Comprehensive Characterization of the Microbiological and Quality Attributes of Traditional Sicilian Canestrato Fresco Cheese
Abstract
1. Introduction
2. Materials and Methods
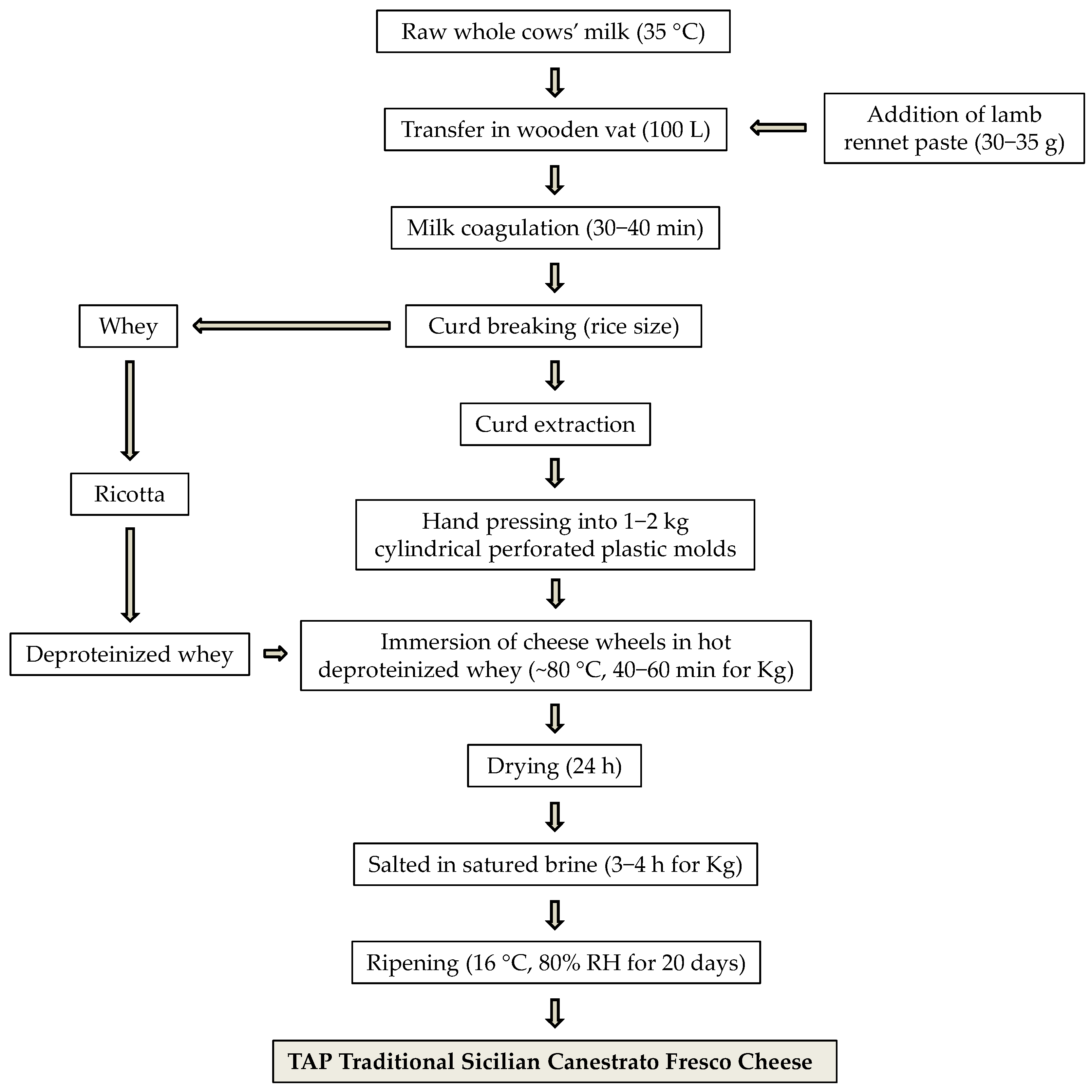
2.1. Sample Collection
2.2. Culture-Dependent Microbiological Assessment of Cheeses
2.3. Culture-Independent Profiling of the Bacterial Community of Cheeses
2.4. Proximate Analysis and Antioxidant Properties of Cheeses
2.5. Determination of Cheese Fatty Acids
2.6. Volatile Organic Compound Analysis
2.7. Sensory Analysis
2.8. Statistical Analyses
3. Results and Discussion
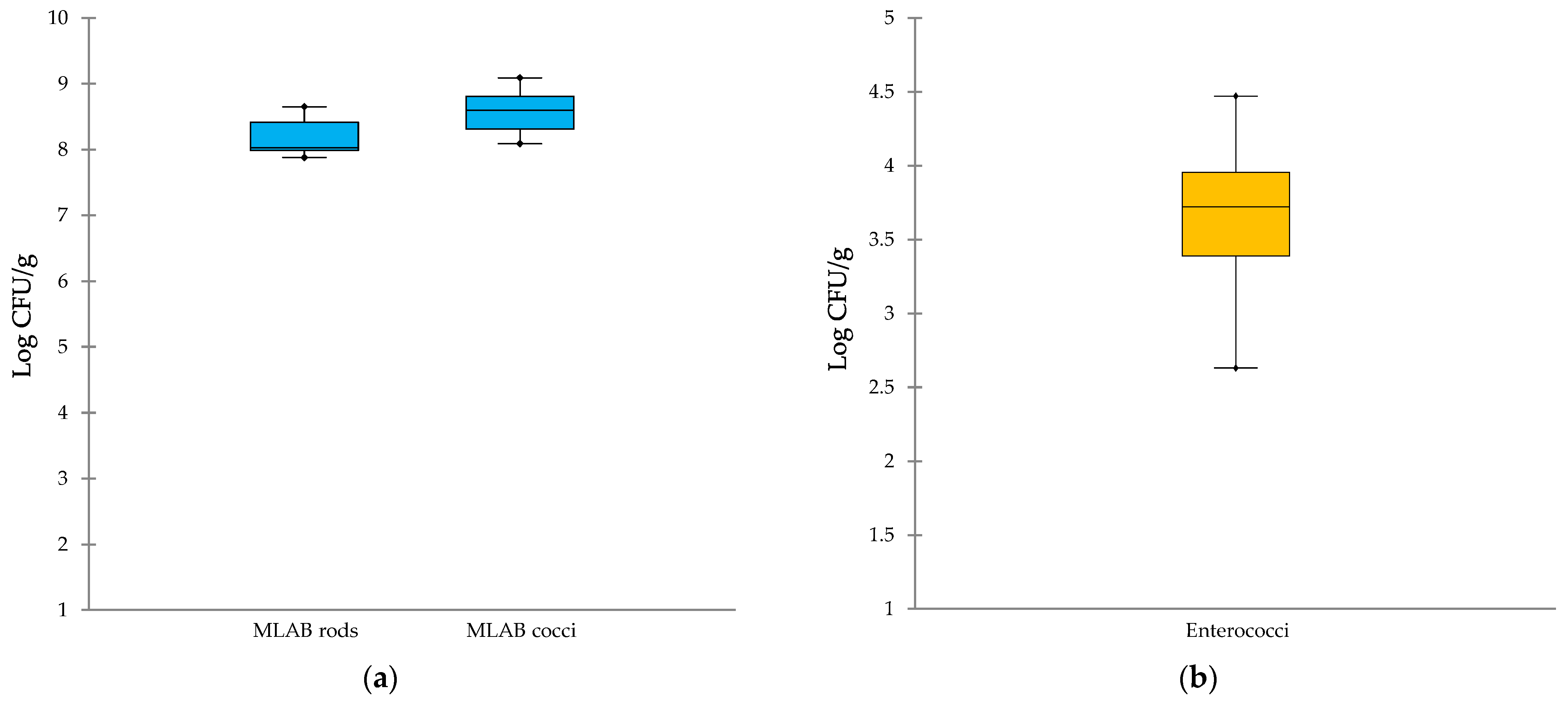
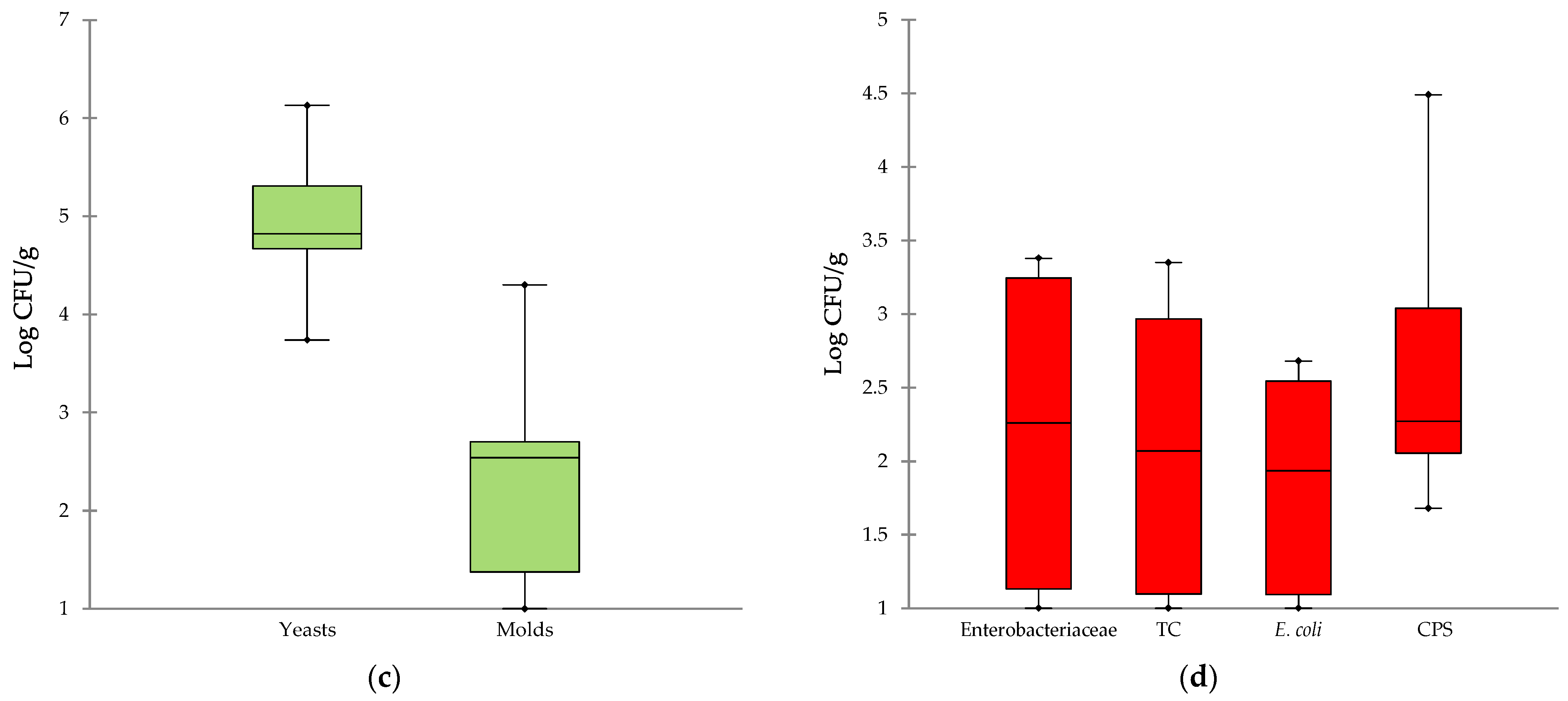
3.1. Microbiological Profile of Cheeses
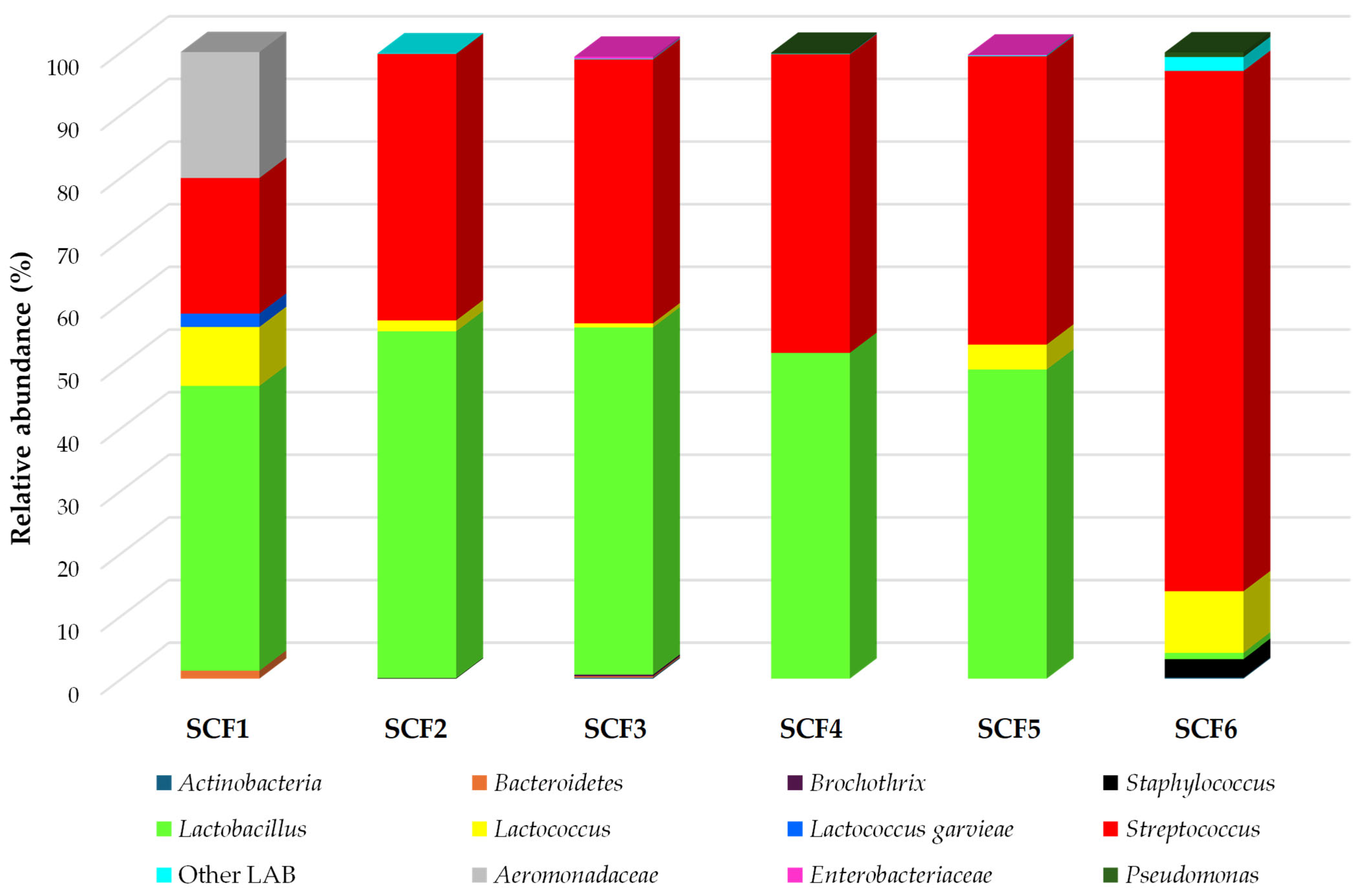
3.2. Composition of Cheese Bacterial Communities
3.3. Proximate Composition and Antioxidant Properties of Cheeses
3.4. Fatty Acid Composition of Cheeses
3.5. Volatilome Composition
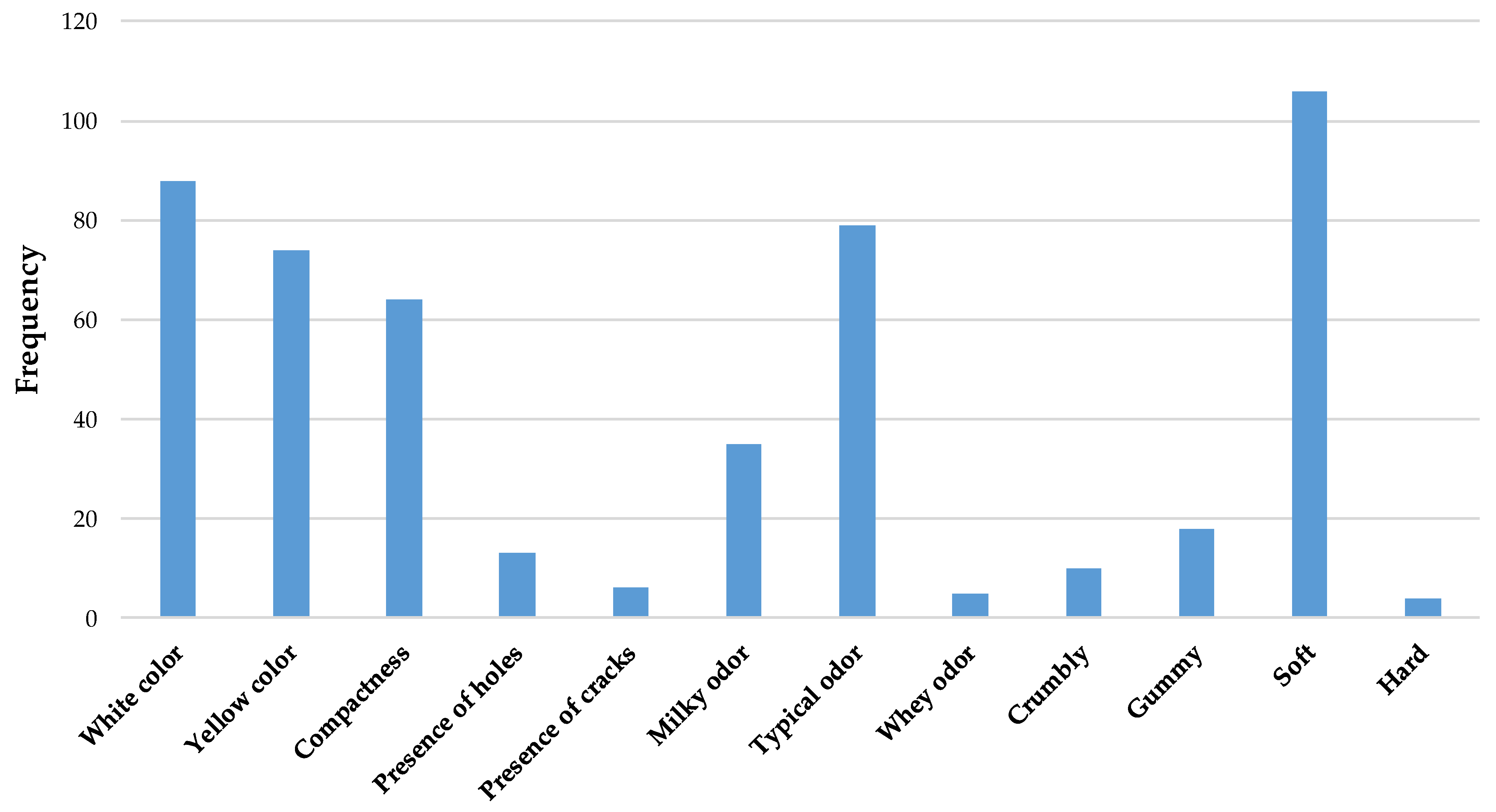
3.6. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angulo, J.T.; Barría, L.T.; Ferrari, C.B.; Valenzuela, S.; Aravena, S.C.G.; Chepo, M.M. The Artisanal Cheese Factory in the Context of the Araucanía Region–Chile. Int. J. Membr. Sci. Technol. 2023, 10, 1414–1423. [Google Scholar] [CrossRef]
- D’Angelo, E.A. Resource-Based Perspective to Assess Firms’ Profitability in the Food Industry: Evidence from the Italian Cheese Industry. Eur. Sci. J. 2018, 14, 1–12. [Google Scholar] [CrossRef][Green Version]
- Ruta, S.; Belvedere, G.; Licitra, G.; Nero, L.A.; Caggia, C.; Randazzo, C.L.; Caccamo, M. Recent Insights on the Multifaceted Roles of Wooden Tools in Cheese-Making: A Review of Their Impacts on Safety and Final Traits of Traditional Cheeses. Int. J. Food Microbiol. 2025, 435, 111179. [Google Scholar] [CrossRef]
- Cruciata, M.; Gaglio, R.; Todaro, M.; Settanni, L. Ecology of Vastedda della valle del Belìce cheeses: A review and recent findings to stabilize the traditional production. Food Rev. Int. 2019, 35, 90–103. [Google Scholar] [CrossRef]
- ISMEA; Fondazione Qualivita. Rapporto Ismea–Qualivita sulle Produzioni Agroalimentari e Vitivinicole Italiane DOP, IGP e STG; Edizioni Qualivita: Siena, Italy, 2024; ISBN 978-88-96530-58-0. Available online: https://www.qualivita.it/attivita/rapporto-ismea-qualivita-2024/ (accessed on 14 July 2025).
- Gazzetta Ufficiale della Repubblica Italiana. Decreto Ministeriale 29 Febbraio 2024: Aggiornamento dell’Elenco Nazionale dei Prodotti Agroalimentari Tradizionali ai sensi dell’Art. 12, Comma 1, della Legge 12 Dicembre 2016, n. 238 (24A01305). G.U. Serie Gen. 2024, 61. Available online: https://www.gazzettaufficiale.it/eli/id/2024/03/13/24A01305/SG (accessed on 14 July 2025).
- Calmiere dei Latticini; Archivio Comunale di Palermo: Palermo, Italy, 1407; Volume 22, p. 44.
- Calmiere Imposto dai Giurati e Probiviri per la Vendita al Minuto nei Mercati; Archivio Comunale di Palermo: Palermo, Italy, 1412; Volume 23, p. 2.
- Assolatte. Formaggi Freschi Italiani: Primosale; Associazione Italiana Lattiero Casearia: Milano, Italy, 2022. [Google Scholar]
- ONAF. Organizzazione Nazionale Assaggiatori di Formaggi. Scheda tecnica: Canestrato Vacchino. Available online: https://www.onaf.it/uploads/public/15327_canestrato-vacchino.pdf (accessed on 14 July 2025).
- Donnelly, C.W. Growth and survival of microbial pathogens in cheese. In Cheese: Chemistry, Physics and Microbiology; Chapman & Hall: London, UK, 2004; pp. 541–560. [Google Scholar]
- Giammanco, G.M.; Pepe, A.; Aleo, A.; D’Agostino, V.; Milone, S.; Mammina, C. Microbiological Quality of Pecorino siciliano “Primosale” Cheese on Retail Sale in the Street Markets of Palermo, Italy. New Microbiol. 2011, 34, 179–185. [Google Scholar]
- Regione Siciliana. Assessorato dell’Agricoltura e delle Foreste. Decreto 28 dicembre 1998. Riconoscimento di prodotti a base di latte come prodotti storici fabbricati tradizionalmente. Gazz. Uff. Reg. Sicil. 1999, Parte I, 15. Available online: http://www.gurs.regione.sicilia.it/Gazzette/g99-6-b.htm (accessed on 14 July 2025).
- ISO 6579-1; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 11290-1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Busetta, G.; Garofalo, G.; Ponte, M.; Barbera, M.; Alfonzo, A.; Franciosi, E.; Francesca, N.; Frusteri, G.; Piazzese, D.; Bonanno, A.; et al. Replacing Preservative E 252 with Powdered Dried Sumac (Rhus coriaria L.) Fruits in “Suino Nero dei Nebrodi” Salamis: Effects on Microbiological, Physicochemical, and Antioxidant Properties. Food Microbiol. 2025, 127, 104684. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Scatassa, M.L.; Miraglia, V.; Carrozzo, A.; Ducato, B.; Lazzara, F.; Todaro, M.; Mancuso, I. Determination of Chemical Parameters by Means of NIR Technology (FoodScan™ Dairy Analyser) in “Caciocavallo Palermitano” Cheese Samples. In Proceedings of the XIV Congresso Nazionale S.I.Di.L.V., Sorrento, Italy, 24–26 October 2012; Società Italiana di Diagnostica di Laboratorio Veterinaria (SIDiLV): Parma, Italy, 2012; pp. 482–483. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Axidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Mannino, G.; Perrone, A.; Campobenedetto, C.; Schittone, A.; Bertea, C.M.; Gentile, C. Phytochemical Profile and Antioxidative Properties of Plinia trunciflora fruits: A New Source of Nutraceuticals. Food Chem. 2020, 307, 125515. [Google Scholar] [CrossRef] [PubMed]
- De Jong, C.; Badings, H.T. Determination of Free Fatty Acids in Milk and Cheese: Procedures for Extraction, Clean-Up, and Capillary Gas Chromatographic Analysis. J. High Resolut. Chromatogr. 1990, 13, 94–98. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Tweed, J.K.S. Isomerisation of cis-9 trans-11 Conjugated Linoleic Acid (CLA) to trans-9 trans-11 CLA During Acidic Methylation can be Avoided by a Rapid Base Catalysed Methylation of Milk Fat. J. Dairy Res. 2008, 75, 354–356. [Google Scholar] [CrossRef][Green Version]
- Ares, G.; Jaeger, S.R. Check-All-That-Apply Questions: Influence of Attribute Order on Sensory Product Characterization. Food Qual. Prefer. 2013, 28, 141–153. [Google Scholar] [CrossRef]
- Ares, G.; Barreiro, C.; Deliza, R.; Giménez, A.; Gámbaro, A. Application of a Check-All-That-Apply Question to the Development of Chocolate Milk Desserts. J. Sens. Stud. 2010, 25, 67–86. [Google Scholar] [CrossRef]
- Delarue, J.; Sieffermann, J.M. Sensory Mapping Using Flash Profile. Comparison with a Conventional Descriptive Method for the Evaluation of the Flavor of Fruit Dairy Products. Food Qual. Prefer. 2004, 15, 383–392. [Google Scholar] [CrossRef]
- Quintieri, L.; Caputo, L.; Brasca, M.; Fanelli, F. Recent Advances in the Mechanisms and Regulation of QS in Dairy Spoilage by Pseudomonas spp. Foods 2021, 10, 3088. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Garofalo, G.; Busetta, G.; Gannuscio, R.; Di Rosa, A.R.; Scatassa, M.L.; Cardamone, C.; Mancuso, I.; Franciosi, E.; Rando, F.; et al. Reduction of PDO Pecorino Siciliano Cheese Making Duration: Microbial Dynamics and Quality Attributes Deriving from Replacing Whey Permeate with Hot Water During Cooking. Int. J. Food Microbiol. 2024, 410, 110481. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Kim, S.H. Recent Biotechnological Trends in Lactic Acid Bacterial Fermentation for Food Processing Industries. Syst. Microbiol. Biomanuf. 2022, 2, 14–40. [Google Scholar] [CrossRef]
- Bondi, M.; Laukova, A.; de Niederhausern, S.; Messi, P.; Papadopoulou, C.; Economou, V. Controversial Aspects Displayed by Enterococci: Probiotics or Pathogens? BioMed Res. Int. 2020, 2020, 9816185. [Google Scholar] [CrossRef]
- Graham, K.; Stack, H.; Rea, R. Safety, Beneficial and Technological Properties of Enterococci for Use in Functional Food Applications—A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3836–3861. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Teso-Pérez, C.; López-Gazcón, A.; Peralta-Sánchez, J.M.; Martínez-Bueno, M.; Valdivia, E.; Fárez-Vidal, M.E.; Martín-Platero, A.M. Bacteriocin-Producing Enterococci Modulate Cheese Microbial Diversity. Microb. Ecol. 2024, 87, 175. [Google Scholar] [CrossRef]
- Gaglio, R.; Barbaccia, P.; Barbera, M.; Restivo, I.; Attanzio, A.; Maniaci, G.; Di Grigoli, A.; Francesca, N.; Tesoriere, L.; Bonanno, A.; et al. The Use of Winery By-Products to Enhance the Functional Aspects of the Fresh Ovine “Primosale” Cheese. Foods 2021, 10, 461. [Google Scholar] [CrossRef]
- Minj, J.; Sudhakaran, A.V.; Kumari, A. The Role of Yeast and Molds in Dairy Industry: An Update. In Dairy Processing: Advanced Research to Applications; Minj, J., Sudhakaran, A.V., Kumari, A., Eds.; Springer: Singapore, 2020; pp. 243–262. [Google Scholar]
- Lavoie, K.; Touchette, M.; St-Gelais, D.; Labrie, S. Characterization of the Fungal Microflora in Raw Milk and Specialty Cheeses of the Province of Quebec. Dairy Sci. Technol. 2012, 92, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Banjara, N.; Suhr, M.J.; Hallen-Adams, H.E. Diversity of Yeast and Mold Species from a Variety of Cheese Types. Curr. Microbiol. 2015, 70, 792–800. [Google Scholar] [CrossRef]
- Montel, M.C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional Cheeses: Rich and Diverse Microbiota with Associated Benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Current Pathogenic Escherichia coli Foodborne Outbreak Cases and Therapy Development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Jagadeesan, B.; Gerner-Smidt, P.; Allard, M.W.; Leuillet, S.; Winkler, A.; Xiao, Y.; Chaffron, S.; Van Der Vossen, J.; Tang, S.; Katase, M.; et al. The Use of Next Generation Sequencing for Improving Food Safety: Translation into Practice. Food Microbiol. 2019, 79, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Barbaccia, P.; Bonanno, A.; Ponte, M.; Di Gerlando, R.; Franciosi, E.; Di Grigoli, A.; Gaglio, R. Evolution of Indigenous Starter Microorganisms and Physicochemical Parameters in Spontaneously Fermented Beef, Horse, Wild Boar and Pork Salamis Produced under Controlled Conditions. Food Microbiol. 2020, 87, 103385. [Google Scholar] [CrossRef]
- Logares, R.; Audic, S.; Bass, D.; Bittner, L.; Boutte, C.; Christen, R.; Claverie, J.M.; Decelle, J.; Dolan, J.R.; Dunthorn, M.; et al. Patterns of Rare and Abundant Marine Microbial Eukaryotes. Curr. Biol. 2014, 24, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and Disadvantages of Non-Starter Lactic Acid Bacteria from Traditional Fermented Foods: Potential Use as Starters or Probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef]
- Yeluri Jonnala, B.R.; McSweeney, P.L.H.; Sheehan, J.J.; Cotter, P.D. Sequencing of the Cheese Microbiome and Its Relevance to Industry. Front. Microbiol. 2018, 9, 1020. [Google Scholar] [CrossRef]
- Settanni, L.; Busetta, G.; Puccio, V.; Licitra, G.; Franciosi, E.; Botta, L.; Di Gerlando, R.; Todaro, M.; Gaglio, R. In-Depth Investigation of the Safety of Wooden Shelves Used for Traditional Cheese Ripening. Appl. Environ. Microbiol. 2021, 87, e01524-21. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, T.; Fagan, C.C.; O’Donnell, C.P.; Downey, G. Application of Near and Mid-Infrared Spectroscopy to Determine Cheese Quality and Authenticity. Food Bioprocess Technol. 2008, 1, 117–129. [Google Scholar] [CrossRef]
- De Marchi, M.; Toffanin, V.; Cassandro, M.; Penasa, M. Invited Review: Midinfrared Spectroscopy as Phenotyping Tool for Milk Traits. J. Dairy Sci. 2014, 97, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Neviani, E.; Fox, P. The Most Traditional and Popular Italian Cheeses. In The Cheeses of Italy: Science and Technology; Gobbetti, M., Neviani, E., Fox, P., Eds.; Springer: Cham, Switzerland, 2018; pp. 99–274. [Google Scholar]
- Cutrim, C.S.; Cortez, M.A.S. A Review on Polyphenols: Classification, Beneficial Effects and Their Application in Dairy Products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choińska, R.; Marszałek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the Correlation Between the Molecular Structure and Biological Activities of Metal–Phenolic Compound Complexes: Research and Description of the Role of Metal Ions in Improving the Antioxidant Activities of Phenolic Compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef]
- Nájera, A.I.; Nieto, S.; Barron, L.J.R.; Albisu, M. A Review of the Preservation of Hard and Semi-Hard Cheeses: Quality and Safety. Int. J. Environ. Res. Public Health 2021, 18, 9789. [Google Scholar] [CrossRef]
- Franceschi, P.; Formaggioni, P.; Brasca, M.; Natrella, G.; Faccia, M.; Malacarne, M.; Summer, A. Fatty Acids Composition and Lipolysis of Parmigiano Reggiano PDO Cheese: Effect of the Milk Cooling Temperature at the Farm. Anim. Biosci. 2023, 36, 132–143. [Google Scholar] [CrossRef]
- Faccia, M.; Natrella, G.; Gambacorta, G.; Trani, A. Cheese Ripening in Nonconventional Conditions: A Multiparameter Study Applied to Protected Geographical Indication Canestrato di Moliterno Cheese. J. Dairy Sci. 2022, 105, 140–153. [Google Scholar] [CrossRef]
- Litrenta, F.; Lopreiato, V.; Potortì, A.G.; Lo Turco, V.; Randazzo, C.L.; Nava, V.; Cavallo, C.; Rando, R.; Di Bella, G.; Liotta, L. The Identification of Potential Nutritional and Sensory Markers for the Authentication of an Innovative Canestrato Cheese Based on Plant-Derived Rennet. Dairy 2024, 5, 828–841. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; Volume 1, p. 271. [Google Scholar]
- Careri, M.; Spagnoli, S.; Panari, G.; Zannoni, M.; Barbieri, G. Chemical Parameters of the Non-Volatile Fraction of Ripened Parmigiano-Reggiano Cheese. Int. Dairy J. 1996, 6, 147–155. [Google Scholar] [CrossRef]
- Boland, M.; MacGibbon, A.; Hill, J. Designer Milks for the New Millennium. Livest. Prod. Sci. 2001, 72, 99–109. [Google Scholar] [CrossRef]
- Bittante, G.; Amalfitano, N.; Tagliapietra, F.; Schiavon, S.; Cipolat-Gotet, C.; Stocco, G. Characterization of the Detailed Fatty Acid Profiles of a Large Number of Types of Cheese from the Mountains and Plains. Foods 2024, 13, 4040. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant Milk Fat Plasticity: Nutritional Control of Saturated, Polyunsaturated, Trans and Conjugated Fatty Acids. Ann. Zootech. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Diezhandino, I.; Fernández, D.; Abarquero, D.; Prieto, B.; Renes, E.; Fresno, J.M.; Tornadijo, M.E. Changes in the Concentration and Profile of Free Fatty Acids during the Ripening of a Spanish Blue-Veined Cheese Made from Raw and Pasteurized Cow and Goat Milk. Dairy 2023, 4, 222–234. [Google Scholar] [CrossRef]
- Jenkins, T.C.; McGuire, M.A. Major Advances in Nutrition: Impact on Milk Composition. J. Dairy Sci. 2006, 89, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Manuelian, C.L.; Currò, S.; Penasa, M.; Cassandro, M.; De Marchi, M. Characterization of Major and Trace Minerals, Fatty Acid Composition, and Cholesterol Content of Protected Designation of Origin Cheeses. J. Dairy Sci. 2017, 100, 3384–3395. [Google Scholar] [CrossRef]
- Sulejmani, E.; Hayaloglu, A.A. Influence of Starter Culture on Nitrogen Fraction and Volatile Compounds in Beaten Cow’s Milk Cheese. J. Food Process. Preserv. 2020, 44, e14689. [Google Scholar] [CrossRef]
- Wolf, I.V.; Perotti, M.C.; Zalazar, C.A. Composition and Volatile Profiles of Commercial Argentinean Blue Cheeses. J. Sci. Food Agric. 2011, 91, 385–393. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Sampaio, P. Volatile Fraction of DOP “Castelo Branco” Cheese: Influence of Breed. Food Chem. 2009, 112, 1053–1059. [Google Scholar] [CrossRef]
- McSweeney, P.L.; Sousa, M.J. Biochemical Pathways for the Production of Flavour Compounds in Cheeses during Ripening: A Review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key Odorants in Various Cheese Types as Determined by Gas Chromatography-Olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, F.; Song, Y.; Lou, Y.; Zhao, A.; Liu, Y.; Peng, H.; Hui, Y.; Ren, R.; Wang, B. Physicochemical and Textural Characteristics and Volatile Compounds of Semihard Goat Cheese as Affected by Starter Cultures. J. Dairy Sci. 2021, 104, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Pinho, O.; Pérès, C.; Ferreira, I.M.P.L.V.O. Solid-Phase Microextraction of Volatile Compounds in “Terrincho” Ewe Cheese: Comparison of Different Fibers. J. Chromatogr. A 2003, 1011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ziino, M.; Condurso, C.; Romeo, V.; Giuffrida, D.; Verzera, A. Characterization of “Provola dei Nebrodi”, a Typical Sicilian Cheese, by Volatiles Analysis Using SPME-GC/MS. Int. Dairy J. 2005, 15, 585–593. [Google Scholar] [CrossRef]
- Tekin, A.; Hayaloglu, A.A. Understanding the Mechanism of Ripening Biochemistry and Flavour Development in Brine Ripened Cheeses. Int. Dairy J. 2023, 137, 105508. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.J.; Wang, Y.D.; Cao, Y.P.; Wang, B.; Liu, Y. The Key Aroma Compounds and Sensory Characteristics of Commercial Cheddar Cheeses. J. Dairy Sci. 2021, 104, 7555–7571. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, W.; Yu, H.; Yuan, J.; Tian, H. Characterization of Major Odor-Active Compounds Responsible for Nutty Flavor in Cheddar Cheese According to Chinese Taste. Flavour Fragr. J. 2021, 36, 171–181. [Google Scholar] [CrossRef]
- Gan, H.H.; Yan, B.; Linforth, R.S.T.; Fisk, I.D. Development and Validation of an APCI-MS/GC–MS Approach for the Classification and Prediction of Cheddar Cheese Maturity. Food Chem. 2016, 190, 442–447. [Google Scholar] [CrossRef]
- Beltrán Sanahuja, A.; Pesci de Almeida, R.; Igler Marí, K.A.; Lamadrid, M.C.; Valdés García, A.; Nadal, E.S. Sensory Attributes and Instrumental Chemical Parameters of Commercial Spanish Cured Ewes’ Milk Cheeses: Insights into Cheese Quality Figures. Foods 2023, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Martín Miguélez, J.M.; Martín, I.; Robledo, J.; Ventanas, S.; Córdoba, J.J. Effect of Artisanal Processing on Volatile Compounds and Sensory Characteristics of Traditional Soft-Ripened Cheeses Matured with Selected Lactic Acid Bacteria. Foods 2025, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Trani, A.; Gambacorta, G.; Loizzo, P.; Cassone, A.; Faccia, M. Chemical and Sensory Characteristics of Canestrato di Moliterno Cheese Manufactured in Spring. J. Dairy Sci. 2016, 99, 6080–6085. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, V. (Ed.) Manuale Lattiero Caseario; Tecniche Nuove: Milano, Italy, 2011. [Google Scholar]


| Parameters | Samples | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| SCF1 | SCF2 | SCF3 | SCF4 | SCF5 | SCF6 | |||
| Moisture (%) | 36.11 | 36.55 | 35.42 | 36.48 | 34.81 | 37.52 | 0.27 | 0.707 |
| Protein (%) | 28.37 | 25.81 | 27.63 | 26.78 | 26.02 | 25.56 | 0.28 | 0.572 |
| Fat (%) | 28.71 | 27.42 | 27.30 | 26.38 | 30.82 | 27.87 | 0.30 | 0.187 |
| NaCl (%) | 2.03 | 1.53 | 1.58 | 1.74 | 1.73 | 1.91 | 0.04 | 0.153 |
| TPC (mg GAE/100 g FW) | 130.06 | 130.45 | 126.41 | 130.84 | 140.04 | 132.87 | 0.84 | 0.125 |
| FRAP (µM TE/100 g FW) | 18.71 | 21.51 | 19.14 | 21.16 | 20.02 | 18.99 | 0.20 | 0.057 |
| Fatty Acids (g/100 g FA) | Samples | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| SCF1 | SCF2 | SCF3 | SCF4 | SCF5 | SCF6 | |||
| Caproic acid (C6:0) | 2.41 | 2.40 | 2.46 | 2.44 | 2.39 | 2.48 | 0.02 | 0.781 |
| Caprylic acid (C8:0) | 1.61 | 1.72 | 1.70 | 1.74 | 1.69 | 1.63 | 0.01 | 0.083 |
| Capric acid (C10:0) | 3.75 | 3.71 | 3.85 | 3.72 | 3.80 | 3.79 | 0.02 | 0.622 |
| Lauric acid (C12:0) | 4.38 | 4.49 | 4.40 | 4.50 | 4.39 | 4.46 | 0.02 | 0.855 |
| Myristic acid (C14:0) | 12.90 | 12.85 | 12.95 | 12.80 | 12.83 | 13.00 | 0.06 | 0.995 |
| Pentadecanoic acid (C15:0) | 1.35 | 1.40 | 1.36 | 1.39 | 1.38 | 1.37 | 0.01 | 0.889 |
| Palmitic acid (C16:0) | 34.26 | 34.39 | 34.19 | 34.80 | 34.44 | 34.66 | 0.15 | 0.990 |
| Palmitoleic acid (C16:1) | 1.95 | 2.00 | 1.92 | 1.98 | 1.96 | 2.01 | 0.01 | 0.771 |
| Stearic acid (C18:0) | 11.77 | 11.82 | 11.77 | 11.68 | 11.80 | 11.76 | 0.04 | 0.858 |
| Oleic acid [C18:1 (cis)] | 20.65 | 20.70 | 21.02 | 20.40 | 20.55 | 20.45 | 0.10 | 0.950 |
| Oleic acid [C18:1 (trans)] | 1.55 | 1.50 | 1.46 | 1.4 | 1.42 | 1.49 | 0.01 | 0.196 |
| Linoleic acid (C18:2) | 2.92 | 2.91 | 2.89 | 2.86 | 2.90 | 2.85 | 0.01 | 0.972 |
| ∑SCFA | 7.91 | 7.83 | 8.01 | 7.90 | 7.88 | 7.96 | n.e. | n.e. |
| ∑MCFA | 17.31 | 17.34 | 17.48 | 17.39 | 17.20 | 17.56 | n.e. | n.e. |
| ∑LCFA | 74.46 | 74.72 | 74.61 | 74.58 | 74.45 | 74.59 | n.e. | n.e. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisana, C.; Caccamo, M.; Barbera, M.; Marino, G.; Serio, G.; Franciosi, E.; Settanni, L.; Gaglio, R.; Caggia, C. Comprehensive Characterization of the Microbiological and Quality Attributes of Traditional Sicilian Canestrato Fresco Cheese. Foods 2025, 14, 3123. https://doi.org/10.3390/foods14173123
Pisana C, Caccamo M, Barbera M, Marino G, Serio G, Franciosi E, Settanni L, Gaglio R, Caggia C. Comprehensive Characterization of the Microbiological and Quality Attributes of Traditional Sicilian Canestrato Fresco Cheese. Foods. 2025; 14(17):3123. https://doi.org/10.3390/foods14173123
Chicago/Turabian StylePisana, Chiara, Margherita Caccamo, Marcella Barbera, Giovanni Marino, Graziella Serio, Elena Franciosi, Luca Settanni, Raimondo Gaglio, and Cinzia Caggia. 2025. "Comprehensive Characterization of the Microbiological and Quality Attributes of Traditional Sicilian Canestrato Fresco Cheese" Foods 14, no. 17: 3123. https://doi.org/10.3390/foods14173123
APA StylePisana, C., Caccamo, M., Barbera, M., Marino, G., Serio, G., Franciosi, E., Settanni, L., Gaglio, R., & Caggia, C. (2025). Comprehensive Characterization of the Microbiological and Quality Attributes of Traditional Sicilian Canestrato Fresco Cheese. Foods, 14(17), 3123. https://doi.org/10.3390/foods14173123









