3.8.1. Specific Volume
The specific volume of bread tends to increase with the rising saturation level of fatty acid chains present in the formulation. This effect is attributed to the behavior of saturated fats during baking, as they melt upon heating and promote the proper expansion of gas cells, thereby facilitating greater gas retention and contributing to a more aerated crumb structure [
80]. In this context, bread formulated with buriti and acuri oils exhibited higher specific volumes as the substitution levels increased, which was directly associated with lower firmness values, as shown in
Table 7.
In general, the incorporation of lipids enhances the specific volume of bread up to a certain threshold, beyond which adverse effects may occur. Ropciuc et al. (2022), for instance, reported that hemp oil supplementation at concentrations between 5% and 10% improved technological quality, including higher specific volume, while additions above 10% resulted in a decline in this parameter [
81]. Similarly, Mikolasova et al. (2022) found that the inclusion of just 1.5% sunflower oil increased the specific volume of bread from 3.30 cm
3/g (control sample) to 4.39 cm
3/g, demonstrating the positive impact of low concentrations of vegetable oils on bread structure [
82].
Although textural and volumetric properties may differ significantly between gluten-containing and gluten-free breads, research has shown consistent effects of lipid addition across both systems. Mancebo et al. (2017), for example, observed a substantial increase in the specific volume of gluten-free bread supplemented with vegetable oils compared to lipid-free controls [
83]. The authors also noted a direct correlation between increased specific volume and reduced hardness, suggesting that the presence of oil contributes to softer bread textures. Zhang et al. (2021) further confirmed that the addition of soybean, corn, or rapeseed oils significantly enhanced dough specific volume and resulted in softer textures and more homogeneous crumb structures [
84].
3.8.2. Colorimetry of Oils and Breads
The colorimetric results of the oils, based on the CIELab system, are presented in
Table 8. Soybean oil, used as the reference standard, exhibited higher L* values compared to buriti and acuri oils, which reflects its lighter and more translucent appearance. Both buriti and acuri oils showed darker coloration than soybean oil, as evidenced by their lower L* values, an indicator of lightness on a scale from 0 (black) to 100 (white). This difference is likely attributed to the presence of carotenoid pigments, as well as potential suspended solids derived from the fruit pulp.
The a* and b* parameters represent the chromaticity coordinates of the samples. Positive a* values, observed in both acuri and buriti oils, indicate a tendency toward red hues, while positive b* values suggest a shift toward yellow, in contrast to blue tones. Notably, buriti oil displayed higher b* and lower a* values than acuri oil, indicating a more intense yellow hue and less pronounced red coloration.
These findings are consistent with those reported by Marcelino et al. [
45], who attributed the characteristic yellow color of buriti oil to its high β-carotene content.
The colorimetric results of the control bread and the samples formulated with buriti and acuri oils at four levels of substitution are presented in
Table 8. Despite the chromatic differences between the oils, the color behavior of the breads showed particular patterns. Although statistically significant differences in L* values were observed between the control and the bread containing buriti and acuri oils, the values remained relatively close, suggesting that the incorporation of these oils did not substantially alter the overall appearance of the breads. Notably, the bread made with 100% buriti oil exhibited a darker color compared to the control. In contrast, bread formulated with acuri oil became progressively lighter with increasing substitution levels, which may be attributed to the markedly higher carotenoid content in buriti oil compared to acuri oil (
Table 4).
Furthermore, breads prepared with both oils exhibited an increase in a* values (indicating a more reddish hue) and b* values (more yellowish hue) as the oil concentration increased. This effect is likely due to the high levels of carotenoids in buriti and acuri oils, which intensify the final product’s coloration.
3.8.3. Texture Profile Analysis (TPA)
Lipids play multiple roles in baking, functioning not only as an energy source and carrier of bioactive compounds but also as key modulators of bread texture, volume, and shelf life. Their plasticity, determined by the ratio between solid and liquid lipid fractions, has a direct impact on dough structure by enhancing softness, chewability, and crumb staling delay. This is primarily due to the formation of starch–lipid complexes that inhibit retrogradation [
83,
84,
85]. Triglycerides rich in saturated fatty acids confer greater plasticity to the lipid phase, facilitating the development of a more stable protein network during baking, which ultimately results in softer and more voluminous loaves [
82].
In Brazil, soybean oil remains the predominant lipid source in approximately 67% of packaged bread formulations, followed by margarine, hydrogenated vegetable fats, or other vegetable oils [
86]. This preference is largely driven by its wide availability, low cost, and satisfactory technological performance. However, soybean oil, rich in polyunsaturated fatty acids, exhibits low oxidative stability and a reduced content of bioactive compounds, thereby limiting its nutritional potential [
87].
In the present study, substituting soybean oil with buriti and acuri oils significantly influenced the textural parameters of the bread, particularly reducing firmness and chewiness in samples formulated with 100% acuri oil (
Table 9). This effect is likely related to the high saturated fatty acid content of acuri oil (54.23%), which enhances plasticity, improves oxidative stability, and may extend product shelf life. A similar trend was observed in bread made with buriti oil, which, despite containing a lower proportion of saturated fatty acids (19.10%), also led to a significant reduction in firmness and chewiness compared to the control, indicating a softer crumb structure (
Table 9). However, elasticity was reduced in bread with higher concentrations of buriti oil, suggesting a more compact texture and reduced resilience after compression. Although lower elasticity is not desirable in all bread types, it is generally acceptable in sandwich breads, which naturally possess a less elastic crumb.
Regarding cohesiveness, a parameter related to crumb fragmentation, a slight but significant increase was observed in bread containing buriti oil, whereas no significant effect was found in those formulated with acuri oil. This suggests that the type of oil may have a limited influence on this attribute.
Although no previous studies were found investigating the use of buriti or acuri oils in bread formulations, instrumental texture parameters reported for other types of breads and lipids may serve as useful comparisons. For example, Zhang et al. [
84] reported an increase in specific volume and a reduction in firmness in Chinese steamed breads when vegetable shortening was partially replaced with oleic acid-rich peanut oil (46% oleic acid) [
84]. In contrast, substitution with soybean oil led to lower viscosity and elasticity, rendering it a less suitable alternative for improving dough performance. Additionally, Gerits et al. [
87] demonstrated that unsaturated lipids are capable of interacting with gluten proteins, particularly glutenins, thereby enhancing the viscoelastic network and improving the rheological properties of wheat doughs. It is important to note, however, that gluten-related characteristics were not assessed in the present study [
87].
Beyond their effects on texture, lipids also contribute to the development of volatile compounds responsible for flavor and aroma. Wu et al. [
88] observed increased levels of aldehydes, ketones, and furans in sourdough breads enriched with corn oil, further supporting the functional role of lipids in sensory quality and consumer acceptance.
Taken together, these findings highlight the potential of buriti and acuri oils as promising alternatives to soybean oil in bakery applications. Their incorporation may improve both technological and sensory attributes of bread while also offering added nutritional value and supporting sustainable supply chains based on native Brazilian fruits.
3.8.4. Principal Components Analysis (PCA) of Color and Texture Evaluations
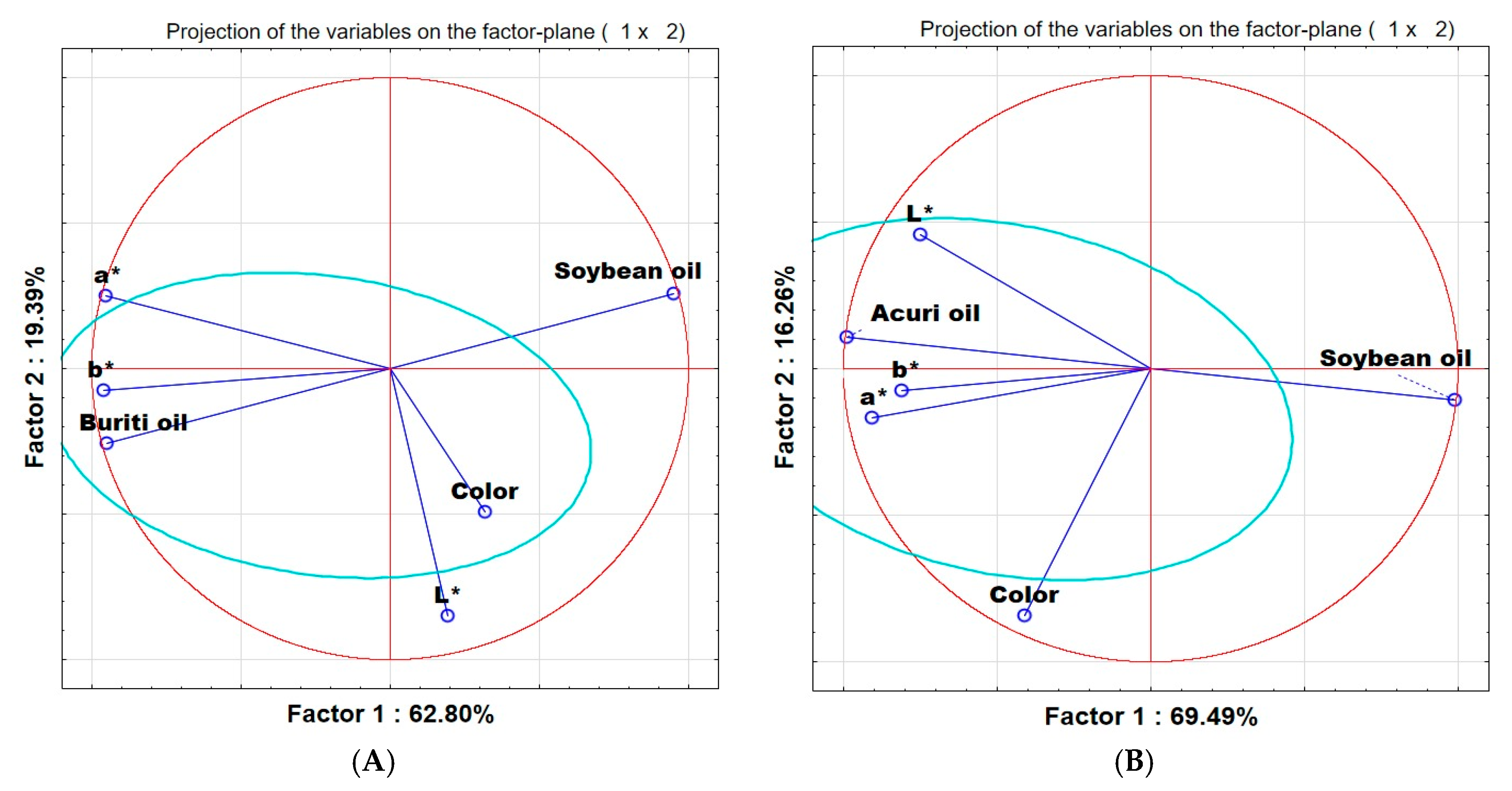
The principal component analysis of the sensory and instrumental evaluations of the control breads and those containing buriti and acuri oils is presented in
Figure 2A,B. The control breads are represented by the vector “Soybean oil,” while the terms “buriti oil” and “acuri oil” refer to the native oils used in the formulations.
Principal component analysis (PCA) shown in
Figure 2A, comparing buriti oil breads (BB) and the control bread (soybean oil), indicates that sensory perception of color by consumers was more strongly associated with lightness (L*) than with the chromatic coordinates a* and b*. The BB samples are positioned in the lower-left quadrant, aligned with lower L* values and opposite to a* and b*, reflecting a darker appearance and reduced saturation in warm tones, consistent with their carotenoid-rich visual profile.
Similarly, in the PCA of breads containing acuri oil (AB), a weak correlation was observed between lightness and chromatic coordinates, while a* and b* displayed a positive correlation. In
Figure 2B, however, the variable “Color” appears distinctly separated from the instrumental parameters, which may indicate that panelists gave greater weight to subjective aspects of color, such as saturation, vividness, or hue, not necessarily captured by L*, a*, or b* individually. The AB samples are aligned with lower a* and b* values and higher L* values, suggesting a paler, less saturated, but lighter color when compared, for example, to the BB samples.
With respect to bread texture, the principal component analysis (
Figure 3) revealed that overall acceptability and sensory texture were more strongly associated with the use of buriti oil, whereas instrumental texture parameters showed no clear relationship with oil type. In the case of breads containing acuri oil, overall acceptability was also associated with the oil, but no correlation was found with instrumental measurements. These findings indicate that other factors, such as fermentation time, gluten strength, and flour characteristics, may have influenced the observed parameters, underscoring the need for further in-depth studies.
3.8.5. Fourier-Transform Infrared Spectroscopy (FT-IR)
Infrared spectroscopy, often used in conjunction with other analytical techniques, is a standard method for determining the identity of oils and detecting potential adulteration. It also serves to confirm the predominant fatty acid composition of oils.
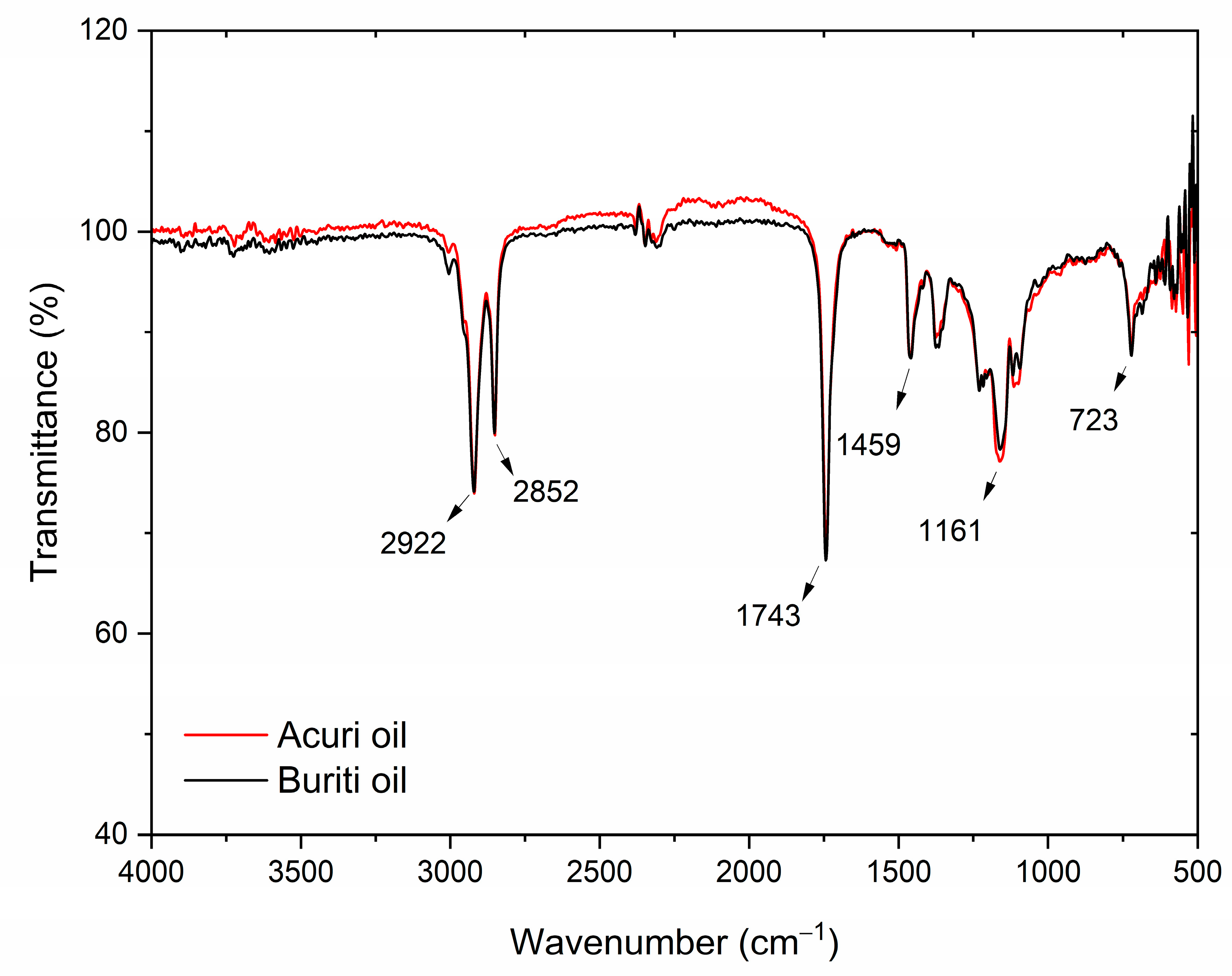
Figure 4 shows the FT-IR spectra of crude buriti and acuri oils, which were found to be largely similar. The strong absorption bands observed at 1741 cm
−1 for acuri oil and 1743 cm
−1 for buriti oil are characteristic of carbonyl (C=O) stretching vibrations found in triglyceride esters, the primary constituents of vegetable oils [
8,
87,
88,
89]. These bands are consistent with the presence of long-chain fatty acids, as expected in buriti and acuri oils.
Additionally, the band observed at 1459 cm
−1 is primarily associated with the bending vibration of methylene (–CH
2–) groups within fatty acid chains and is a typical signature of lipid materials such as triglycerides. According to Silva et al. (2024), the spectral range between 1630 and 1755 cm
−1, typical of carboxylic acids and ester, may also reflect contributions from phenolic acids in buriti oil [
46]. The intense bands at 2922 cm
−1 and 2852 cm
−1 are attributed to asymmetric and symmetric stretching vibrations of CH
2 groups, respectively, indicating the presence of saturated hydrocarbon chains in the oil molecules, corresponding to the palmitic acid content of both oils [
89]. The absorption band at 3005 cm
−1 corresponds to C–H stretching in =C–H (sp
2) bonds, characteristic of unsaturated fatty acids and likely associated with oleic acid. Albuquerque et al. [
90] reported the same band in the spectrum of pure oleic acid, and similar vibrational patterns have been observed in other vegetable oils commonly used in the food industry, such as soybean, palm, and palm kernel oils [
46,
90].
Moreover, multiple bands appearing in the region between 1100 and 1500 cm
−1, especially the peak at 1161 cm
−1, are possibly associated with molecular arrangements resembling those of triolein, indicating structural similarities between acuri and buriti oils and olive oil [
91]. Previous studies have shown that buriti oil contains approximately 91.4% triacylglycerols, of which 28.8% is triolein, while olive oil contains around 32.5% triolein, suggesting that buriti oil may exhibit biological properties similar to those of olive oil [
33]. Finally, the bands at 723 cm
−1 are attributed to out-of-plane bending vibrations associated with cis-double bonds between carbon atoms, which characterize unsaturated fatty acids in their natural cis configuration.
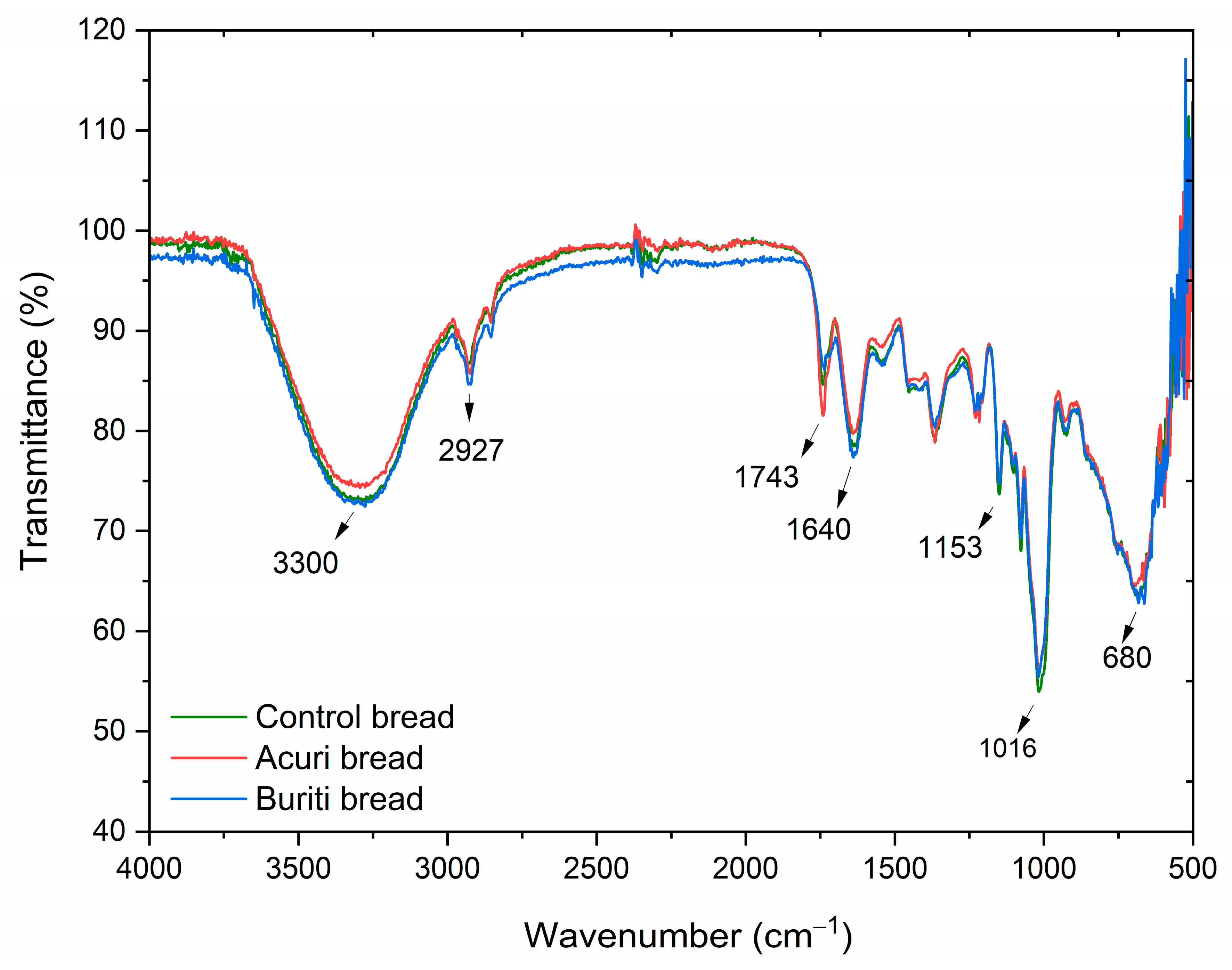
The infrared spectra of control bread, BB100, and AB100 samples are shown in
Figure 4 and
Figure 5, respectively. The broad absorption band near 3300 cm
−1 is attributed to the presence of water in the bread matrices. The bands at 2920 and 1743 cm
−1 correspond to the C–H and C=O stretching vibrations of lipids, respectively, and are associated with the incorporation of vegetable oils into the dough formulations [
92]. The overall overlap among the spectra suggests no major structural differences between the control, BB100, and AB100 samples. However, a slight reduction in the intensity of the 1743 cm
−1 band was observed in the bread containing 100% buriti oil, which may be related to partial oxidation or degradation of fatty acid esters during breadmaking.
The absorption bands at 1153 and 1016 cm
−1 are typical of starch transformations that occur during baking and subsequent cooling, corresponding to the (C–O–C) stretching of glycosidic ester bonds and asymmetric (C–O and C–C) stretching, respectively. In particular, the band at 1016 cm
−1 is associated with starch retrogradation and reflects the recrystallization of amylopectin [
93,
94]. The superimposition of spectra at this wavelength across BB100, AB100, and the control suggests that lipid source substitution did not significantly affect starch retrogradation, the primary mechanism of bread staling. Likewise, no unique absorption bands were identified in the buriti or acuri bread samples that were absent in the control, indicating that the molecular structure of key functional groups remained unaffected by the lipid replacement. These findings suggest that the breads enriched with buriti and acuri oils preserved similar structural, visual, and sensory properties compared to the control, supporting their potential acceptability in commercial bakery applications, an outcome also supported by instrumental and sensory analyses.
3.8.6. Thermogravimetric Analysis of Breads (TGA)
Thermogravimetric analysis (TGA) was performed to assess the thermal stability of the control bread and the bread containing a 100% substitution of soybean oil with buriti (BB100) and acuri oil (AB100), within the temperature range of 30 °C to 600 °C. The TGA curves and differential thermal analysis (DTA) are shown in
Figure 6. The first weight-loss stage, observed between 30 °C and 120 °C, corresponds to the evaporation of water retained in the bread matrix. The BB100 sample retained a higher amount of water in the bread compared to the other formulations, as evidenced by the smoother TGA curve with greater mass loss in the initial stage, and by the more pronounced endothermic peak observed in the DTA curve. These results suggest that bread formulated with native fruit oils exhibited greater water retention capacity, likely due to differences in the lipid matrix structure or specific interactions between lipids and starch components in the wheat flour [
92,
95].
The second weight-loss phase, observed between 257 °C and 305 °C in all three samples, is attributed to the thermal degradation of carbohydrates, proteins, and lipids. The BB100 and AB100 samples exhibited lower total mass loss (22.33% and 22.56%, respectively) compared to the control (25.47%), indicating greater thermal resistance that can be attributed to the presence of antioxidant bioactives from buriti and acuri oils. In the final degradation stage, near 500 °C, the TGA curves of the BB100 and AB100 samples (represented by red and black lines, respectively) end at higher residual mass levels compared to the control bread. This behavior highlights the presence of thermally stable compounds in the native fruit oils, possibly derived from antioxidant constituents, that persist even after bread baking.
Cheng et al. [
96] reported that the addition of canola oil to white bread increased moisture retention as oil concentration increased. However, the thermal decomposition temperature of carbohydrates and proteins remained unchanged, indicating no significant influence of oil presence on this stage of thermal stability. Additionally, the authors observed an endothermic peak near 400 °C, associated with the degradation of unsaturated fatty acids, a feature that was also evident in the DTA curves of the control, BB100, and AB100 samples in the present study, where peaks appeared between 410 °C and 500 °C (
Figure 6) [
96]. Marcelino et al. (2022) attributed the decomposition peak observed between 276 °C and 479 °C in pure buriti oil to the presence of long-chain fatty acids [
45].
The results clearly demonstrate that replacing soybean oil with buriti and acuri oils markedly influences the thermal stability profile of bread, affecting both moisture retention and the decomposition behavior of organic components. These modifications should be taken into account in industrial applications involving thermal processing or extended storage. Moreover, the findings reinforce the functional and technological potential of these regional oils as promising ingredients in bakery formulations.