Mechanism Insights in Freeze–Thaw Process Impacting Cold Denaturation of Gluten Proteins During Frozen Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gluten Samples
2.3. Water-Holding and Oil-Holding Capacity Analysis
2.4. Freezable Water Content Analysis
2.5. Surface Hydrophobicity Analysis
2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
2.7. Raman Spectroscopy
2.8. Fluorescence Quenching Experiments
2.9. Molecular Dynamics Simulations
2.10. Statistical Analysis
3. Results
3.1. Changes in Water-Holding and Oil-Holding Capacities
3.2. Changes in Freezable Water Content
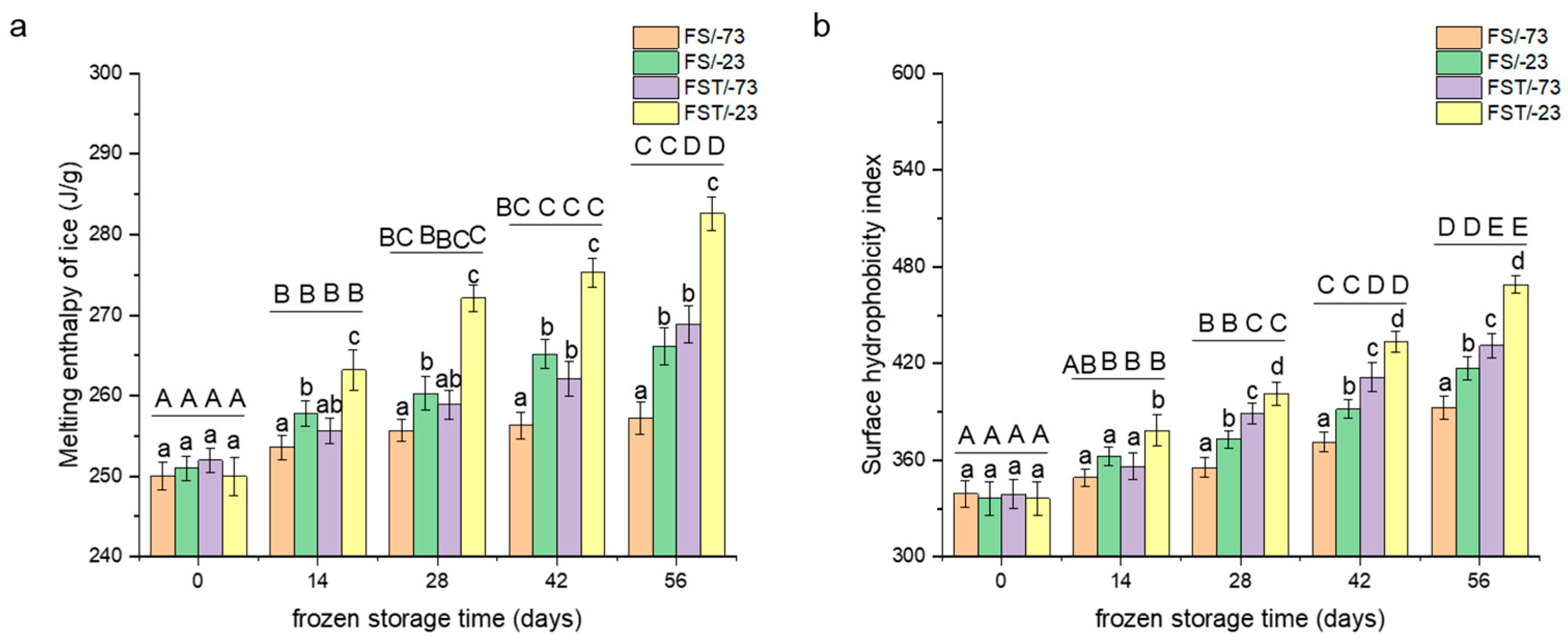
3.3. Changes in Surface Hydrophobicity
3.4. Changes in Secondary Structure
3.5. Changes in Aromatic Amino Acid Microenvironment
3.6. Fluorescence Quenching Analysis
3.7. Molecular Dynamics Simulation Analysis
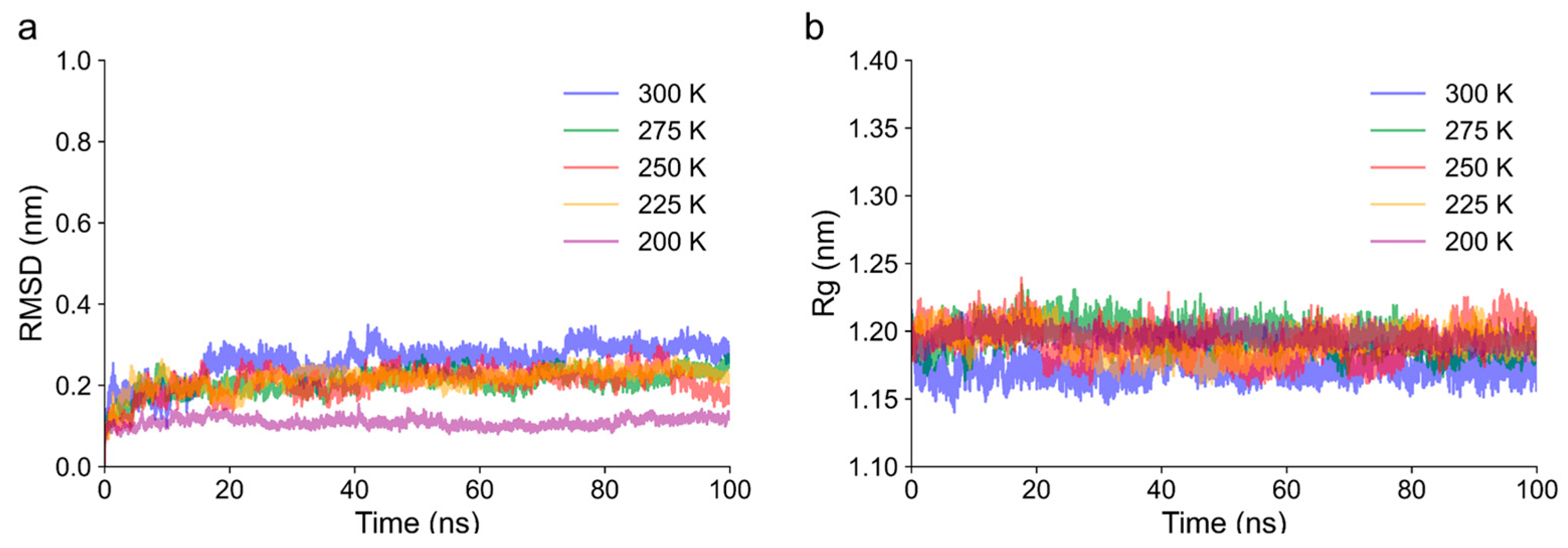
3.7.1. Structural Stability and Flexibility
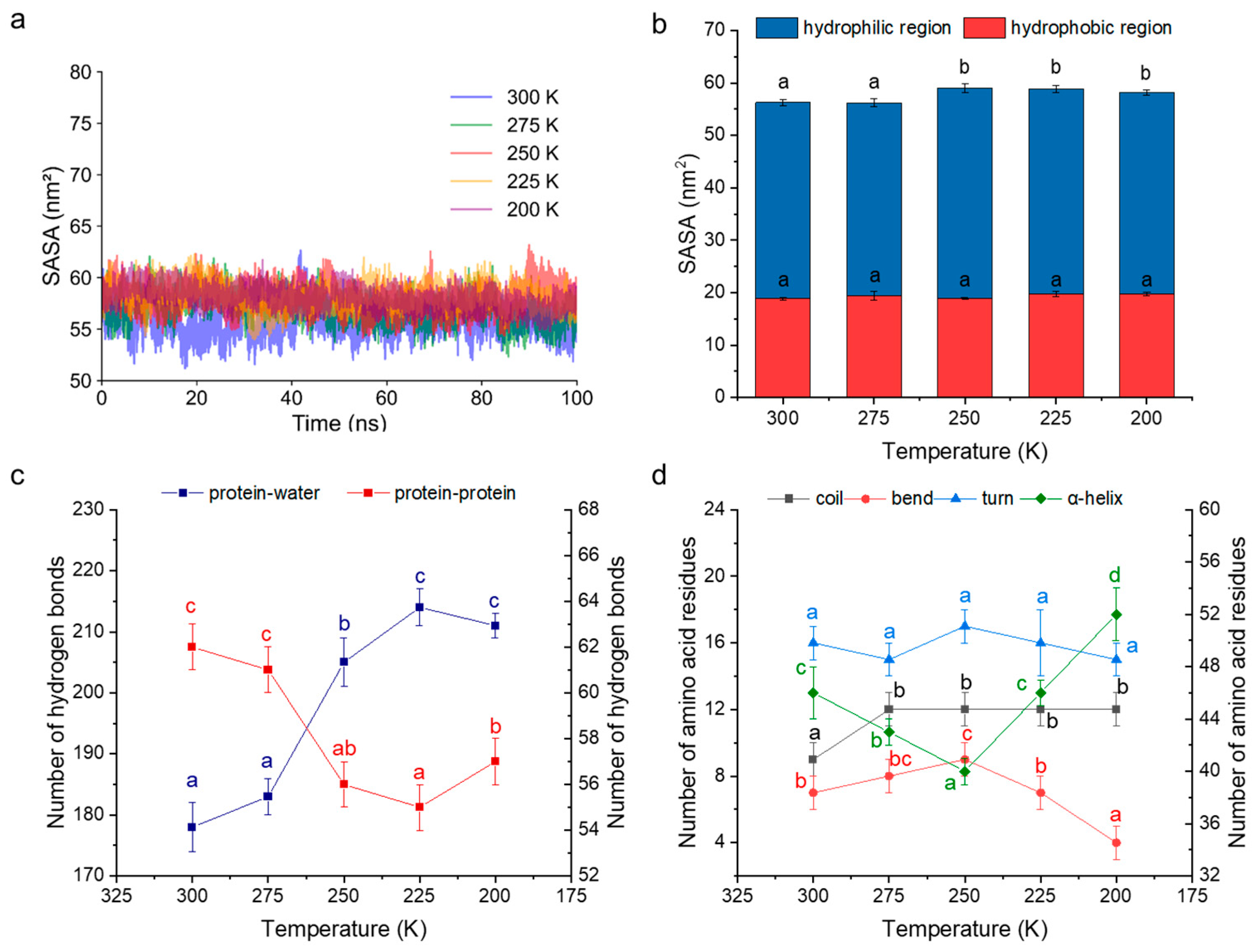
3.7.2. Interaction with Water Molecules
3.7.3. Conformational Transformation Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHC | Water-holding capacity |
| OHC | Oil-holding capacity |
| ΔH | Melting enthalpy |
| MD | Molecular dynamics |
| RMSD | Root-mean-square deviation |
| Rg | Radius of gyration |
| SASA | Solvent-accessible surface area |
References
- Arsiccio, A.; McCarty, J.; Pisano, R.; Shea, J.-E. Heightened cold-denaturation of proteins at the ice-water interface. J. Am. Chem. Soc. 2020, 142, 5722–5730. [Google Scholar] [CrossRef]
- Dinani, S.T.; Carrillo, M.F.C.; Boom, R.; van der Goot, A.J. Quality improvement of plant-based meat alternatives by addition of iota carrageenan to pea protein-wheat gluten blend. Eur. Food Res. Technol. 2023, 249, 1637–1654. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, M.; Bai, J.; Liu, X.; Yang, X.; Duan, X. Preparation and properties of high-soluble wheat gluten protein-based meat analogues. J. Sci. Food Agric. 2024, 104, 42–50. [Google Scholar] [CrossRef]
- Webb, D.; Li, Y.; Alavi, S. Chemical and physicochemical features of common plant proteins and their extrudates for use in plant-based meat. Trends Food Sci. Technol. 2023, 131, 129–138. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Zhang, J.; Liu, S.; Tian, Y.; Cheng, T.; Guo, Z.; Wang, Z. Improve the fiber structure and texture properties of plant-based meat analogues by adjusting the ratio of soy protein isolate (SPI) to wheat gluten (WG). Food Chem. X 2024, 24, 101962. [Google Scholar] [CrossRef]
- Xiang, N.; Yuen, J.S.; Stout, A.J.; Rubio, N.R.; Chen, Y.; Kaplan, D.L. 3D porous scaffolds from wheat glutenin for cultured meat applications. Biomaterials 2022, 285, 121543. [Google Scholar] [CrossRef]
- Wang, P.; Zou, M.; Li, D.; Zhou, Y.; Jiang, D.; Yang, R.; Gu, Z. Conformational rearrangement and polymerization behavior of frozen-stored gluten during thermal treatment. Food Hydrocoll. 2020, 101, 105502. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, L.; Zeng, J.; Gao, H.; Ma, H. Effects of different freezing temperatures on the molecular structure of gluten proteins. J. Food Meas. Charact. 2024, 18, 2259–2267. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, H.; Tian, X.; Huang, K.; Liu, Y.; Zeng, J.; Wang, M.; Qin, Y. Effect of freeze-thaw cycles at different temperatures on the properties of gluten proteins in unfermented dough. Cereal Chem. 2022, 99, 1039–1048. [Google Scholar] [CrossRef]
- Jia, G.; Chen, Y.; Sun, A.; Orlien, V. Control of ice crystal nucleation and growth during the food freezing process. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2433–2454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, H.; Li, C.; Ban, X.; Gu, Z.; Lu, Y.; Li, Z. Short-clustered maltodextrin mediates stabilization of gluten proteins during frozen storage compared to trehalose and guar gum. Food Chem. 2025, 476, 143387. [Google Scholar] [CrossRef]
- Park, J.K.; Patel, M.; Piao, Z.; Park, S.-J.; Jeong, B. Size and shape control of ice crystals by amphiphilic block copolymers and their implication in the cryoprotection of mesenchymal stem cells. ACS Appl. Mater. Interfaces 2021, 13, 33969–33980. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, K.-X. Physicochemical and fermentation properties of pre-fermented frozen dough: Comparative study of frozen storage and freeze-thaw cycles. Food Hydrocoll. 2023, 136, 108253. [Google Scholar] [CrossRef]
- Tang, W.; Ye, L.; Han, T.; He, J.; Liu, J. Effect of chitosan with different molecular weights on the freeze-thaw stability of gluten protein: Protein structures, functional characteristics, and cryo-protective mechanism. Food Hydrocoll. 2025, 160, 110763. [Google Scholar] [CrossRef]
- Tang, W.; Lin, X.; Ye, L.; He, J.; Wang, Z.; Tang, J.; Liu, J.; Zhao, P. Effect of pectin with different esterification degree on the freeze-thaw stability of gluten protein: Structures, functional properties, and cryoprotective mechanism. Food Chem. 2025, 465, 142040. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Ban, X.; Cheng, L.; Hong, Y.; Gu, Z.; Li, Z. Alleviative effect of short-clustered maltodextrin on the quality deterioration of frozen dough: Compared with trehalose and guar gum. Food Hydrocoll. 2021, 118, 106791. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Ban, X.; Cheng, L.; Hong, Y.; Gu, Z.; Li, Z. New insights into the alleviating role of starch derivatives on dough quality deterioration caused by freeze. Food Chem. 2021, 362, 130240. [Google Scholar] [CrossRef]
- Han, C.; Ma, M.; Li, M.; Sun, Q. Further interpretation of the underlying causes of the strengthening effect of alkali on gluten and noodle quality: Studies on gluten, gliadin, and glutenin. Food Hydrocoll. 2020, 103, 105661. [Google Scholar] [CrossRef]
- Wang, P.; Xu, L.; Nikoo, M.; Ocen, D.; Wu, F.; Yang, N.; Jin, Z.; Xu, X. Effect of frozen storage on the conformational, thermal and microscopic properties of gluten: Comparative studies on gluten-, glutenin- and gliadin-rich fractions. Food Hydrocoll. 2014, 35, 238–246. [Google Scholar] [CrossRef]
- Khrustalev, V.V.; Poboinev, V.V.; Stojarov, A.N.; Khrustaleva, T.A. Microenvironment of tryptophan residues in proteins of four structural classes: Applications for fluorescence and circular dichroism spectroscopy. Eur. Biophys. J. 2019, 48, 523–537. [Google Scholar] [CrossRef]
- Li, Y.; Kong, H.; Li, C.; Ban, X.; Gu, Z.; Lu, Y.; Li, Z. Mitigating the effects of starch derivatives on cold denaturation of gluten protein: Insights from hydration capacity and conformation behavior. J. Agric. Food Chem. 2024, 72, 26451–26461. [Google Scholar] [CrossRef]
- Bailey, M.; Hallett, J. Growth rates and habits of ice crystals between −20 °C and −70 °C. J. Atmos. Sci. 2004, 61, 514–544. [Google Scholar] [CrossRef]
- Petzold, G.; Aguilera, J.M. Ice morphology: Fundamentals and technological applications in foods. Food Biophys. 2009, 4, 378–396. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, G.; Xie, J. Alleviative effects of carboxymethyl chitosan on the quality deterioration of frozen rice dough during freeze thaw cycles. Food Hydrocoll. 2024, 149, 109599. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, H.; Xu, X.; Xu, D. Deterioration mechanisms and quality improvement methods in frozen dough: An updated review. Trends Food Sci. Technol. 2024, 143, 104251. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Qualitative composition. Cereal Chem. 2022, 100, 23–35. [Google Scholar] [CrossRef]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef]
- Ma, Y.; Hong, T.; Chen, Y.; Wu, F.; Xu, X.; Jin, Z. The conformational rearrangement and microscopic properties of wheat gluten following superheated steam treatment. Food Control 2022, 137, 108924. [Google Scholar] [CrossRef]
- Liu, M.; Chen, G.; Zhang, H.; Yu, Q.; Mei, X.; Kan, J. Heat-induced inulin-gluten gel: Insights into the influences of inulin molecular weight on the rheological and structural properties of gluten gel to molecular and physicochemical characteristics. Food Hydrocoll. 2021, 111, 106397. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Y.; Zhang, S.; Liu, M.; He, B.; Wu, X.; Yin, H.; Zhang, X.; Wang, J. Physicochemical properties and conformational structures of pre-cooked wheat gluten during freeze-thaw cycles affected by curdlan. Food Hydrocoll. 2024, 147, 109381. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins—A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, M.; Wang, L.; Qian, H.; Li, Y. Unveiling the structural and physico-chemical properties of glutenin macropolymer under frozen storage: Studies on experiments and molecular dynamics simulation. Food Res. Int. 2024, 197 Pt 1, 115252. [Google Scholar] [CrossRef]
- Mátyus, L.; Szöllősi, J.; Jenei, A. Steady-state fluorescence quenching applications for studying protein structure and dynamics. J. Photochem. Photobiol. B Biol. 2006, 83, 223–236. [Google Scholar] [CrossRef]
- Kim, S.B.; Palmer, J.C.; Debenedetti, P.G. Computational investigation of cold denaturation in the Trp-cage miniprotein. Proc. Natl. Acad. Sci. USA 2016, 113, 8991–8996. [Google Scholar] [CrossRef]
- Raghunathan, S. Solvent accessible surface area-assessed molecular basis of osmolyte-induced protein stability. RSC Adv. 2024, 14, 25031–25041. [Google Scholar] [CrossRef]
- Bitonti, A.; Puglisi, R.; Meli, M.; Martin, S.R.; Colombo, G.; Temussi, P.A.; Pastore, A. Recipes for inducing cold denaturation in an otherwise stable protein. J. Am. Chem. Soc. 2022, 144, 7198–7207. [Google Scholar] [CrossRef]






| Samples | Ksv (×104 L/mol) | R2 | Kb (×105 L/mol) | n | R2 |
|---|---|---|---|---|---|
| Control | 3.054 ± 0.17 a | 0.99 | 2.57 ± 0.02 ab | 1.171 ± 0.02 a | 0.98 |
| FS/-73 | 3.160 ± 0.16 ab | 0.99 | 2.49 ± 0.09 a | 1.161 ± 0.04 a | 0.99 |
| FS/-23 | 3.251 ± 0.10 abc | 0.99 | 2.69 ± 0.09 c | 1.167 ± 0.03 a | 0.99 |
| FST/-73 | 3.397 ± 0.11 bc | 0.99 | 2.70 ± 0.03 c | 1.172 ± 0.03 a | 0.99 |
| FST/-23 | 3.486 ± 0.08 c | 0.99 | 2.67 ± 0.03 bc | 1.160 ± 0.03 a | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, Y.; Chen, S.; Li, M.; Zhang, X.; Lu, Y. Mechanism Insights in Freeze–Thaw Process Impacting Cold Denaturation of Gluten Proteins During Frozen Storage. Foods 2025, 14, 3103. https://doi.org/10.3390/foods14173103
Li Y, Sun Y, Chen S, Li M, Zhang X, Lu Y. Mechanism Insights in Freeze–Thaw Process Impacting Cold Denaturation of Gluten Proteins During Frozen Storage. Foods. 2025; 14(17):3103. https://doi.org/10.3390/foods14173103
Chicago/Turabian StyleLi, Yang, Yilin Sun, Shuya Chen, Mingfei Li, Xiaowei Zhang, and Yujie Lu. 2025. "Mechanism Insights in Freeze–Thaw Process Impacting Cold Denaturation of Gluten Proteins During Frozen Storage" Foods 14, no. 17: 3103. https://doi.org/10.3390/foods14173103
APA StyleLi, Y., Sun, Y., Chen, S., Li, M., Zhang, X., & Lu, Y. (2025). Mechanism Insights in Freeze–Thaw Process Impacting Cold Denaturation of Gluten Proteins During Frozen Storage. Foods, 14(17), 3103. https://doi.org/10.3390/foods14173103






