Carbon Dots from Dried German Chamomile Flower and Its Residual Biomass: Characteristics, Bioactivities, Cytotoxicity and Its Preservative Effect on the Refrigerated Precooked Baby Clam (Paphia undulata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Microbial Media and Baby Clam Samples
2.2. Synthesis of CDs from Dried German Chamomile Flower and Residual Biomass
2.3. Characterization of CDs
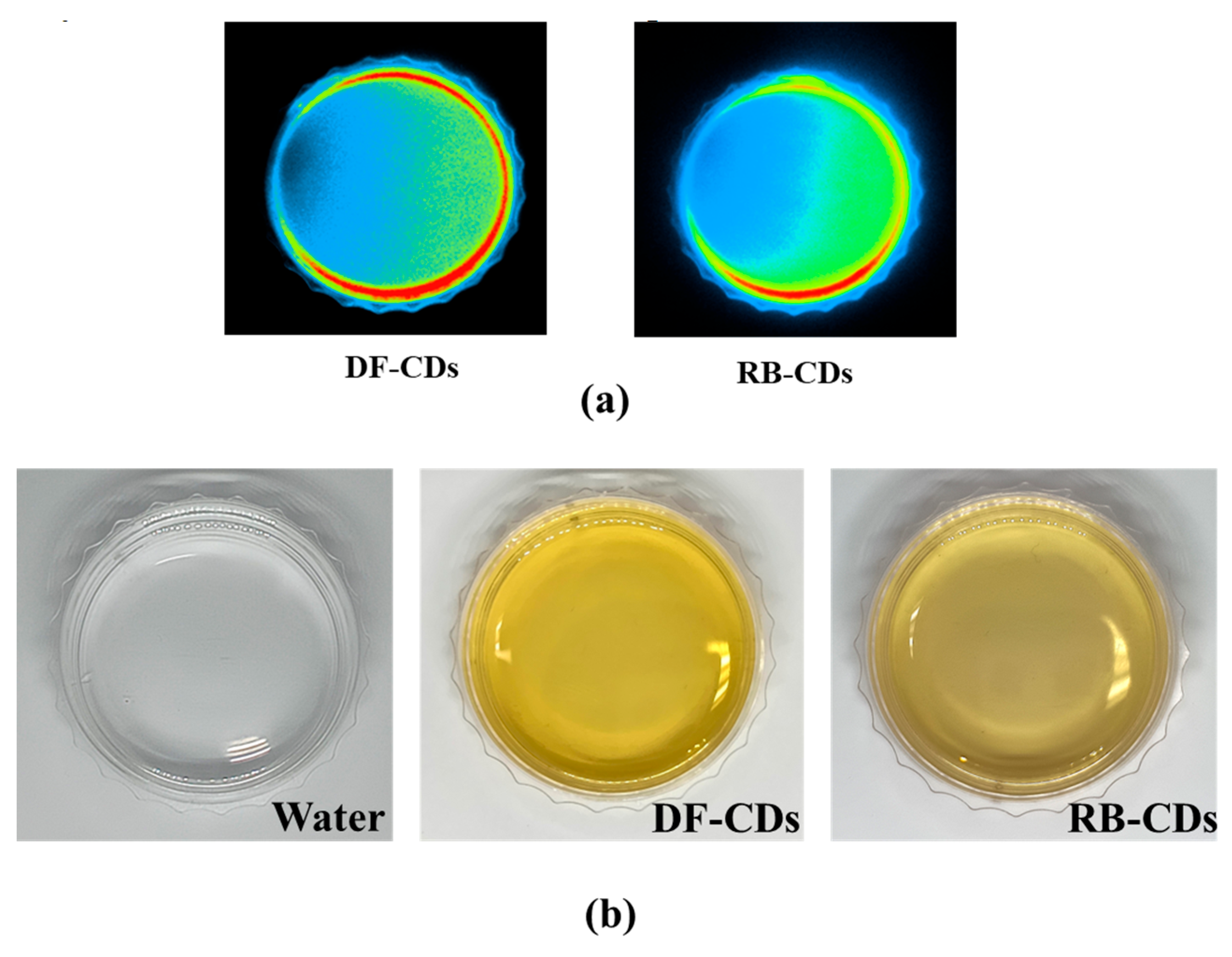
2.3.1. Visual Appearance and Color
2.3.2. UV-Vis Spectrophotometric and Spectrofluorometric Spectra
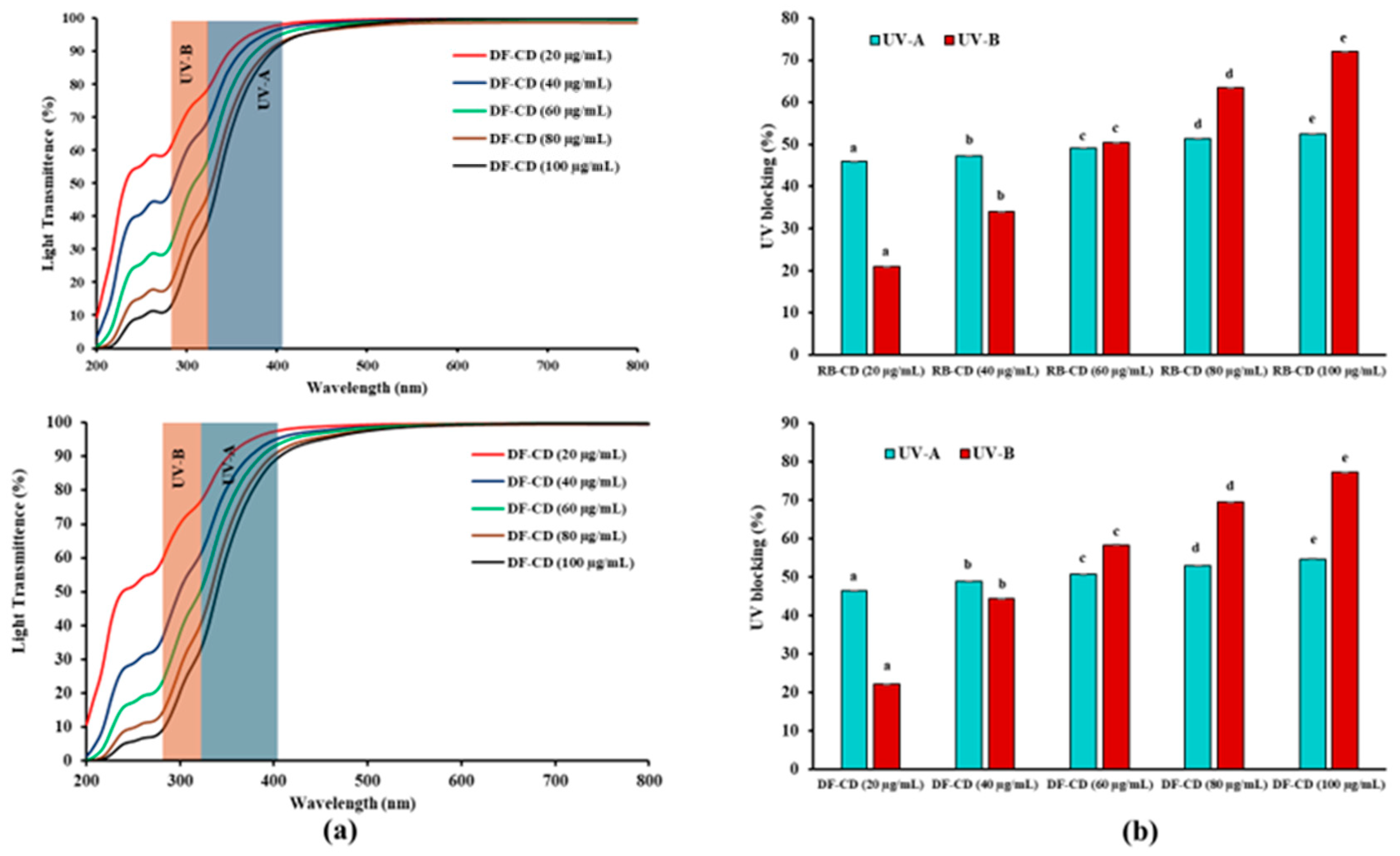
2.3.3. UV Blocking Property
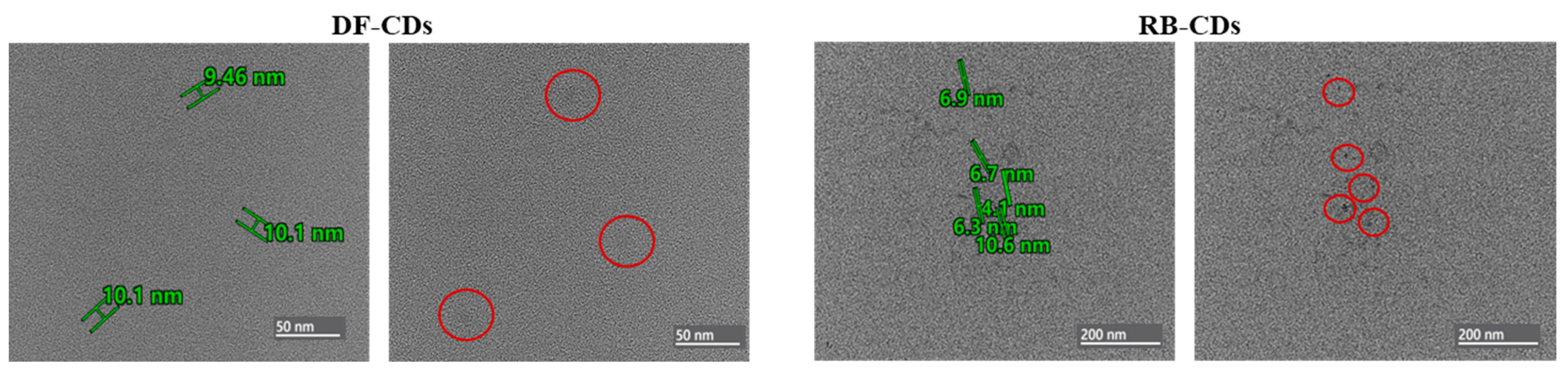
2.3.4. TEM and Particle Size
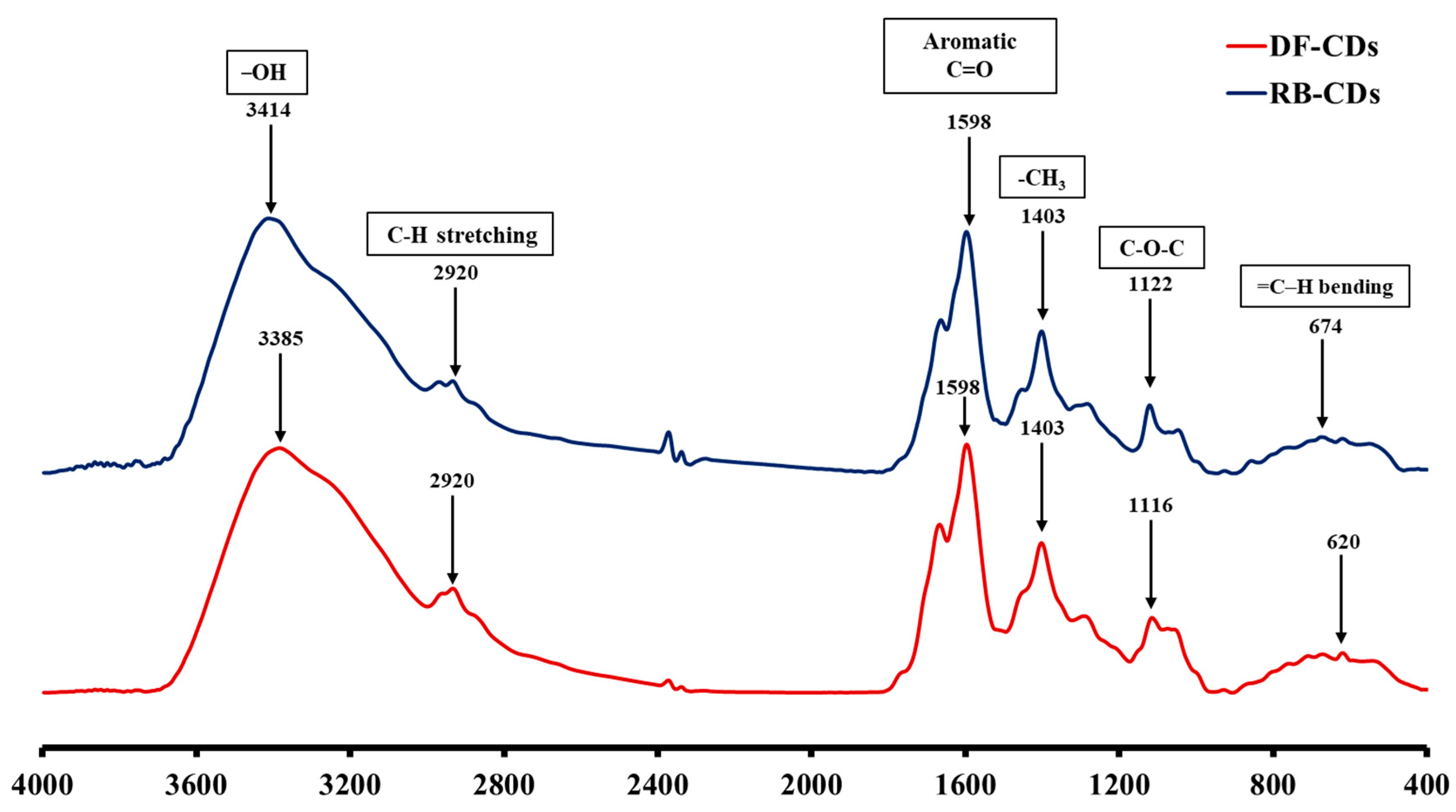
2.3.5. Fourier Transform Infrared (FTIR) Spectra
2.4. Antioxidant Activity
2.4.1. 2,2-Diphenyl-1-Picrylhydrazyl Radical Scavenging Activity (DPPH-RSA)
2.4.2. 2,2′-Azino-Bis (3-Ethyl Benzothiazoline-6-Sulfonic Acid) Radical Scavenging Activity (ABTS-RSA)
2.4.3. Ferric Reducing Antioxidant Power (FRAP)
2.4.4. Metal Chelating Activity (MCA)
2.5. Antimicrobial Activity
2.5.1. Bacterial Strains, Culture Conditions and Cell Suspension
2.5.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
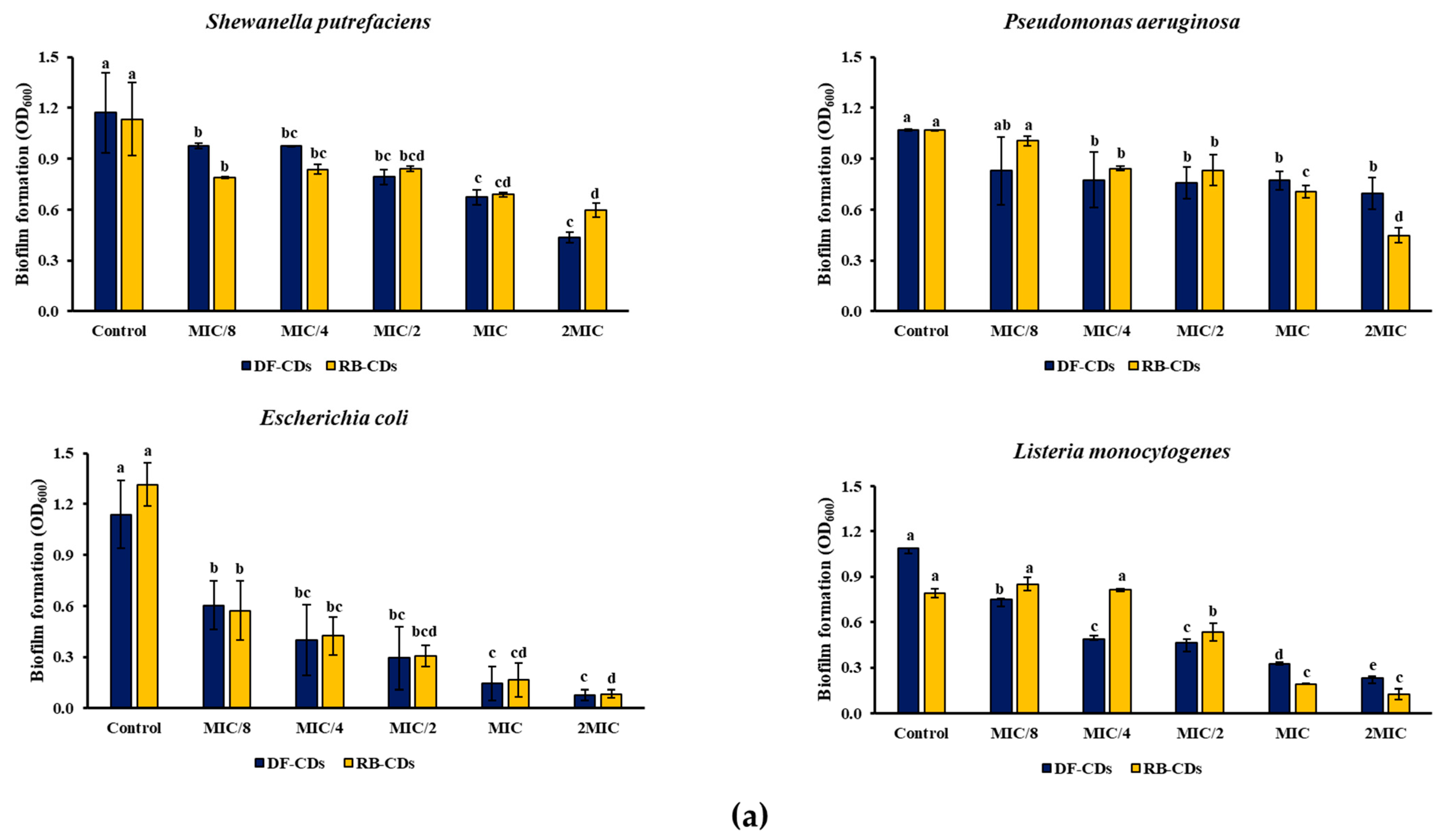
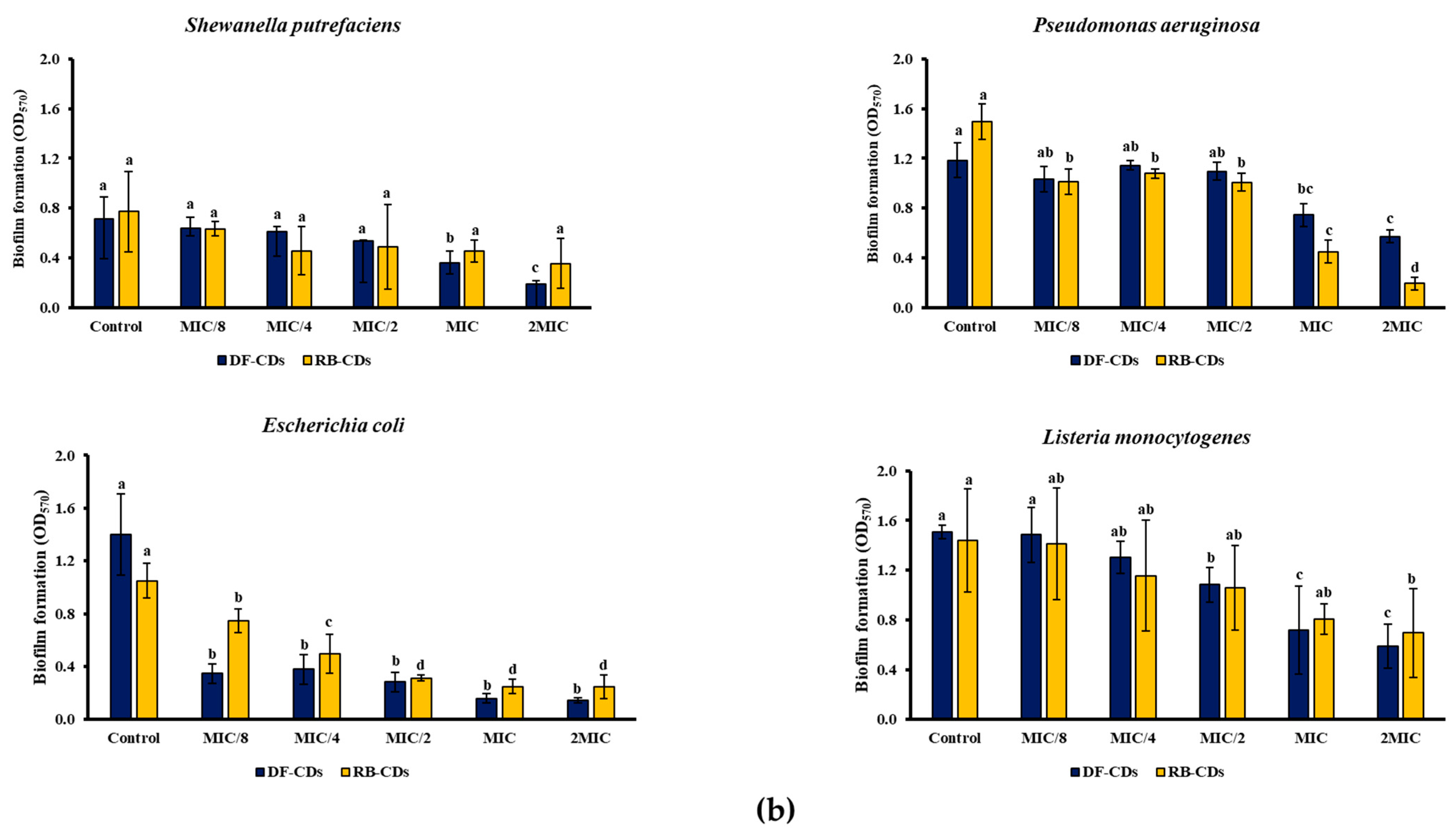
2.5.3. Bacterial Growth and Biofilm Formation
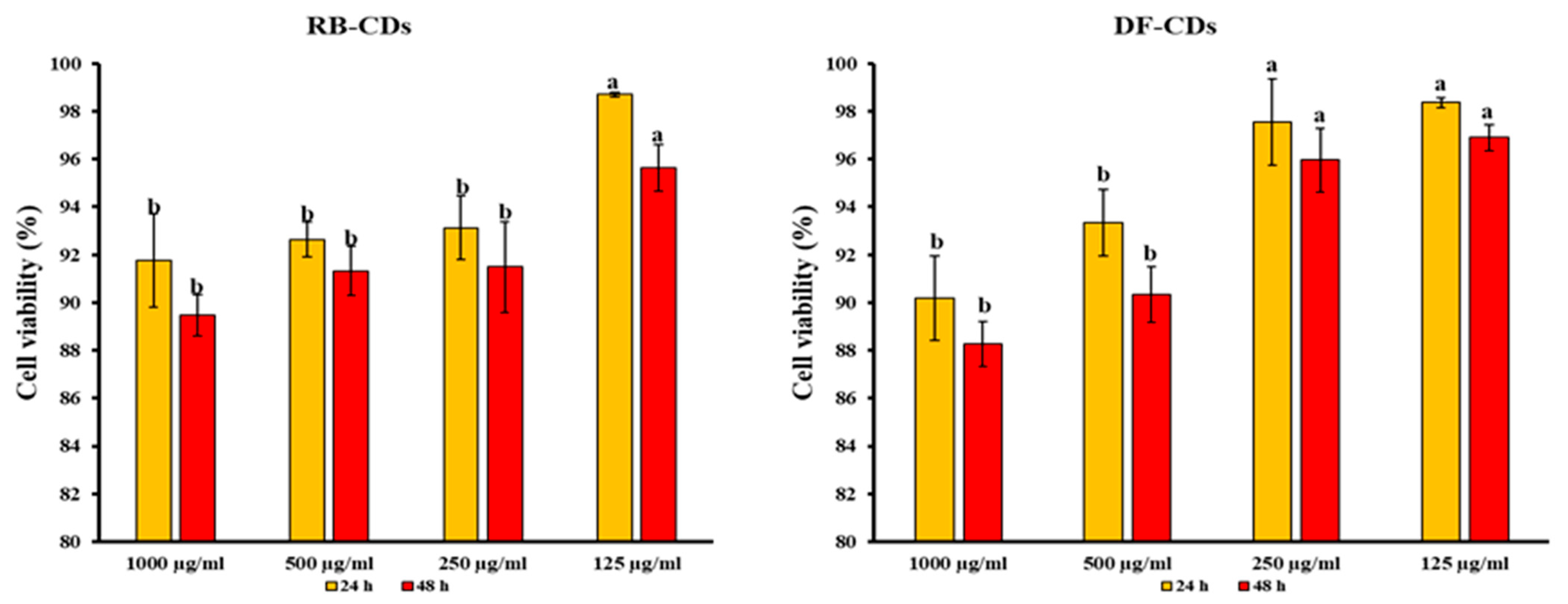
2.6. Cytotoxicity
2.7. Evaluation of Preservative Effectiveness of CDs on Precooked Baby Clam During Refrigerated Storage
2.7.1. Preparation of Precooked Edible Portion of Baby Clam Treated with CDs
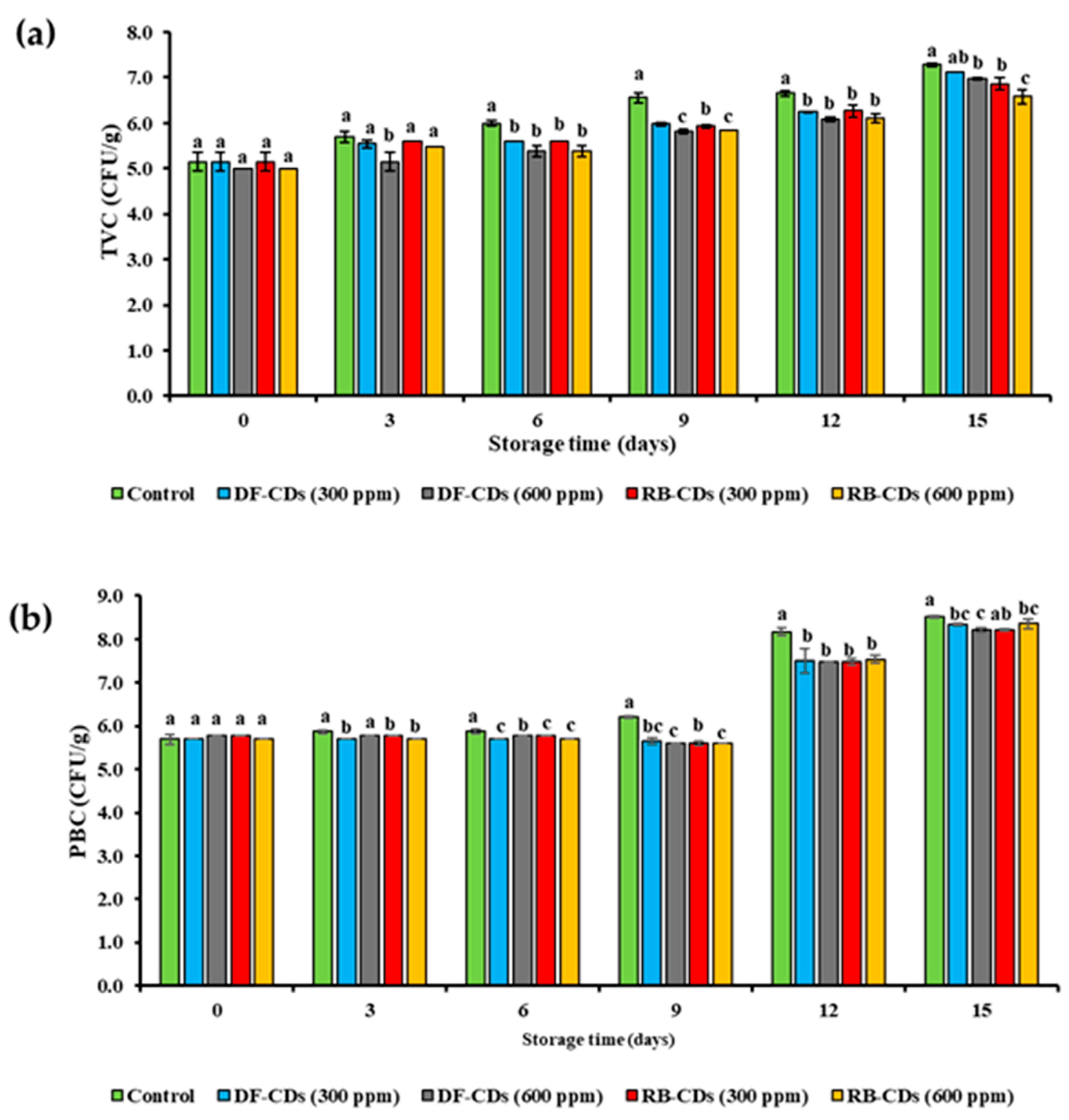
2.7.2. Microbiological Analyses
2.7.3. Chemical Analyses
2.8. Statistical Analyses
3. Results and Discussion
3.1. Characteristics of Synthesized Carbon Dots
3.1.1. Visual Appearance and Color
3.1.2. UV-Vis Spectrophotometric and Photoluminescence Spectra
3.1.3. UV Blocking Property
3.1.4. TEM and Particle Size
3.1.5. FTIR Spectra
3.2. Antioxidant Activities
3.3. Antimicrobial Activities
3.3.1. MIC and MBC
3.3.2. Bacterial Growth and Biofilm Formation
3.4. Cytotoxicity of Synthesized CDs
3.5. Use of CDs as Preservative of Precooked Baby Clam Stored at Refrigerated Temperature
3.5.1. TVC and PBC
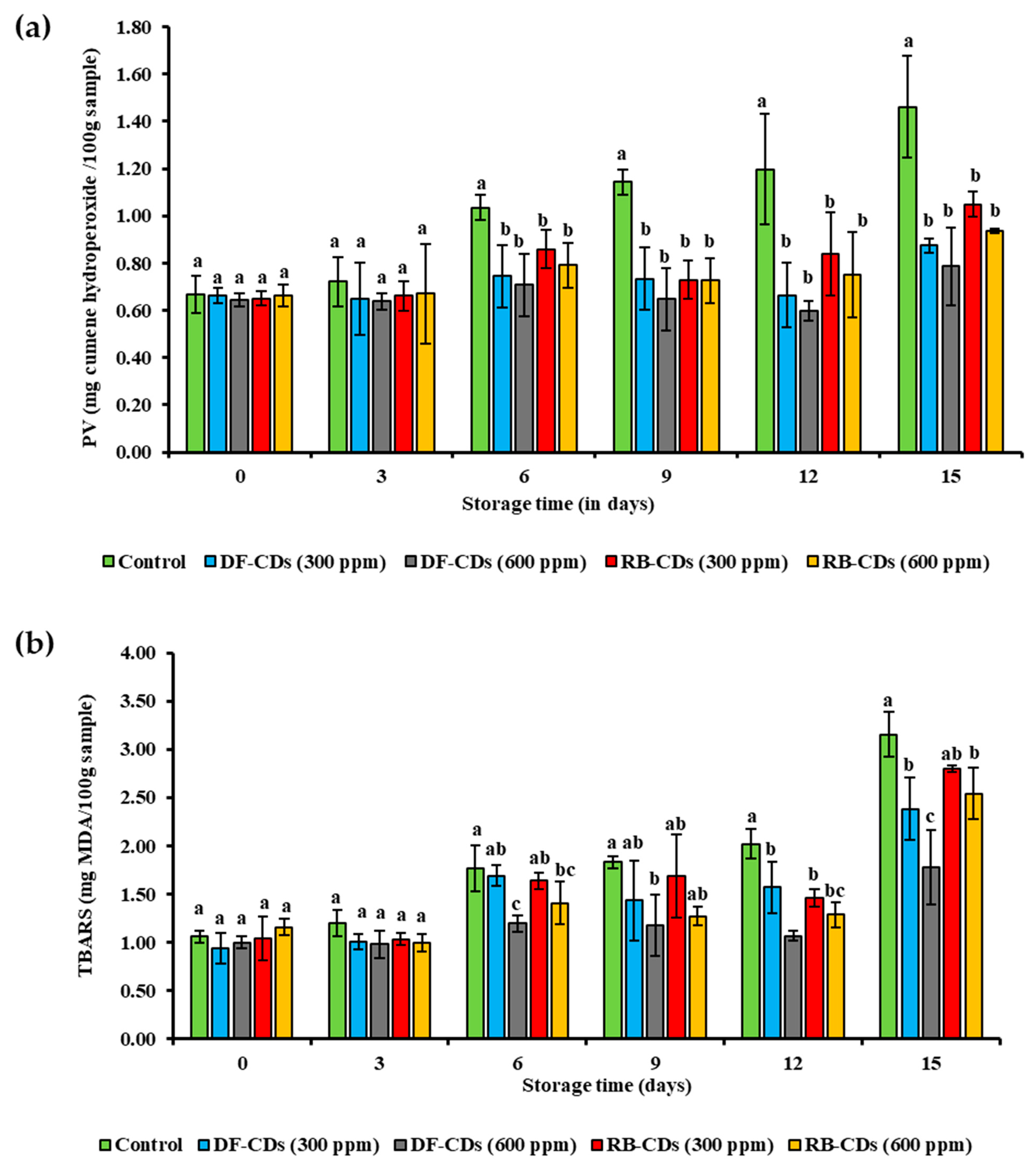
3.5.2. TBARS and PV
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barzegar, F.; Nabizadeh, S.; Kamankesh, M.; Ghasemi, J.B.; Mohammadi, A. Recent advances in natural product-based nanoemulsions as promising substitutes for hazardous synthetic food additives: A new revolution in food processing. Food Bioprocess Technol. 2024, 17, 1087–1108. [Google Scholar] [CrossRef]
- Obahiagbon, E.G.; Ogwu, M.C. Organic food preservatives: The shift towards natural alternatives and sustainability in the global south’s markets. In Food Safety and Quality in the Global South; Springer: Berlin/Heidelberg, Germany, 2024; pp. 299–329. [Google Scholar]
- El Alami El Hassani, N.; Baraket, A.; Alem, C. Recent advances in natural food preservatives: A sustainable solution for food safety and shelf life extension. J. Food Meas. Charact. 2025, 19, 293–315. [Google Scholar] [CrossRef]
- Willer, D.F.; Nicholls, R.J.; Aldridge, D.C. Opportunities and challenges for upscaled global bivalve seafood production. Nat. Food 2021, 2, 935–943. [Google Scholar] [CrossRef]
- Samarajeewa, U. Emerging challenges in maintaining marine food-fish availability and food safety. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4734–4757. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: A review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Xiao, Z.; Luo, Y. Sustainable nanotechnology for food preservation: Synthesis, mechanisms, and applications of zinc oxide nanoparticles. J. Agric. Food Res. 2025, 19, 101743. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Ndraha, N.; Wu, R.-S.; Liu, H.-Y.; Lin, S.-W.; Yang, K.-M.; Lin, H.-Y. An overview of the potential of food-based carbon dots for biomedical applications. Int. J. Mol. Sci. 2023, 24, 16579. [Google Scholar] [CrossRef]
- Guan, X.; Li, Z.; Geng, X.; Lei, Z.; Karakoti, A.; Wu, T.; Kumar, P.; Yi, J.; Vinu, A. Emerging trends of carbon-based quantum dots: Nanoarchitectonics and applications. Small 2023, 19, 2207181. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, Y.; Liu, Y.; Xiao, Y.; Hu, H.; Liang, Y.; Zheng, M. Surface chemical functionality of carbon dots: Influence on the structure and energy storage performance of the layered double hydroxide. RSC Adv. 2021, 11, 10785–10793. [Google Scholar] [CrossRef]
- Li, D.; Qu, Y.; Zhang, X.; Zheng, W.; Rogach, A.L.; Qu, S. Supra-(carbon dots) with versatile morphologies and promising optical properties. Chem. Eng. J. 2023, 454, 140069. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.; Qu, X.; Yang, B.; Lu, S. Carbon dots in bioimaging, biosensing and therapeutics: A comprehensive review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Khosravanian, A.; Moslehipour, A.; Ashrafian, H. A review on bioimaging, biosensing, and drug delivery systems based on graphene quantum dots. Prog. Chem. Biochem. Res. 2021, 4, 44. [Google Scholar]
- Ge, J.; Jia, Q.; Liu, W.; Lan, M.; Zhou, B.; Guo, L.; Zhou, H.; Zhang, H.; Wang, Y.; Gu, Y. Carbon dots with intrinsic theranostic properties for bioimaging, red-light-triggered photodynamic/photothermal simultaneous therapy in vitro and in vivo. Adv. Healthc. Mater. 2016, 5, 665–675. [Google Scholar] [CrossRef]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-derived carbon dots and their applications. Energy Environ. Mater. 2019, 2, 172–192. [Google Scholar] [CrossRef]
- Strickland, S.; Fourroux, L.; Pappas, D. Effect of precursors on carbon dot functionalization and applications: A review. Analyst 2025, 150, 1448–1469. [Google Scholar] [CrossRef] [PubMed]
- El Mihyaoui, A.; Esteves da Silva, J.C.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A review of ethnomedicinal use, phytochemistry and pharmacological uses. Life 2022, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Kerbab, K.; Sanah, I.; Djeghim, F.; Belattar, N.; Santoro, V.; D’Elia, M.; Rastrelli, L. Nutritional composition, physicochemical properties, antioxidant activity, and sensory quality of Matricaria chamomilla-enriched wheat bread. Foods 2025, 14, 838. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, T.; Zhou, L.; Han, G.; Shen, Y.; Ke, C. Thermal tolerance traits of the undulated surf clam Paphia undulata based on heart rate and physiological energetics. Aquaculture 2019, 498, 343–350. [Google Scholar] [CrossRef]
- Thailand Department of Fisheries, Fisheries Statistics of Thailand: Fisheries Development and Planning Division. 2023. Available online: https://www4.fisheries.go.th/en (accessed on 23 April 2025).
- Zhang, P.; Yongo, E.; Feng, H.; Pan, S.; Sun, A.; Zhou, L.; Guo, Z.; Ke, C. Effects of substrate, temperature, salinity, size and transportation on burrowing capacity of juvenile undulated surf clam Paphia undulata. Aquac. Res. 2022, 53, 2796–2805. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Hu, X.; Huang, X.; Zhang, X.; Zou, X.; Shi, J. Preparation of edible antibacterial films based on corn starch/carbon nanodots for bioactive food packaging. Food Chem. 2024, 444, 138467. [Google Scholar] [CrossRef]
- Kumar, L.; Gaikwad, K.K. Carbon dots for food packaging applications. Sustain. Food Technol. 2023, 1, 185–199. [Google Scholar]
- Bora, B.; Yin, T.; Zhang, B.; Altan, C.O.; Benjakul, S. Comparison between Indian and commercial chamomile essential oils: Chemical compositions, antioxidant activities and preventive effect on oxidation of Asian seabass visceral depot fat oil. Food Chem. X 2025, 26, 102292. [Google Scholar] [CrossRef]
- Murugan, G.; Nilsuwan, K.; Kim, J.T.; Rhim, J.W.; Yin, T.; Zhang, B.; Benjakul, S. Bambara Groundnut Pericarp–Derived Carbon Dots: Reinforcement of Gelatin/PLA Bilayer Films and Their Function as Preservative in Packed Asian Seabass Slices. J. Food Sci. 2025, 90, e70469. [Google Scholar] [CrossRef] [PubMed]
- Koutchma, T.; Popović, V.; Ros-Polski, V.; Popielarz, A. Effects of ultraviolet light and high-pressure processing on quality and health-related constituents of fresh juice products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 844–867. [Google Scholar] [CrossRef]
- Wu, T.; Li, M.; Li, T.; Zhao, Y.; Yuan, J.; Zhao, Y.; Tian, X.; Kong, R.; Zhao, Y.; Kong, H.; et al. Natural biomass-derived carbon dots as a potent solubilizer with high biocompatibility and enhanced antioxidant activity. Front. Mol. Biosci. 2023, 10, 1284599. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, N.; Tan, G.; Tevlek, A.; Arslan, G.; Zengin, G.; Sargin, I. Dead Cell Discrimination with Red Emissive Carbon Quantum Dots from the Medicinal and Edible Herb Echinophora tenuifolia. J. Fluoresc. 2025, 1–18. [Google Scholar] [CrossRef]
- Tariq, M.; Singh, A.; Varshney, N.; Samanta, S.K.; Sk, M.P. Biomass-derived carbon dots as an emergent antibacterial agent. Mater. Today Commun. 2022, 33, 104347. [Google Scholar] [CrossRef]
- Sharma, M.H.; Palamae, S.; Yingkajorn, M.; Benjakul, S.; Singh, A.; Buatong, J. Multidrug-resistance of Vibrio species in bivalve mollusks from Southern Thailand: Isolation, identification, pathogenicity, and their sensitivity toward chitooligosaccharide-epigallocatechin-3-gallate conjugate. Foods 2024, 13, 2375. [Google Scholar] [CrossRef]
- Lucero-Mejía, J.E.; Romero-Gómez, S.d.J.; Hernández-Iturriaga, M. A new classification criterion for the biofilm formation index: A study of the biofilm dynamics of pathogenic Vibrio species isolated from seafood and food contact surfaces. J. Food Sci. 2020, 85, 2491–2497. [Google Scholar] [CrossRef]
- Saetang, J.; Sukkapat, P.; Mittal, A.; Julamanee, J.; Khopanlert, W.; Maneechai, K.; Nazeer, R.A.; Sangkhathat, S.; Benjakul, S. Proteome analysis of the antiproliferative activity of the novel chitooligosaccharide–gallic acid conjugate against the SW620 colon cancer cell line. Biomedicines 2023, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Palamae, S.; Patil, U.; Sinlapapanya, P.; Hong, H.; Zhao, Y.; Zhang, B.; Benjakul, S. Impact of high-pressure processing on prevention of quality loss and spoilage bacteria diversity in precooked baby clam (Paphia undulata) during refrigerated storage. Foods 2025, 14, 1421. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.P.; Hultin, H.O. Contributions of blood and blood components to lipid oxidation in fish muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Osman, M.A.; El-Said, W.A.; Othman, A.A.; Abd-Elrahim, A.G. Influence of thermally induced structural and morphological changes, and UV irradiation on photoluminescence and optical absorption behavior of CdS nanoparticles. J. Phys. D Appl. Phys. 2016, 49, 165302. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.-H.; Chen, X.-B.; Wei, J.-S.; Li, X.-B.; Xiong, H.-M. Surface states of carbon dots and their influences on luminescence. J. Appl. Phys. 2020, 127, 231101. [Google Scholar] [CrossRef]
- Khan, A.; Ezati, P.; Rhim, J.-W. Chitosan/gelatin-based multifunctional film integrated with green tea carbon dots to extend the shelf life of pork. Food Packag. Shelf Life 2023, 37, 101075. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Ahmed, S.R.; Sherazee, M.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Carbon dot biopolymer-based flexible functional films for antioxidant and food monitoring applications. ACS Appl. Polym. Mater. 2022, 4, 9323–9340. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef]
- Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic profile by HPLC-PDA-MS of Greek chamomile populations and commercial varieties and their antioxidant activity. Foods 2021, 10, 2345. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Omer, K.M.; Hawaiz, F.E. Deep insights to explain the mechanism of carbon dot formation at various reaction times using the hydrothermal technique: FT-IR, 13 C-NMR, 1 H-NMR, and UV-visible spectroscopic approaches. RSC Adv. 2023, 13, 14340–14349. [Google Scholar] [CrossRef]
- Semsey, D.; Nguyen, D.H.; Törős, G.; Papp, V.; Pénzes, J.; Vida, T.; Béni, Á.; Rai, M.; Prokisch, J. Analysis of fluorescent carbon nanodots synthesized from spices through thermal processes treatment. Nanomaterials 2025, 15, 625. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.H.; Gedanken, A. Green synthesis of multifunctional carbon dots with antibacterial activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef]
- Siddique, A.B.; Hossain, S.M.; Pramanick, A.K.; Ray, M. Excitation dependence and independence of photoluminescence in carbon dots and graphene quantum dots: Insights into the mechanism of emission. Nanoscale 2021, 13, 16662–16671. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Ren, L.; Li, S.; Feng, M.; Zhang, Q.; Qi, R.; Qin, Z.; Zhang, J.; Jiang, L. The enhanced photoluminescence properties of carbon dots derived from glucose: The effect of natural oxidation. Nanomaterials 2024, 14, 970. [Google Scholar] [CrossRef]
- Qiu, J.; Ye, W.; Chen, C.; Xu, Z.; Hu, C.; Zhuang, J.; Dong, H.; Lei, B.; Hu, G.; Liu, Y. Toward efficient broad-spectrum UV absorption of carbon dots: Facile preparation, performance characterization and its application as UV absorbers. J. Ind. Eng. Chem. 2023, 117, 442–449. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Moon, J.-Y.; Lee, Y.-C. Plant extract-derived carbon dots as cosmetic ingredients. Nanomaterials 2023, 13, 2654. [Google Scholar] [CrossRef] [PubMed]
- Sabukuttan, D. Hydrothermal Synthesis and Physicochemical Characterization of Carbon Quantum Dots from Marine Cyanobacteria Leptolyngbya sp. nov. KMBMA-1. BioNanoScience 2025, 15, 1–13. [Google Scholar] [CrossRef]
- Ponnusamy, A.; Murugan, G.; Mittal, A.; Saetang, J.; Prodpran, T.; Rhim, J.-W.; Benjakul, S. Carbon dots derived from polyphenols by hydrothermal carbonization: Spectral, antioxidant, and antimicrobial properties and cytotoxicity assessment. Food Biophys. 2025, 20, 89. [Google Scholar] [CrossRef]
- Wang, C.; Xing, Y.; Shi, K.; Wang, S.; Xia, Y.; Li, J.; Gui, X. Chemical structure characteristics and model construction of coal with three kinds of coalification degrees. ACS Omega 2023, 9, 1881–1893. [Google Scholar] [CrossRef]
- Amaral, V.A.; Alves, T.R.; de Souza, J.F.; Batain, F.; de Moura Crescencio, K.M.; Soeiro, V.S.; de Barros, C.T.; Chaud, M.V. Phenolic compounds from Psidium guajava (Linn.) leaves: Effect of the extraction-assisted method upon total phenolics content and antioxidant activity. Biointerface Res. Appl. Chem. 2021, 11, 9346–9357. [Google Scholar]
- Kumar, S.; Sharma, J. Stable phase CdS nanoparticles for optoelectronics: A study on surface morphology, structural and optical characterization. Mater. Sci. Poland 2016, 34, 368–373. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, A.; Lee, H.-J. The antioxidant properties of green carbon dots: A review. Environ. Chem. Lett. 2025, 23, 1–49. [Google Scholar] [CrossRef]
- Hussen, E.M.; Endalew, S.A. In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina. BMC Complement. Med. Ther. 2023, 23, 146. [Google Scholar] [CrossRef]
- Berker, K.I.; Güçlü, K.; Tor, İ.; Apak, R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Avila-Calderón, E.D.; Ruiz-Palma, M.d.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer membrane vesicles of gram-negative bacteria: An outlook on biogenesis. Front. Microbiol. 2021, 12, 557902. [Google Scholar] [CrossRef] [PubMed]
- Paitan, Y. Current trends in antimicrobial resistance of Escherichia coli. In Escherichia Coli, A Versatile Pathogen; Springer: Berlin/Heidelberg, Germany, 2018; pp. 181–211. [Google Scholar]
- Odeyemi, O.A.; Stratev, D.; Onyeaka, H.; Dewi, F.R.; Amin, M. Detection of Seafood Spoilage Microorganisms. In Handbook of Seafood and Seafood Products Analysis; CRC Press: Boca Raton, FL, USA, 2024; pp. 545–559. [Google Scholar]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Wang, H. Application of carbon dots in food preservation: A critical review for packaging enhancers and food preservatives. Crit. Rev. Food Sci. Nutr. 2023, 63, 6738–6756. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, S.; Arumugam, L.; Srirengan, S.P.; Pitchan, R.; Sevugan, P.; Kannan, K.; Pitchan, G.; Hegde, T.A.; Gandhirajan, V. Biocompatible carbon quantum dots derived from sugarcane industrial wastes for effective nonlinear optical behavior and antimicrobial activity applications. ACS Omega 2020, 5, 30363–30372. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wang, Z.; Wu, F.-G. Carbon dots for killing microorganisms: An update since 2019. Pharmaceuticals 2022, 15, 1236. [Google Scholar] [CrossRef]
- Hao, S.; Qi, Y.; Zhang, Z. Influence of light conditions on the antibacterial performance and mechanism of waterborne fluorescent coatings based on waterproof long afterglow phosphors/PDMS composites. Polymers 2023, 15, 3873. [Google Scholar] [CrossRef]
- de Lima, M.S.; Schio, A.L.; Aguzzoli, C.; de Souza, W.V.; Roesch-Ely, M.; Leidens, L.M.; Boeira, C.D.; Alvarez, F.; Elois, M.A.; Fongaro, G.; et al. Visible Light-Driven Photocatalysis and Antibacterial Performance of a Cu-TiO2 Nanocomposite. ACS Omega 2024, 9, 47122–47134. [Google Scholar] [CrossRef]
- Dörr, T.; Moynihan, P.J.; Mayer, C. Bacterial cell wall structure and dynamics. Front. Microbiol. 2019, 10, 2051. [Google Scholar] [CrossRef]
- Cui, F.; Ning, Y.; Wang, D.; Li, J.; Li, X.; Li, T. Carbon dot-based therapeutics for combating drug-resistant bacteria and biofilm infections in food preservation. Crit. Rev. Food Sci. Nutr. 2024, 64, 203–219. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Meena, R.; Bohidar, H.B.; Sharma, S.K.; Abdellattif, M.H.; Saravanan, M.; Rajamani, P. Comparative in vitro cytotoxicity study of carbon dot-based organometallic nanoconjugates: Exploration of their cell proliferation, uptake, and localization in cancerous and normal cells. Oxid. Med. Cell. Longev. 2022, 2022, 3483073. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Bagheri, Z.; Ehtesabi, H.; Fatahi, Z.; Tavana, H.; Latifi, H. Effect of carbonization degree of carbon dots on cytotoxicity and photo-induced toxicity to cells. Heliyon 2019, 5, e02940. [Google Scholar] [CrossRef] [PubMed]
- Daby, T.P.M.; Modi, U.; Yadav, A.K.; Bhatia, D.; Solanki, R. Bioimaging and therapeutic applications of multifunctional carbon quantum dots: Recent progress and challenges. Next Nanotechnol. 2025, 8, 100158. [Google Scholar] [CrossRef]
- Wright, A.C.; Fan, Y.; Baker, G.L. Nutritional value and food safety of bivalve molluscan shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- Swanson, K.M.J. Fish and Seafood Products. In Microorganisms in Foods 8: Use of Data for Assessing Process Control and Product Acceptance; Springer: Berlin/Heidelberg, Germany, 2011; pp. 107–133. [Google Scholar]
- Khan, M.A.; Parrish, C.C.; Shahidi, F. Effects of environmental characteristics of aquaculture sites on the quality of cultivated Newfoundland blue mussels (Mytilus edulis). J. Agric. Food Chem. 2006, 54, 2236–2241. [Google Scholar] [CrossRef]
- Sahu, V.; Sahoo, S.K. Biogenic synthesis of carbon dots with inbuilt biological activity. Next Nanotechnol. 2024, 5, 100034. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Dragoev, S.G. Lipid peroxidation in muscle foods: Impact on quality, safety and human health. Foods 2024, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- de Jorge Gouvêa, F.; de Oliveira, V.S.; Mariano, B.J.; Takenaka, N.A.R.; Gamallo, O.D.; da Silva Ferreira, M.; Saldanha, T. Natural antioxidants as strategy to minimize the presence of lipid oxidation products in canned fish: Research progress, current trends and future perspectives. Food Res. Int. 2023, 173, 113314. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-R.; Qiu, Y.-T.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Purification and characterization of antioxidant peptides derived from protein hydrolysate of the marine bivalve mollusk Tergillarca granosa. Mar. Drugs 2019, 17, 251. [Google Scholar] [CrossRef]

| Sample Name | L* | a* | b* |
|---|---|---|---|
| DF-CDs | 85.05 ± 0.25 | −1.24 ± 0.02 | 40.14 ± 0.06 |
| RB-CDs | 90.25 ± 0.16 | −1.35 ± 0.03 | 33.94 ± 0.11 |
| Sample Name | DPPH-RSA (µmol TE/mL) | ABTS-RSA (µmol TE/mL) | FRAP (µmol TE/mL) | MCA (µmol EE/mL) |
|---|---|---|---|---|
| DF-CDs | 56.42 ± 0.60 a | 95.51± 5.38 a | 136.47 ± 3.79 a | 16.46 ± 2.75 a |
| RB-CDs | 54.60 ± 1.16 a | 91.77 ± 9.16 b | 130.98 ± 1.02 b | 14.68 ± 1.72 a |
| Sample Name | MIC | MBC | ||
|---|---|---|---|---|
| DF-CDs | RB-CDs | DF-CDs | RB-CDs | |
| Shewanella putrefaciens | 8 mg/mL | 8 mg/mL | 8 mg/mL | 8 mg/mL |
| Pseudomonas aeruginosa | 4 mg/mL | 4 mg/mL | 8 mg/mL | 8 mg/mL |
| Escherichia coli | 8 mg/mL | 8 mg/mL | 16 mg/mL | 16 mg/mL |
| Listeria monocytogenes | 2 mg/mL | 2 mg/mL | 16 mg/mL | 16 mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bora, B.; Palamae, S.; Zhang, B.; Yin, T.; Kim, J.T.; Rhim, J.-W.; Benjakul, S. Carbon Dots from Dried German Chamomile Flower and Its Residual Biomass: Characteristics, Bioactivities, Cytotoxicity and Its Preservative Effect on the Refrigerated Precooked Baby Clam (Paphia undulata). Foods 2025, 14, 3130. https://doi.org/10.3390/foods14173130
Bora B, Palamae S, Zhang B, Yin T, Kim JT, Rhim J-W, Benjakul S. Carbon Dots from Dried German Chamomile Flower and Its Residual Biomass: Characteristics, Bioactivities, Cytotoxicity and Its Preservative Effect on the Refrigerated Precooked Baby Clam (Paphia undulata). Foods. 2025; 14(17):3130. https://doi.org/10.3390/foods14173130
Chicago/Turabian StyleBora, Birinchi, Suriya Palamae, Bin Zhang, Tao Yin, Jun Tae Kim, Jong-Whan Rhim, and Soottawat Benjakul. 2025. "Carbon Dots from Dried German Chamomile Flower and Its Residual Biomass: Characteristics, Bioactivities, Cytotoxicity and Its Preservative Effect on the Refrigerated Precooked Baby Clam (Paphia undulata)" Foods 14, no. 17: 3130. https://doi.org/10.3390/foods14173130
APA StyleBora, B., Palamae, S., Zhang, B., Yin, T., Kim, J. T., Rhim, J.-W., & Benjakul, S. (2025). Carbon Dots from Dried German Chamomile Flower and Its Residual Biomass: Characteristics, Bioactivities, Cytotoxicity and Its Preservative Effect on the Refrigerated Precooked Baby Clam (Paphia undulata). Foods, 14(17), 3130. https://doi.org/10.3390/foods14173130










