Causal Inference Framework Reveals Mediterranean Diet Superiority and Inflammatory Mediation Pathways in Mortality Prevention: A Comparative Analysis of Nine Common Dietary Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Dietary Indices
2.3. Ascertainment of Inflammatory Biomarkers
2.4. Outcome Ascertainment
2.5. Covariate Assessment
2.6. Causal Inference Framework
2.7. General Statistical Analysis
3. Results
3.1. Sample Population Characteristics
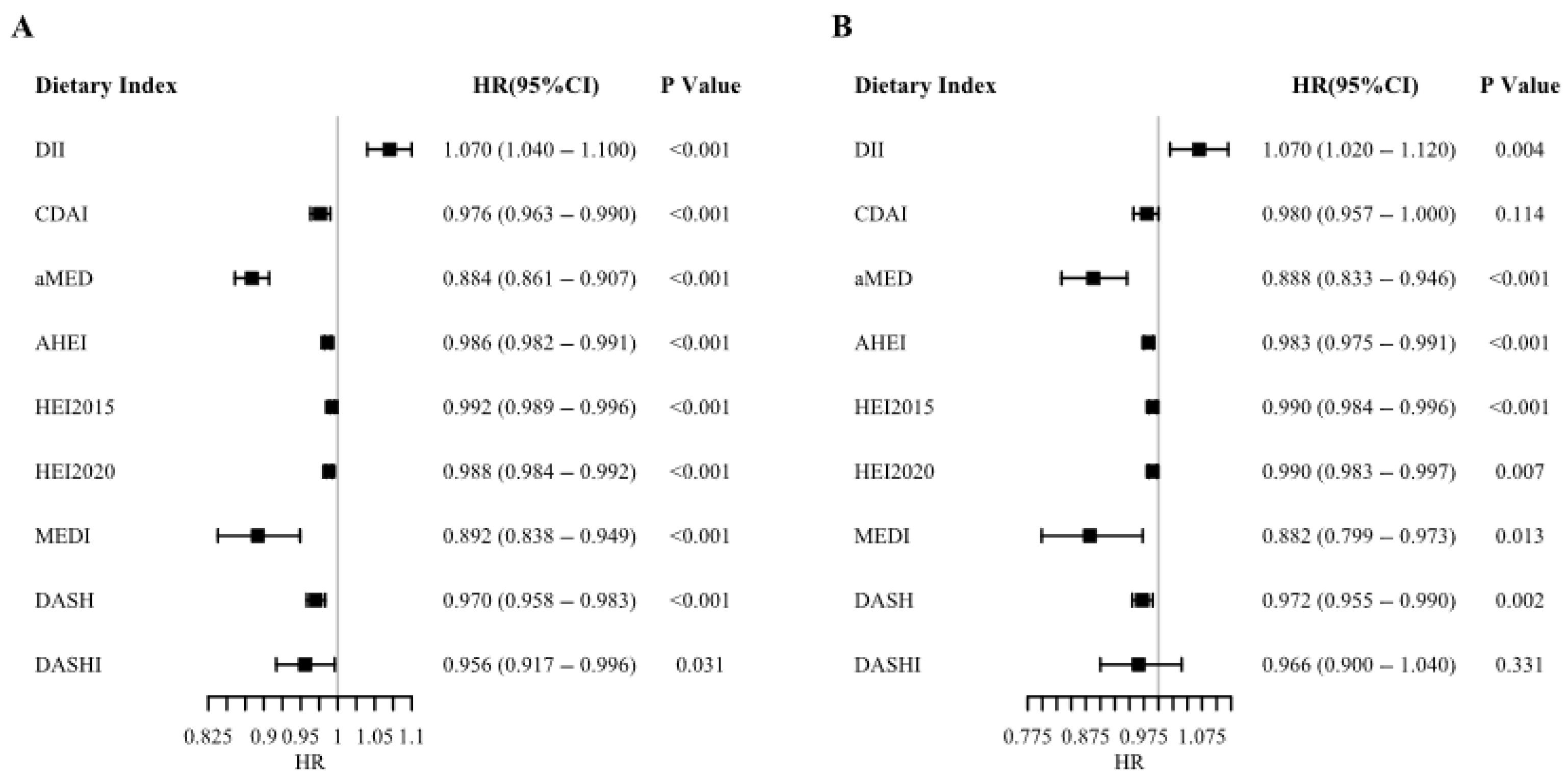
3.2. Causal Effects of Dietary Quality on Mortality Outcomes
3.3. Dose–Response Relationships Through Restricted Cubic Splines
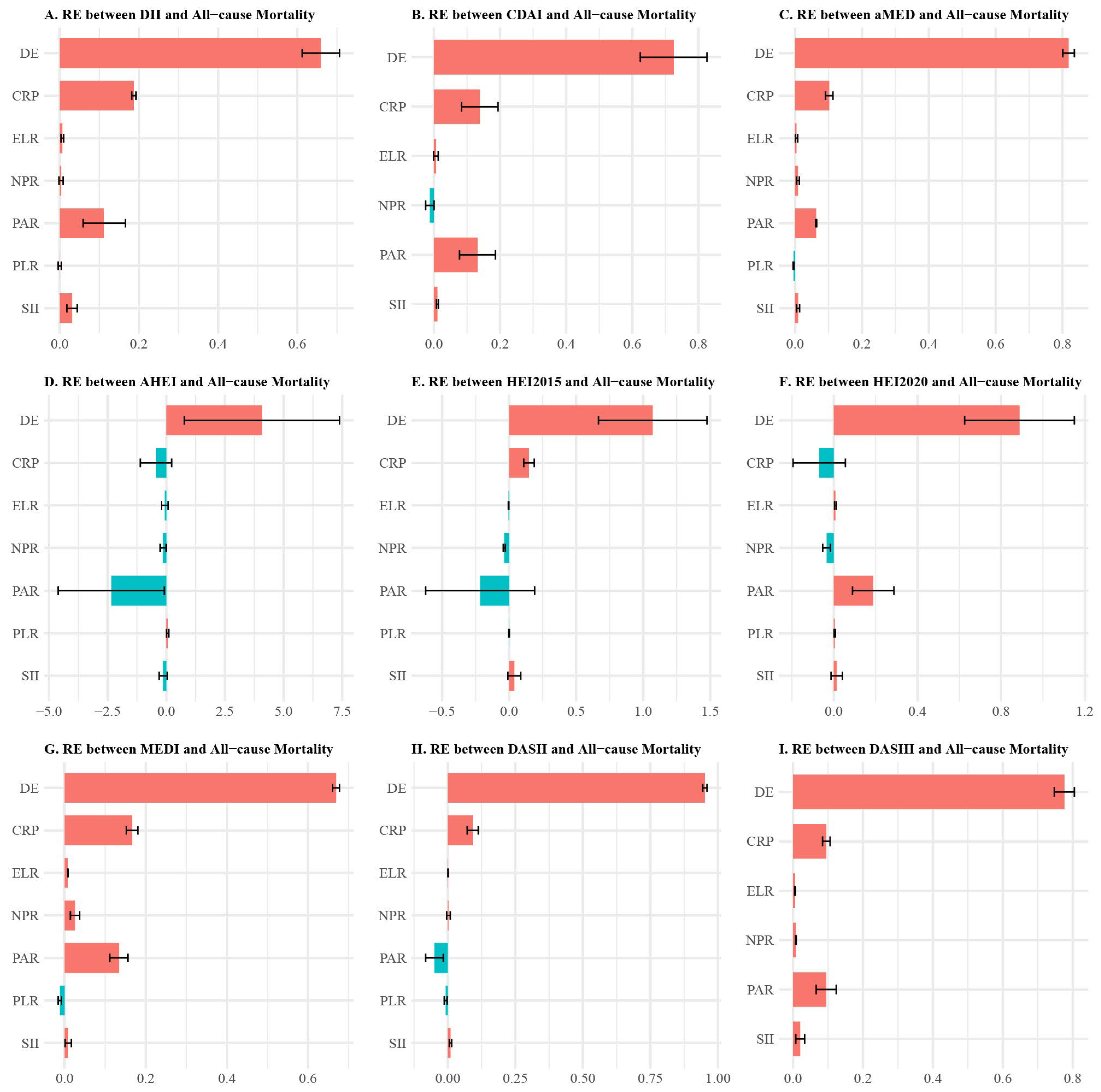
3.4. Inflammatory Mediation Pathways
3.5. Sensitivity Analysis for Unmeasured Confounding
4. Discussion
4.1. Principal Findings and Causal Evidence
4.2. Mechanistic Insights Through Multiple Mediation Analysis
4.3. Mediterranean Diet Superiority and Mechanistic Basis
4.4. DASH Diet Findings and Clinical Implications
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DGA | Dietary Guidelines for Americans |
| RCT | Randomized Controlled Trial |
| DII | Dietary Inflammatory Index |
| MED | Mediterranean Diet |
| HEI | Healthy Eating Index |
| AHEI | Alternative Healthy Eating Index |
| DASH | Dietary Approaches to Stop Hypertension |
| DASHI | Dietary Approaches to Stop Hypertension Index |
| PLR | Platelet-to-Lymphocyte Ratio |
| LMR | Lymphocyte-to-Monocyte Ratio |
| PAR | Platelet-to-Albumin Ratio |
| SII | Systemic Inflammation Index |
| NPR | Neutrophil-to-Platelet Ratio |
| ELR | Eosinophil-to-Lymphocyte Ratio |
| TyG | Triglyceride-Glucose Index |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| NHANES | National Health and Nutrition Examination Survey |
| CRP | C-reactive Protein |
References
- Zhang, Q.; Xiao, S.; Jiao, X.; Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 2023, 22, 279. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar]
- Zhang, J.; Feng, Y.; Yang, X.; Li, Y.; Wu, Y.; Yuan, L.; Li, T.; Hu, H.; Li, X.; Huang, H.; et al. Dose-Response Association of Dietary Inflammatory Potential with All-Cause and Cause-Specific Mortality. Adv. Nutr. 2022, 13, 1834–1845. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Hebert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)—Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef]
- Fadnes, L.T.; Okland, J.M.; Haaland, O.A.; Johansson, K.A. Estimating impact of food choices on life expectancy: A modeling study. PLoS Med. 2022, 19, e1003889. [Google Scholar]
- Olier, I.; Zhan, Y.; Liang, X.; Volovici, V. Causal inference and observational data. BMC Med. Res. Methodol. 2023, 23, 227. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.L. Target Trial Emulation to Improve Causal Inference from Observational Data: What, Why, and How? J. Am. Soc. Nephrol. 2023, 34, 1305–1314. [Google Scholar] [CrossRef]
- Kawahara, T.; Shiba, K.; Tsuchiya, A. Application of Causal Inference Methods in the Analysis of Observational Neurosurgical Data: G-Formula and Marginal Structural Model. World Neurosurg. 2022, 161, 310–315. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar]
- Xu, W.; Luo, G.; Meng, W.; Zhai, X.; Zheng, K.; Wu, J.; Li, Y.; Xing, A.; Li, J.; Li, Z.; et al. MRAgent: An LLM-based automated agent for causal knowledge discovery in disease via Mendelian randomization. Brief. Bioinform. 2025, 26, bbaf140. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Zhang, J.; Li, M.; Zhang, Z.; Zhu, Y.; Zeng, X.F.; Li, H.; Wang, Y.; Wang, S.F.; et al. Association of dietary inflammatory potential with risk of overall and cause-specific mortality. Br. J. Nutr. 2022, 127, 1878–1887. [Google Scholar] [PubMed]
- Zheng, X.; Ge, Y.Z.; Ruan, G.T.; Lin, S.Q.; Chen, Y.; Liu, C.A.; Xie, H.L.; Song, M.M.; Liu, T.; Wang, Z.W.; et al. Association between the Dietary Inflammatory Index and All-Cause Mortality in Adults with Obesity. Ann. Nutr. Metab. 2023, 79, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.J.; Hodge, R.A.; Dunlop, A.L.; Lee, M.M.; Bui, L.; Liang, D.; Ferranti, E.P. Dietaryindex: A User-Friendly and Versatile R Package for Standardizing Dietary Pattern Analysis in Epidemiological and Clinical Studies. Am. J. Clin. Nutr. 2024, 120, 1165–1174. [Google Scholar] [CrossRef]
- Maugeri, A.; Hruskova, J.; Jakubik, J.; Kunzova, S.; Sochor, O.; Barchitta, M.; Agodi, A.; Bauerova, H.; Medina-Inojosa, J.R.; Vinciguerra, M. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: A cross-sectional assessment in the Kardiovize study. Free Radic. Biol. Med. 2019, 131, 274–281. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Chen, Y.; Fang, W.; Li, X.; Wang, R.; Liu, J.; Ma, X. The association between sedentary behavior, exercise, and sleep disturbance: A mediation analysis of inflammatory biomarkers. Front. Immunol. 2022, 13, 1080782. [Google Scholar]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Cao, Y.; Li, P.; Zhang, Y.; Qiu, M.; Li, J.; Ma, S.; Yan, Y.; Li, Y.; Han, Y. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: Results from NHANES. Front. Immunol. 2023, 14, 1087345. [Google Scholar]
- Dahabreh, I.J.; Bibbins-Domingo, K. Causal Inference About the Effects of Interventions from Observational Studies in Medical Journals. JAMA 2024, 331, 1845–1853. [Google Scholar] [CrossRef]
- Tennant, P.W.G.; Murray, E.J.; Arnold, K.F.; Berrie, L.; Fox, M.P.; Gadd, S.C.; Harrison, W.J.; Keeble, C.; Ranker, L.R.; Textor, J.; et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: Review and recommendations. Int. J. Epidemiol. 2021, 50, 620–632. [Google Scholar]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple imputation by chained equations: What is it and how does it work? Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef]
- Yu, Q.; Medeiros, K.L.; Wu, X.; Jensen, R.E. Nonlinear Predictive Models for Multiple Mediation Analysis: With an Application to Explore Ethnic Disparities in Anxiety and Depression Among Cancer Survivors. Psychometrika 2018, 83, 991–1006. [Google Scholar] [CrossRef]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Rheims, S.; Herbillon, V.; Villeneuve, N.; Auvin, S.; Napuri, S.; Cances, C.; Berquin, P.; Castelneau, P.; Nguyen The Tich, S.; Villega, F.; et al. ADHD in childhood epilepsy: Clinical determinants of severity and of the response to methylphenidate. Epilepsia 2016, 57, 1069–1077. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Danielewicz, A.; Hoffmann, G.; Schwingshackl, L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2020, 120, 1998–2031.e1915. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, R.; Zuo, Y.; Qiao, C.; Jiang, W.; Cheng, W.; Wei, W.; Liu, Z.; Geng, Y.; Dong, Y. The association of circulating systemic inflammation with premature death and the protective role of the Mediterranean diet: A large prospective cohort study of UK biobank. BMC Public Health 2024, 24, 1449. [Google Scholar] [CrossRef]
- Karhade, A.V.; Shah, K.C.; Shah, A.A.; Ogink, P.T.; Nelson, S.B.; Schwab, J.H. Neutrophil to lymphocyte ratio and mortality in spinal epidural abscess. Spine J. 2019, 19, 1180–1185. [Google Scholar] [CrossRef]
- Xu, B.; Wu, Q.; La, R.; Lu, L.; Abdu, F.A.; Yin, G.; Zhang, W.; Ding, W.; Ling, Y.; He, Z.; et al. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc. Diabetol. 2024, 23, 212. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, Y.; Gu, Y.; Xia, X. The clinical value of nutritional and inflammatory indicators in predicting pneumonia among patients with intracerebral hemorrhage. Sci. Rep. 2024, 14, 16171. [Google Scholar] [CrossRef]
- Shan, Z.; Wang, F.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; et al. Healthy Eating Patterns and Risk of Total and Cause-Specific Mortality. JAMA Intern. Med. 2023, 183, 142–153. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, N.E.; Cruijsen, E.; Visseren, F.L.; van der Schouw, Y.T.; Geleijnse, J.M.; Koopal, C. Compliance with the DASH diet and risk of all-cause and cardiovascular mortality in patients with myocardial infarction. Clin. Nutr. 2023, 42, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Liu, R.; Cheng, H.; Xu, L.; Wang, L.; He, C.; Wei, Q. Longer-Term Effects of Cardiac Telerehabilitation on Patients with Coronary Artery Disease: Systematic Review and Meta-Analysis. JMIR mHealth uHealth 2023, 11, e46359. [Google Scholar] [CrossRef]
- Banks, E.; Joshy, G.; Korda, R.J.; Stavreski, B.; Soga, K.; Egger, S.; Day, C.; Clarke, N.E.; Lewington, S.; Lopez, A.D. Tobacco smoking and risk of 36 cardiovascular disease subtypes: Fatal and non-fatal outcomes in a large prospective Australian study. BMC Med. 2019, 17, 128. [Google Scholar] [CrossRef] [PubMed]


| Total | Alive | CVD Mortality | p Value | All-Causal Mortality | p Value | |
|---|---|---|---|---|---|---|
| Age, mean (SE) | 47.07 (0.23) | 45.46 (0.22) | 70.18 (0.49) | <0.0001 | 66.89 (0.35) | <0.0001 |
| Sex, N (%) | 0.004 | <0.0001 | ||||
| Female | 17,271 (51.34) | 15,791 (51.68) | 434 (45.88) | 1480 (47.12) | ||
| Male | 16,610 (48.66) | 14,580 (48.32) | 621 (54.12) | 2030 (52.88) | ||
| Race, N (%) | <0.0001 | <0.0001 | ||||
| Mexican American | 5326 (8.36) | 5038 (8.74) | 72 (3.37) | 288 (3.65) | ||
| Non-Hispanic Black | 7253 (11.00) | 6512 (11.00) | 228 (11.84) | 741 (11.04) | ||
| Non-Hispanic White | 14,601 (67.98) | 12,437 (67.05) | 660 (78.68) | 2164 (79.38) | ||
| Other Hispanic | 3183 (5.40) | 3003 (5.66) | 60 (2.82) | 180 (2.17) | ||
| Other Race | 3518 (7.27) | 3381 (7.56) | 35 (3.29) | 137 (3.75) | ||
| Education, N (%) | <0.0001 | <0.0001 | ||||
| <High school | 3488 (5.24) | 2894 (5.40) | 185 (13.70) | 594 (12.38) | ||
| High school | 8555 (20.96) | 7216 (22.50) | 399 (38.63) | 1339 (38.55) | ||
| >High school | 18,185 (61.99) | 16,815 (72.10) | 406 (47.66) | 1370 (49.07) | ||
| Marital status, N (%) | <0.0001 | <0.0001 | ||||
| Married | 20,315 (63.85) | 18,575 (64.86) | 505 (49.86) | 1740 (51.73) | ||
| Single | 13,556 (36.12) | 11,787 (35.14) | 550 (50.14) | 1769 (48.27) | ||
| Smoke, N (%) | <0.0001 | <0.0001 | ||||
| No | 18,635 (55.04) | 17,276 (56.42) | 470 (45.33) | 1359 (38.18) | ||
| Yes | 15,236 (44.94) | 13,086 (43.58) | 585 (54.67) | 2150 (61.82) | ||
| Diabetes, N (%) | <0.0001 | <0.0001 | ||||
| No | 24,368 (77.15) | 22,514 (79.91) | 524 (53.03) | 1854 (56.55) | ||
| Yes | 8871 (21.58) | 7223 (20.09) | 530 (46.97) | 1648 (43.45) | ||
| Hypertension, N (%) | <0.0001 | <0.0001 | ||||
| No | 19,739 (62.83) | 18,758 (65.46) | 237 (23.50) | 981 (30.49) | ||
| Yes | 14,140 (37.17) | 11,612 (34.54) | 818 (76.50) | 2528 (69.51) | ||
| CVD, N (%) | <0.0001 | <0.0001 | ||||
| No | 30,286 (91.63) | 27,973 (93.54) | 591 (59.07) | 2313 (68.22) | ||
| Yes | 3591 (8.36) | 2394 (6.46) | 464 (40.93) | 1197 (31.78) | ||
| Stroke, N (%) | <0.0001 | <0.0001 | ||||
| No | 32,567 (97.11) | 29,503 (97.90) | 890 (86.46) | 3064 (88.61) | ||
| Yes | 1273 (2.79) | 838 (2.10) | 160 (13.54) | 435 (11.39) | ||
| ASCVD, N (%) | <0.0001 | <0.0001 | ||||
| No | 30,611 (92.35) | 28,164 (94.02) | 638 (63.06) | 2447 (71.97) | ||
| Yes | 3265 (7.64) | 2202 (5.98) | 417 (36.94) | 1063 (28.03) | ||
| Heart attack, N (%) | <0.0001 | <0.0001 | ||||
| No | 32,402 (96.60) | 29,422 (97.55) | 839 (81.40) | 2980 (86.25) | ||
| Yes | 1432 (3.30) | 912 (0.45) | 213 (18.60) | 520 (13.75) | ||
| DII, mean (SE) | 1.39 (0.03) | 1.37 (0.03) | 1.65 (0.07) | <0.0001 | 1.68 (0.04) | <0.0001 |
| HEI2015, mean (SE) | 50.71 (0.19) | 50.63 (0.19) | 52.20 (0.53) | 0.004 | 51.71 (0.35) | 0.002 |
| DASH, mean (SE) | 2.38 (0.02) | 2.37 (0.02) | 2.54 (0.07) | 0.01 | 2.49 (0.04) | 0.001 |
| CDAI, mean (SE) | 0.84 (0.04) | 0.90 (0.04) | 0.06 (0.13) | <0.0001 | 0.08 (0.08) | <0.0001 |
| MED, mean (SE) | 3.50 (0.02) | 3.50 (0.02) | 3.56 (0.06) | 0.22 | 3.47 (0.04) | 0.48 |
| AHEI, mean (SE) | 39.08 (0.19) | 39.12 (0.20) | 39.05 (0.49) | 0.24 | 38.66 (0.32) | 0.14 |
| HEI2020, mean (SE) | 51.46 (0.18) | 51.40 (0.18) | 53.08 (0.52) | 0.004 | 52.17 (0.33) | 0.02 |
| MEDI, mean (SE) | 3.59 (0.02) | 3.59 (0.02) | 3.49 (0.05) | <0.001 | 3.48 (0.03) | <0.001 |
| DASHI, mean (SE) | 3.52 (0.01) | 3.52 (0.01) | 3.62 (0.06) | 0.16 | 3.55 (0.03) | 0.24 |
| PLR, M (IQR) | 118.57 (94.50, 148.57) | 118.18 (94.41, 147.50) | 130.00 (98.67, 178.00) | <0.0001 | 125.29 (95.93, 168.62) | <0.0001 |
| LMR, M (IQR) | 3.80 (3.00, 4.80) | 3.83 (3.00, 4.83) | 3.00 (2.20, 4.00) | <0.0001 | 3.17 (2.33, 4.20) | <0.0001 |
| PAR, M (IQR) | 33.62 (16.51, 65.71) | 34.81 (17.50, 67.30) | 16.64 (5.30, 36.17) | <0.0001 | 18.49 (6.09, 42.45) | <0.0001 |
| NPR, M (IQR) | 0.02 (0.01, 0.02) | 0.02 (0.01, 0.02) | 0.02 (0.01, 0.02) | <0.0001 | 0.02 (0.01, 0.02) | <0.0001 |
| ELR, M (IQR) | 0.08 (0.05, 0.13) | 0.08 (0.05, 0.13) | 0.11 (0.06, 0.17) | <0.0001 | 0.10 (0.06, 0.15) | <0.0001 |
| CRP, M (IQR) | 0.18 (0.07, 0.43) | 0.17 (0.07, 0.41) | 0.26 (0.11, 0.60) | <0.0001 | 0.24 (0.10, 0.57) | <0.0001 |
| TyG, mean (SE) | 8.60 (0.01) | 8.58 (0.01) | 8.84 (0.03) | <0.0001 | 8.81 (0.02) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Wang, Q.; Liu, X.; Zhou, M.; Feng, Z.; Ma, X.; Li, J.; Gan, R.; Wang, X.; Li, K. Causal Inference Framework Reveals Mediterranean Diet Superiority and Inflammatory Mediation Pathways in Mortality Prevention: A Comparative Analysis of Nine Common Dietary Patterns. Foods 2025, 14, 3122. https://doi.org/10.3390/foods14173122
Lin J, Wang Q, Liu X, Zhou M, Feng Z, Ma X, Li J, Gan R, Wang X, Li K. Causal Inference Framework Reveals Mediterranean Diet Superiority and Inflammatory Mediation Pathways in Mortality Prevention: A Comparative Analysis of Nine Common Dietary Patterns. Foods. 2025; 14(17):3122. https://doi.org/10.3390/foods14173122
Chicago/Turabian StyleLin, Jianlin, Qiletian Wang, Xiaoxia Liu, Miao Zhou, Zhongwen Feng, Xiuling Ma, Junrong Li, Renyou Gan, Xu Wang, and Kefeng Li. 2025. "Causal Inference Framework Reveals Mediterranean Diet Superiority and Inflammatory Mediation Pathways in Mortality Prevention: A Comparative Analysis of Nine Common Dietary Patterns" Foods 14, no. 17: 3122. https://doi.org/10.3390/foods14173122
APA StyleLin, J., Wang, Q., Liu, X., Zhou, M., Feng, Z., Ma, X., Li, J., Gan, R., Wang, X., & Li, K. (2025). Causal Inference Framework Reveals Mediterranean Diet Superiority and Inflammatory Mediation Pathways in Mortality Prevention: A Comparative Analysis of Nine Common Dietary Patterns. Foods, 14(17), 3122. https://doi.org/10.3390/foods14173122








