Comparative Analysis of Flavor Quality of Beef with Tangerine Peel Reheated by Stir-Frying, Steaming and Microwave
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.2.1. Preparation of Beef with Tangerine Peel
2.2.2. Reheating Process
2.3. Moisture Content and Thiobarbituric Acid Reactive Substance Value
2.4. Gas Chromatography–Mass Spectrometry Analysis
2.5. Odor Activity Value Analysis
2.6. Gas Chromatography–Ion Mobility Spectrometry Analysis
2.7. Determination of Free Amino Acids
2.8. Determination of Nucleotides
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
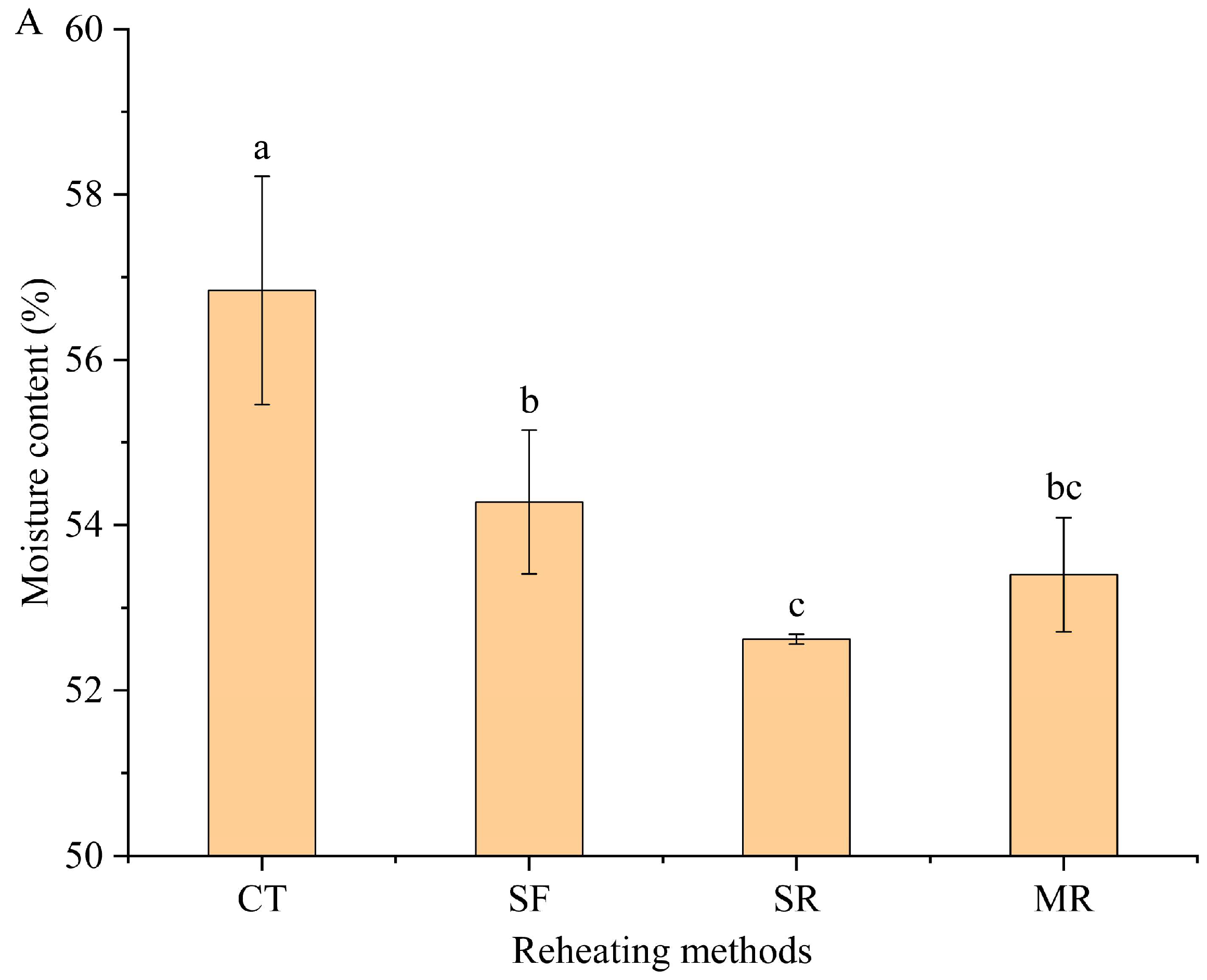
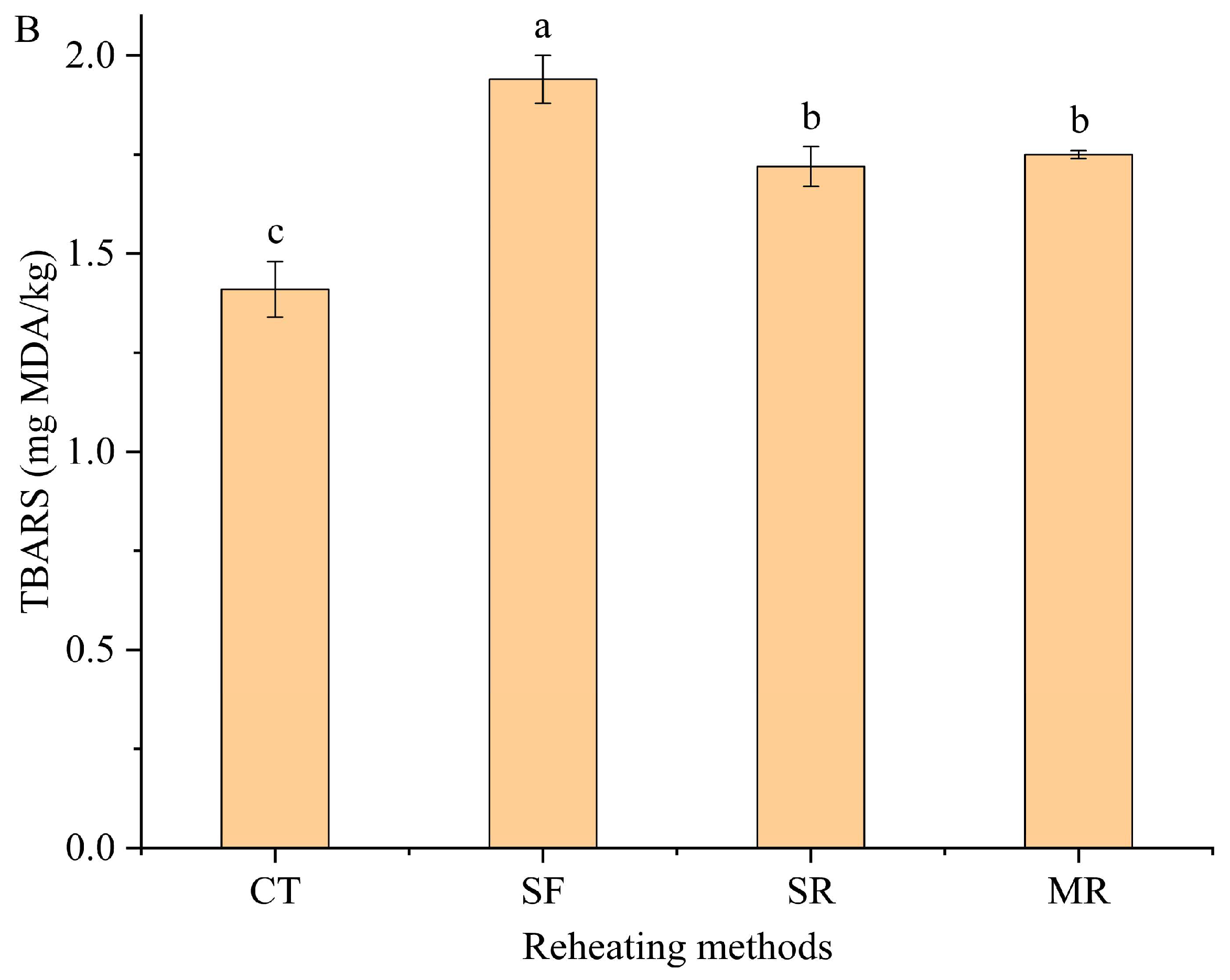
3.1. Moisture Content and Lipid Oxidation
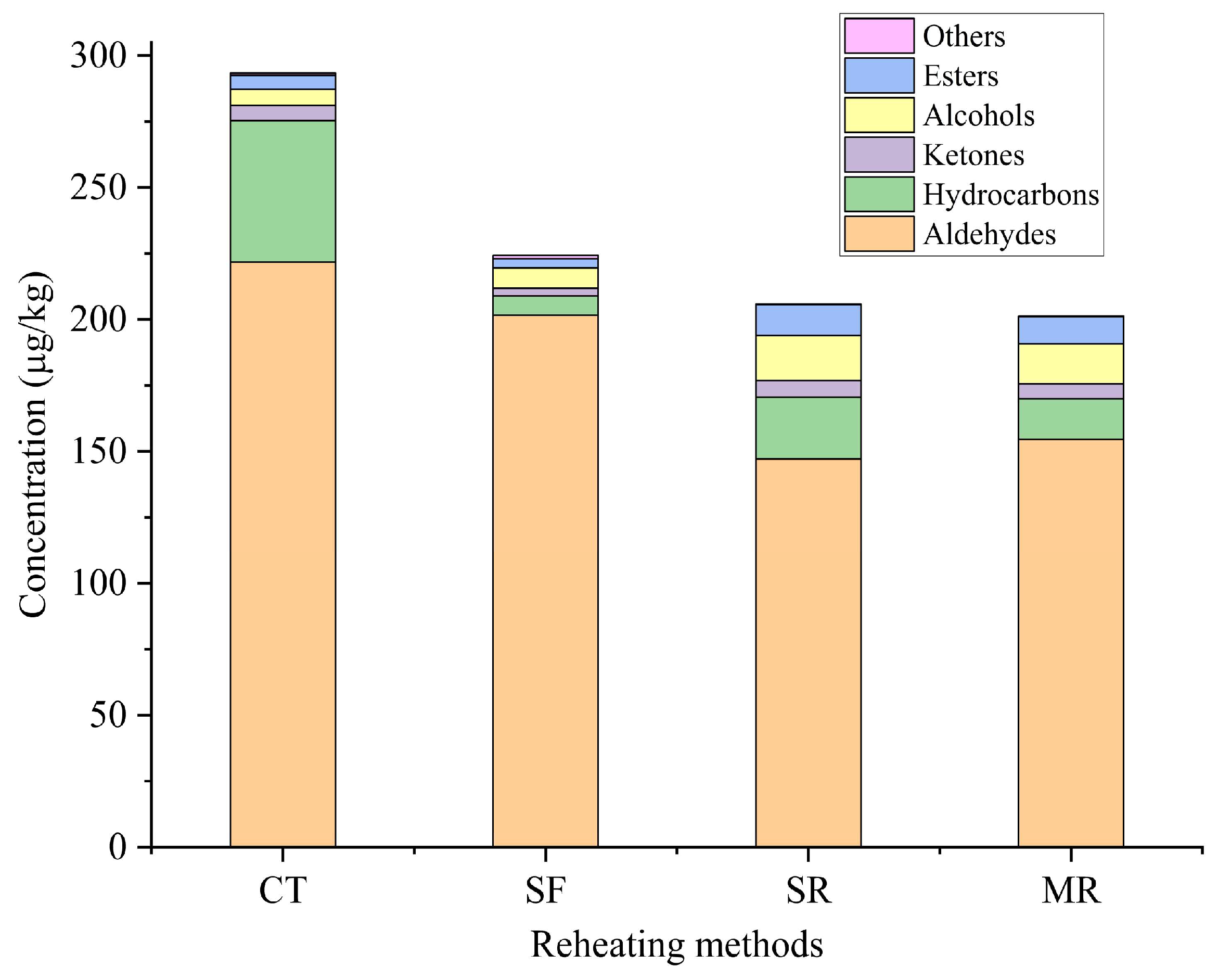
3.2. Volatile Compounds of Different Reheated Samples by Gas Chromatography–Mass Spectrometry
3.3. Odor-Active Compounds of Reheated Beef Samples
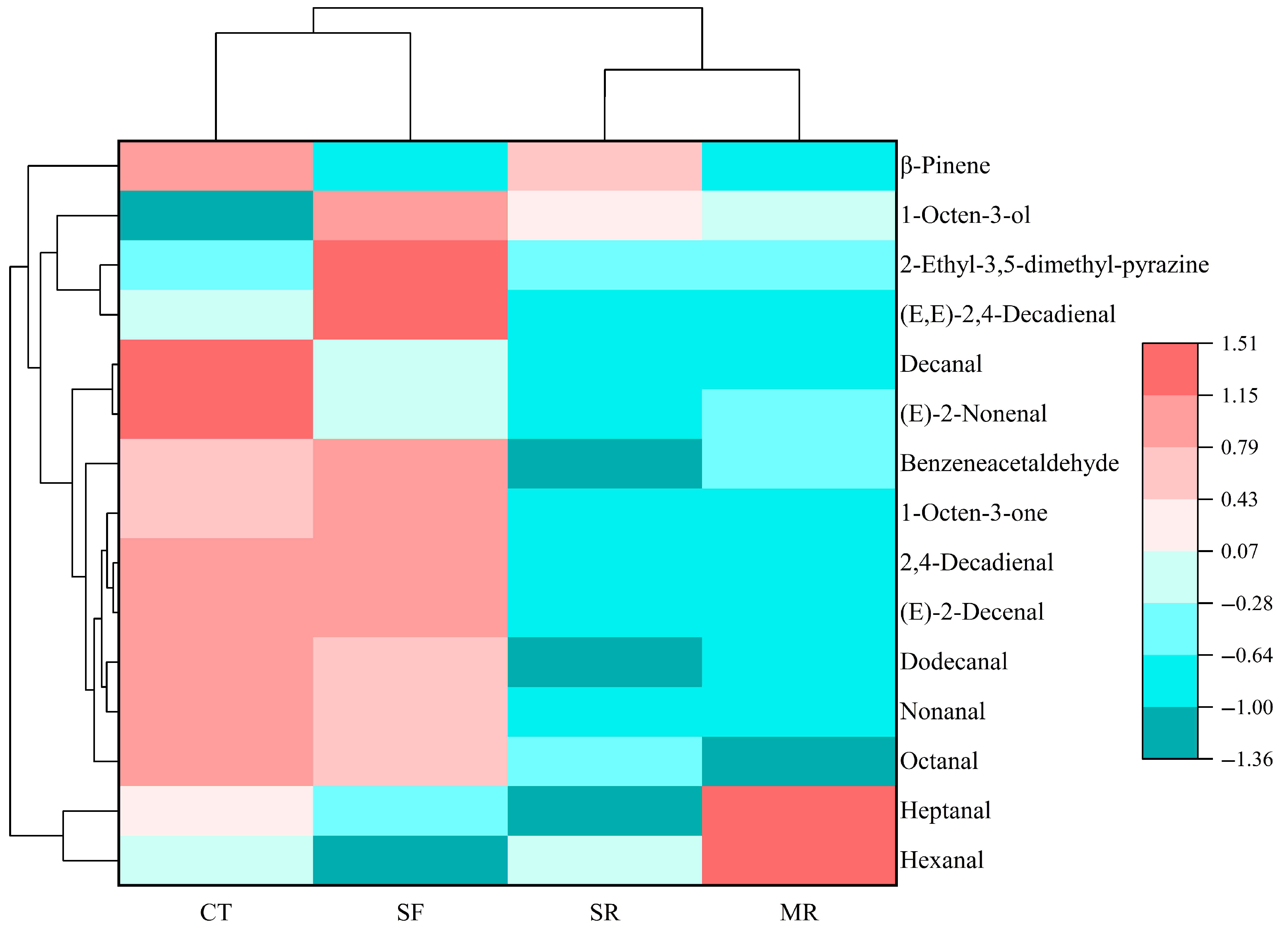
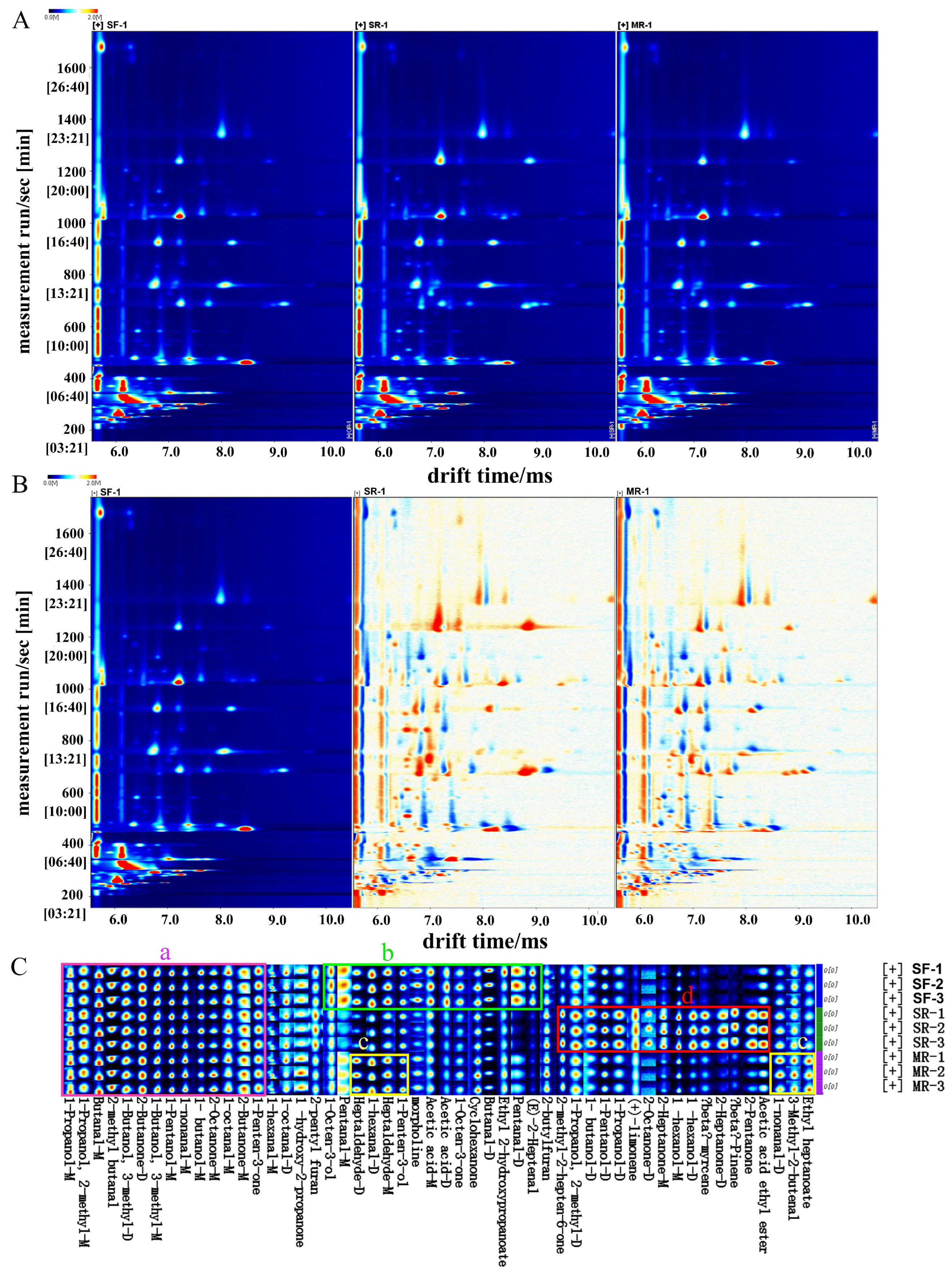
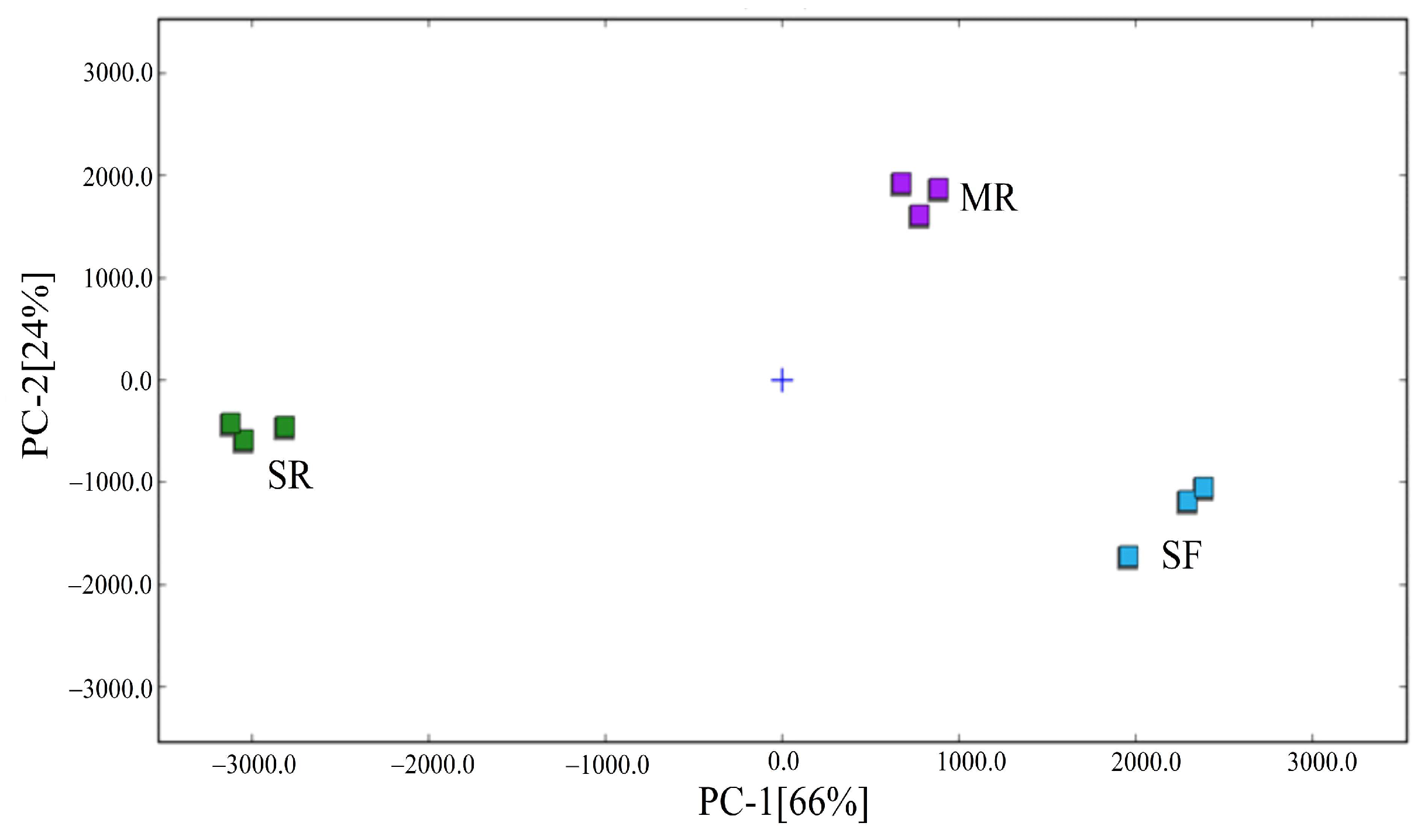
3.4. Changes of Volatile Organic Compounds by Gas Chromatography–Ion Mobility Spectrometry
3.5. Free Amino Acid Concentration in Reheated Beef Samples
3.6. Nucleotides Concentration in Reheated Beef Samples
3.7. Sensory Evaluation of Different Reheated Beef Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Control without reheating treatment |
| SF | Stir-frying reheating |
| SR | Steaming reheating |
| MR | Microwave reheating |
| GC–MS | Gas chromatography–mass spectrometry |
| OAV | Odor activity value |
| GC–IMS | Gas chromatography–ion mobility spectrometry |
| PCA | Principal component analysis |
| TBARS | Thiobarbituric acid reactive substance |
| FAAs | Free amino acids |
| HPLC | High-performance liquid chromatography |
| ANOVA | Analysis of variance |
References
- Chen, J.; Zhang, Y.; Ren, Y.; Chen, X.; Feng, Y.; Zhang, Y.; Yin, J.; Liu, G. The formation mechanism and control strategies of warmed-over flavor in prepared dishes: A comprehensive review and future perspectives. Trends Food Sci. Technol. 2024, 153, 104746. [Google Scholar] [CrossRef]
- Hu, J.; Tao, Y.; Jiao, X.; Chen, X.; Zhang, N.; Yan, B.; Tang, X.; Huang, J.; Chen, W.; Fan, D. Current physical processing technologies for salt reduction in prepared dishes. Food Res. Int. 2025, 214, 116653. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, X.; Yan, B.; Zhang, N.; Tao, Y.; Hu, J.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Precooked state based on protein denaturation kinetics impacts moisture status, protein oxidation and texture of prepared chicken breast. Food Chem. 2025, 462, 140994. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, M.; Ju, R.; Mujumdar, A.S.; Wang, H. Advances in prepared dish processing using efficient physical fields: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 4031–4045. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Li, Y.; Liang, J.; Dong, H.; Zhao, W.; Bai, W.; Bi, S. Study on quality of prepared beef with tangerine peel. China Condiment 2024, 49, 135–142. (In Chinese) [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; et al. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]
- Luo, X.; Xiao, S.; Ruan, Q.; Gao, Q.; An, Y.; Hu, Y.; Xiong, S. Differences in flavor characteristics of frozen surimi products reheated by microwave, water boiling, steaming, and frying. Food Chem. 2022, 372, 131260. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Huang, F.; Zhang, C. Effect of reheating methods on eating quality, oxidation and flavor characteristics of Braised beef with potatoes dish. Int. J. Gastron. Food Sci. 2023, 31, 100659. [Google Scholar] [CrossRef]
- Ping, C.; Liu, Y.; Bi, J.; Cai, X.; Li, X.; Qiao, M. Identification of characteristic flavor quality of ceramic-pot sealed meat after reheating based on HS-GC-IMS, bionic sensory combined chemometrics. Food Chem. X 2024, 23, 101640. [Google Scholar] [CrossRef]
- Sheng, J.; Gao, F.; Dong, Y.; Li, Q.; Xu, X.; Wang, H. Evaluating the effects of different preheating and reheating procedures on water-holding capacity and flavor in meat patties. Food Res. Int. 2025, 203, 115849. [Google Scholar] [CrossRef]
- Lee, S.; Jo, K.; Park, M.K.; Choi, Y.; Jung, S. Role of lipids in beef flavor development: A review of research from the past 20 years. Food Chem. 2025, 475, 143310. [Google Scholar] [CrossRef]
- Wen, R.; Kong, B.; Yin, X.; Zhang, H.; Chen, Q. Characterisation of flavour profile of beef jerky inoculated with different autochthonous lactic acid bacteria using electronic nose and gas chromatography-ion mobility spectrometry. Meat Sci. 2022, 183, 108658. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Ge, S.; Han, L.; Hou, S.; Yuan, Z.; Gui, L.; Sun, S.; Yang, C.; Wang, Z.; Yang, B. Influence of cooking methods on flavor parameters and sensory quality of Tibetan sheep meat examined using an electronic nose, an electronic tongue, GC-IMS, and GC-MS. Foods 2025, 14, 2181. [Google Scholar] [CrossRef]
- Pan, Q.; Shao, X.; Xiao, Q.; Gu, Q.; Chen, C.; Xu, B.; Li, P. Revealing the flavor changes of spiced beef under different thermal treatment temperatures: A complementary approach with GC-IMS and GC-O-MS. Food Chem. 2025, 473, 143074. [Google Scholar] [CrossRef] [PubMed]
- GB-5009.3; Chinese National Standard GB 5009.3-2016. National Food Safety Standard: Determination of Moisture Content in Food. Standards Press of China: Beijing, China, 2016. (In Chinese)
- Zhu, J.; Lin, W.; Sun, Y.; Pan, D.; Xia, Q.; Zhou, C.; He, J. Relationship between flavor characteristics and lipid oxidation in air-dried beef at different roasting stages. Int. J. Gastron. Food Sci. 2024, 37, 100988. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, C.; Chen, M.; Jin, C. The effect of sodium chloride on the physicochemical and textural properties and flavor characteristics of sous vide cooked duck meat. Foods 2023, 12, 3452. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L.J. Odour Threshold: Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Zhan, H.; Hayat, K.; Cui, H.; Hussain, S.; Ho, C.T.; Zhang, X. Characterization of flavor active non-volatile compounds in chicken broth and correlated contributing constituent compounds in muscle through sensory evaluation and partial least square regression analysis. LWT 2020, 118, 108786. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, Y.; Peng, J.; Zhang, Y.; Zhang, W.; Liu, Y. Characterization of stewed beef by sensory evaluation and multiple intelligent sensory technologies combined with chemometrics methods. Food Chem. 2023, 408, 135193. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Shi, H.; Zhao, H.; Zhao, J.; Meng, S.; Shen, S.; Li, J. The effects of different reheating methods on the quality of pre-cooked braised chicken. Foods 2025, 14, 868. [Google Scholar] [CrossRef]
- Mei, H.; Gong, Z.; Ding, H.; Hu, M.; Xu, B.; Wang, W.; Cai, K.; Xu, B. Microwave synergistic steam reheating: A promising method for maintaining flavor and reducing advanced glycation end products in braised pork. LWT 2025, 222, 117633. [Google Scholar] [CrossRef]
- Nuora, A.; Chiang, V.S.C.; Milan, A.M.; Tarvainen, M.; Pundir, S.; Quek, S.Y.; Smith, G.C.; Markworth, J.F.; Ahotupa, M. The impact of beef steak thermal processing on lipid oxidation and postprandial inflammation related responses. Food Chem. 2015, 184, 57–64. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Liu, S.; Hong, P.; Zhou, C.; Zhong, S. Characterization of the effect of different cooking methods on volatile compounds in fish cakes using a combination of GC-MS and GC-IMS. Food Chem. X 2024, 22, 101291. [Google Scholar] [CrossRef]
- Chew, S.C.; How, Y.H.; Chang, L.S.; Tan, C.H.; Chuo, K.M.J.; Wong, S.Y.W.; Degraeve, P.; Nyam, K.L. The impact of cooking methods on the physical, sensory, and nutritional quality of fish. Int. J. Gastron. Food Sci. 2024, 38, 101061. [Google Scholar] [CrossRef]
- Li, C.H.; Bland, J.M.; Bechtel, P.J. Effect of precooking and polyphosphate treatment on the quality of microwave cooked catfish fillets. Food Sci. Nutr. 2017, 5, 812–819. [Google Scholar] [CrossRef]
- Liu, J.; Deng, S.; Wang, J.; Huang, F.; Han, D.; Xu, Y.; Yang, P.; Zhang, C.; Blecker, C. Comparison and elucidation of the changes in the key odorants of precooked stewed beef during cooking-refrigeration-reheating. Food Chem. X 2024, 23, 101654. [Google Scholar] [CrossRef]
- Merlo, T.C.; Lorenzo, J.M.; Saldana, E.; Patinho, I.; Oliveira, A.C.; Menegali, B.S.; Selani, M.M.; Dominguez, R.; Contreras-Castillo, C.J. Relationship between volatile organic compounds, free amino acids, and sensory profile of smoked bacon. Meat Sci. 2021, 181, 108596. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Zhang, Y.; Jiang, Y.; Sun, D.; Piao, C.; Li, T.; Wang, J.; Li, H.; Mu, B.; et al. Evaluation of the flavor profiles of Yanbian-style sauced beef from differently treated raw beef samples. Food Chem. X 2024, 22, 101505. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, M.; Wu, J.; Yu, Y.; Su, W.; Xu, Y.; Yang, J. Flavor characterization of aged Citri Reticulatae pericarpium from core regions: An integrative approach utilizing GC-IMS, GC–MS, E-nose, E-tongue, and chemometrics. Food Chem. 2024, 490, 144995. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Feng, X.; Li, A.; Chen, H.; Peng, X.; Wang, H.; Kan, J. Identification and characterization of aroma profiles of Huaqiao oil from different geographical origins using instruments and sensory analysis. J. Food Compos. Anal. 2024, 129, 106074. [Google Scholar] [CrossRef]
- Ren, X.; Wang, C.; Wang, X.; Su, T.; Yu, Y. Impacts of various reheating methods on crispy chicken: Physicochemical properties, oxidation and flavor profiles. Foods 2025, 14, 1574. [Google Scholar] [CrossRef]
- Yan, K.; Kong, J.; Yu, L.; Yang, J.; Zeng, X.; Bai, W.; Qian, M.; Dong, H. Flavor evolution and identification of the warmed-over flavor (WOF) in pre-cooked goose meat by means of HS-SPME-GC-MS and GC-IMS. Food Chem. 2025, 481, 143979. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, Y.; Lan, H.; Zhang, J.; Gao, Y.; Deng, S. Comparative analysis of quality and flavor profiles in raw and pre-cooked large yellow croaker (Larimichthys crocea) meat post freezing and reheating. Food Chem. 2025, 464, 141865. [Google Scholar] [CrossRef]
- Zhang, L.; Badar, I.H.; Chen, Q.; Xia, X.; Liu, Q.; Kong, B. Changes in flavor, heterocyclic aromatic amines, and quality characteristics of roasted chicken drumsticks at different processing stages. Food Control 2022, 139, 109104. [Google Scholar] [CrossRef]
- Yao, W.; Cai, Y.; Liu, D.; Chen, Y.; Li, J.; Zhang, M.; Chen, N. Analysis of flavor formation during production of Dezhou braised chicken using headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). Food Chem. 2022, 370, 130989. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Xu, B.; Xu, Y.; Yao, Z.; Zhu, B.; Li, X.; Sun, Y. Effects of different thermal treatment temperatures on volatile flavour compounds of water-boiled salted duck after packaging. LWT 2022, 154, 112625. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Li, C.; Xu, B. Insight into the effect of frozen storage on the changes in volatile aldehydes and alcohols of marinated roasted beef meat: Potential mechanisms of their formation. Food Chem. 2022, 385, 132629. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Xing, S.; Tang, C.; Liu, J.; Kan, J.; Dai, Q.; Qian, C. Effect of different thermal methods on the flavor characteristics of Pleurotus geesteranus: The key odor-active compounds and taste components. J. Food Compos. Anal. 2025, 146, 107948. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Kong, S.; Miao, J.; Lai, K. Selection and quantification of volatile indicators for quality deterioration of reheated pork based on simultaneously extracting volatiles and reheating precooked pork. Food Chem. 2023, 419, 135962. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xu, J.; Yang, Q.; Sha, Y.; Jiao, T.; Zhao, S. Effects of yeast cultures on meat quality, flavor composition and rumen microbiota in lambs. Curr. Res. Food Sci. 2024, 9, 100845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, M.; Fang, F.; Fu, C.; Xing, S.; Qian, C.; Liu, J.; Kan, J.; Jin, C. Effect of sous vide cooking treatment on the quality, structural properties and flavor profile of duck meat. Int. J. Gastron. Food Sci. 2022, 29, 100565. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, X.; Wen, R.; Sun, C.; Yu, Q. Changes in meat quality and volatile flavor compounds profile in beef loin during dry-aging. LWT 2024, 205, 116500. [Google Scholar] [CrossRef]
- Lourdes, A.; Janneth, G.; Rocio, G. Ion mobility spectrometry a versatile analytical tool for metabolomics applications in food science. Curr. Metabolomics 2014, 2, 264–271. [Google Scholar] [CrossRef]
- Dong, T.; Wang, S.; Qi, N.; Sun, J.; Chen, H.; Wang, S.; Sun, B. Unraveling the influence of boiling time on aroma generation in Huajiao (Zanthoxylum bungeanum Maxim.) water during boiling through molecular sensory science. Food Chem. X 2024, 24, 101939. [Google Scholar] [CrossRef]
- Zou, Y.; Kang, D.; Liu, R.; Qi, J.; Zhou, G.; Zhang, W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochem. 2018, 46, 36–45. [Google Scholar] [CrossRef]
- Ping, C.; Hu, H.; Bi, J.; Li, X.; Yi, Y.; Qiao, M. Identification of volatile indicators for quality deterioration of Yu-Shiang Shredded pork after reheating based on HS-GC-IMS and intelligent sensory evaluation. Int. J. Gastron. Food Sci. 2025, 40, 101180. [Google Scholar] [CrossRef]
- Xiang, S.; Zou, H.; Liu, Y.; Ruan, R. Effects of microwave heating on the protein structure, digestion properties and Maillard products of gluten. J. Food Sci. Technol. 2020, 57, 2139–2149. [Google Scholar] [CrossRef]
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. Eur. Food Res. Technol. 2015, 241, 157–171. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
| Sensory Attribute | Standard Description | Scores |
|---|---|---|
| Flavor | Obvious meaty and fatty flavor, no peculiar smell | 7–10 |
| Normal meaty and fatty flavor, no peculiar smell | 4–6 | |
| Less meaty and fatty flavor, peculiar smell | 1–3 | |
| Taste | Rich meaty taste, full aftertaste, no bitter and sour taste | 7–10 |
| Moderate meaty taste, no bitter and sour taste | 4–6 | |
| Mild or no meaty taste, bitter and sour taste | 1–3 | |
| Color | Bright color, uniform appearance with gloss | 7–10 |
| Relative bright color, slightly uneven appearance with gloss | 4–6 | |
| Dark color, uneven appearance without gloss | 1–3 | |
| Texture | Complete meat, close texture, moderate hardness | 7–10 |
| Relative complete meat, close texture, softness or hardness | 4–6 | |
| Incomplete meat, loose texture, particular softness or hardness | 1–3 |
| Code | Compounds | RI 1 | RI 2 | Concentration (µg/kg) 3 | |||
|---|---|---|---|---|---|---|---|
| CT | SF | SR | MR | ||||
| Aldehydes (17) | |||||||
| 1 | Hexanal | 802 | 829 | 36.77 ± 0.50 a | 27.57 ± 0.51 a | 36.12 ± 18.50 a | 44.94 ± 8.51 a |
| 2 | Heptanal | 908 | 927 | 10.28 ± 0.57 ab | 9.03 ± 1.32 ab | 6.86 ± 1.49 b | 13.33 ± 4.26 a |
| 3 | Octanal | 1001 | 995 | 29.39 ± 3.92 a | 26.08 ± 7.66 ab | 19.99 ± 2.73 bc | 16.86 ± 1.54 c |
| 4 | Benzeneacetaldehyde | 1044 | 1037 | 4.13 ± 0.13 b | 5.74 ± 0.85 a | nd 4 | 2.04 ± 0.30 c |
| 5 | Nonanal | 1102 | 1085 | 123.52 ± 21.45 a | 113.51 ± 21.43 a | 65.81 ± 13.32 b | 62.51 ± 8.64 b |
| 6 | Citronellal | 1153 | 1131 | 0.28 ± 0.04 a | nd 4 | nd 4 | nd 4 |
| 7 | (E)-2-Nonenal | 1166 | 1135 | 1.61 ± 0.13 a | 0.79 ± 0.19 b | 0.48 ± 0.13 c | 0.59 ± 0.19 bc |
| 8 | Decanal | 1205 | 1181 | 5.02 ± 0.95 a | 3.31 ± 0.95 bc | 2.60 ± 0.14 c | 2.63 ± 0.14 c |
| 9 | 4-(1-Methylethyl)-benzaldehyde | 1240 | 1208 | 0.25 ± 0.02 a | 0.13 ± <0.01 b | 0.26 ± 0.05 a | 0.26 ± 0.02 a |
| 10 | β-Citral | 1238 | 1211 | 1.69 ± 0.29 c | 2.82 ± 0.12 b | 5.73 ± 1.51 a | 3.79 ± 0.19 ab |
| 11 | (E)-2-Decenal | 1263 | 1230 | 2.19 ± 0.69 a | 2.21 ± 0.21 a | 0.72 ± 0.26 b | 0.87 ± 0.16 b |
| 12 | α-Citral | 1270 | 1238 | 1.22 ± 0.15 c | 4.19 ± 0.19 b | 7.94 ± 2.13 a | 5.46 ± 0.35 ab |
| 13 | 2,4-Decadienal | 1309 | 1277 | 0.71 ± 0.07 a | 0.76 ± 0.11 a | nd 4 | nd 4 |
| 14 | 2-Undecenal | 1368 | 1329 | 2.46 ± 0.42 a | 2.02 ± 0.27 b | nd 4 | 0.54 ± 0.14 c |
| 15 | Undecanal | 1310 | 1279 | 0.95 ± 0.12 a | 0.65 ± 0.31 b | 0.38 ± 0.07 b | 0.42 ± 0.04 b |
| 16 | (E,E)-2,4-Decadienal | 1313 | 1277 | 0.63 ± <0.01 b | 2.30 ± 0.09 a | nd 4 | nd 4 |
| 17 | Dodecanal | 1409 | 1378 | 0.61 ± 0.02 a | 0.53 ± 0.53 b | 0.22 ± 0.04 c | 0.28 ± 0.01 c |
| subtotal | 221.71 | 201.64 | 147.11 | 154.52 | |||
| Hydrocarbons (23) | |||||||
| 18 | p-Xylene | 872 | 849 | 0.53 ± 0.08 d | 1.16 ± 0.07 a | 0.74 ± 0.06 c | 0.87 ± 0.02 b |
| 19 | Ethylbenzene | 878 | 841 | 1.14 ± 0.06 b | nd 4 | 0.50 ± 0.19 c | 3.44 ± 1.44 a |
| 20 | o-Xylene | 890 | 854 | 0.12 ± 0.01 c | 0.41 ± 0.01 a | 0.06 ± 0.04 c | 0.22 ± 0.11 b |
| 21 | α-Pinene | 939 | 914 | 0.38 ± 0.08 b | nd 4 | 0.61 ± 0.15 a | 0.20 ± <0.01 c |
| 22 | Camphene | 955 | 928 | 0.78 ± 0.10 b | nd 4 | 1.10 ± 0.31 a | 0.50 ± 0.02 c |
| 23 | β-Pinene | 980 | 973 | 4.17 ± 0.19 a | nd 4 | 2.93 ± 0.17 b | nd 4 |
| 24 | o-Cymene | 1022 | 1005 | 0.52 ± 0.07 c | nd 4 | 1.05 ± 0.07 a | 0.77 ± 0.03 b |
| 25 | p-Cymene | 1025 | 1006 | 0.22 ± 0.03 c | 0.47 ± 0.13 b | nd 4 | 0.62 ± <0.01 a |
| 26 | β-Cymene | 1026 | 1006 | 0.52 ± 0.03 a | nd 4 | 0.33 ± <0.01 b | nd 4 |
| 27 | Sylvestrene | 1027 | 1007 | 0.79 ± 0.02 b | 0.31 ± 0.10 c | 1.42 ± 0.07 a | 0.40 ± 0.24 c |
| 28 | 2-Methyl-decane | 1061 | 1045 | 0.73 ± 0.03 c | 0.70 ± 0.08 c | 1.31 ± 0.10 b | 3.00 ± 1.81 a |
| 29 | 3-Methyl-decane | 1069 | 1052 | 2.38 ± 0.10 a | 0.84 ± 0.03 d | 1.30 ± 0.02 b | 1.17 ± 0.01 c |
| 30 | (E)-β-Ocimene | 1036 | 1022 | 9.25 ± 1.61 a | nd 4 | 1.24 ± 0.33 b | nd 4 |
| 31 | (Z)-β-Ocimene | 1037 | 1030 | 4.15 ± 0.15 a | nd 4 | 1.22 ± 0.12 b | 0.46 ± <0.01 c |
| 32 | 4-Methyl-decane | 1059 | 1040 | 1.16 ± 0.17 a | nd 4 | 0.44 ± 0.06 b | 0.39 ± 0.02 b |
| 33 | γ-Terpinene | 1062 | 1040 | nd 4 | nd 4 | 3.27 ± 0.24 a | nd 4 |
| 34 | α-Terpinolene | 1088 | 1069 | 1.38 ± 0.28 a | nd 4 | 1.38 ± 0.24 a | 1.14 ± 0.20 a |
| 35 | (E,E)-alloocimene | 1140 | 1110 | 5.22 ± 1.33 a | nd 4 | 1.42 ± 0.26 b | 0.75 ± 0.05 c |
| 36 | 3-Methyl-undecane | 1169 | 1149 | 0.63 ± 0.09 c | 0.82 ± 0.14 bc | 1.40 ± 0.11 a | 1.07 ± 0.37 ab |
| 37 | 2,4-Dimethyl-undecane | 1213 | 1185 | 14.61 ± 2.09 a | nd 4 | nd 4 | nd 4 |
| 38 | 2,6-Dimethyl-undecane | 1210 | 1189 | 3.66 ± 0.15 a | 1.13 ± 0.20 b | 1.40 ± 0.18 b | nd 4 |
| 39 | 3-Methyl-tridecane | 1375 | 1336 | 0.47 ± 0.04 a | 0.41 ± 0.12 ab | 0.31 ± 0.03 b | 0.37 ± 0.06 ab |
| 40 | 2,6,11-Trimethyl-dodecane | 1275 | 1294 | 0.90 ± 0.10 a | 0.92 ± 0.06 a | nd 4 | nd 4 |
| subtotal | 53.71 | 7.17 | 23.43 | 15.37 | |||
| Ketones (4) | |||||||
| 41 | 1-Octen-3-one | 980 | 970 | 0.40 ± 0.10 b | 0.53 ± 0.03 a | nd 4 | nd 4 |
| 42 | 6-Methyl-5-hepten-2-one | 986 | 980 | 3.14 ± 0.14 a | 1.35 ± 0.24 c | 2.14 ± 0.28 b | 1.41 ± 0.28 c |
| 43 | α-Piperitone | 1252 | 1222 | 1.53 ± 0.43 b | 1.12 ± 0.32 b | 3.64 ± 0.85 a | 3.92 ± 0.38 a |
| 44 | 2-Undecanone | 1287 | 1267 | 0.62 ± 0.08 a | nd 4 | 0.52 ± 0.14 ab | 0.40 ± 0.06 b |
| subtotal | 5.69 | 3.00 | 6.30 | 5.73 | |||
| Alcohols (4) | |||||||
| 45 | 1-Octen-3-ol | 982 | 973 | nd 4 | 3.65 ± 0.25 a | 2.53 ± 0.42 b | 2.12 ± 0.25 b |
| 46 | Terpinen-4-ol | 1177 | 1153 | 2.43 ± 0.05 b | 1.59 ± 0.30 c | 5.54 ± 1.33 a | 4.81 ± 0.44 a |
| 47 | α-Terpineol | 1189 | 1166 | 3.65 ± 0.31 b | 2.45 ± 0.47 c | 8.35 ± 1.93 a | 7.80 ± 0.48 a |
| 48 | Neodihydrocarveol | 1220 | 1170 | nd 4 | nd 4 | 0.67 ± 0.16 a | 0.32 ± <0.01 b |
| subtotal | 6.08 | 7.69 | 17.09 | 15.05 | |||
| Esters (7) | |||||||
| 49 | Linalyl acetate | 1258 | 1235 | 0.46 ± 0.02 b | 0.45 ± 0.05 b | 0.74 ± 0.19 a | 0.72 ± 0.09 a |
| 50 | Bornyl acetate | 1267 | 1261 | 0.36 ± 0.02 b | 0.24 ± <0.01 c | 0.89 ± 0.22 a | 0.45 ± 0.08 b |
| 51 | Myrtenyl acetate | 1325 | 1300 | 0.23 ± <0.01 a | nd 4 | 0.24 ± 0.06 a | 0.21 ± 0.04 a |
| 52 | 2-Acetoxy-1,8-cineole | 1344 | 1315 | 0.84 ± 0.03 b | 0.51 ± 0.01 c | 1.26 ± 0.27 a | 1.28 ± 0.09 a |
| 53 | α-Terpinyl acetate | 1350 | 1323 | 2.42 ± 0.06 b | 1.50 ± 0.09 c | 5.26 ± 1.22 a | 5.10 ± 0.76 a |
| 54 | β-Citronellol acetate | 1354 | 1327 | 0.27 ± <0.01 b | 0.19 ± 0.07 b | 0.80 ± 0.19 a | 0.65 ± 0.11 a |
| 55 | Geranyl acetate | 1384 | 1356 | 0.78 ± 0.03 c | 0.63 ± 0.19 c | 2.50 ± 0.36 a | 1.87 ± 0.27 b |
| subtotal | 5.36 | 3.52 | 11.69 | 10.28 | |||
| Others (2) | |||||||
| 56 | 3-Methyl-2-(2-methyl-2-butenyl)-furan | 1093 | 1079 | 0.78 ± 0.07 a | 0.26 ± <0.01 b | 0.28 ± 0.03 b | 0.25 ± 0.10 b |
| 57 | 2-Ethyl-3,5-dimethyl-pyrazine | 1095 | 1081 | nd 4 | 0.99 ± 0.01 a | nd 4 | nd 4 |
| subtotal | 0.78 | 1.25 | 0.28 | 0.25 | |||
| Code 1 | Compounds | Odor Descriptions | Odor Threshold (μg/kg) | OAV 2 | |||
|---|---|---|---|---|---|---|---|
| CT | SF | SR | MR | ||||
| 1 | Hexanal | Green, grassy [41] | 4.5 | 8.17 | 6.13 | 8.03 | 9.99 |
| 2 | Heptanal | Fatty, fruity [41] | 3 | 3.43 | 3.01 | 2.29 | 4.44 |
| 3 | Octanal | Citrus, fatty [41] | 0.7 | 41.99 | 37.26 | 28.56 | 24.09 |
| 4 | Benzeneacetaldehyde | Sweet, floral [42] | 4 | 1.03 | 1.44 | nd | <1 |
| 5 | Nonanal | Fatty, floral [41] | 1.1 | 112.29 | 103.19 | 59.83 | 56.83 |
| 7 | (E)-2-Nonenal | Fatty, cucumber [41] | 0.19 | 8.47 | 4.16 | 2.53 | 3.11 |
| 8 | Decanal | Fatty, sweet [25] | 0.3 | 16.73 | 11.03 | 8.67 | 8.77 |
| 11 | (E)-2-Decenal | Oily [25] | 0.3 | 7.30 | 7.37 | 2.40 | 2.90 |
| 13 | 2,4-Decadienal | Oily, fatty [43] | 0.3 | 2.37 | 2.53 | nd | nd |
| 16 | (E,E)-2,4-Decadienal | Fatty, fruity [25] | 0.07 | 9.00 | 32.86 | nd | nd |
| 17 | Dodecanal | Fatty, citrus [44] | 0.53 | 1.15 | 1.00 | <1 | <1 |
| 23 | β-Pinene | Rosin, resin [9] | 6 | <1 | nd | <1 | nd |
| 41 | 1-Octen-3-one | Mushroom, metallic [43] | 0.05 | 8.00 | 10.60 | nd | nd |
| 45 | 1-Octen-3-ol | Mushroom [41] | 1 | nd | 3.65 | 2.53 | 2.12 |
| 57 | 2-Ethyl-3,5-dimethyl-pyrazine | Roasted, caramel [40] | 0.16 | nd | 6.19 | nd | nd |
| Free Amino Acid | Taste Characteristics | Concentration (mg/100 mL) | |||
|---|---|---|---|---|---|
| CT | SF | SR | MR | ||
| Aspartic acid (Asp) | Umami | 0.39 ± 0.01 c | 2.05 ± 0.09 a | 1.84 ± 0.05 b | 2.10 ± 0.04 a |
| Glutamic acid (Glu) | Umami | 3.80 ± 0.29 d | 11.31 ± 0.10 b | 10.02 ± 0.01 c | 12.27 ± 0.67 a |
| Serine (Ser) | Sweet | 0.73 ± 0.12 c | 1.93 ± 0.02 a | 1.58 ± 0.01 b | 1.84 ± 0.06 a |
| Glycine (Gly) | Sweet | 0.18 ± 0.03 c | 1.26 ± 0.03 a | 1.05 ± 0.02 b | 1.25 ± 0.02 a |
| Threonine (Thr) | Sweet | 0.34 ± 0.02 c | 1.54 ± 0.04 a | 1.26 ± 0.05 b | 1.52 ± 0.03 a |
| Alanine (Ala) | Sweet | 1.16 ± 0.03 c | 3.50 ± 0.26 a | 2.92 ± 0.02 b | 3.55 ± 0.31 a |
| Proline (Pro) | Sweet | 0.70 ± 0.01 c | 2.48 ± 0.19 a | 1.64 ± 0.29 b | 1.92 ± 0.07 b |
| Histidine (His) | Bitter | 0.27 ± 0.02 b | 0.53 ± 0.07 a | 0.48 ± 0.04 a | 0.55 ± 0.03 a |
| Arginine (Arg) | Bitter | 9.37 ± 0.01 a | 1.72 ± 0.18 b | 1.28 ± 0.01 d | 1.53 ± 0.09 c |
| Tyrosine (Tyr) | Bitter | nd | 1.53 ± 0.11 a | 1.28 ± 0.09 b | 1.40 ± 0.10 ab |
| Valine (Val) | Bitter | 0.49 ± 0.01 c | 2.05 ± 0.20 a | 1.58 ± 0.07 b | 1.94 ± 0.02 a |
| Methionine (Met) | Bitter | 0.23 ± 0.05 a | 0.14 ± 0.03 b | 0.11 ± 0.01 b | 0.15 ± 0.01 b |
| Phenylalanine (Phe) | Bitter | 0.46 ± 0.01 c | 1.82 ± 0.08 ab | 1.60 ± 0.10 b | 1.93 ± 0.15 a |
| Isoleucine (Ile) | Bitter | 0.47 ± 0.01 c | 1.58 ± 0.12 a | 1.28 ± 0.14 b | 1.58 ± 0.10 a |
| Leucine (Leu) | Bitter | 0.85 ± 0.02 c | 2.78 ± 0.20 a | 2.34 ± 0.15 b | 2.72 ± 0.04 a |
| Lysine (Lys) | Bitter | 0.43 ± 0.07 c | 1.85 ± 0.02 a | 1.43 ± 0.03 b | 1.77 ± 0.02 a |
| Cysteine (Cys-s) | Tasteless | nd | 0.02 ± <0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a |
| Umami FAAs | 4.18 ± 0.29 d | 13.36 ± 0.19 b | 11.86 ± 0.05 c | 14.37 ± 0.71 a | |
| Sweet FAAs | 3.11 ± 0.22 c | 10.71 ± 0.51 a | 8.45 ± 0.27 b | 10.07 ± 0.48 a | |
| Bitter FAAs | 12.54 ± 0.07 bc | 13.98 ± 0.98 a | 11.39 ± 0.55 c | 13.57 ± 0.54 ab | |
| Total | 19.83 ± 0.56 c | 38.09 ± 1.67 a | 31.71 ± 0.87 b | 38.04 ± 1.72 a | |
| 5′-Nucleotide | Concentration (mg/100 g) | |||
|---|---|---|---|---|
| CT | SF | SR | MR | |
| 5′-CMP | nd | 3.96 ± 0.09 a | 2.08 ± 0.07 b | 1.95 ± 0.16 b |
| 5′-AMP | 7.07 ± 0.02 c | 13.63 ± 1.28 a | 8.10 ± 0.25 b | 7.58 ± 0.21 b |
| 5′-UMP | 140.69 ± 0.02 b | 185.97 ± 9.07 a | 142.71 ± 12.61 b | 152.57 ± 4.52 b |
| 5′-GMP | 2.61 ± 0.06 b | 3.13 ± 0.08 a | 2.22 ± 0.08 c | 2.14 ± 0.13 c |
| 5′-IMP | 4.12 ± 0.12 c | 7.61 ± 0.02 a | 4.90 ± 0.22 b | 4.36 ± 0.21 c |
| Total | 154.49 ± 0.03 c | 214.29 ± 10.52 a | 160.01 ± 13.23 b | 168.59 ± 5.21 b |
| Sensory Attributes | CT | SF | SR | MR |
|---|---|---|---|---|
| Flavor | 8.80 ± 0.34 a | 8.11 ± 0.21 b | 6.21 ± 0.32 d | 7.08 ± 0.18 c |
| Taste | 7.50 ± 0.41 a | 7.54 ± 0.30 a | 5.74 ± 0.46 c | 6.52 ± 0.27 b |
| Color | 7.82 ± 0.25 a | 6.87 ± 0.33 c | 7.05 ± 0.28 bc | 7.13 ± 0.15 b |
| Texture | 7.66 ± 0.48 a | 6.63 ± 0.22 b | 6.34 ± 0.20 b | 7.01 ± 0.44 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.; Wang, H.; Chen, H.; Zhu, W.; Qian, C.; Liu, J.; Kan, J.; Zhang, M. Comparative Analysis of Flavor Quality of Beef with Tangerine Peel Reheated by Stir-Frying, Steaming and Microwave. Foods 2025, 14, 3017. https://doi.org/10.3390/foods14173017
Zhu K, Wang H, Chen H, Zhu W, Qian C, Liu J, Kan J, Zhang M. Comparative Analysis of Flavor Quality of Beef with Tangerine Peel Reheated by Stir-Frying, Steaming and Microwave. Foods. 2025; 14(17):3017. https://doi.org/10.3390/foods14173017
Chicago/Turabian StyleZhu, Kaixian, Huaitao Wang, Hongjun Chen, Wenzheng Zhu, Chunlu Qian, Jun Liu, Juan Kan, and Man Zhang. 2025. "Comparative Analysis of Flavor Quality of Beef with Tangerine Peel Reheated by Stir-Frying, Steaming and Microwave" Foods 14, no. 17: 3017. https://doi.org/10.3390/foods14173017
APA StyleZhu, K., Wang, H., Chen, H., Zhu, W., Qian, C., Liu, J., Kan, J., & Zhang, M. (2025). Comparative Analysis of Flavor Quality of Beef with Tangerine Peel Reheated by Stir-Frying, Steaming and Microwave. Foods, 14(17), 3017. https://doi.org/10.3390/foods14173017









