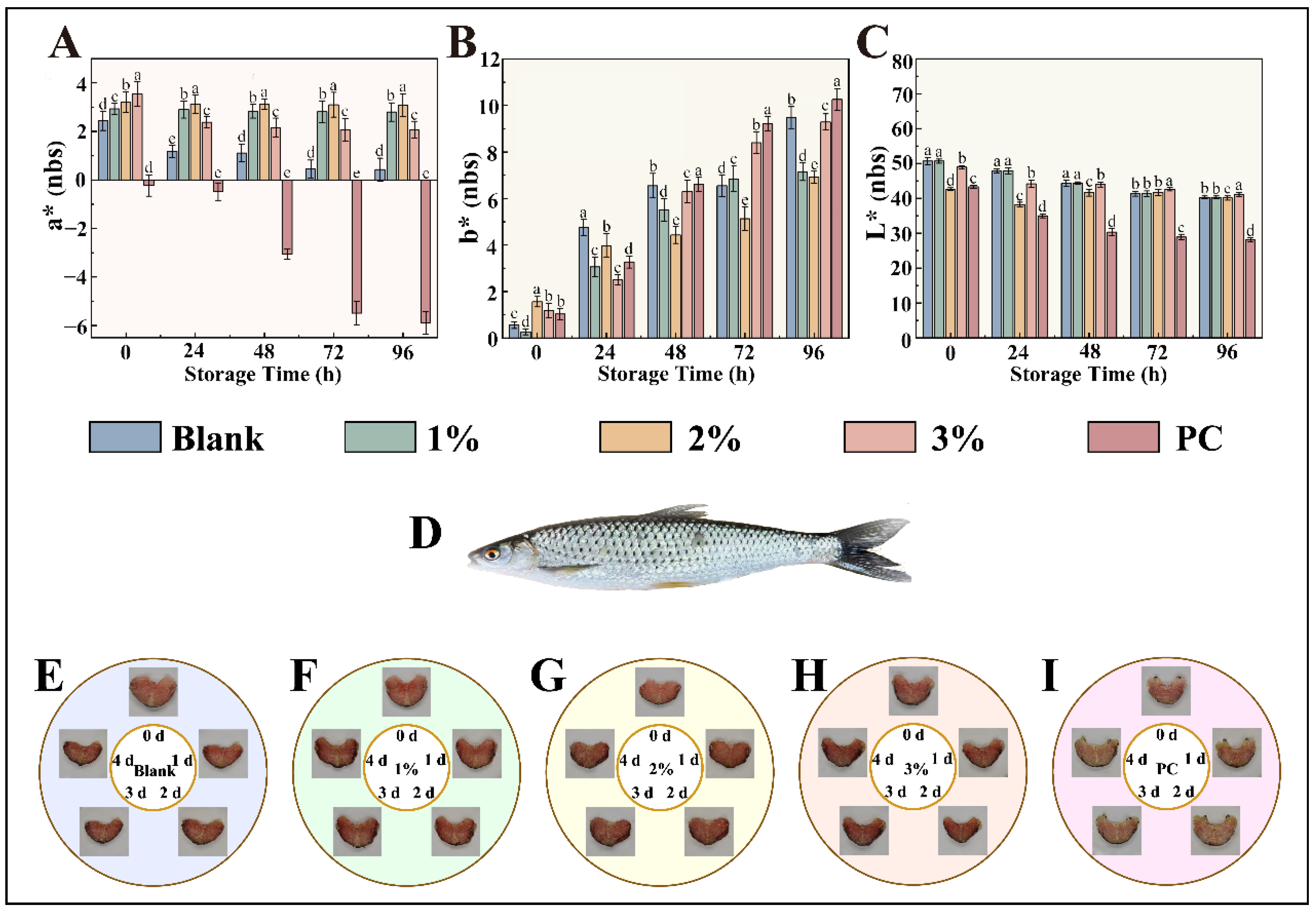
3.6. Preservation Efficacy of Essential Oil Preservative Films on Fish Filets and Their Color Changes During Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, M.; Mei, J.; Xie, J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021, 11, 68. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, F.; Zheng, H.; Zhang, H.; Wen, R.; Li, C. Chromosome-Level Genome Assembly and Annotation of Barbel Chub Squaliobarbus curriculus. Sci. Data 2024, 11, 1453. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Gao, J.; Guo, H.-Y.; Zhu, K.-C.; Liu, B.-S.; Zhang, N.; Zhu, T.-F.; Zhang, D.-C. Influence of Aquaculture Environments on the Muscle Quality of Golden Pompano (Trachinotus ovatus) in the Beibu Gulf: A Multifaceted Analysis of Nutritional, Textural, and Flavor Profiles. LWT 2024, 212, 116957. [Google Scholar] [CrossRef]
- Daskalova, A. Farmed Fish Welfare: Stress, Post-Mortem Muscle Metabolism, and Stress-Related Meat Quality Changes. Int. Aquat. Res. 2019, 11, 113–124. [Google Scholar] [CrossRef]
- Erikson, U.; Uglem, S.; Greiff, K. Freeze-Chilling of Whitefish: Effects of Capture, On-Board Processing, Freezing, Frozen Storage, Thawing, and Subsequent Chilled Storage—A Review. Foods 2021, 10, 2661. [Google Scholar] [CrossRef]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Sun, D.-W. Effects of Freezing on Cell Structure of Fresh Cellular Food Materials: A Review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, X.; Zhang, Z.; Long, G.; Lyu, F.; Cai, Y.; Liu, J.; Ding, Y. Effects of Immersion Freezing on Ice Crystal Formation and the Protein Properties of Snakehead (Channa argus). Foods 2020, 9, 411. [Google Scholar] [CrossRef]
- Mahato, S.; Zhu, Z.; Sun, D.-W. Glass Transitions as Affected by Food Compositions and by Conventional and Novel Freezing Technologies: A Review. Trends Food Sci. Technol. 2019, 94, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Miyazaki, R.; Saitou, S.; Hirasaka, K.; Takeshita, S.; Tachibana, K.; Taniyama, S. The Effect of Ice Crystals Formations on the Flesh Quality of Frozen Horse Mackerel (Trachurus japonicus). J. Texture Stud. 2018, 49, 485–491. [Google Scholar] [CrossRef]
- Guo, C.; Guo, H. Progress in the Degradability of Biodegradable Film Materials for Packaging. Membranes 2022, 12, 500. [Google Scholar] [CrossRef]
- Huang, C.; Liao, Y.; Zou, Z.; Chen, Y.; Jin, M.; Zhu, J.; Abdalkarim, S.Y.H.; Zhou, Y.; Yu, H.-Y. Novel Strategy to Interpret the Degradation Behaviors and Mechanisms of Bio- and Non-Degradable Plastics. J. Clean. Prod. 2022, 355, 131757. [Google Scholar] [CrossRef]
- Raddadi, N.; Fava, F. Biodegradation of Oil-Based Plastics in the Environment: Existing Knowledge and Needs of Research and Innovation. Sci. Total Environ. 2019, 679, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Doğan, G.; İzci, L. Effects on Quality Properties of Smoked Rainbow Trout (Oncorhynchus mykiss) Fillets of Chitosan Films Enriched with Essential Oils. J. Food Process. Preserv. 2017, 41, e12757. [Google Scholar] [CrossRef]
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An Overview of Biodegradable Packaging in Food Industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef]
- Yan, P.; Lan, W.; Xie, J. Characterizations of Two Physically Modified Sodium Alginate Composite Films Doped with Gallic Acid and Their Preservation of Refrigerated Sea Bass (Lateolabrax maculatus). Food Chem. 2025, 474, 143147. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. A Novel Method of Preparing Stable Zein Nanoparticle Dispersions for Encapsulation of Peppermint Oil. Food Hydrocoll. 2015, 43, 593–602. [Google Scholar] [CrossRef]
- Lisitsyn, A.; Semenova, A.; Nasonova, V.; Polishchuk, E.; Revutskaya, N.; Kozyrev, I.; Kotenkova, E. Approaches in Animal Proteins and Natural Polysaccharides Application for Food Packaging: Edible Film Production and Quality Estimation. Polymers 2021, 13, 1592. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; Nashy, E.-S.H.A.; Othman, A.M. Novel Natural Composite Films as Packaging Materials with Enhanced Properties. Int. J. Biol. Macromol. 2019, 136, 774–784. [Google Scholar] [CrossRef]
- Stoleru, E.; Brebu, M. Stabilization Techniques of Essential Oils by Incorporation into Biodegradable Polymeric Materials for Food Packaging. Molecules 2021, 26, 6307. [Google Scholar] [CrossRef] [PubMed]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer Nano-Particles and Natural Nano-Carriers for Nano-Encapsulation of Phenolic Compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]
- Kuspradini, H.; Putri, A.S.; Sukaton, E.; Mitsunaga, T. Bioactivity of Essential Oils from Leaves of Dryobalanops Lanceolata, Cinnamomum Burmannii, Cananga Odorata, and Scorodocarpus Borneensis. Agric. Agric. Sci. Procedia 2016, 9, 411–418. [Google Scholar] [CrossRef]
- Ervina, M.; Lie, H.S.; Diva, J.; Caroline; Tewfik, S.; Tewfik, I. Optimization of Water Extract of Cinnamomum Burmannii Bark to Ascertain Its in Vitro Antidiabetic and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2019, 19, 101152. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Fang, Z.; Liu, Y. Preparation and Characterization of Blended Cloves/Cinnamon Essential Oil Nanoemulsions. LWT 2017, 75, 316–322. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical Properties and Antioxidant Activity of Gelatin-Sodium Alginate Edible Films with Tea Polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Jeyaratnam, N.; Nour, A.H.; Kanthasamy, R.; Nour, A.H.; Yuvaraj, A.R.; Akindoyo, J.O. Essential Oil from Cinnamomum Cassia Bark through Hydrodistillation and Advanced Microwave Assisted Hydrodistillation. Ind. Crops Prod. 2016, 92, 57–66. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, X.; Chen, M.; Wang, J.; Qiu, D.; Liu, Z.; Huang, Y.; Zhang, L.; Liu, Z. An Environmentally Friendly Production Method: The Pectin and Essential Oil from the Waste Peel of Juvenile Pomelo (Citrus maxima ‘Shatian Yu’) Were Extracted Simultaneously in One Step with an Acid-Based Deep Eutectic Solvent. LWT 2024, 206, 116622. [Google Scholar] [CrossRef]
- Bao, M.; Mu, H.; Niu, B.; Liu, R.; Chen, H.; Chen, H.; Wang, L.; Han, Y.; Wang, G.; Gao, H. High UV-Shielding Polyvinyl Alcohol/Chitosan-Based Transparent Bioplastic Film for Food Preservation. Food Packag. Shelf Life 2025, 47, 101421. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, M.; Ruiz-Montoya, M.; Giraldez, I.; López, R.; Madejón, E.; Díaz, M.J. Use of Electronic Nose and GC-MS in Detection and Monitoring Some VOC. Atmos. Environ. 2012, 51, 278–285. [Google Scholar] [CrossRef]
- Pennazza, G.; Fanali, C.; Santonico, M.; Dugo, L.; Cucchiarini, L.; Dachà, M.; D’Amico, A.; Costa, R.; Dugo, P.; Mondello, L. Electronic Nose and GC–MS Analysis of Volatile Compounds in Tuber Magnatum Pico: Evaluation of Different Storage Conditions. Food Chem. 2013, 136, 668–674. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, B.; Zhang, X.; Ma, Z.; Feng, X. Incorporating Portulaca Oleracea Extract Endows the Chitosan-Starch Film with Antioxidant Capacity for Chilled Meat Preservation. Food Chem. X 2023, 18, 100662. [Google Scholar] [CrossRef]
- Kuttithodi, A.M.; Narayanankutty, A.; Visakh, N.U.; Job, J.T.; Pathrose, B.; Olatunji, O.J.; Alfarhan, A.; Ramesh, V. Chemical Composition of the Cinnamomum Malabatrum Leaf Essential Oil and Analysis of Its Antioxidant, Enzyme Inhibitory and Antibacterial Activities. Antibiotics 2023, 12, 940. [Google Scholar] [CrossRef]
- Kaewthong, P.; Waiyagan, K.; Wattanachant, S. Imaging Analysis by Digital Camera for Separating Broiler Breast Meat with Low Water-Holding Capacity. J. Poult. Sci. 2017, 54, 253–261. [Google Scholar] [CrossRef]
- Baixas-Nogueras, S.; Bover-Cid, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Effect of Gutting on Microbial Loads, Sensory Properties, and Volatile and Biogenic Amine Contents of European Hake (Merluccius merluccius Var. mediterraneus) Stored in Ice. J. Food Prot. 2009, 72, 1671–1676. [Google Scholar] [CrossRef]
- Spanget-Larsen, J.; Hansen, B.K.V.; Hansen, P.E. OH Stretching Frequencies in Systems with Intramolecular Hydrogen Bonds: Harmonic and Anharmonic Analyses. Chem. Phys. 2011, 389, 107–115. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, A.; Zheng, Y.; Zhu, X.; Hu, Y.; Qu, X. Scented Solutions: Harnessing Lavender Essential Oil Liposomes for Enhanced Plywood Performance. Sustain. Chem. Pharm. 2024, 42, 101826. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Petrisor, G.; Oprea, O.-C.; Trușcǎ, R.-D.; Ficai, A.; Andronescu, E.; Hudita, A.; Holban, A.M. Antimicrobial Hydroxyethyl-Cellulose-Based Composite Films with Zinc Oxide and Mesoporous Silica Loaded with Cinnamon Essential Oil. Pharmaceutics 2024, 16, 1225. [Google Scholar] [CrossRef] [PubMed]
- Munhuweyi, K.; Caleb, O.J.; Lennox, C.L.; van Reenen, A.J.; Opara, U.L. In Vitro and in Vivo Antifungal Activity of Chitosan-Essential Oils against Pomegranate Fruit Pathogens. Postharvest Biol. Technol. 2017, 129, 9–22. [Google Scholar] [CrossRef]
- Liang, Q.; Cao, P.; Lu, H.; Du, Y.; Kang, L.; Ma, H.; Ren, X. Ultrasonic Engineering of Zein-Curcumin Nanoparticles/Sodium Alginate Composite Films for Active Food Packaging Applications. Int. J. Biol. Macromol. 2025, 315, 144268. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.; Li, Y.; Wu, X.; Wang, Y.; Xia, X. Effect of Clove Essential Oil Nanoemulsion on Physicochemical and Antioxidant Properties of Chitosan Film. Int. J. Biol. Macromol. 2024, 263, 130286. [Google Scholar] [CrossRef]
- Rodriguez-Caturla, M.Y.; Margalho, L.P.; Graça, J.S.; Pia, A.K.R.; Xavier, V.L.; Noronha, M.F.; Cabral, L.; Lemos-Junior, W.J.F.; Castillo, C.J.C.; Sant’Ana, A.S. Bacterial Dynamics and Volatile Metabolome Changes of Vacuum-Packaged Beef with Different PH during Chilled Storage. Int. J. Food Microbiol. 2025, 427, 110955. [Google Scholar] [CrossRef]
- Velázquez-Contreras, F.; Zamora-Ledezma, C.; López-González, I.; Meseguer-Olmo, L.; Núñez-Delicado, E.; Gabaldón, J.A. Cyclodextrins in Polymer-Based Active Food Packaging: A Fresh Look at Nontoxic, Biodegradable, and Sustainable Technology Trends. Polymers 2022, 14, 104. [Google Scholar] [CrossRef]
- Martin, D.; Joly, C.; Dupas-Farrugia, C.; Adt, I.; Oulahal, N.; Degraeve, P. Volatilome Analysis and Evolution in the Headspace of Packed Refrigerated Fish. Foods 2023, 12, 2657. [Google Scholar] [CrossRef]
- Qu, Y.; Yun, J.; Li, Y.; Ai, D.; Zhang, W. Microbial Succession and Its Correlation with the Dynamics of Flavor Compounds Involved in the Fermentation of Longxi Bacon. Front. Microbiol. 2023, 14, 1234797. [Google Scholar] [CrossRef]
- Skočibušić, M.; Bezić, N. Phytochemical Analysis and in Vitro Antimicrobial Activity of Two Satureja Species Essential Oils. Phyther. Res. 2004, 18, 967–970. [Google Scholar] [CrossRef]
- Budiastuti; Andini, Y.W.; Cahyasarl, I.A.; Primaharinastiti, R.; Sukardiman. Standardization Bark of Cinnamomum Burmannii Nees Ex Bl. from Five Areas of Indonesia. Pharmacogn. J. 2020, 12, 578–588. [Google Scholar] [CrossRef]
- Chairunnisa; Tamhid, H.A.; Nugraha, A.T. Gas Chromatography—Mass Spectrometry Analysis and Antibacterial Activity of Cinnamomum Burmanii Essential Oil to Staphylococcus Aureus and Escherichia Coli by Gaseous Contact. AIP Conf. Proc. 2017, 1823, 020073. [Google Scholar] [CrossRef]
- Chen, X.; Liu, P.; Wang, J.; He, X.; Wang, J.; Chen, H.; Wang, G. TMT-Based Quantitative Proteomics Revealed the Antibacterial Mechanism of Cinnamaldehyde against MRSA. J. Proteome Res. 2024, 23, 4637–4647. [Google Scholar] [CrossRef]
- Rachmawati, A.; Thohari, I.; Al Awwaly, K.U.; Apriliyani, M.W. Antimicrobial Activity of Edible Film with Cinnamon Essential Oil as Antimicrobial Packaging. J. Ilmu dan Teknol. Has. Ternak 2023, 18, 120–129. [Google Scholar] [CrossRef]
- Maniki, E.; Kostoglou, D.; Paterakis, N.; Nikolaou, A.; Kourkoutas, Y.; Papachristoforou, A.; Giaouris, E. Chemical Composition, Antioxidant, and Antibiofilm Properties of Essential Oil from Thymus capitatus Plants Organically Cultured on the Greek Island of Lemnos. Molecules 2023, 28, 1154. [Google Scholar] [CrossRef] [PubMed]
- Thiansilakul, Y.; Benjakul, S.; Richards, M.P. The Effect of Different Atmospheric Conditions on the Changes in Myoglobin and Colour of Refrigerated Eastern Little Tuna (Euthynnus affinis) Muscle. J. Sci. Food Agric. 2011, 91, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Erdağ, M.; Ayvaz, Z. The Use of Color to Determine Fish Freshness: European Seabass (Dicentrarchus labrax). J. Aquat. Food Prod. Technol. 2021, 30, 847–867. [Google Scholar] [CrossRef]
- Karamucki, T.; Jakubowska, M.; Rybarczyk, A.; Gardzielewska, J. The Influence of Myoglobin on the Colour of Minced Pork Loin. Meat Sci. 2013, 94, 234–238. [Google Scholar] [CrossRef]
- Zhong, H.; Wei, S.; Kang, M.; Sun, Q.; Xia, Q.; Wang, Z.; Han, Z.; Liu, Y.; Liu, M.; Liu, S. Effects of Different Storage Conditions on Microbial Community and Quality Changes of Greater Amberjack (Seriola dumerili) Fillets. LWT 2023, 179, 114640. [Google Scholar] [CrossRef]
| Sample | Film Thickness (mm) | Mean Tensile Strength (MPa) | Elongation at Break (%) | Light Transmittance (%) |
|---|---|---|---|---|
| Blank film | 0.17 ± 0.01 | 6.66 ± 0.06 | 0.66 ± 0.01 | 32.28 ± 0.08 |
| Film (1% EO) | 0.20 ± 0.01 | 9.73 ± 0.03 | 1.43 ± 0.03 | 33.91 ± 0.11 |
| Film (2% EO) | 0.22 ± 0.01 | 9.15 ± 0.03 | 1.53 ± 0.03 | 34.59 ± 0.09 |
| Film (3% EO) | 0.23 ± 0.01 | 9.50 ± 0.05 | 1.37 ± 0.02 | 35.97 ± 0.07 |
| No. | Compounds A | Chemical Structure | RI B | CAS | RA (%) C |
|---|---|---|---|---|---|
| 1 | 2-Hexenal,(E)- | C6H10O | 850 | 006728-26-3 | 0.14 |
| 2 | 3-Hexen-1-ol,(E)- | C6H12O | 853 | 000928-97-2 | 0.60 |
| 3 | Tricyclo [2.2.1.0(2,6)]heptane,1,7,7-trimethyl- | C10H16 | 928 | 000508-32-7 | 0.02 |
| 4 | Bicyclo [3.1.0]hex-2-ene,2-methyl-5-(1-methylethyl)- | C10H16 | 941 | 002867-05-2 | 1.53 |
| 5 | (1R)-2,6,6-Trimethylbicyclo [3.1.1]hept-2-ene | C10H16 | 1010 | 007785-70-8 | 5.10 |
| 6 | Camphene | C10H16 | 1079 | 000079-92-5 | 1.57 |
| 7 | Bicyclo [3.1.0]hexane,4-methylene-1-(1-methylethyl)- | C10H16 | 1109 | 003387-41-5 | 5.78 |
| 8 | β-Pinene | C10H16 | 1119 | 000127-91-3 | 2.96 |
| 9 | β-Myrcene | C10H16 | 1148 | 000123-35-3 | 2.87 |
| 10 | α-Phellandrene | C10H16 | 1155 | 000099-83-2 | 4.97 |
| 11 | 3-Carene | C10H16 | 1156 | 013466-78-9 | 0.69 |
| 12 | 1,3-Cyclohexadiene,1-methyl-4-(1-methylethyl)- | C10H16 | 1166 | 000099-86-5 | 0.56 |
| 13 | p-Cymene | C10H14 | 1241 | 000099-87-6 | 0.10 |
| 14 | Benzene,1-methyl-3-(1-methylethyl)- | C10H14 | 1244 | 000535-77-3 | 6.40 |
| 15 | 1,3,6-Octatriene,3,7-dimethyl-,(Z)- | C10H16 | 1245 | 003338-55-4 | 0.40 |
| 16 | γ-Terpinene | C10H16 | 1248 | 000099-85-4 | 0.58 |
| 17 | Bicyclo [3.1.0]hexan-2-ol,2-methyl-5-(1-methylethyl)-,(1α,2α,5α)- | C10H18O | 1444 | 017699-16-0 | 0.09 |
| 18 | Cyclohexene,3-methyl-6-(1-methylethylidene)- | C10H16 | 1447 | 000586-63-0 | 0.07 |
| 19 | Cyclohexene,1-methyl-4-(1-methylethylidene)- | C10H16 | 1449 | 000586-62-9 | 0.84 |
| 20 | Linalool | C10H18O | 1498 | 000078-70-6 | 1.15 |
| 21 | (E)-4,8-Dimethylnona-1,3,7-triene | C11H18 | 1499 | 019945-61-0 | 0.06 |
| 22 | Fenchol | C10H18O | 1548 | 001632-73-1 | 0.05 |
| 23 | 2-Cyclohexen-1-ol,1-methyl-4-(1-methylethyl)-,cis- | C10H18O | 1593 | 029803-82-5 | 0.13 |
| 24 | (+)-2-Bornanone | C10H16O | 1598 | 000464-49-3 | 0.37 |
| 25 | Borneol | C10H18O | 1653 | 000507-70-0 | 10.81 |
| 26 | Isoborneol | C10H18O | 1659 | 000124-76-5 | 2.68 |
| 27 | Terpinen-4-ol | C10H18O | 1663 | 000562-74-3 | 2.92 |
| 28 | α-Terpineol | C10H18O | 1668 | 000098-55-5 | 5.29 |
| 29 | 2-Cyclohexen-1-ol,3-methyl-6-(1-methylethyl)-,trans- | C10H18O | 1675 | 016721-39-4 | 0.09 |
| 30 | 2,6-Octadienal,3,7-dimethyl-,(Z)- | C10H16O | 1676 | 000106-26-3 | 0.25 |
| 31 | 2,6-Octadienal,3,7-dimethyl-,(E)- | C10H16O | 1718 | 000141-27-5 | 0.40 |
| 32 | Cinnamaldehyde,(E)- | C9H8O | 1725 | 014371-10-9 | 0.25 |
| 33 | Bornylacetate | C12H20O2 | 1733 | 000076-49-3 | 2.23 |
| 34 | 4-Terpinenylacetate | C12H20O2 | 1738 | 004821-04-9 | 0.08 |
| 35 | 2,6-Octadien-1-ol,3,7-dimethyl-,propanoate,(Z)- | C13H22O2 | 1758 | 000105-91-9 | 0.12 |
| 36 | α-Cubebene | C15H24 | 1762 | 017699-14-8 | 0.04 |
| 37 | Propanoicacid,2-methyl-,3,7-dimethyl-2,6-octadienylester,(Z)- | C14H24O2 | 1766 | 002345-24-6 | 0.29 |
| 38 | Cyclohexane,1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-,[1S-(1α,2β,4β)]- | C15H24 | 1769 | 000515-13-9 | 0.05 |
| 39 | Tridecanal | C13H26O | 1782 | 010486-19-8 | 0.11 |
| 40 | Caryophyllene | C15H24 | 1788 | 000087-44-5 | 0.90 |
| 41 | Aceticacid, cinnamylester | C11H12O2 | 1792 | 000103-54-8 | 0.23 |
| 42 | Humulene | C15H24 | 1804 | 006753-98-6 | 0.26 |
| 43 | β-Copaene | C15H24 | 1815 | 018252-44-3 | 0.07 |
| 44 | Bicyclogermacrene | C15H24 | 1821 | 067650-90-2 | 0.51 |
| 45 | Cyclohexanemethanol,4-ethenyl-α,α,4-trimethyl-3-(1-methylethenyl)-,[1R-(1α,3α,4β)]- | C15H26O | 1866 | 000639-99-6 | 1.30 |
| 46 | 1,6,10-Dodecatrien-3-ol,3,7,11-trimethyl-,(E)- | C15H26O | 1869 | 040716-66-3 | 0.37 |
| 47 | 1H-Cycloprop[e]azulen-7-ol, decahydro-1,1,7-trimethyl-4-methylene-,[1ar-(1aα,4aα,7β,7aβ,7bα)]- | C15H24O | 1894 | 006750-60-3 | 1.74 |
| 48 | Caryophylleneoxide | C15H24O | 1937 | 001139-30-6 | 0.93 |
| 49 | Guaiol | C15H26O | 2065 | 000489-86-1 | 1.39 |
| 50 | (1R,3E,7E,11R)-1,5,5,8-Tetramethyl-12-oxabicyclo [9.1.0]dodeca-3,7-diene | C15H24O | 2068 | 019888-34-7 | 0.36 |
| 51 | Tetradecanal | C14H28O | 2072 | 000124-25-4 | 0.31 |
| 52 | Naphthalene,1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methylethenyl)-,[1R-(1α,7β,8aα)]- | C15H24 | 2123 | 004630-07-3 | 0.04 |
| 53 | Caryophylla-4(12),8(13)-dien-5α-ol | C15H24O | 2285 | 019431-79-9 | 0.10 |
| 54 | 2-Naphthalenemethanol,1,2,3,4,4a,5,6,8a-octahydro-α,α,4a,8-tetramethyl-,[2R-(2α,4aα,8aβ)]- | C15H26O | 2289 | 000473-16-5 | 0.78 |
| 55 | 5-Azulenemethanol,1,2,3,3a,4,5,6,7-octahydro-α,α,3,8-tetramethyl-,[3S-(3α,3aβ,5α)]- | C15H26O | 2296 | 022451-73-6 | 0.60 |
| Total identified compounds | 100% | ||||
| Total terpene hydrocarbons | 34.23% | ||||
| Total oxygenated terpenes | 34.00% | ||||
| Total non-terpenoid | 31.77% |
| Storage Duration (Days) | BC | 1% EO | 2% EO | 3% EO | Positive Control |
|---|---|---|---|---|---|
| 0 | 3.52 ± 0.08 | 3.56 ± 0.10 | 3.44 ± 0.11 | 3.57 ± 0.07 | 3.41 ± 0.17 |
| 2 | 7.10 ± 0.16 | 5.94 ± 0.64 | 5.83 ± 0.72 | 4.78 ± 0.58 | 5.14 ± 0.71 |
| 4 | 9.07 ± 0.39 | 7.79 ± 0.90 | 6.70 ± 0.73 | 6.75 ± 0.44 | 6.23 ± 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lai, J.; Dai, X.; Huang, F.; Guan, L.; Wen, R. Design and Evaluation of a Cinnamomum burmannii Essential Oil-Loaded Preservative Film for Enhancing the Quality and Shelf Life of Squaliobarbus curriculus Filets. Foods 2025, 14, 3139. https://doi.org/10.3390/foods14173139
Zhang X, Lai J, Dai X, Huang F, Guan L, Wen R. Design and Evaluation of a Cinnamomum burmannii Essential Oil-Loaded Preservative Film for Enhancing the Quality and Shelf Life of Squaliobarbus curriculus Filets. Foods. 2025; 14(17):3139. https://doi.org/10.3390/foods14173139
Chicago/Turabian StyleZhang, Xiaonan, Jiayi Lai, Xiaoxiao Dai, Feng Huang, Lei Guan, and Rushu Wen. 2025. "Design and Evaluation of a Cinnamomum burmannii Essential Oil-Loaded Preservative Film for Enhancing the Quality and Shelf Life of Squaliobarbus curriculus Filets" Foods 14, no. 17: 3139. https://doi.org/10.3390/foods14173139
APA StyleZhang, X., Lai, J., Dai, X., Huang, F., Guan, L., & Wen, R. (2025). Design and Evaluation of a Cinnamomum burmannii Essential Oil-Loaded Preservative Film for Enhancing the Quality and Shelf Life of Squaliobarbus curriculus Filets. Foods, 14(17), 3139. https://doi.org/10.3390/foods14173139






