Nano Emulsion of Essential Oils Loaded in Chitosan Coating for Controlling Anthracnose in Tomatoes (Solanum lycopersicum) During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fungal Cultures
2.3. Inhibition Rate of EOs and CS by the Contact Method
2.4. The Effect of Nano-EO and CS on the Growth of C. gloeosporioides In Vitro
Development of Nano-(T)-EO
2.5. Development of CS and Nano (T)-EO Edible Coatings with Antifungal Properties
2.6. Inhibition of Spore Germination
2.7. In Vivo Antifungal Activity of Nano-(T)-EO-CS Coating Formulation
2.8. Scanning Electron Microscopy (SEM)
2.9. Defence Response and Antioxidant Activity
2.10. Statistical Analysis
3. Results and Discussions
3.1. Nano (T)-EO-CS on Inhibition Rate of C. gloeosporioides Mycelia
3.2. Nano-(T)-EO-CS Coating on the Inhibition of C. gloeosporioides in Inoculated Fruits
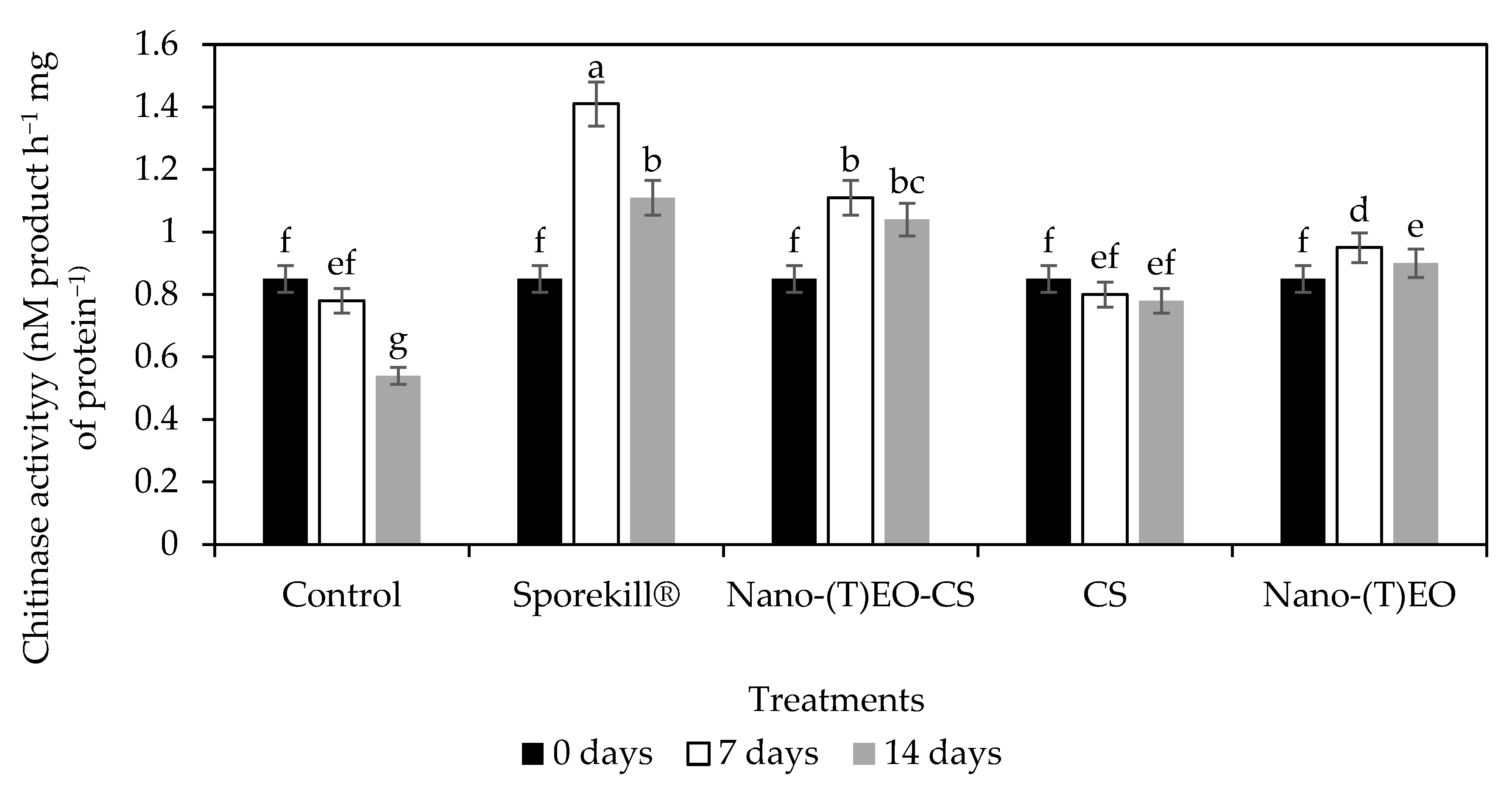
3.3. Nano-(T)-EO-CS Coating on Chitinase Activity
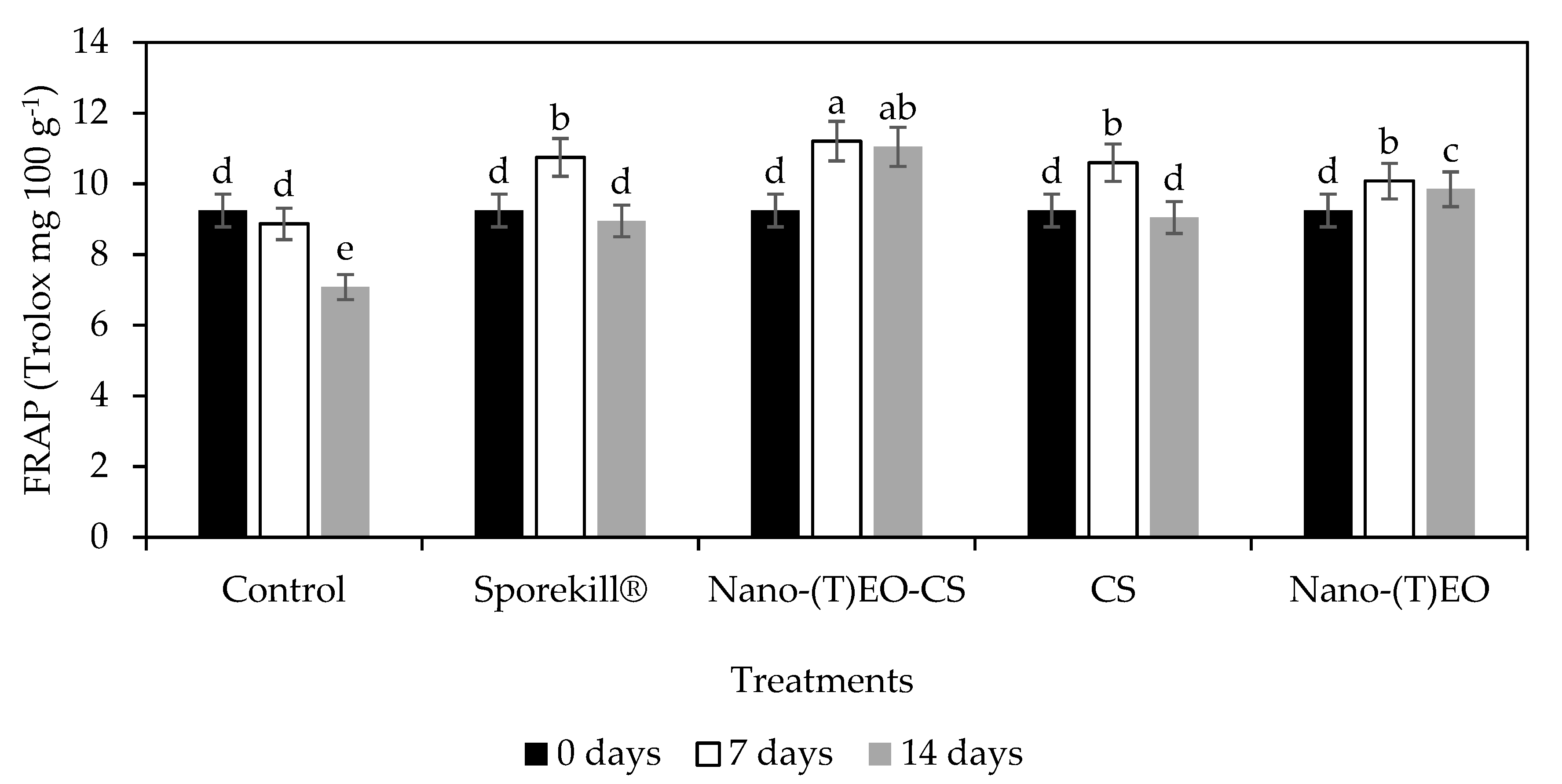
3.4. Nano-(T)-EO-CS Coating on Total Phenols and Antioxidant Power
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Agricultural Production Statistics 2010–2023. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/df90e6cf-4178-4361-97d4-5154a9213877/content (accessed on 18 December 2024).
- Li, C.; Tao, J.; Zhang, H. Peach gum polysaccharides-based edible coatings extend shelf life of cherry tomatoes. Biotech 2017, 7, 168. [Google Scholar] [CrossRef]
- Kim, K.H.; Yoon, J.B.; Park, H.-G.; Park, E.W.; Kim, Y.H. Structural modifications and programmed cell death of chili pepper fruit related to resistance responses to Colletotrichum gloeosporioides infection. Phytopathology 2004, 94, 1295–1304. [Google Scholar] [CrossRef]
- Camagay, A.V.; Kendall, N.; Connolly, M.K. Quaternary ammonium compound toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Moumni, M.; Romanazzi, G.; Najar, B.; Pistelli, L.; Ben Amara, H.; Mezrioui, K.; Karous, O.; Chaieb, I.; Allagui, M.B. Antifungal activity and chemical composition of seven essential oils to control the main seedborne fungi of cucurbits. Antibiotics 2021, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Wills, R.B.; Bowyer, M.C.; Golding, J.B.; Kirkman, T.; Pristijono, P. Potential control of postharvest fungal decay of citrus fruits by crude or photochemically changed essential oils—A review. Food Rev. Int. 2023, 40, 1029–1046. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Walla, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L.Y. A review of regulatory standards and advances in essential oils as antimicrobials in foods. J. Food Prot. 2022, 86, 100025. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, Z.; Saba, M.K.; Nourbakhsh, H.; Amini, J. Efficiency of nanoemulsion of essential oils to control Botrytis cinerea on strawberry surface and prolong fruit shelf life. Int. J. Food Microbiol. 2023, 384, 109979. [Google Scholar] [CrossRef]
- Pandey, V.K.; Islam, R.U.L.; Shams, R.; Hussain Dar, A. A comprehensive review on the application of essential oils as bioactive compounds in Nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, N.; Kumar, M.; Kumar, V.; Wang, S.; Abd-Elsalam, K.A. Nanomaterials act as plant defense mechanism. In Nanotechnology: Food and Environmental Paradigm; Springer: Singapore, 2017; pp. 253–269. [Google Scholar]
- de Souza, E.L.; Lundgren, G.A.; de Oliveira, K.Á.R.; Berger, L.R.R.; Magnani, M. An analysis of the published literature on the effects of edible coatings formed by polysaccharides and essential oils on postharvest microbial control and overall quality of fruit. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 1947–1967. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S.; Qazi, I.M.; Durrani, Y.; Sarkhosh, A.; Hussain, I.; Brecht, J.K. Pre-storage chitosan-thyme oil coating control anthracnose in mango fruit. Sci. Hortic. 2021, 284, 110139. [Google Scholar] [CrossRef]
- Morales-Rabanales, Q.N.; Coyotl-Pérez, W.A.; Rubio-Rosas, E.; Cortes-Ramírez, G.S.; Ramírez, J.F.; Villa-Ruano, N. Antifungal properties of hybrid films containing the essential oil of Schinus molle: Protective effect against postharvest rot of tomato. Food Control. 2022, 134, 108766. [Google Scholar] [CrossRef]
- Maswanganye, L.T.C.; Kesavan Pillai, S.; Sivakumar, D. Chitosan coating loaded with spearmint essential oil nanoemulsion for antifungal protection in soft citrus (Citrus reticulata) fruits. Coatings 2025, 15, 105. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Kahlmeter, G.; Guinea, J.; Meletiadis, J. How to: Perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E.Def 11.0, exemplified by Trichophyton. Clin. Microbiol. Infect. 2021, 27, 55–60. [Google Scholar] [CrossRef]
- Elshamy, S.; Khadizatul, K.; Uemura, K.; Nakajima, M.; Neves, M.A. Chitosan-based film incorporated with essential oil nanoemulsion foreseeing enhanced antimicrobial effect. J. Food Sci. Technol. 2021, 58, 3314–3327. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D.; Wijeratnam, R.W.; Wijesundera, R.L.C. Effect of GRAS compounds on mycelial growth, pectic enzyme activity and disease severity of postharvest pathogens on Rambutan (Nephelium lappaceum). Phytoparasitica 2001, 29, 135–141. [Google Scholar] [CrossRef]
- Malfatti, A.D.; Mallmann, G.C.; Oliveira Filho, L.C.; Carniel, L.S.; Cruz, S.P.; Klauberg-Filho, O. Ecotoxicological test to assess effects of herbicides on spore germination of Rhizophagus clarus and Gigaspora albida. Ecotoxicol. Environ. Saf. 2021, 207, 111599. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thomson, K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef]
- Bello, M.A.; Ruiz-León, Y.; Sandoval-Sierra, J.V.; Rezinciuc, S.; Diéguez-Uribeondo, J. Scanning electron microscopy (SEM) protocols for problematic plant, oomycete, and fungal samples. J. Vis. Exp. 2017, 120, 55031. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Enhancing the defence related and antioxidant enzymes activities in avocado cultivars with essential oil vapours. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Živković, S.; Stevanović, M.; Đurović, S.; Ristić, D.; Stošić, S. Antifungal activity of chitosan against Alternaria alternata and Colletotrichum gloeosporioides. Pestic. Fitomed. 2018, 33, 197–204. [Google Scholar] [CrossRef]
- Ayon Reyna, L.E.; Uriarte Gastelum, Y.G.; Camacho Diaz, B.H.; Tapia Maruri, D.; Lopez Lopez, M.E.; Lopez Velazquez, J.G.; Vega Garcia, M.O. Antifungal activity of a chitosan and mint essential oil coating on the development of Colletotrichum gloeosporioides in papaya using macroscopic and microscopic analysis. Food Bioprocess Technol. 2022, 15, 368–378. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and their potential applications in food industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Gidado, M.J.; Gunny, A.A.; Gopinath, S.C.; Ali, A.; Wongs-Aree, C.; Salleh, N.H. Challenges of postharvest water loss in fruits: Mechanisms, influencing factors, and effective control strategies—A comprehensive review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- de Bellaire, L.D.; Chillet, M.; Dubois, C.; Mourichon, X. Importance of different sources of inoculum and dispersal methods of conidia of Colletotrichum musae, the causal agent of banana anthracnose, for fruit contamination. Plant Pathol. 2008, 49, 782–790. [Google Scholar] [CrossRef]
- Vizcaino, I.S.; Rosas, E.R.; Almanza, E.A.; Castro, M.M.; Cocoletzi, H.H. Ultrasonic production of chitosan nanoparticles and their application against Colletotrichum gloeosporioides present in the Ataulfo mango. Polymers 2024, 16, 3058. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Lv, Y.; Wang, W.; Huang, J.; Zhang, D. Synergistic effect of acetic acid and chitosan against Aspergillus flavus. Int. J. Biol. Macromol. 2024, 281, 136548. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- de Billerbeck, V.G.; Roques, C.G.; Bessière, J.M.; Fonvieille, J.L.; Dargent, R. Effects of Cymbopogon nardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can. J. Microbiol. 2001, 47, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Helal, G.A.; Sarhan, M.M.; Abu Shahla, A.N.; Abou El-Khair, E.K. Effects of Cymbopogon citratus L. essential oil on the growth, lipid content and morphogenesis of Aspergillus niger ML2-strain. J. Basic Microbiol. 2006, 46, 456–469. [Google Scholar] [CrossRef]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 2015, 99, 37–43. [Google Scholar] [CrossRef]
- Amoozegaran, A.; Dehghan, H.; Homami, S.S.; Hashemi, S.A. Efficacy of an edible coating, containing thyme essential oil, to control Fusarium oxysporum and the quality of tomato fruits. J. Food Meas. Charact. 2022, 16, 3760–3767. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action mechanisms of effectors in plant-pathogen interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Gupta, A.; Prusty, M.R.; Kumar, M. Elicitation of fruit fungi infection and its protective response to improve the postharvest quality of fruits. Stresses 2023, 3, 231–255. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Int. 1999, 32, 407–412. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]

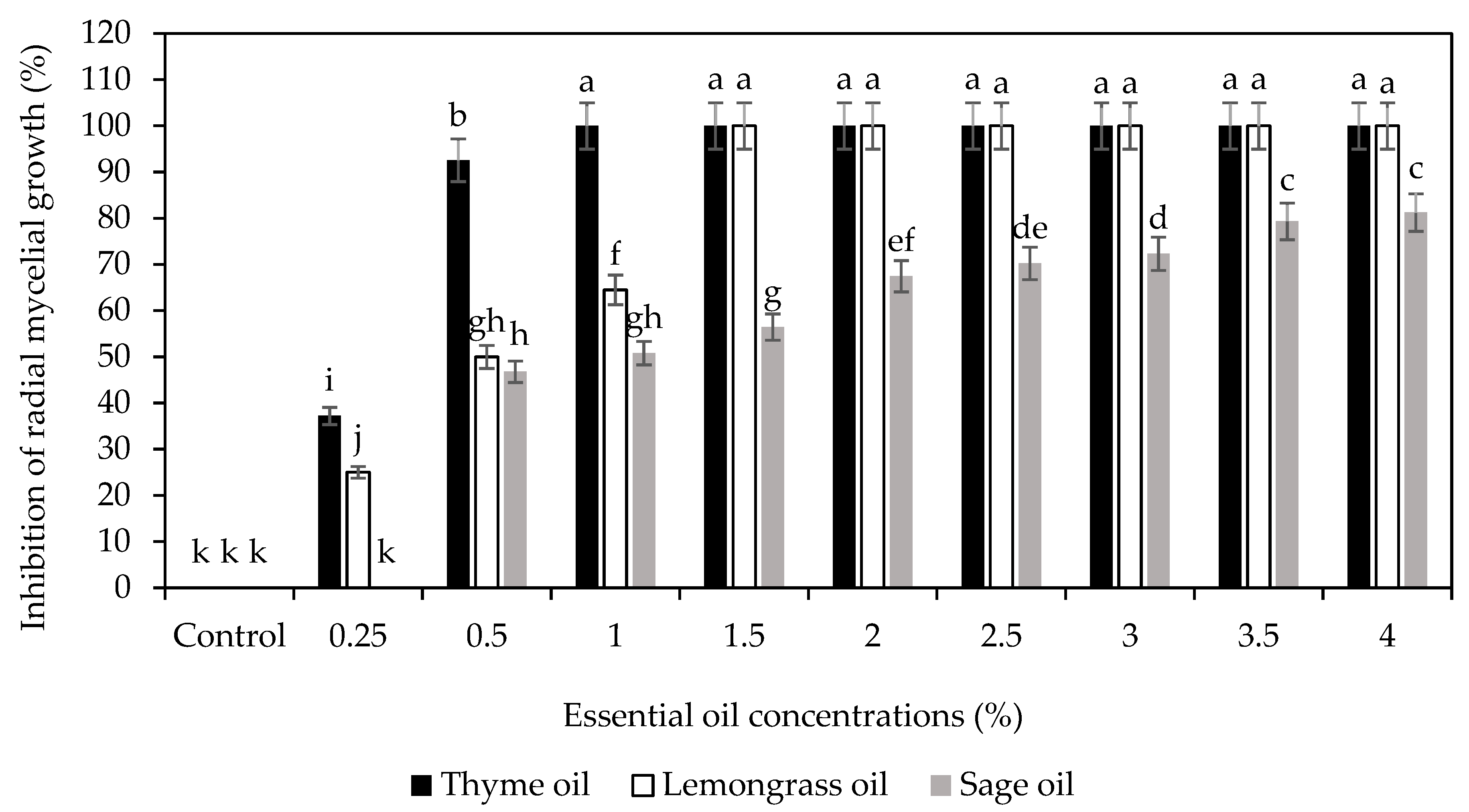

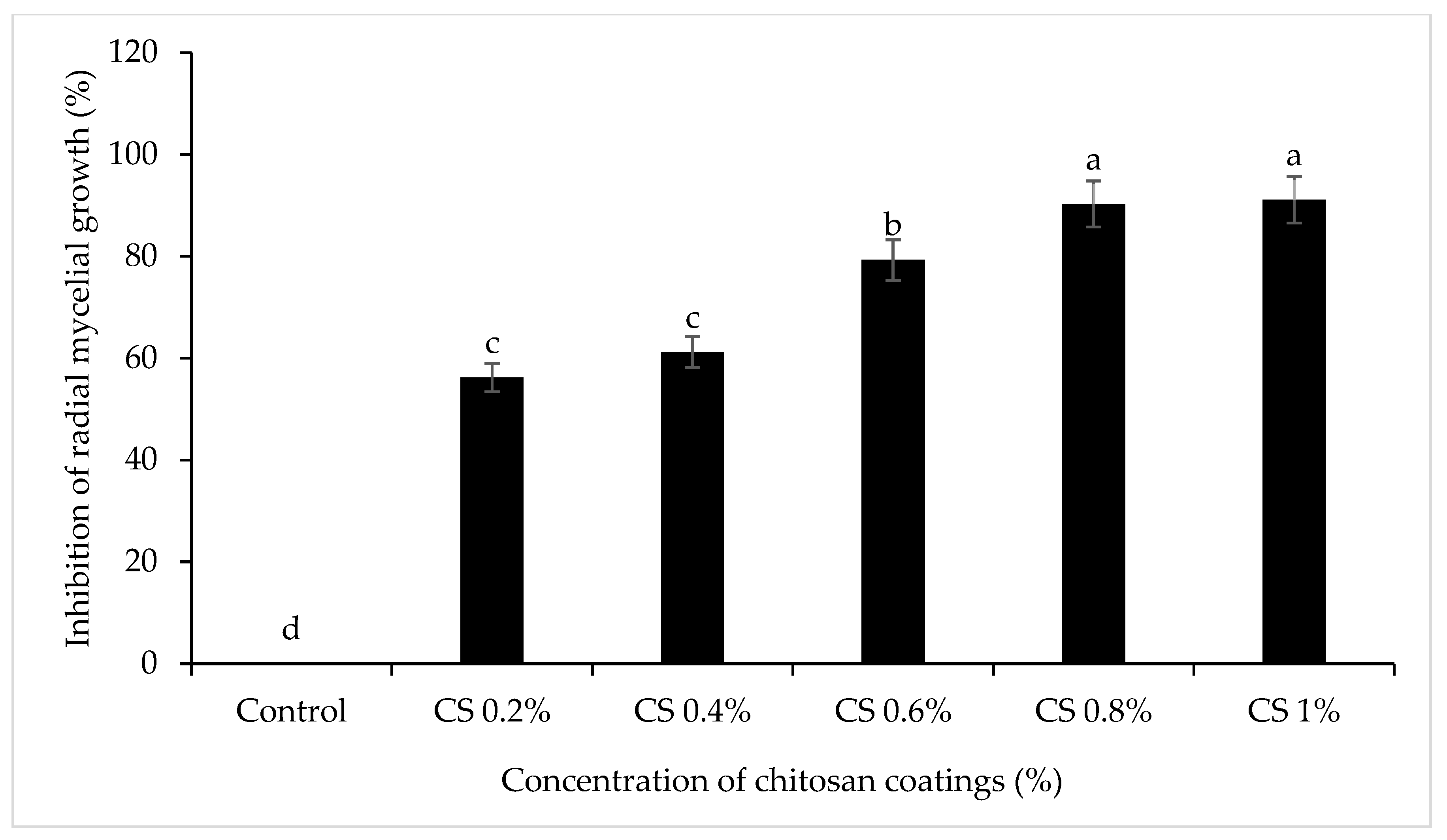




| Treatments | Concentrations | Colletotrichum gloeosporioides | ||
|---|---|---|---|---|
| Mycelia Growth (mm) | Inhibition (%) | Nature of Toxicity | ||
| Nano-(T)-EO | 0.25% | 38 ± 2 b | 38 | Fungistatic |
| 0.5% | 4.5 ± 0.5 g | 93 | Fungistatic | |
| 1% | 0 ± 0.00 h | 100 | Fungistatic | |
| CS | 0.2% | 26.5 ± 0.5 c | 56.19 | Fungistatic |
| 0.4% | 23.5 ± 0.5 d | 61.15 | Fungistatic | |
| 0.6 | 12.5 ± 0.5 e | 79.33 | Fungistatic | |
| 0.8% | 6 ± 1.00 f | 90.30 | Fungistatic | |
| 1% | 5.4 ± 0.0 f | 91.20 | Fungistatic | |
| Nano-(TEO-CS) | 1% + 0.8% | 0 ± 0.00 h | 100 | Fungistatic |
| Control | 59 ± 0.03 a | 0 | Fungistatic | |
| Sporekill® | 1.5% | 0 ± 0.00 h | 100 | Fungicidal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumede, S.; Mpai, S.; Kesavan Pillai, S.; Sivakumar, D. Nano Emulsion of Essential Oils Loaded in Chitosan Coating for Controlling Anthracnose in Tomatoes (Solanum lycopersicum) During Storage. Foods 2025, 14, 3038. https://doi.org/10.3390/foods14173038
Gumede S, Mpai S, Kesavan Pillai S, Sivakumar D. Nano Emulsion of Essential Oils Loaded in Chitosan Coating for Controlling Anthracnose in Tomatoes (Solanum lycopersicum) During Storage. Foods. 2025; 14(17):3038. https://doi.org/10.3390/foods14173038
Chicago/Turabian StyleGumede, Sibahle, Semakaleng Mpai, Sreejarani Kesavan Pillai, and Dharini Sivakumar. 2025. "Nano Emulsion of Essential Oils Loaded in Chitosan Coating for Controlling Anthracnose in Tomatoes (Solanum lycopersicum) During Storage" Foods 14, no. 17: 3038. https://doi.org/10.3390/foods14173038
APA StyleGumede, S., Mpai, S., Kesavan Pillai, S., & Sivakumar, D. (2025). Nano Emulsion of Essential Oils Loaded in Chitosan Coating for Controlling Anthracnose in Tomatoes (Solanum lycopersicum) During Storage. Foods, 14(17), 3038. https://doi.org/10.3390/foods14173038








