The Effect of Sodium Benzoate on the Gut Microbiome Across Age Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Ex Vivo Culturing Experiments
2.2. Bacterial Cell Counts
2.3. Environmental pH, Gas Measurement, and Short-Chain Fatty Acid (SCFA) and Branched-Chain Fatty Acid (BCFA) Quantification
2.4. DNA Extraction, Library Preparation, and Sequencing
2.5. Read-Based Taxonomic and Functional Profiling
2.6. Taxon and Pathway Association Testing
2.7. Other Analyses and Visualizations
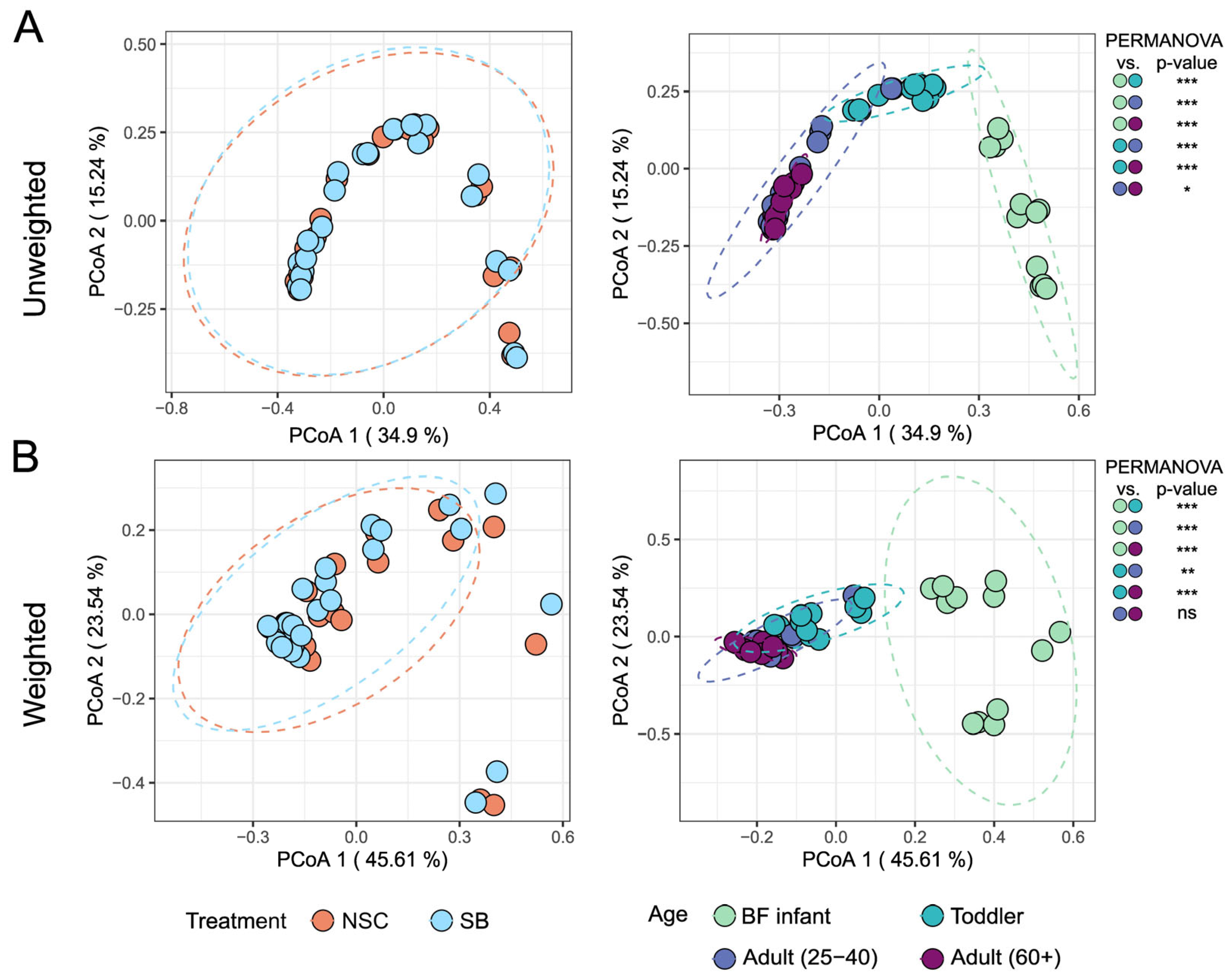
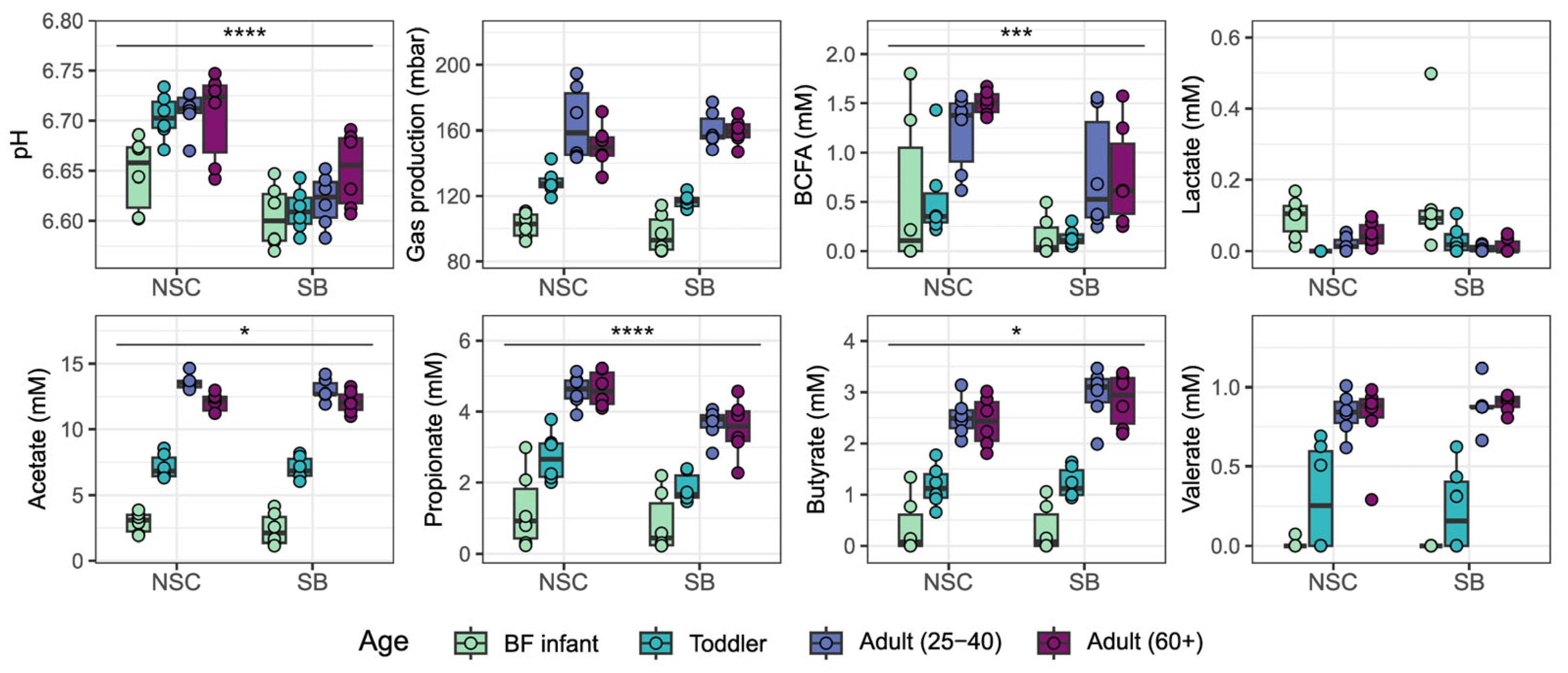
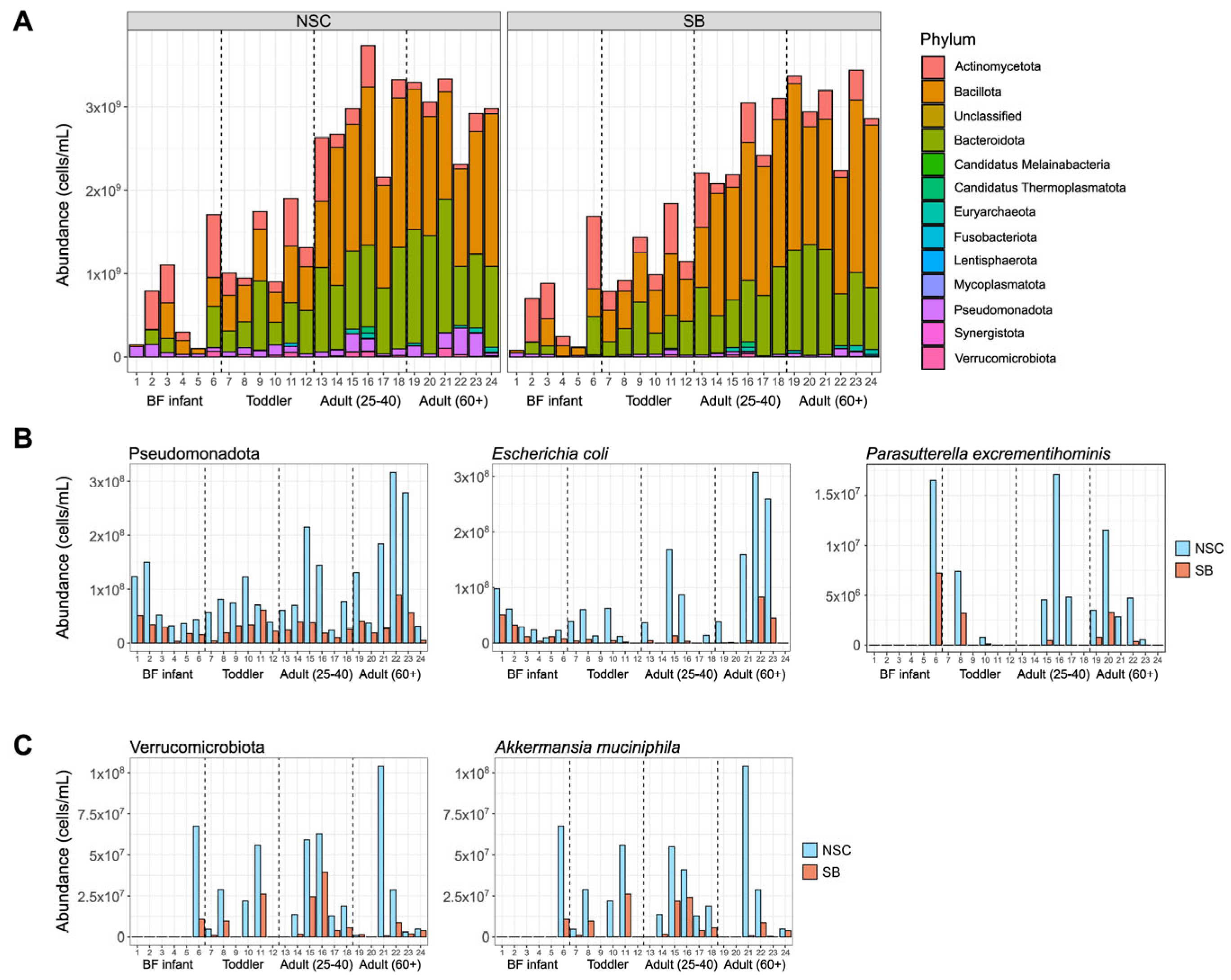
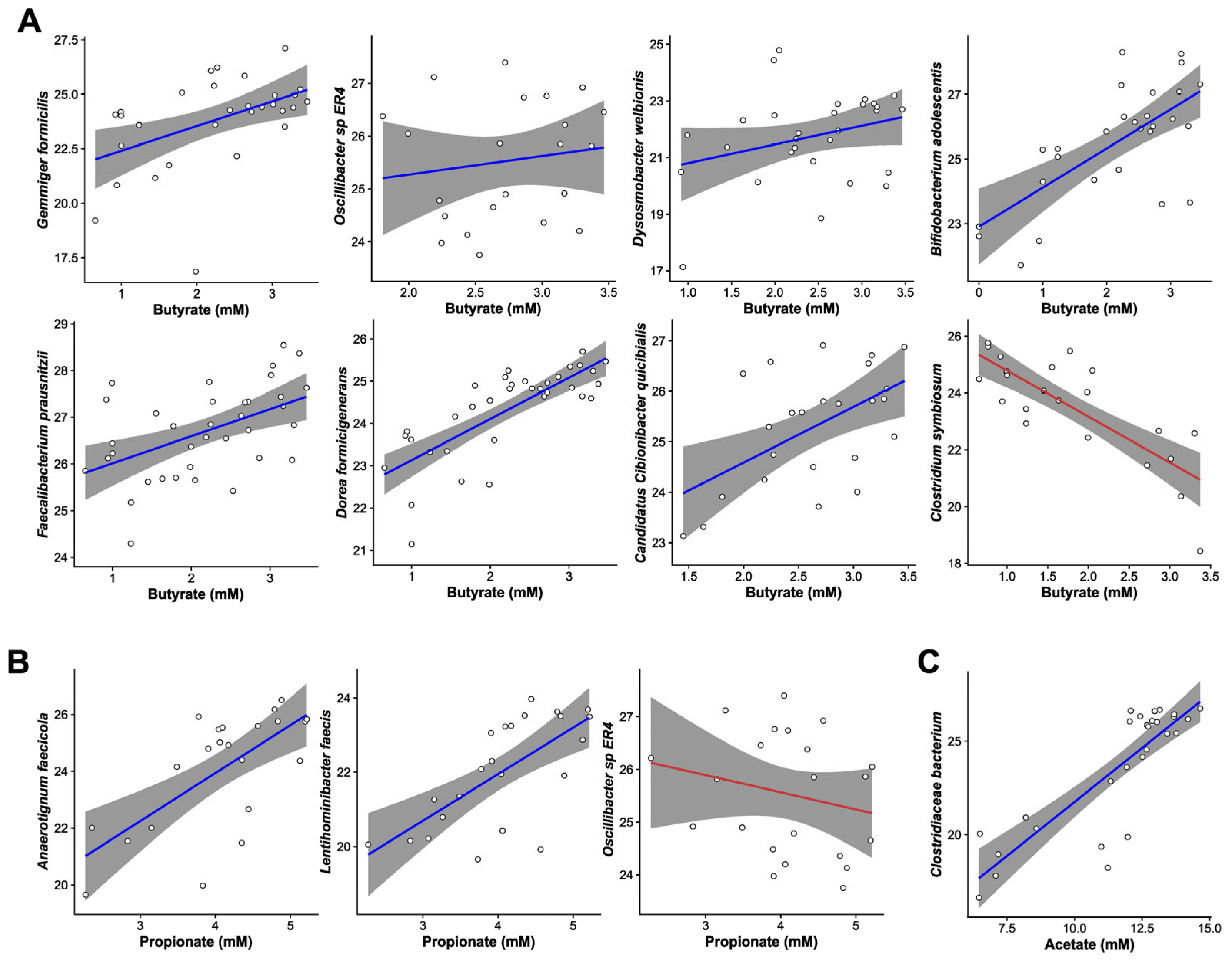
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SB | sodium benzoate |
| NSC | no substrate control |
| ADI | acceptable daily intake |
| BF | breastfed |
| SCFA | short-chain fatty acids |
| BCFA | branched-chain fatty acids |
| SIFR | Systemic Intestinal Fermentation Research |
| KO | KEGG ortholog |
| PCoA | principal coordinate analysis |
References
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Guidance for submission for food additive evaluations. EFSA J. 2012, 10, 2760. [Google Scholar] [CrossRef]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.K.; Chang, C.T.; Wada, N.; Ho, H.C.; Lo, Y.L.; Tsai, S.P.; Chen, R.A.; Huang, H.S.; Liu, P.Y.; et al. Common dietary emulsifiers promote metabolic disorders and intestinal microbiota dysbiosis in mice. Commun. Biol. 2024, 7, 749. [Google Scholar] [CrossRef]
- Wibbertmann, A.; Kielhorn, J.; Koennecker, G.; Mangelsdorf, I.; Melber, C. Benzoic Acid and Sodium Benzoate; United Nations Environment Programme, and the World Health Organization, Ed.; WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- De Villiers, M.M. Antimicrobial Preservatives. In A Practical Guide to Contemporary Pharmacy Practice, 3rd ed.; Thompson, J.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- International Program on Chemical Safety. Concise International Chemical Assessment Document; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources (ANS). Scientific Opinion on the re-evaluation of benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) as food additives. EFSA J. 2016, 14, 4433. [Google Scholar] [CrossRef]
- Guggenbuhl, P.; Séon, A.; Quintana, A.P.; Nunes, C.S. Effects of dietary supplementation with benzoic acid (VevoVitall®) on the zootechnical performance, the gastrointestinal microflora and the ileal digestibility of the young pig. Livest. Sci. 2007, 108, 218–221. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- O’Donnell, M.M.; Rea, M.C.; Shanahan, F.; Ross, R.P. The Use of a Mini-Bioreactor Fermentation System as a Reproducible, High-Throughput ex vivo Batch Model of the Distal Colon. Front. Microbiol. 2018, 9, 01844. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Maathuis, A.; Heilig, H.G.H.J.; Venema, K.; de Vos, W.M.; Smidt, H. Evaluating the microbial diversity of an in vitro model of the human large intestine by phylogenetic microarray analysis. Microbiology 2010, 156, 3270–3281. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gérard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; et al. Microbial Community Development in a Dynamic Gut Model Is Reproducible, Colon Region Specific, and Selective forBacteroidetesandClostridiumCluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Deyaert, S.; Thabuis, C.; Perreau, C.; Bajic, D.; Wintergerst, E.; Joossens, M.; Firrman, J.; Walsh, D.; Baudot, A. Bridging preclinical and clinical gut microbiota research using the ex vivo SIFR® technology. Front. Microbiol. 2023, 14, 1131662. [Google Scholar] [CrossRef]
- Lemons, J.M.S.; Narrowe, A.B.; Firrman, J.; Mahalak, K.K.; Liu, L.; Higgins, S.; Moustafa, A.M.; Baudot, A.; Deyaert, S.; Van den Abbeele, P. The food additive butylated hydroxyanisole minimally affects the human gut microbiome ex vivo. Food Chem. 2025, 473, 143037. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives; WHO; Food and Agriculture Organization of the United Nations; International Programme on Chemical Safety. Toxicological evaluation of certain food additives and contaminants. In Proceedings of the 37th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA), Geneva, Switzerland, 5–14 June 1990. [Google Scholar]
- Van den Abbeele, P.; Detzel, C.; Rose, A.; Deyaert, S.; Baudot, A.; Warner, C. Serum-Derived Bovine Immunoglobulin Stimulates SCFA Production by Specific Microbes in the Ex Vivo SIFR® Technology. Microorganisms 2023, 11, 659. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Deyaert, S.; Albers, R.; Baudot, A.; Mercenier, A. Carrot RG-I Reduces Interindividual Differences between 24 Adults through Consistent Effects on Gut Microbiota Composition and Function Ex Vivo. Nutrients 2023, 15, 2090. [Google Scholar] [CrossRef]
- Bushnell, B. BBMap-Bushnell B.—Sourceforge.Net/Projects/Bbmap/. Available online: https://sourceforge.net/projects/bbmap/files/ (accessed on 20 July 2025).
- Blanco-Miguez, A.; Beghini, F.; Cumbo, F.; McIver, L.J.; Thompson, K.N.; Zolfo, M.; Manghi, P.; Dubois, L.; Huang, K.D.; Thomas, A.M.; et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. 2023, 41, 1633–1644. [Google Scholar] [CrossRef]
- Beghini, F.; McIver, L.J.; Blanco-Miguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 2021, 10, 65088. [Google Scholar] [CrossRef]
- Nickols, W.A.; Kuntz, T.; Shen, J.; Maharjan, S.; Mallick, H.; Franzosa, E.A.; Thompson, K.N.; Nearing, J.T.; Huttenhower, C. MaAsLin 3: Refining and extending generalized multivariate linear models for meta-omic association discovery. bioRxiv 2024. [Google Scholar] [CrossRef]
- Martinez Arbizu, P. Pairwiseadonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.4. 2020. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 20 July 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Oksanen, J.S.G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; Wagner, H.; et al. Weedon Vegan: Community Ecology Package, R package version 2.6-4; CRAN: Vienna, Austria, 2022.
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Env. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Salmond, C.V.; Kroll, R.G.; Booth, I.R. The Effect of Food Preservatives on pH Homeostasis in Escherichia coli. Microbiology 1984, 130, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A.; Wiggins, D.; Stubbs, M.; Sols, A.; Bedoya, F. Studies on the mechanism of the antifungal action of benzoate. Biochem. J. 1983, 214, 657–663. [Google Scholar] [CrossRef]
- Warth, A.D. Mechanism of action of benzoic acid on Zygosaccharomyces bailii: Effects on glycolytic metabolite levels, energy production, and intracellular pH. Appl. Environ. Microbiol. 1991, 57, 3410–3414. [Google Scholar] [CrossRef]
- Kolmos, H.J. The bactericidal action of benzoic acid and sodium acetate on the gram-negative flora of dialysis fluid. Acta Pathol. Microbiol. Scand B 1976, 84B, 259–264. [Google Scholar] [CrossRef]
- Ioannou, A.; Berkhout, M.D.; Geerlings, S.Y.; Belzer, C. Akkermansia muciniphila: Biology, microbial ecology, host interactions and therapeutic potential. Nat. Rev. Microbiol. 2025, 23, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Hrncirova, L.; Hudcovic, T.; Sukova, E.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Human gut microbes are susceptible to antimicrobial food additives in vitro. Folia Microbiol. 2019, 64, 497–508. [Google Scholar] [CrossRef]
- Hrncirova, L.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Food Preservatives Induce Proteobacteria Dysbiosis in Human-Microbiota Associated Nod2-Deficient Mice. Microorganisms 2019, 7, 383. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R.; Drake, H.L. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, 00395-21. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Q.; Li, S.; Du, D.; Yu, W.; Guo, J.; Zhao, Z.; Yu, X.; Ma, F.; Sun, P. Effect of Dietary Benzoic Acid Supplementation on Growth Performance, Rumen Fermentation, and Rumen Microbiota in Weaned Holstein Dairy Calves. Animals 2024, 14, 2823. [Google Scholar] [CrossRef]
- Giannenas, I.; Papaneophytou, C.P.; Tsalie, E.; Pappas, I.; Triantafillou, E.; Tontis, D.; Kontopidis, G.A. Dietary Supplementation of Benzoic Acid and Essential Oil Compounds Affects Buffering Capacity of the Feeds, Performance of Turkey Poults and Their Antioxidant Status, pH in the Digestive Tract, Intestinal Microbiota and Morphology. Asian-Australas. J. Anim. Sci. 2014, 27, 225–236. [Google Scholar] [CrossRef]
- Gong, H.; Yang, Z.; Celi, P.; Yan, L.; Ding, X.; Bai, S.; Zeng, Q.; Xu, S.; Su, Z.; Zhuo, Y.; et al. Effect of benzoic acid on production performance, egg quality, intestinal morphology, and cecal microbial community of laying hens. Poult. Sci. 2021, 100, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, S.W. Dietary Intervention of Benzoic Acid for Intestinal Health and Growth of Nursery Pigs. Animals 2024, 14, 2394. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Chen, J.L.; Zheng, P.; Zhang, C.; Yu, B.; He, J.; Yu, J.; Luo, J.Q.; Mao, X.B.; Huang, Z.Q.; Chen, D.W. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Physiol. Anim. Nutr. 2016, 101, 1137–1146. [Google Scholar] [CrossRef]
- Gossling, J.; Moore, W.E.C. Gemmiger formicilis, n.gen., n.sp., an Anaerobic Budding Bacterium from Intestines. Int. J. Syst. Bacteriol. 1975, 25, 202–207. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H.; Shade, A. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2, 00130-17. [Google Scholar] [CrossRef]
- Le Roy, T.; Moens de Hase, E.; Van Hul, M.; Paquot, A.; Pelicaen, R.; Regnier, M.; Depommier, C.; Druart, C.; Everard, A.; Maiter, D.; et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut 2022, 71, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. [Google Scholar] [CrossRef]
- Firrman, J.; Friedman, E.S.; Hecht, A.; Strange, W.C.; Narrowe, A.B.; Mahalak, K.; Wu, G.D.; Liu, L.; Fraser, C.M. Preservation of conjugated primary bile acids by oxygenation of the small intestinal microbiota in vitro. mBio 2024, 15, 00943-24. [Google Scholar] [CrossRef] [PubMed]

| Phylum | Species | Log2fold Change | q-Value |
|---|---|---|---|
| Bacillota | Ruminococcus lactaris | 1.71 ± 0.33 | 8.67 × 10−3 |
| Bacillota | Anaerostipes hadrus * | 1.42 ± 0.37 | 0.036 |
| Bacillota | Fusicatenibacter saccharivorans * | 0.96 ± 0.26 | 0.042 |
| Bacillota | Oscillibacter sp. ER4 | 0.79 ± 0.15 | 0.013 |
| Bacillota | Gemmiger formicilis * | 0.72 ± 0.18 | 0.036 |
| Bacillota | Dysosmobacter welbionis * | 0.64 ± 0.15 | 0.021 |
| Bacillota | Faecalibacterium Prausnitzii * | 0.48 ± 0.07 | 8.26 × 10−5 |
| Bacillota | Candidatus Cibionibacter quicibialis * | 0.32 ± 0.04 | 1.33 × 10−4 |
| Bacteroidota | GGB1567 SGB2154 * | −0.26 ± 0.01 | 0.042 |
| Bacteroidota | Bacteroides ovatus | −0.46 ± 0.12 | 0.042 |
| Bacteroidota | Alistipes putredinis * | −0.53 ± 0.11 | 0.015 |
| Bacteroidota | Alistipes finegoldii * | −0.85 ± 0.13 | 2.74 × 10−3 |
| Bacteroidota | Phocaeicola dorei * | −1.17 ± 0.16 | 1.33 × 10−4 |
| Bacillota | Lentihominibacter faecis * | −2.17 ± 0.41 | 9.13 × 10−4 |
| Pseudomonadota | Parasutterella excrementihominis * | −2.26 ± 0.36 | 0.016 |
| Pseudomonadota | Escherichia coli * | −2.89 ± 0.46 | 4.13 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemons, J.M.S.; Firrman, J.; Mahalak, K.K.; Liu, L.; Narrowe, A.B.; Higgins, S.; Moustafa, A.M.; Baudot, A.; Deyaert, S.; Van den Abbeele, P. The Effect of Sodium Benzoate on the Gut Microbiome Across Age Groups. Foods 2025, 14, 2949. https://doi.org/10.3390/foods14172949
Lemons JMS, Firrman J, Mahalak KK, Liu L, Narrowe AB, Higgins S, Moustafa AM, Baudot A, Deyaert S, Van den Abbeele P. The Effect of Sodium Benzoate on the Gut Microbiome Across Age Groups. Foods. 2025; 14(17):2949. https://doi.org/10.3390/foods14172949
Chicago/Turabian StyleLemons, Johanna M. S., Jenni Firrman, Karley K. Mahalak, LinShu Liu, Adrienne B. Narrowe, Stephanie Higgins, Ahmed M. Moustafa, Aurélien Baudot, Stef Deyaert, and Pieter Van den Abbeele. 2025. "The Effect of Sodium Benzoate on the Gut Microbiome Across Age Groups" Foods 14, no. 17: 2949. https://doi.org/10.3390/foods14172949
APA StyleLemons, J. M. S., Firrman, J., Mahalak, K. K., Liu, L., Narrowe, A. B., Higgins, S., Moustafa, A. M., Baudot, A., Deyaert, S., & Van den Abbeele, P. (2025). The Effect of Sodium Benzoate on the Gut Microbiome Across Age Groups. Foods, 14(17), 2949. https://doi.org/10.3390/foods14172949








