Evaluation of Quality Parameters in Canned Pork Enriched with 1% Freeze-Dried Cell-Free Supernatant of Lacticaseibacillus paracasei B1 and Reduced Sodium Nitrite Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Lyophilized Cell Free Supernatant (CFS)
2.2. Meat Product Preparation
2.3. Physicochemical (pH, aw, ORP) Properties
2.4. Antioxidant Activity of Meat Product Extract
2.4.1. Radical Scavenging Activity
2.4.2. Determination of Reducing Power
2.5. Thiobarbituric Acid Reactive Substance (TBARS) Status
2.6. Fatty Acid (FAA) Status
2.7. Instrumental Color (L*a*b*)
2.8. Texture Profile Analysis (TPA)
2.9. Microbiological Analysis
2.9.1. Microorganism Identification Using Mass Spectrometry
2.9.2. MALDI-TOF Biotyper Matrix Solution
2.10. Statistical Analysis
3. Results
4. Discussion
4.1. Physiochemical Parameters
4.2. Oxidative Stability
4.3. Color and Texture
4.4. Microbiology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deveci, G.; Tek, N.A. N-Nitrosamines: A potential hazard in processed meat products. J. Sci. Food Agric. 2024, 104, 2551–2560. [Google Scholar] [CrossRef]
- Dissanayake, K.; Rifky, M.; Zokirov, K.; Jesfar, M.; Farmonov, J.; Ermat, S.; Makhmayorov, J.; Samadiy, M. Impact of curing salt (nitrites) on the processed meat products and its alternatives: A review. New Mater. Compd. Appl. 2024, 8, 254–264. [Google Scholar] [CrossRef]
- Halagarda, M.; Wójciak, K.M. Health and safety aspects of traditional European meat products. A review. Meat Sci. 2022, 184, 108623. [Google Scholar] [CrossRef]
- EU Commission. Commission regulation (EU) n° 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) n° 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union 2011, L295, 1–177. [Google Scholar]
- Ferysiuk, K.; Wójciak, K.M. The possibility of reduction of synthetic preservative E 250 in canned pork. Foods 2020, 9, 1869. [Google Scholar] [CrossRef]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Ozturk, B.; Sengun, I.Y. Effects of cell-free supernatants produced by lactic acid bacteria on the safety and quality of poultry meat. Br. Poult. Sci. 2025, 1–13. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Mardani, K.; Ezati, P. Efficacy of lyophilized cell-free supernatant of Lactobacillus salivarius (Ls-BU2) on Escherichia coli and shelf life of ground beef. Vet. Res. Forum 2019, 10, 193. [Google Scholar] [PubMed]
- Masebe, R.D.; Thantsha, M.S. Anti-biofilm activity of cell free supernatants of selected lactic acid bacteria against Listeria monocytogenes isolated from avocado and cucumber fruits, and from an avocado processing plant. Foods 2022, 11, 2872. [Google Scholar] [CrossRef] [PubMed]
- Alimi, J.O.; Ogunbanwo, S.T.; Alimi, J.P.; Gbadamosi, T.A.; Adarabierin, I.A.G. Influence of cell-free supernatant of lactic acid bacteria as additive in preservation of pineapple juice. Food Environ. Saf. J. 2025, 24, 11–20. [Google Scholar] [CrossRef]
- Jutinico-Shubach, A.; Gutiérrez-Cortés, C.; Suarez, H. Antilisterial activity of chitosan-based edible coating incorporating cell-free supernatant from Pediococcus pentosaceus 147 on the preservation of fresh cheese. J. Food Process. Preserv. 2020, 44, e14715. [Google Scholar] [CrossRef]
- Cavallo, N.; De Angelis, M.; Calasso, M.; Quinto, M.; Mentana, A.; Minervini, F.; Cappelle, S.; Gobbetti, M. Microbial cell-free extracts affect the biochemical characteristics and sensorial quality of sourdough bread. Food Chem. 2017, 237, 159–168. [Google Scholar] [CrossRef]
- Dai, L.; Yu, P.; Ma, P.; Chen, C.; Ma, J.; Zhang, J.; Huang, B.; Xin, Z.; Zheng, X.; Tang, T. Effects of the supernatant of Chlorella vulgaris cultivated under different culture modes on lettuce (Lactuca sativa L.) growth. Front. Nutr. 2024, 11, 1437374. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, L.; Gu, L.; Lv, Y.; Li, J.; Yang, Y.; Meng, X. Cell-Free Supernatant of Lactiplantibacillus plantarum 90: A Clean Label Strategy to Improve the Shelf Life of Ground Beef Gel and Its Bacteriostatic Mechanism. Foods 2023, 12, 4053. [Google Scholar] [CrossRef]
- İncili, G.K.; Akgöl, M.; Karatepe, P.; Üner, S.; Tekin, A.; Kanmaz, H.; Kaya, B.; Çalicioğlu, M.; Hayaloğlu, A.A. Quantification of bioactive metabolites derived from cell-free supernatant of Pediococcus acidilactici and screening their protective properties in frankfurters. Probiot. Antimicrob. Proteins 2025, 17, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S.; Eskandari, M.H.; Shekarforoush, S.S. Antimicrobial activity of cell-free supernatant of lactic acid bacteria on spoilage bacteria of vacuum-packed sliced emulsion-type sausages. Iran. J. Vet. Res. 2024, 25, 182. [Google Scholar]
- Srour, B.; Chazelas, E.; Debras, C.; Druesne-Pecollo, N.; Agaesse, C.; Szabo de Edelenyi, F.; Sellem, L.; Kesse-Guyot, E.; Deschasaux-Tanguy, M.; Touvier, M. Nitrites and nitrates from additives and natural sources and risk of cardiovascular outcomes. Eur. J. Public Health 2022, 32 (Suppl. 3), ckac129.491. [Google Scholar] [CrossRef]
- Jeong, C.H.; Ko, H.I.; Lee, M.E.; Min, S.G.; Lee, M.A.; Kim, T.W. Combination approach of paired starter culture and lactic acid on inhibiting autochthonous lactic acid bacteria for extending kimchi shelf life. Food Control 2024, 157, 110167. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of Browning reaction: Antioxidative activities of products of Browning reaction prepared from Glucosamine. Nutr. Mag. 1987, 44, 307–315. [Google Scholar]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- AOAC. Helrich, K., Ed.; Official method 969.33. Fatty acids in oils and fats. Preparation of methyl esters. Boron trifluoride. In Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC International: Arlington, VA, USA, 1990; pp. 963–964. [Google Scholar]
- Commission Internationale de l’Eclairage (CIE). Bureau Central de la CIE, Paris. 1978. [Google Scholar]
- Kačániová, M.; Čmiková, N.; Ban, Z.; Garzoli, S.; Elizondo-Luevano, J.H.; Ben Hsouna, A.; Ben Saad, R.; Bianchi, A.; Venturi, F.; Kluz, M.I.; et al. Enhancing the Shelf Life of Sous-Vide Red Deer Meat with Piper nigrum Essential Oil: A Study on Antimicrobial Efficacy against Listeria monocytogenes. Molecules 2024, 29, 4179. [Google Scholar] [CrossRef]
- Ferysiuk, K.; Wójciak, K.M.; Trząskowska, M. Fortification of low-nitrite canned pork with willow herb (Epilobium angustifolium L.). Int. J. Food Sci. Technol. 2022, 57, 4194–4210. [Google Scholar] [CrossRef]
- Estévez, M. Critical overview of the use of plant antioxidants in the meat industry: Opportunities, innovative applications and future perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Leistner, L.; Gorris, L.G. Food preservation by hurdle technology. Trends Food Sci. Technol. 1995, 6, 41–46. [Google Scholar] [CrossRef]
- Aksu, M.I.; Erdemir, E.; Çakıcı, N. Changes in the physico-chemical and microbial quality during the production of Pastırma cured with different levels of sodium nitrite. Korean J. Food Sci. Anim. Resour. 2016, 36, 617. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A.; Wójciak, K.M. Impact of sodium nitrite reduction on lipid oxidation and antioxidant properties of cooked meat products. Antioxidants 2019, 9, 9. [Google Scholar] [CrossRef]
- Guo, J.; Li, Z.; Zhang, Y.; Tian, X.; Shao, L.; Wang, W. Comparative Study of the Antibacterial Effects of S-Nitroso-N-acetylcysteine and Sodium Nitrite against Escherichia coli and Their Application in Beef Sausages. Foods 2024, 13, 2383. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate–nitrite–nitric oxide pathway: A mechanism of hypoxia and anoxia tolerance in plants. Int. J. Mol. Sci. 2022, 23, 11522. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.; Barba, F.J.; Lorenzo, J.M. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef]
- Roshanzamir, T.; Basiri, S.; Shekarforoush, S.S.; Gholamhosseini, A. Bio preservation Strategies: Evaluating the Efficacy of Lactic Acid cell-free Supernatants in Extending the Refrigerated Shelf Life of Shrimp. Appl. Food Res. 2025, 5, 101047. [Google Scholar] [CrossRef]
- Karwowska, M.; Wójciak, K.M.; Dolatowski, Z.J. The influence of acid whey and mustard seed on lipid oxidation of organic fermented sausage without nitrite. J. Sci. Food Agric. 2015, 95, 628–634. [Google Scholar] [CrossRef]
- Asadi, Z.; Abbasi, A.; Ghaemi, A.; Montazeri, E.A.; Akrami, S. Investigating the properties and antibacterial, antioxidant, and cytotoxicity activity of postbiotics derived from Lacticaseibacillus casei on various gastrointestinal pathogens in vitro and in food models. GMS Hyg. Infect. Control 2024, 19, Doc60. [Google Scholar]
- Morrissey, P.A.; Tichivangana, J.Z. The antioxidant activities of nitrite and nitrosylmyoglobin in cooked meats. Meat Sci. 1985, 14, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.H.; Zhang, W.G. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Stasiak, D.M.; Kęska, P. The influence of different levels of sodium nitrite on the safety, oxidative stability, and colour of minced roasted beef. Sustainability 2019, 11, 3795. [Google Scholar] [CrossRef]
- Hernández, J.D.D.; Castell, A.; Arroyo-Manzanares, N.; Guillén, I.; Vizcaíno, P.; López-García, I.; Hernández-Córdoba, M.; Viñas, P. Toward nitrite-free curing: Evaluation of a new approach to distinguish real uncured meat from cured meat made with nitrite. Foods 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, C.; Jia, X.; Guo, Y.; Lei, N.; Hackman, R.M.; Chen, L.; Zhou, G. Influence of sodium nitrite on protein oxidation and nitrosation of sausages subjected to processing and storage. Meat Sci. 2016, 116, 260–267. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Q.; He, L.; Zhang, Q.; Ma, J. Effects of nitrite concentrations on the quality and protein oxidation of salted meat. J. Food Sci. 2022, 87, 3978–3994. [Google Scholar] [CrossRef]
- Govari, M.; Pexara, A. Nitrates and Nitrites in meat products. J. Hell. Vet. Med. Soc. 2015, 66, 127–140. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3564–3578. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, T.; Xiong, Z.; Yuan, L.; Hong, H.; Gao, R.; Bao, Y. Oxidation affects dye binding of myofibrillar proteins via alteration in net charges mediated by a reduction in isoelectric point. Food Res. Int. 2023, 163, 112204. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.-H.; Wan, J.; Zhou, Y.-L.; Zhou, Y.; Zhu, Q.-J.; Liu, L.-G.; Chen, D.; Huang, Y.-P.; Gu, S.; Li, M.-M. Mediated curing strategy: An overview of salt reduction for dry-cured meat products. Food Rev. Int. 2023, 39, 4565–4580. [Google Scholar]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary supplementation of postbiotics mitigates adverse impacts of heat stress on antioxidant enzyme activity, total antioxidant, lipid peroxidation, physiological stress indicators, lipid profile and meat quality in broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research update on the impact of lactic acid bacteria on the substance metabolism, flavor, and quality characteristics of fermented meat products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control, Botulism—Annual Epidemiological Report for 2022. 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/botulism-annual-epidemiological-report-2022 (accessed on 1 June 2025).
- Fayaz, H.; Ahmad, S.R.; Hussain, S.A.; Sofi, A.H.; Nazir, T. Nitrite Reduction/Replacement in Processed Meat Products. In Hand Book of Processed Functional Meat Products; Springer Nature: Cham, Switzerland, 2024; pp. 251–289. [Google Scholar]
- Glass, K.A.; Golden, M.C.; Sindelar, J.J.; Wanless, B.J. Comparison of growth inhibition of Clostridium perfringens, Clostridium botulinum, and Bacillus cereus during extended cooling of uncured poultry. Meat Muscle Biol. 2024, 8, 17650. [Google Scholar]
- Nikolova, A.S.; Belichovska, D. Possibilities of nitrite replacement in meat products. Knowl. Int. J. 2022, 51, 465–470. [Google Scholar]
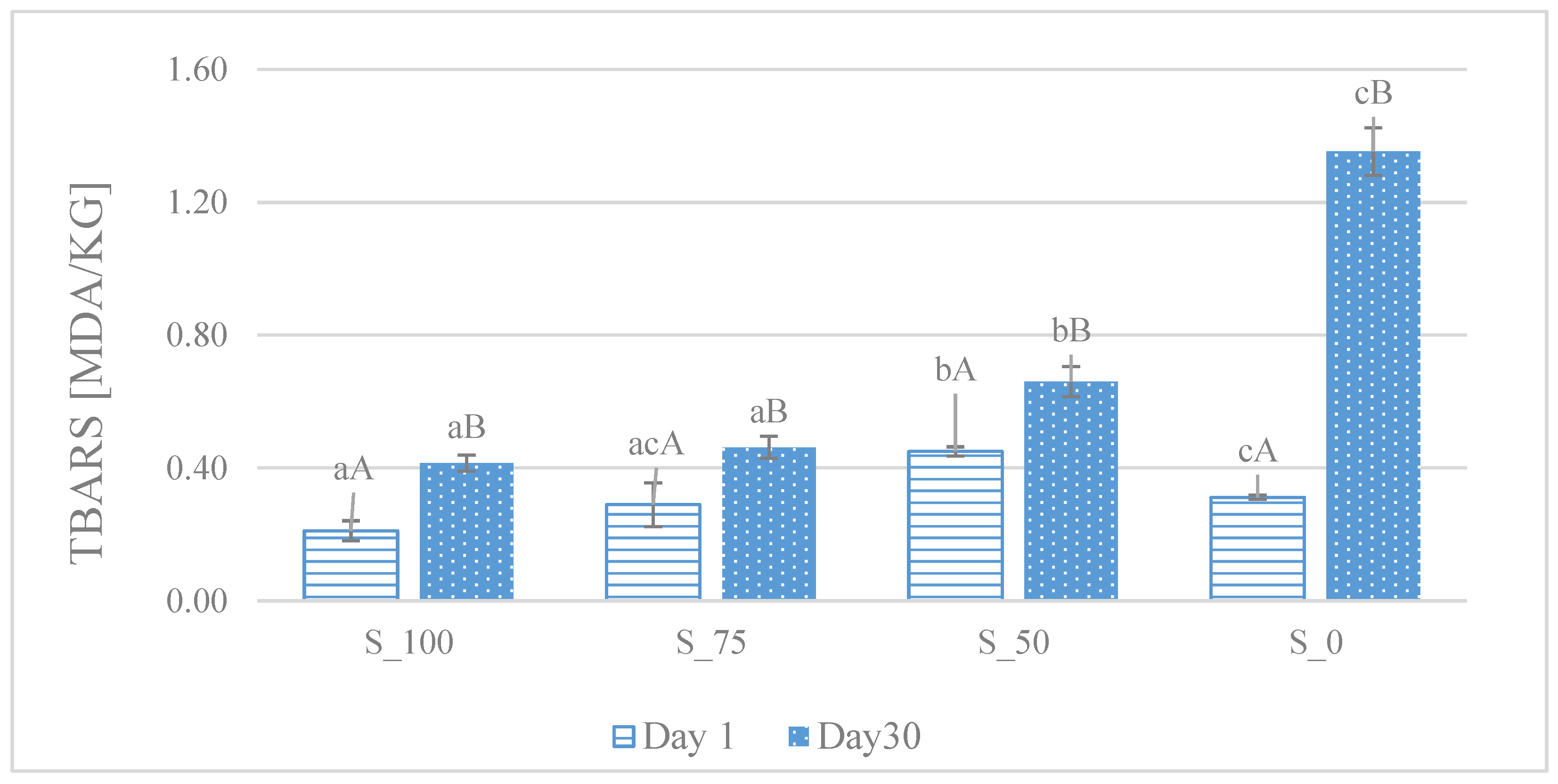
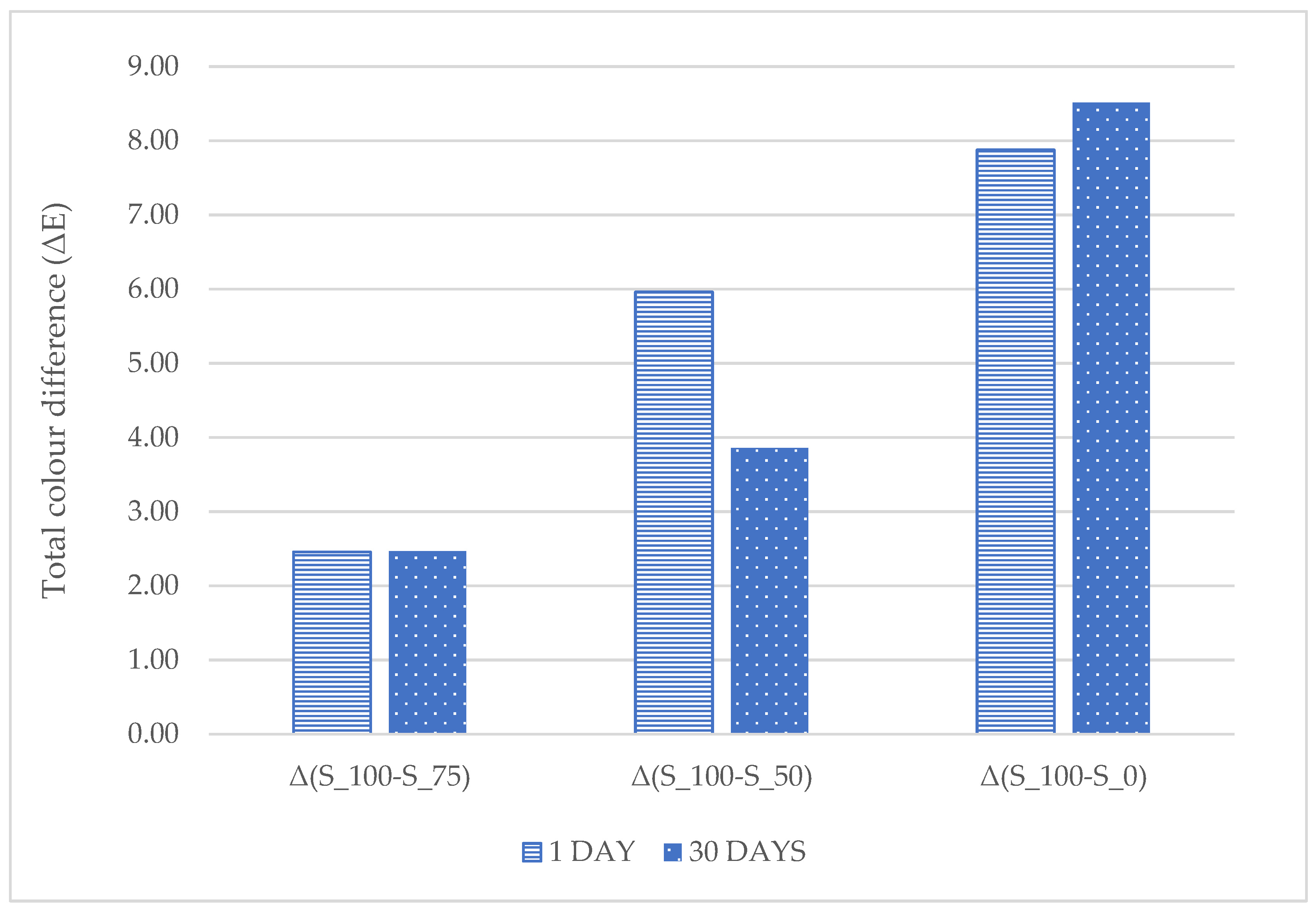
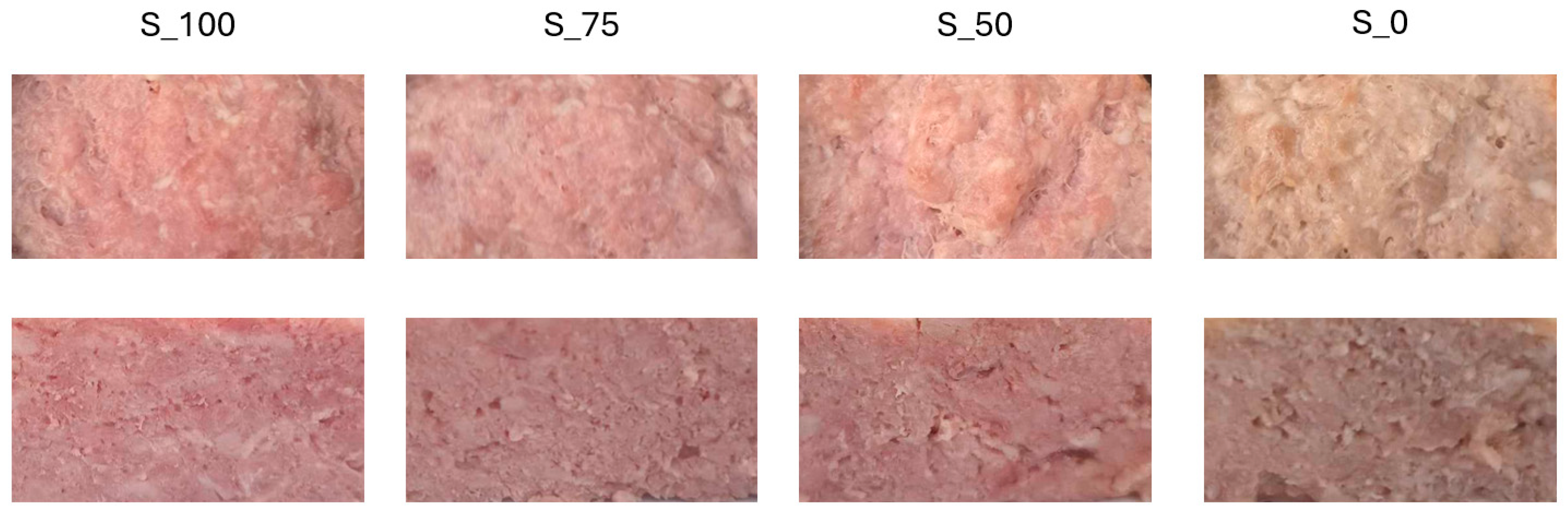
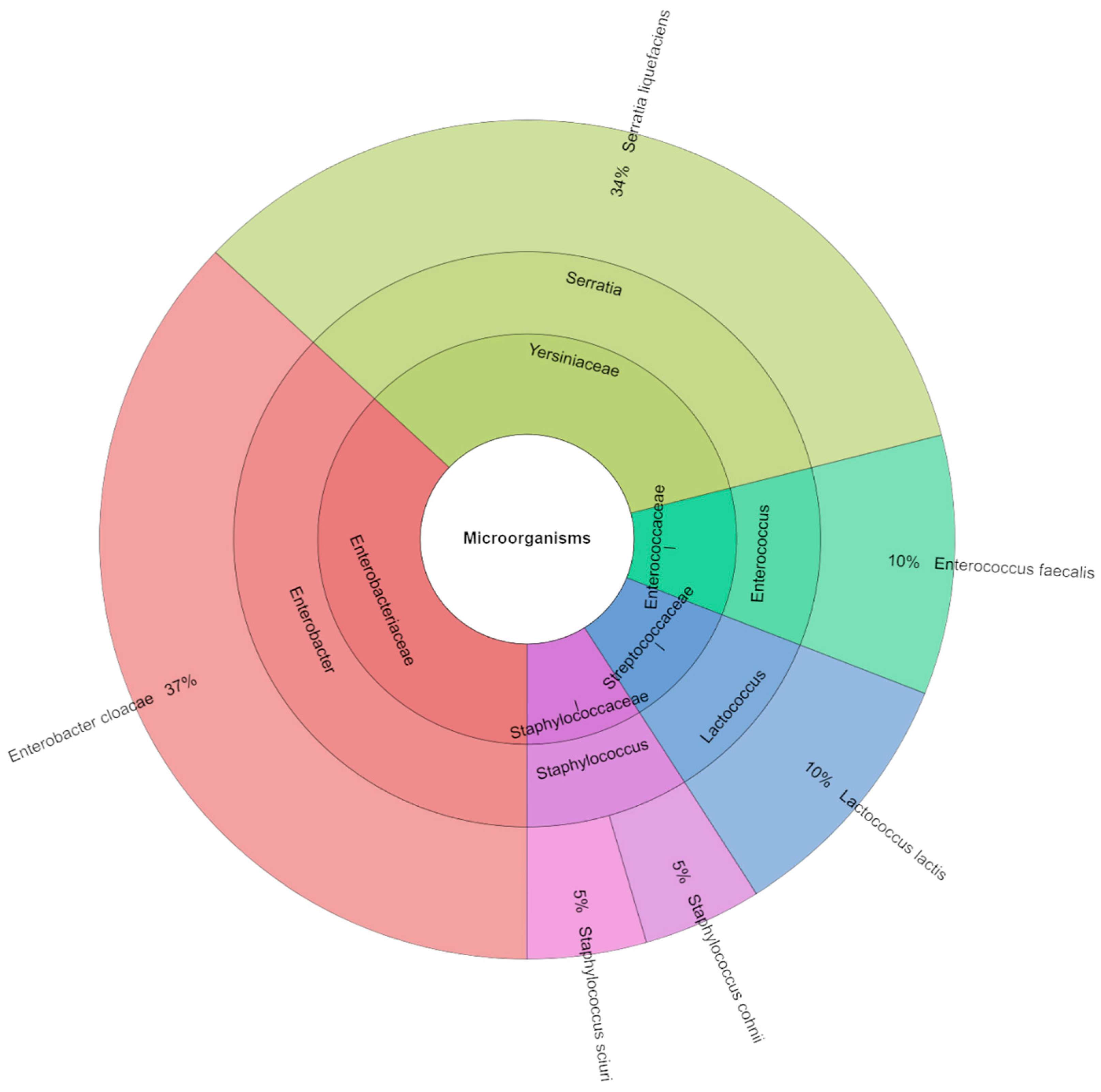
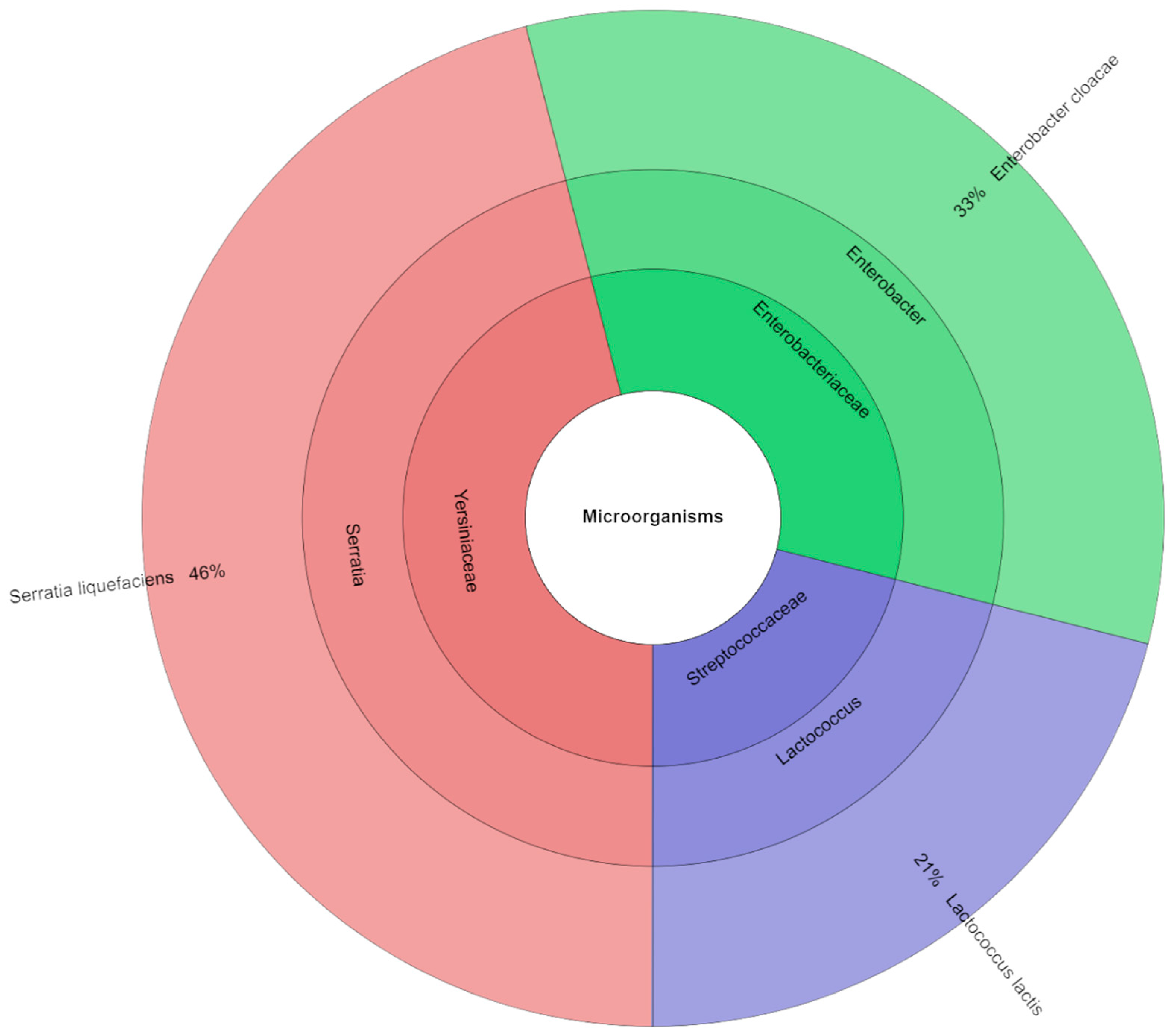
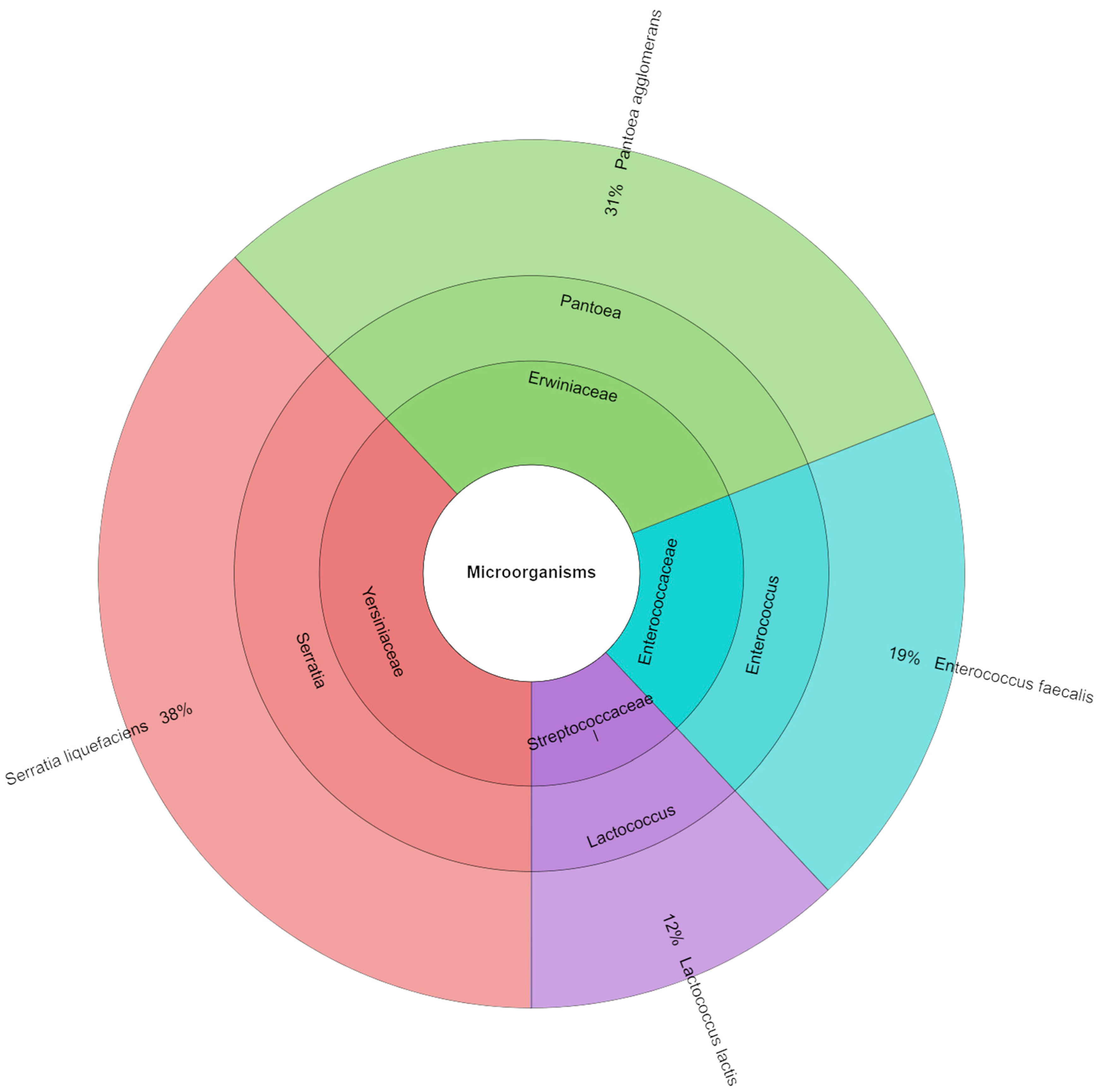
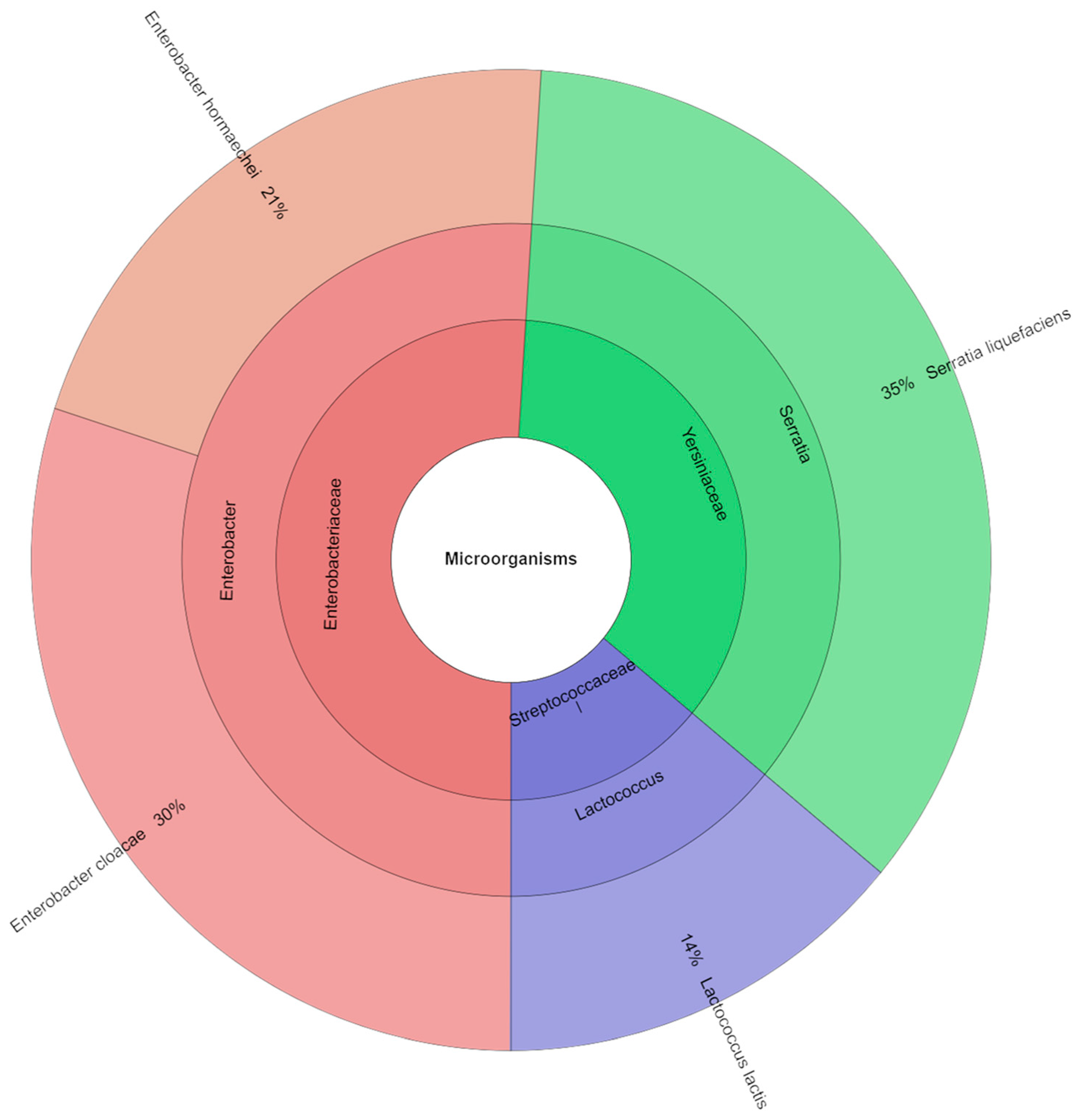
| Storage [Day] | S_100 | S_75 | S_50 | S_0 |
|---|---|---|---|---|
| water activity | ||||
| 1 | 0.985 ± 0.002 aA | 0.980 ± 0.002 aA | 0.985 ± 0.004 aA | 0.987 ± 0.04 aA |
| 30 | 0.967 ± 0.02 aB | 0.968 ± 0.001 aB | 0.964 ± 0.001 aB | 0.958 ± 0.002 bB |
| pH | ||||
| 1 | 5.968 ± 0.049 aA | 5.870 ± 0.021 bA | 5.835 ± 0.005 cA | 5.782 ± 0.013 cA |
| 30 | 6.165 ± 0.049 aB | 6.063 ± 0.072 abB | 6.070 ± 0.010 abB | 6.057 ± 0.006 bB |
| oxidation-reduction potential | ||||
| 1 | 342.933 ± 7.574 aA | 339.633 ± 1.528 aA | 340.400 ± 2.265 aA | 352.167 ± 5.543 aA |
| 30 | 393.100 ± 3.253 aB | 408.457 ± 3.669 bcB | 400.500 ± 5.027 abB | 414.800 ± 6.843 cB |
| Fatty Acid | S_100 | S_75 | S_50 | S_0 |
|---|---|---|---|---|
| C6:0 | 0.08 ± 0.10 a | 0.09 ± 0.005 a | 0.09 ± 0.002 a | 0.08 ± 0.002 a |
| C8:0 | 0.08 ± 0.05 a | 0.08 ± 0.002 a | 0.08 ± 0.010 a | 0.90 ± 0.010 b |
| C10:0 | 0.13 ± 0.02 a | 0.30 ± 0.005 b | 0.14 ± 0.05 c | 0.11 ± 0.005 d |
| C12:0 | 0.11 ± 0.010 a | 0.11 ± 0.005 a | 0.11 ± 0.05 a | 0.10 ± 0.002 a |
| C14:0 | 1.69 ± 0.005 a | 1.59 ± 0.010 b | 1.66 ± 0.008 c | 1.61 ± 0.005 d |
| C14:1n5 | 0.02 ± 0.005 a | 0.02 ± 0.005 a | 0.02 ± 0.002 a | 0.02 ± 0.005 a |
| C15:0 | 0.048 ± 0.008 a | 0.047 ± 0.006 a | 0.05 ± 0.030 a | 0.05 ± 0.005 a |
| C16:0 | 29.56 ± 0.002 a | 28.53 ± 0.002 b | 29.19 ± 0.005 c | 29.11 ± 0.002 d |
| C16:1n7 | 2.98 ± 0.030 a | 3.06 ± 0.010 b | 3.06 ± 0.010 b | 2.88 ± 0.002 c |
| C17:0 | 0.23 ± 0.005 a | 0.24 ± 0.005 ab | 0.25 ± 0.005 b | 0.23 ± 0.10 a |
| C17:1n7 | 0.17 ± 0.002 a | 0.20 ± 0.10 b | 0.20 ± 0.005 b | 0.17 ± 0.005 a |
| C18:0 | 16.19 ± 0.010 a | 15.24 ± 0.002 b | 15.50 ± 0.010 c | 16.39 ± 0.005 d |
| C18:1n9c + C18:1n9t | 39.14 ± 0.010 a | 40.40 ± 0.002 b | 36.64 ± 0.005 c | 39.92 ± 0.005 d |
| C18:2n6c + C18:2n6t | 2.1 ± 0.005 a | 2.81 ± 0.030 b | 2.08 ± 0.005 a | 2.39 ± 0.002 c |
| C18:3n3 (ALPHA) | 0.04 ± 0.002 a | 0.09 ± 0.002 b | 0.04 ± 0.002 a | 0.07 ± 0.005 c |
| C20:0 | 0.21 ± 0.010 a | 0.20 ± 0.030 a | 0.20 ± 0.010 a | 0.22 ± 0.005 a |
| C20:1n15 | 0.77 ± 0.005 a | 0.77 ± 0.005 a | 0.77 ± 0.005 a | 0.78 ± 0.002 a |
| C20:1n9 | 0.12 ± 0.010 a | 0.15 ± 0.002 b | 0.11 ± 0.010 a | 0.13 ± 0.010 ab |
| C20:3n3 | 0.03 ± 0.005 a | 0.03 ± 0.005 a | 0.02 ± 0.008 a | 0.05 ± 0.005 b |
| C22:0 | 0.07 ± 0.010 a | 0.07 ± 0.005 a | 0.05 ± 0.005 b | 0.06 ± 0.002 a |
| C24:1n9 | 0.42 ± 0.005 a | 0.33 ± 0.010 b | 0.37 ± 0.002 c | 0.32 ± 0.00 b |
| SUMMARY | ||||
| SFA | 48.48 ± 0.027 a | 46.35 ± 0.287 b | 47.40 ± 0.027 c | 48.50 ± 0.287 a |
| MUFA | 43.62 ± 0.046 a | 44.93 ± 0.046 b | 44.17 ± 0.037 c | 44.22 ± 0.046 c |
| PUFA | 2.08 ± 0.035 a | 2.97 ± 0.033 b | 2.24 ± 0.013 c | 2.55 ± 0.033 d |
| OMEGA3 | 0.07 ± 0.08 a | 0.14 ± 0.001 b | 0.07 ± 0.009 a | 0.12 ± 0.001 c |
| OMEGA6 | 2.10 ± 0.042 a | 2.83 ± 0.040 b | 2.17 ± 0.042 a | 2.43 ± 0.040 c |
| OMEGA9 | 39.68 ± 0.092 a | 40.88 ± 0.024 b | 40.12 ± 0.052 c | 40.37 ± 0.024 d |
| Storage [Day] | S_100 | S_75 | S_50 | S_0 |
|---|---|---|---|---|
| ABTS [%] | ||||
| 1 | 77.08 ± 1.19 cA | 73.23 ± 4.52 cA | 57.52 ± 3.39 bA | 47.13 ± 2.73 aA |
| 30 | 72.71 ± 3.52 cB | 61.10 ± 2.09 bA | 58.60 ± 1.54 bB | 54.64 ± 1.42 aB |
| RP [A700] | ||||
| 1 | 0.16 ± 0.07 cA | 1.60 ± 0.01 bA | 1.62 ± 0.06 bA | 1.40 ± 0.05 aA |
| 30 | 1.66 ± 0.02 bB | 1.68 ± 0.03 bB | 1.71 ± 0.02 bB | 1.54 ± 0.01 aB |
| Storage [Day] | S_100 | S_75 | S_50 | S_0 |
|---|---|---|---|---|
| lightness (L*) | ||||
| 1 | 66.09 ± 1.70 aA | 66.09 ± 1.70 aA | 67.59 ± 2.54 aA | 67.31 ± 2.70 aA |
| 30 | 65.21 ± 1.31 aA | 65.21 ± 1.31 aA | 67.43 ± 1.58 bA | 69.99 ± 1.56 cB |
| redness (a*) | ||||
| 1 | 9.15 ± 0.82 aA | 9.15 ± 0.82 aA | 8.07 ± 0.45 cA | 3.61 ± 0.52 dA |
| 30 | 9.34 ± 0.74 aA | 9.34 ± 0.74 aA | 8.18 ± 0.69 bA | 5.11 ± 0.61 cB |
| yellowness (b*) | ||||
| 1 | 9.23 ± 0.60 aA | 9.23 ± 0.60 aA | 12.60 ± 0.49 bA | 14.69 ± 0.96 cA |
| 30 | 9.90 ± 0.87 aA | 9.90 ± 0.87 aA | 12.83 ± 0.50 bA | 15.52 ± 0.31 cA |
| chroma (C*) | ||||
| 1 | 13.01 ± 1.44 aA | 13.01 ± 1.44 aA | 14.96 ± 1.42 bA | 15.15 ± 1.71 bA |
| 30 | 13.67 ± 0.91 aA | 13.67 ± 0.91 aA | 15.24 ± 0.41 bA | 16.35 ± 0.29 cA |
| hue angle (H°) | ||||
| 1 | 45.30 ± 1.44 aA | 45.30 ± 1.44 aA | 57.43 ± 1.42 cA | 76.20 ± 1.71 dA |
| 30 | 46.95 ± 2.47 aA | 46.95 ± 2.47 aA | 57.50 ± 2.78 cA | 71.86 ± 2.08 dB |
| Storage [Day] | S_100 | S_75 | S_50 | S_0 |
|---|---|---|---|---|
| Hardness [g] | ||||
| 1 | 2053.44 ± 26.73 aA | 2269.14 ± 55.08 bA | 2833.60 ± 51.42 cA | 2917.84 ± 58.36 dA |
| 30 | 1656.90 ± 47.43 aB | 1552.31 ± 47.43 abB | 1727.12 ± 93.30 acB | 2296.78 ± 28.21 dB |
| Cohesion [-] | ||||
| 1 | 0.349 ± 0.02 aA | 0.332 ± 0.03 aA | 0.351 ± 0.01 aA | 0.270 ± 0.03 bA |
| 30 | 0.586 ± 0.01 aB | 0.657 ± 0.03 aB | 0.653 ± 0.06 aB | 0.582 ± 0.07 aB |
| Springiness [%] | ||||
| 1 | 70.695 ± 0.61 aA | 68.04 ± 0.96 bA | 62.904 ± 1.49 cA | 76.230 ± 2.98 dA |
| 30 | 85.313 ± 0.35 aB | 84.076 ± 0.87 abB | 82.918 ± 0.48 bB | 80.116 ± 0.77 cA |
| Gumminess | ||||
| 1 | 718.43 ± 4.41 aA | 760.37 ± 3.07 bA | 808.77 ± 2.02 cA | 868.23 ± 14.36 dA |
| 30 | 970.218 ± 10.71 aB | 1007.521 ± 7.67 bB | 1234.153 ± 11.13 cB | 1413.439 ± 19.18 dB |
| Chewiness | ||||
| 1 | 503.29 ± 7.24 aA | 512.16 ± 8.64 aA | 537.23 ± 6.56 bA | 636.695 ± 7.43 cA |
| 30 | 829.627 ± 4.78 aB | 859.012 ± 3.65 aB | 1008.642 ± 7.11 bB | 922.153 ± 2.85 cB |
| Microbiological Criterium | S_100 | S_75 | S_50 | S_0 |
|---|---|---|---|---|
| Coliforms bacteria | 1.56 ± 0.09 a | 1.63 ± 0.13 a | 1.65 ± 0.12 a | 1.68 ± 0.15 a |
| Total viable count | 1.70 ± 0.06 a | 1.76 ± 0.09 a | 1.68 ± 0.20 a | 1.58 ± 0.11 a |
| Lactic acid bacteria | 1.68 ± 0.15 a | 1.65 ± 0.17 a | 1.52 ± 0.29 a | 1.56 ± 0.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kęska, P.; Kačániová, M.; Stadnik, J.; Wójciak, K.; Zielińska, D. Evaluation of Quality Parameters in Canned Pork Enriched with 1% Freeze-Dried Cell-Free Supernatant of Lacticaseibacillus paracasei B1 and Reduced Sodium Nitrite Content. Foods 2025, 14, 3080. https://doi.org/10.3390/foods14173080
Kęska P, Kačániová M, Stadnik J, Wójciak K, Zielińska D. Evaluation of Quality Parameters in Canned Pork Enriched with 1% Freeze-Dried Cell-Free Supernatant of Lacticaseibacillus paracasei B1 and Reduced Sodium Nitrite Content. Foods. 2025; 14(17):3080. https://doi.org/10.3390/foods14173080
Chicago/Turabian StyleKęska, Paulina, Miroslava Kačániová, Joanna Stadnik, Karolina Wójciak, and Dorota Zielińska. 2025. "Evaluation of Quality Parameters in Canned Pork Enriched with 1% Freeze-Dried Cell-Free Supernatant of Lacticaseibacillus paracasei B1 and Reduced Sodium Nitrite Content" Foods 14, no. 17: 3080. https://doi.org/10.3390/foods14173080
APA StyleKęska, P., Kačániová, M., Stadnik, J., Wójciak, K., & Zielińska, D. (2025). Evaluation of Quality Parameters in Canned Pork Enriched with 1% Freeze-Dried Cell-Free Supernatant of Lacticaseibacillus paracasei B1 and Reduced Sodium Nitrite Content. Foods, 14(17), 3080. https://doi.org/10.3390/foods14173080










