Development of Conjugated Linoleic Acid Nanostructured Lipid Carriers and Their Synergistic Efficacy with Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CLA-NLC and Cur-CLA-NLC
2.3. Determination of Particle Size and PDI
2.4. Microstructure, Spectral, and Thermal Stability Analysis
2.4.1. Fourier Transform Infrared Spectroscopy (FT-IR) Measurements
2.4.2. X-Ray Diffraction (XRD) Measurements
2.4.3. Differential Scanning Calorimeter (DSC) Measurements
2.4.4. Transmission Electron Microscope (TEM) Measurements
2.5. The Bioaccessibility of Curcumin
2.6. Determination of Solubility of Curcumin in Lipid Phase
2.7. Determination of Antioxidant Activities
2.7.1. DPPH Radical Scavenging Activity
2.7.2. ABTS Radical Scavenging Activity
2.7.3. Ferric Reducing Antioxidant Power (FRAP)
2.8. Determination of Peroxide Value
2.9. Cytotoxicity of Cur, CLA-NLC, and Cur-CLA-NLC
2.10. Statistical Analysis
3. Results and Discussion
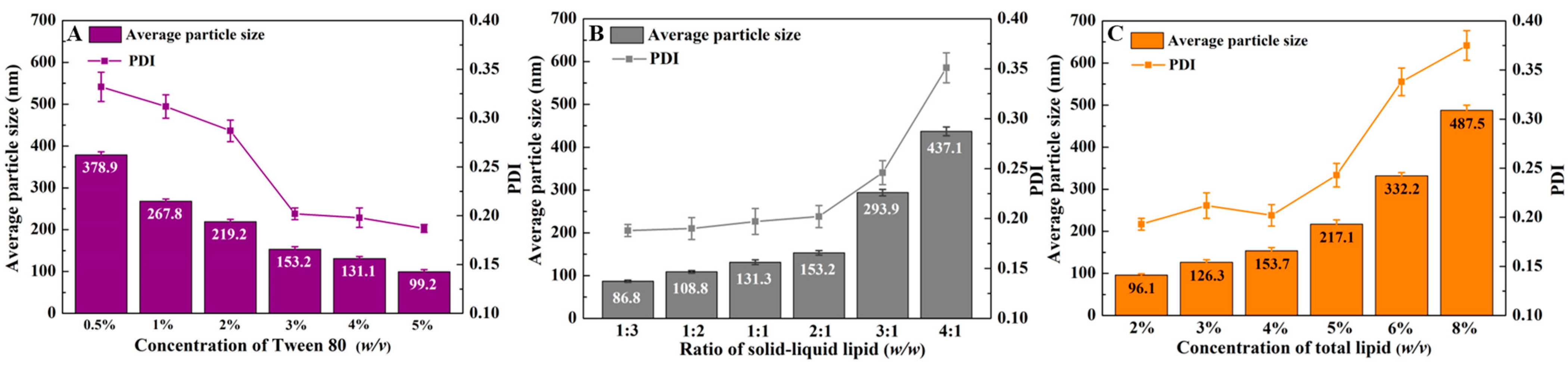
3.1. The Construction of CLA-NLC and Cur-CLA-NLC
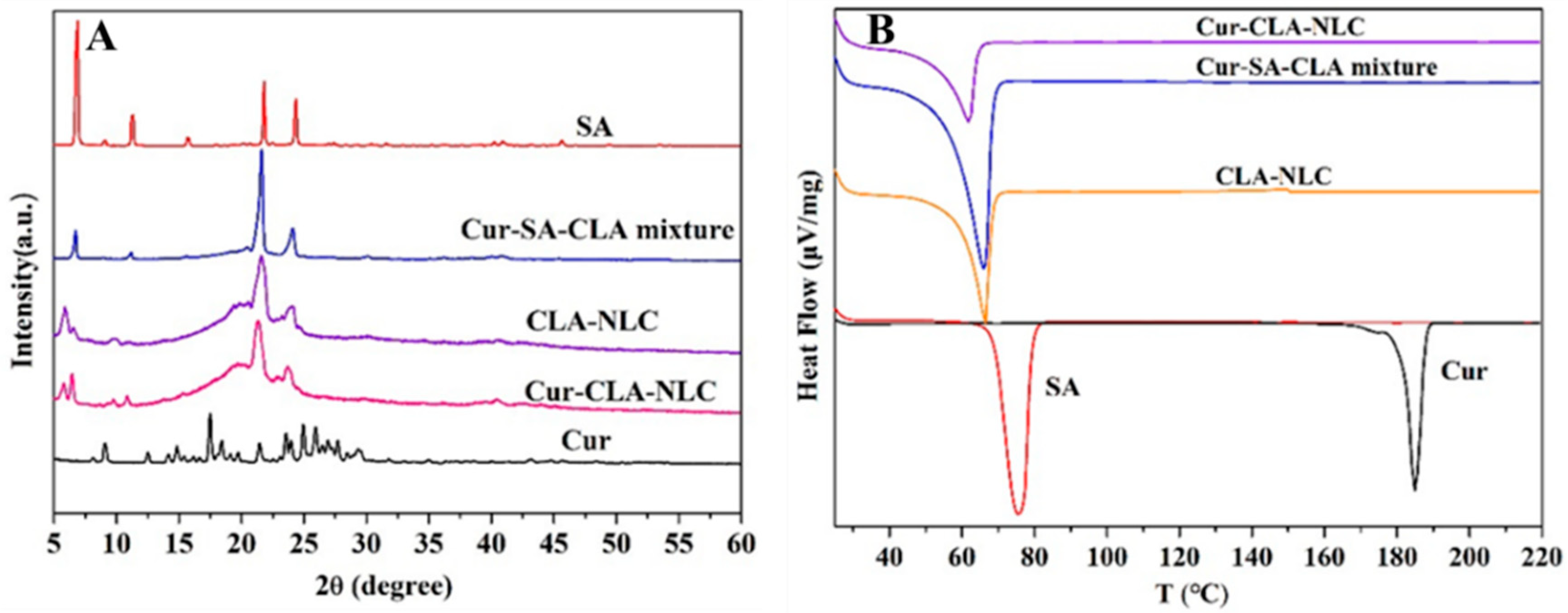
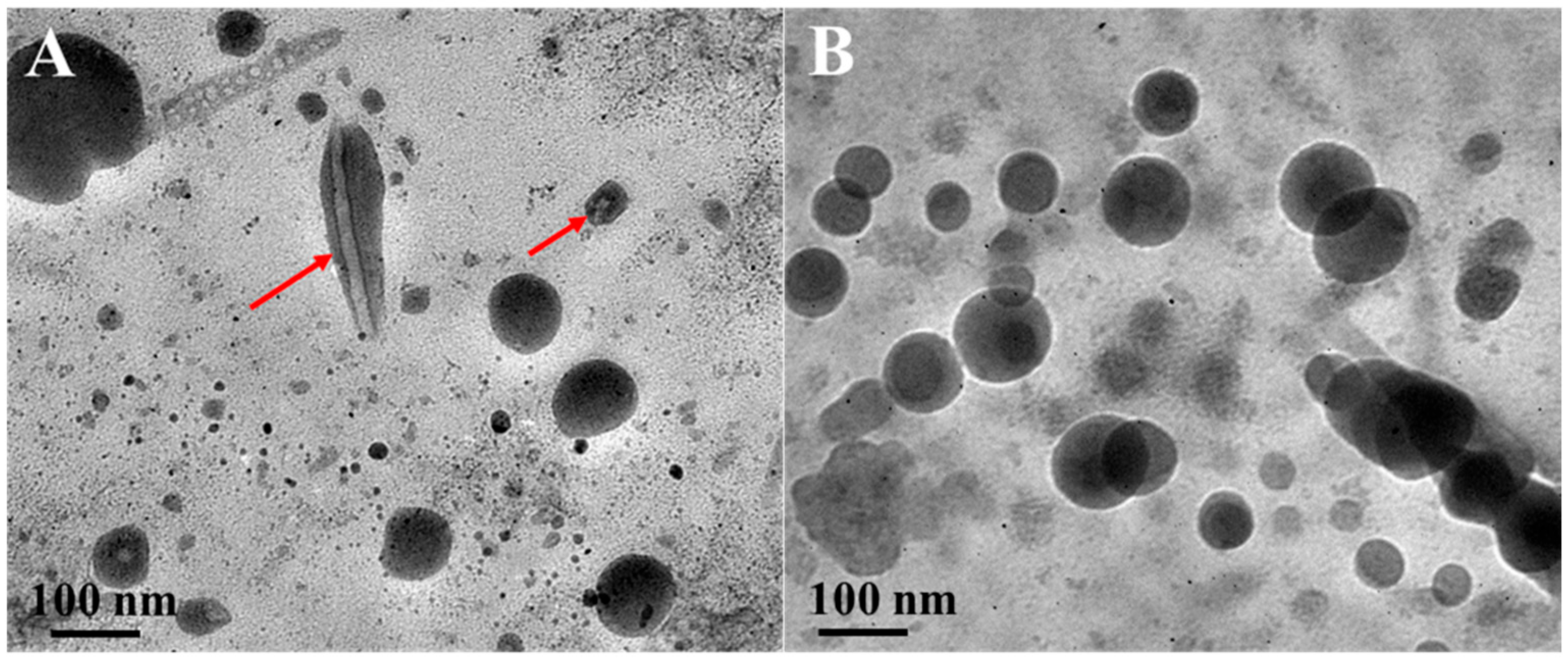
3.2. Characterization and Thermal Stability of CLA-NLC and Cur-CLA-NLC
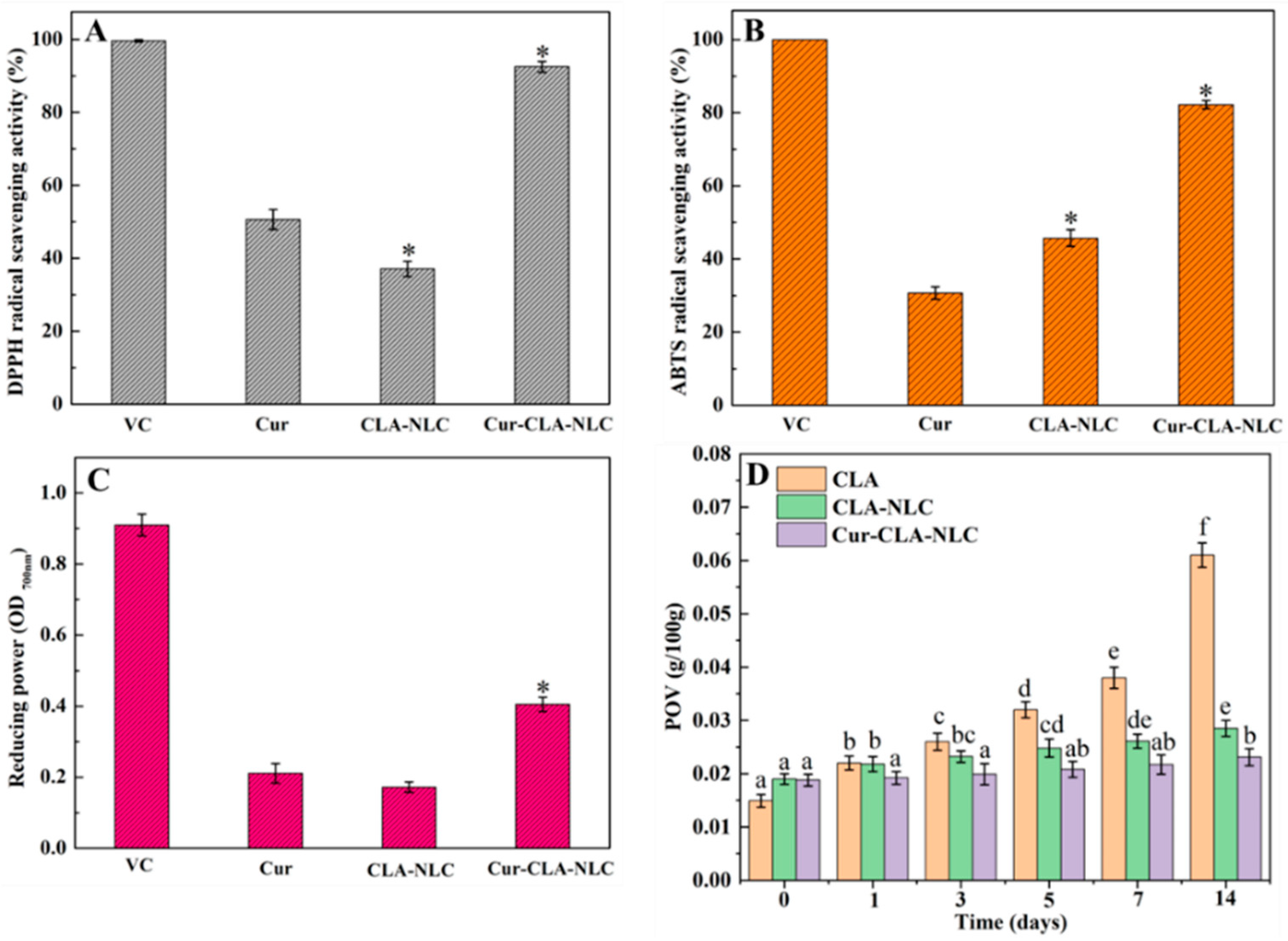
3.3. Bioaccessibility, Antioxidant Activity, and Anti-Lipid Peroxidation Ability of Cur-CLA-NLC
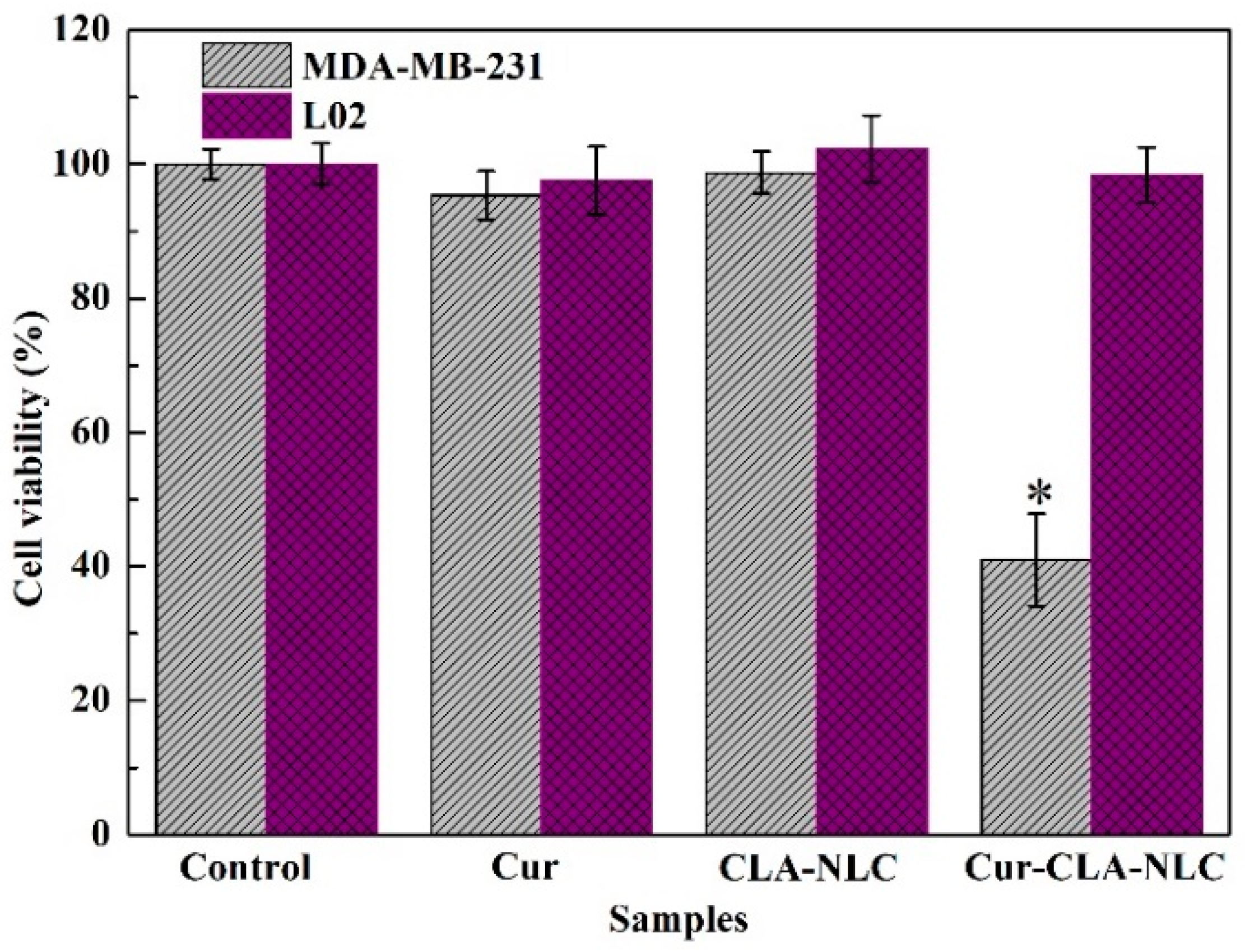
3.4. The Cytocompatibility of CLA-NLC and Cur-CLA-NLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic) |
| CLA | Conjugated linoleic acid |
| Cur | Curcumin |
| DPPH | 1,1-Diphenyl-2-picryl hydrazyl radical |
| DSC | Differential Scanning Calorimeter |
| FRAP | Ferric reducing antioxidant power |
| FT-IR | Fourier infrared spectroscopy |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NLC | Nanostructured lipid carrier |
| PBS | Phosphate-Buffered Saline |
| PDI | Polydispersity index |
| SA | Stearic acid |
| TEM | Transmission Electron Microscope |
| UV-Vis | Ultraviolet–visible spectroscopy |
| XRD | X-ray diffraction |
References
- Sun, Q.; Lv, M.; Li, Y. Nanotechnology-based drug delivery systems for curcumin and its derivatives in the treatment of cardiovascular diseases. J. Funct. Foods 2024, 122, 106476. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, Z.; Tsai, S.; Zhang, H.; Li, Y.; Yuan, F.; Wang, Q. Curcumin-loaded pickering emulsion formed by ultrasound and stabilized by metal organic framework optimization. Foods 2021, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, T.; Chen, L.; Zhao, R.; Ye, X.; Wang, X.; Wu, D.; Hu, J. High Internal Phase Emulsions Stabilized with Ultrasound-Modified Spirulina Protein for Curcumin Delivery. Foods 2024, 13, 1324. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.C.; Rezaei, A.; Qamar, M.; Assadpour, E.; Esatbeyoglu, T.; Jafari, S.M. Lipid-based nanodelivery systems of curcumin: Recent advances, approaches, and applications. Food Chem. 2025, 463, 141193. [Google Scholar] [CrossRef]
- Ghasemi, M.A.G.; Hamishehkar, H.; Javadi, A.; Homayouni-Rad, A.; Jafarizadeh-Malmiri, H. Natural-based edible nanocomposite coating for beef meat packaging. Food Chem. 2024, 435, 137582. [Google Scholar] [CrossRef]
- Kaewmalun, S.; Yata, T.; Kitiyodom, S.; Yostawonkul, J.; Namdee, K.; Kamble, M.T.; Pirarat, N. Clove oil-nanostructured lipid carriers: A platform of herbal anesthetics in Whiteleg Shrimp (Penaeus vannamei). Foods 2022, 11, 3162. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, Z.; Huang, W.; Zhao, X.; Xu, L.; Teng, C.; Li, Y. Hyaluronic acid-decorated lipid nanocarriers as novel vehicles for curcumin: Improved stability, cellular absorption, and anti-inflammatory effects. Food Chem. 2025, 463, 141420. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Dai, Z.; Zhang, Z.; Feng, L.; Nie, M.; Liu, C.; Li, D.; Zhang, M. Study on the relationship between lutein bioaccessibility and in vitro lipid digestion of nanostructured lipid carriers with different interface structures. Food Hydrocoll. 2023, 139, 108569. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Zhao, S.; Zhang, F.; Meng, X.; Liu, B. Highly stable nanostructured lipid carriers containing can-delilla wax for d-limonene encapsulation: Preparation, characterization and antifungal activity. Food Hydrocoll. 2023, 145, 109101. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid lipid nanoparticles vs. nanostructured lipid carriers: A comparative review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Zaky, M.F.; Megahed, M.A.; Hammady, T.M.; Gad, S.; Ghorab, M.M.; El-Say, K.M. Tailoring apixaban in nanostructured lipid carrier enhancing its oral bioavailability and anticoagulant activity. Pharmaceutics 2022, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Cakir, N.; Altas, B.O.; Kalaycioglu, G.D.; Mustafaoglu, N.; Aydogan, N. Development and Characterization of DHA-Integrated Nanostructured Lipid Carrier Formulations for Enhanced Cellular Binding and Uptake. Colloids Surf. Physicochem. Eng. Aspects 2025, 720, 137122. [Google Scholar] [CrossRef]
- La Torre, C.; Caputo, P.; Cione, E.; Fazio, A. Comparing nutritional values and Bioactivity of Kefir from different types of animal milk. Molecules 2024, 29, 2710. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Li, L.; Xia, Y.; Hu, X.; Fang, Y. The incorporated hydrogel of chitosan-oligoconjugated linoleic acid vesicles and the protective sustained release for curcumin in the gel. Int. J. Biol. Macromol. 2023, 227, 17–26. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of curcuminoid bioaccessibility from turmeric by a nanostructured lipid carrier system. Food Chem. 2018, 251, 51–57. [Google Scholar] [CrossRef]
- Moraes, S.; Marinho, A.; Lima, S.; Granja, A.; Araújo, J.P.; Reis, S.; Sousa, C.T.; Nunes, C. Targeted nanostructured lipid carriers for doxorubicin oral delivery. Int. J. Pharm. 2021, 592, 120029. [Google Scholar] [CrossRef]
- Behbahani, E.S.; Ghaedi, M.; Abbaspour, M.; Rostamizadeh, K.; Dashtian, K. Curcumin loaded nanostructured lipid carriers: In vitro digestion and release studies. Polyhedron 2019, 164, 113–122. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Li, L.; Hu, X.; Fang, Y.; Xia, Y. Synergistic effect on antioxidant activity of vitamin C provided with acidic vesiculation of hybrid fatty acids. J. Funct. Foods 2021, 85, 104647. [Google Scholar] [CrossRef]
- Madhavi, B.G.K.; Wijethunga, A.M.; Okagu, O.D.; Sun, X. Defatted Wheat Germ Protein-Derived Peptides Showed Multiple Biological Activities from the Stomach to Small Intestine: In Silico and In Vitro Approaches. J. Agr. Food Chem. 2024, 72, 20527–20536. [Google Scholar] [CrossRef]
- Carpenter, J.; George, S.; Saharan, V.K. Curcumin encapsulation in multilayer oil-in-water emulsion: Synthesis using ultrasonication and studies on stability and antioxidant and release activities. Langmuir 2019, 35, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Mengíbar, M.; Miralles, B.; Heras, Á. Use of soluble chitosans in Maillard reaction products with β-lactoglobulin. Emulsifying and antioxidant properties. LWT 2017, 75, 440–446. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Oxidative stability and in vitro digestion of menhaden oil emulsions with whey protein: Effects of EGCG conjugation and interfacial cross-linking. Food Chem. 2018, 265, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.-H.; Léonard, M.-L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Luo, C.; Wu, B.; Lin, Z.; Yuan, L.; Wang, H. Determination of Peroxide Value in the Plastic Resin. Asian J. Chem. 2013, 25, 4506–4508. [Google Scholar] [CrossRef]
- Sohail, R.; Abbas, S.R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Vicente, A.A.; Pinheiro, A.C. Incorporation of curcumin-loaded lipid-based nano delivery systems into food: Release behavior in food simulants and a case study of application in a beverage. Food Chem. 2023, 405, 134740. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; Wang, M.; Sun, Z.; Chang, C.; Ying, Y.; Li, D.; Zheng, H. Effect of emulsifier type on camellia oil-based nanostructured lipid carriers for delivery of curcumin. Food Chem. 2025, 482, 144193. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Chideme, N.; De Vaal, P.L. Effect of liquid viscosity and surface tension on the spray droplet size and the measurement thereof. J. Appl. Fluid Mechan. 2024, 17, 2652–2672. [Google Scholar]
- Velmurugan, R.; Selvamuthukumar, S. Development and optimization of ifosfamide nanostructured lipid carriers for oral delivery using response surface methodology. Appl. Nanosci. 2016, 6, 159–173. [Google Scholar] [CrossRef]
- Ma, Y.; Li, C.; Xiu, W.; Wang, X. In vivo and in vitro evaluation of stability and antioxidant activity of lyco-pene-nanostructured lipid carriers. Food Sci. Biotechnol. 2023, 32, 833–845. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Cerqueira, M.A.; Gonçalves, C.; Amado, I.R.; Teixeira, J.A.; Pastrana, L. Encapsulation of vitamin D3 using rhamnolipids-based nanostructured lipid carriers. Food Chem. 2023, 427, 136654. [Google Scholar] [CrossRef]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N. Injectable and in situ-formable thiolated chitosan-coated liposomal hydrogels as curcumin carriers for prevention of in vivo breast cancer recurrence. ACS Appl. Mater. Interf. 2020, 12, 17936–17948. [Google Scholar] [CrossRef]
- Kovačević, A.B.; Müller, R.H.; Keck, C.M. Formulation development of lipid nanoparticles: Improved lipid screening and development of tacrolimus loaded nanostructured lipid carriers (NLC). Int. J. Pharm. 2020, 576, 118918. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.S.; Farzadnia, F.; Aghajani, A.; Ahmadzadeh NobariAzar, F.; Pezeshki, A. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications. Food Sci. Nutr. 2020, 8, 4185–4195. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Reguant, J.; Romero, M.-P.; Macià, A.; Motilva, M.-J. Effect of fat content on the digestibility and bioac-cessibility of cocoa polyphenol by an in vitro digestion model. J. Agr. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, N.; Liu, H.; Qiu, T.; Chen, C.; Liu, X.; Zhu, Y. Extraction of curcuminoids from Curcuma longa L. by fatty acid-based ionic liquid aqueous solution: Experimental and mechanism study. Food Chem. 2025, 464, 141605. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, M.; Li, X.; Song, Y.; Wang, Y.; Hong, P.; Zhou, C. Development of curcumin-loaded hyaluronic acid complexes for curcumin intestinal delivery: Preparation, characterization, antioxidant, and in vitro digestive properties. LWT 2024, 214, 117164. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, C.; Zhao, J.; Guo, F.; Qin, J.; Wang, Y. Hyaluronic acid modulates techno-functional and digestion properties of heat-induced ginkgo seed protein isolate gel. Food Biosci. 2024, 59, 104204. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhang, Y.; Gao, B.; Li, Y.; He, X.; Sun, J.; Choe, U.; Chen, P.; Blaustein, R.A.; et al. Chemical composition of turmeric (Curcuma longa L.) ethanol extract and its antimicrobial activities and free radical scavenging capacities. Foods 2024, 13, 1550. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, H.; Yang, B.; Zhao, J.; Chen, W. Structural identification and antioxidant activity of trans-9, trans-11, cis-15-conjugated linolenic acid converted by probiotics. Food Res. Int. 2024, 184, 114258. [Google Scholar] [CrossRef]
- Ros, M.; Riesco-Llach, G.; Polonio-Alcalá, E.; Morla-Barcelo, P.M.; Ruiz-Martínez, S.; Feliu, L.; Planas, M.; Puig, T. Inhibition of Cancer stem-like cells by Curcumin and other polyphenol derivatives in MDA-MB-231 TNBC cells. Int. J. Mol. Sci. 2024, 25, 7446. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Gong, M.; Wu, G.; Jin, J.; Wang, X.; Jin, Q. Conjugated linolenic acid (CLnA) vs conjugated linoleic acid (CLA): A comprehensive review of potential advantages in molecular characteristics, health benefits, and production techniques. J. Agric. Food Chem. 2024, 72, 5503–5525. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.; Singh, T.; Tyurina, Y.Y.; Tyurin, V.A.; Samovich, S.; Nicolas, E.; Maslar, K.; Zhou, Y.; Cai, K.Q.; Tan, Y. Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1. Nat. Commun. 2021, 12, 2244. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ye, X.; Zhai, D.; Dai, W.; Wu, Y.; Chen, J.; Chen, W. Curcumin-loaded nanostructured lipid carrier induced apoptosis in human HepG2 cells through activation of DR5/caspases-mediated extrinsic apoptosis pathway. Acta Pharm. 2020, 70, 227–237. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Huang, X.; Liu, Y.; Zheng, G.; Yang, W.; Li, B. Development of Conjugated Linoleic Acid Nanostructured Lipid Carriers and Their Synergistic Efficacy with Curcumin. Foods 2025, 14, 3104. https://doi.org/10.3390/foods14173104
Liu H, Huang X, Liu Y, Zheng G, Yang W, Li B. Development of Conjugated Linoleic Acid Nanostructured Lipid Carriers and Their Synergistic Efficacy with Curcumin. Foods. 2025; 14(17):3104. https://doi.org/10.3390/foods14173104
Chicago/Turabian StyleLiu, Huan, Xingyu Huang, Yuxiu Liu, Guangming Zheng, Wei Yang, and Bo Li. 2025. "Development of Conjugated Linoleic Acid Nanostructured Lipid Carriers and Their Synergistic Efficacy with Curcumin" Foods 14, no. 17: 3104. https://doi.org/10.3390/foods14173104
APA StyleLiu, H., Huang, X., Liu, Y., Zheng, G., Yang, W., & Li, B. (2025). Development of Conjugated Linoleic Acid Nanostructured Lipid Carriers and Their Synergistic Efficacy with Curcumin. Foods, 14(17), 3104. https://doi.org/10.3390/foods14173104




