Co-Regulation of Very Fast Chilling Treatment and the Follow-Up Storage Temperature on Meat Tenderness Through Glycolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. pH
2.3. Shear Force
2.4. Degradation Degree of Desmin and Troponin T
2.5. Contents of Glycolytic Metabolites
2.6. Protein Phosphorylation and Acetylation Levels
2.7. Activity of Glycolytic Enzymes
2.8. Statistical Analysis
3. Results
3.1. The Tenderness of Meat at Different Storage Temperature
3.2. The Glycolytic Rates of Meat at Different Storage Temperature
3.3. Protein Phosphorylation Levels at Different Storage Temperature
3.4. Protein Acetylation Levels at Different Storage Temperature
3.5. Activity of Glycolytic Enzymes at Different Storage Temperatures
4. Discussion
4.1. Effect of Storage Temperature on Meat Tenderness
4.2. Effect of Storage Temperature on Glycolysis
4.3. Effect of Storage Temperature on Glycolytic Enzymes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VFC | very fast chilling |
| WHC | water-holding capacity |
| PYGM | glycogen phosphorylase |
| PFKM | phosphofructokinase |
| PGK | phosphoglycerate kinase |
| ALDOA | aldolase |
| LDH | lactate dehydrogenase |
| TPI1 | triosephosphate isomerase |
| PVDF | polyvinylidene fluoride membrane |
| NC | nitrocellulose membrane |
| GLM | general linear model |
References
- Wang, H.; Qin, Y.; Li, J.; Xu, X.; Zhou, G. Edible quality of soft-boiled chicken processing with chilled carcass was better than that of hot-fresh carcass. Food Sci. Nutr. 2019, 7, 797–804. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Zhao, Q.; Yang, Y.; Li, F.; Qin, Y.; Liu, X.; Yue, X.; Zhang, J. Integrated lipidomics and targeted metabolomics analyses reveal changes in flavor precursors in psoas major muscle of castrated lambs. Food Chem. 2020, 333, 127451. [Google Scholar] [CrossRef]
- Sukumaran, A.; Holtcamp, A.; Campbell, Y.; Burnett, D.; Schilling, M.; Din, T. Technological characteristics of pre- and post-rigor deboned beef mixtures from Holstein steers and quality attributes of cooked beef sausage. Meat Sci. 2018, 145, 71–78. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, D.; Zhang, H.; Li, X.; Fang, F.; Liang, C.; Wang, Z. Effect of postmortem phases on lamb meat quality: A physicochemical, microstructural and water mobility approach. Food Sci. Anim. Resour. 2021, 41, 802. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R. Very fast chilling of beef and tenderness-A report from an EU concerted action. Meat Sci. 1996, 43, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Watt, D.; Herring, H.K. Rapid chilling of beef carcasses utilizing ammonia and cryogenic systems: Effects on shrink and tenderness. J. Anim. Sci. 1974, 38, 928–934. [Google Scholar] [CrossRef]
- Jacob, R.; Rosenvold, K.; North, M.; Kemp, R.; Warner, R.; Geesink, G. Rapid tenderisation of lamb M. longissimus with very fast chilling depends on rapidly achieving sub-zero temperatures. Meat Sci. 2012, 92, 16–23. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, D.; Wen, X.; Li, X.; Chen, L.; Zheng, X.; Fang, F.; Li, J.; Hou, C. Effects of chilling rate on the freshness and microbial community composition of lamb carcasses. LWT–Food Sci. Technol. 2022, 153, 112559. [Google Scholar] [CrossRef]
- Kim, Y.; Ma, D.; Setyabrata, D.; Farouk, M.; Lonergan, S.; Huff-Lonergan, E.; Hunt, M. Understanding postmortem biochemical processes and post-harvest aging factors to develop novel smart-aging strategies. Meat Sci. 2018, 144, 74–90. [Google Scholar] [CrossRef]
- Yan, T.; Hou, C.; Wang, Z.; Li, X.; Chen, L.; Liang, C.; Xu, Y.; Zang, D. Effects of chilling rate on progression of rigor mortis in postmortem lamb meat. Food Chem. 2021, 373, 131463. [Google Scholar] [CrossRef]
- Warner, R.; Jacob, R.; Rosenvold, K.; Rochfort, S.; Trenerry, C.; Plozza, T.; Mcdonagh, M. Altered post-mortem metabolism identified in very fast chilled lamb M. longissimus thoracis et lumborum using metabolomic analysis. Meat Sci. 2015, 108, 155–164. [Google Scholar] [CrossRef]
- Bai, Y.; Ren, C.; Hou, C.; Chen, L.; Wang, Z.; Li, X.; Zhang, D. Phosphorylation and acetylation responses of glycolytic enzymes in meat to different chilling rates. Food Chem. 2023, 421, 135896. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.; Eikevik, T.; Rustad, T.; Kolsaker, K. Superchilling of food: A review. J. Food Eng. 2011, 107, 141–146. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, C.; Ye, X.; Hu, Y.; Chen, S.; Liu, D. A critical review on superchilling preservation technology in aquatic product. J. Integr. Agric. 2014, 13, 2788–2806. [Google Scholar] [CrossRef]
- Warner, R.; Kearney, G.; Hopkins, D.; Jacob, R. Retail colour stability of lamb meat is influenced by breed type, muscle, packaging and iron concentration. Meat Sci. 2017, 129, 28–37. [Google Scholar] [CrossRef]
- Ren, C.; Hou, C.; Li, Z.; Li, X.; Bai, Y.; Zhang, D. Effects of temperature on protein phosphorylation in postmortem muscle. J. Sci. Food Agric. 2019, 100, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Yamauchi, K. Analysis of global and gene-specific acetylation of histones in the liver of American bullfrog (Rana catesbeiana) tadpoles acclimated to low temperature. J. Therm. Biol. 2019, 84, 488–495. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, D.; Zheng, X.; Wen, X.; Yan, T.; Zhang, Z.; Hou, C. Effects of different storage temperatures on the physicochemical properties and bacterial community structure of fresh lamb meat. Food Sci. Anim. Resour. 2021, 41, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.; Toohey, E.; Warner, R.; Kerr, M.; Van, R. Measuring the shear force of lamb meat cooked from frozen samples: Comparison of two laboratories. Anim. Prod. Sci. 2010, 50, 382–385. [Google Scholar] [CrossRef]
- Koohmaraie, M. Biochemical factors regulating the toughening and tenderization processes of meat. Meat Sci. 1996, 43, 193–201. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Moir, A.; Perry, S. The sites of phosphorylation of rabbit cardiac troponin I by adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Effect of interaction with troponin C. Biochem. J. 1977, 167, 333–343. [Google Scholar] [CrossRef]
- Maddock, K.; Huff-Lonergan, E.; Rowe, L.; Lonergan, S. Effect of pH and ionic strength on μ- and m-calpain inhibition by calpastatin1. J. Anim. Sci. 2005, 83, 1370–1376. [Google Scholar] [CrossRef]
- Lian, T.; Wang, L.; Liu, Y. A new insight into the role of calpains in post-mortem meat tenderization in domestic animals: A review. Asian Australasian. J. Anim. Sci. 2013, 26, 443–454. [Google Scholar] [CrossRef]
- Shen, Q.; Du, M. Role of AMP-activated protein kinase in the glycolysis of postmortem muscle. J. Sci. Food Agric. 2005, 85, 2401–2406. [Google Scholar] [CrossRef]
- Chen, X.; Luo, X.; Zhu, L.; Liang, R.; Dong, P.; Yan, X.; Niu, L.; Hopkins, D.; Ga, S.; Mao, Y.; et al. The underlying mechanisms of the effect of superchilling on the tenderness of beef Longissimus lumborum. Meat Sci. 2022, 194, 108976. [Google Scholar] [CrossRef]
- Okitani, A.; Ichinose, N.; Koza, M.; Yamanaka, K.; Migita, K.; Matsuishi, M. AMP and IMP dissociate actomyosin into actin and myosin. Biosci. Biotech. Bioch. 2008, 72, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, S.; Lin, Y.; Xu, W.; Ye, D.; Xiong, Y.; Zhao, S.; Guan, K. Acetylation negatively regulates glycogen phosphorylase by recruiting protein phosphatase 1. Cell Metab. 2012, 15, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Yasykova, M.; Petukhov, S.; Muronetz, V. Phosphorylation of lactate dehydrogenase by protein kinases. Biochemistry 2000, 65, 1192–1196. [Google Scholar] [CrossRef]
- Fu, Q.; Yu, Z. Phosphoglycerate kinase 1 (PGK1) in cancer: A promising target for diagnosis and therapy. Life Sci. 2020, 256, 117863. [Google Scholar] [CrossRef]
- Duncan, L.; Shay, C.; Teg, Y. PGK1: An essential player in modulating tumor metabolism. In Physical Exercise and Natural and Synthetic Products in Health and Disease; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2343, pp. 57–70. [Google Scholar] [CrossRef]
- Leite, A.; Gomes, A.; Sousa, A.; Fontes, M.; Moretti, N. Effect of lysine acetylation on the regulation of Trypanosoma brucei glycosomal aldolase activity. Biochem. J. 2020, 477, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Dihazi, H.; Kessler, R.; Müller, G.; Eschrich, K. Lysine 3 acetylation regulates the phosphorylation of yeast 6-phosphofructo-2-kinase under hypo-osmotic stress. Biol. Chem. 2005, 386, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liu, R.; Li, J.; Wang, Y.; Tan, L.; Li, X.; Qian, X.; Zhang, C.; Xia, Y.; Xu, D.; et al. EGFR phosphorylated platelet isoform of phosphofructokinase 1 promotes PI3K activation. Mol. Cell. 2018, 70, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.; Locasale, J.; Swanson, K.; Sharfi, H.; Heffron, G.; Amador-Noguez, D.; Christofk, H.; Wagner, G.; Rabinowitz, J.; Asara, J.; et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 2010, 329, 1492–1499. [Google Scholar] [CrossRef]
- Lu, Z.; Hunter, T. Metabolic kinases moonlighting as protein kinases. Trends Biochem. Sci. 2018, 43, 301–310. [Google Scholar] [CrossRef]

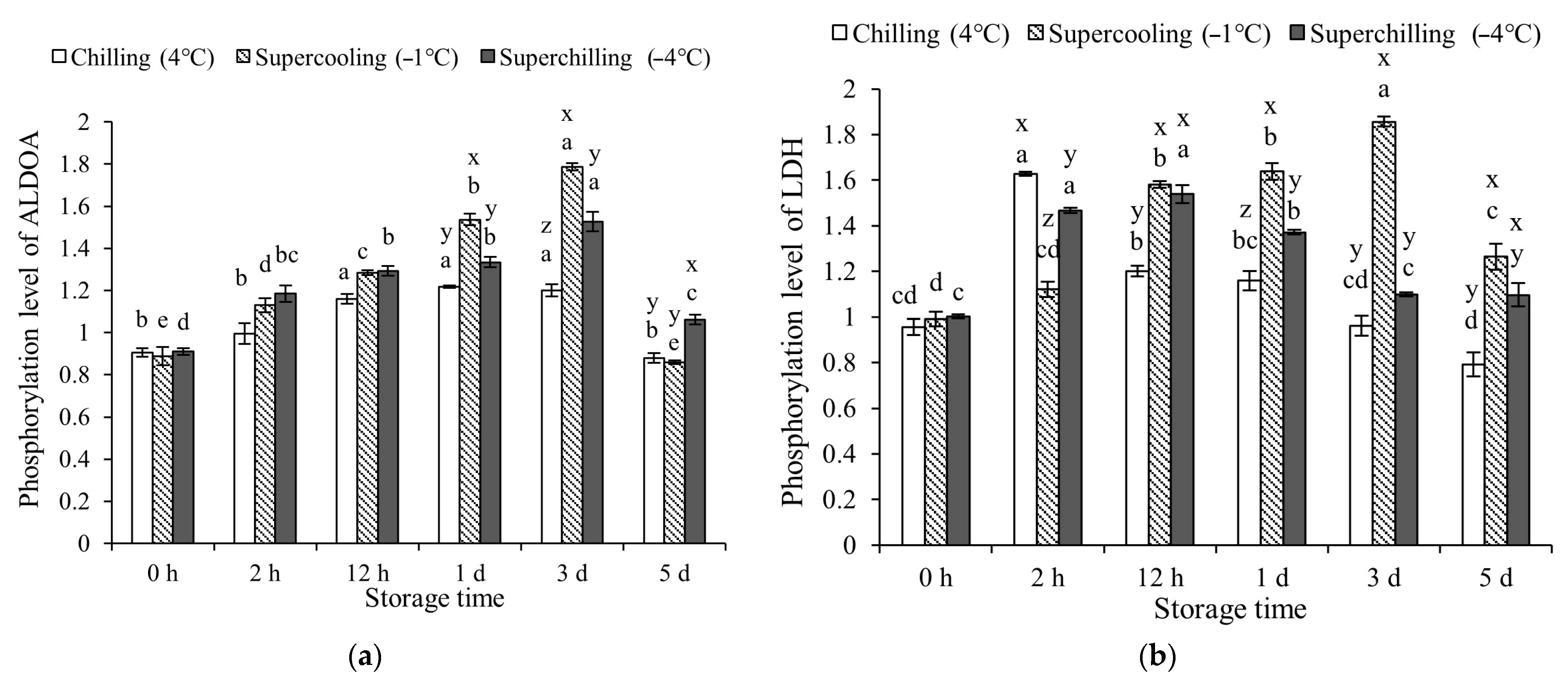
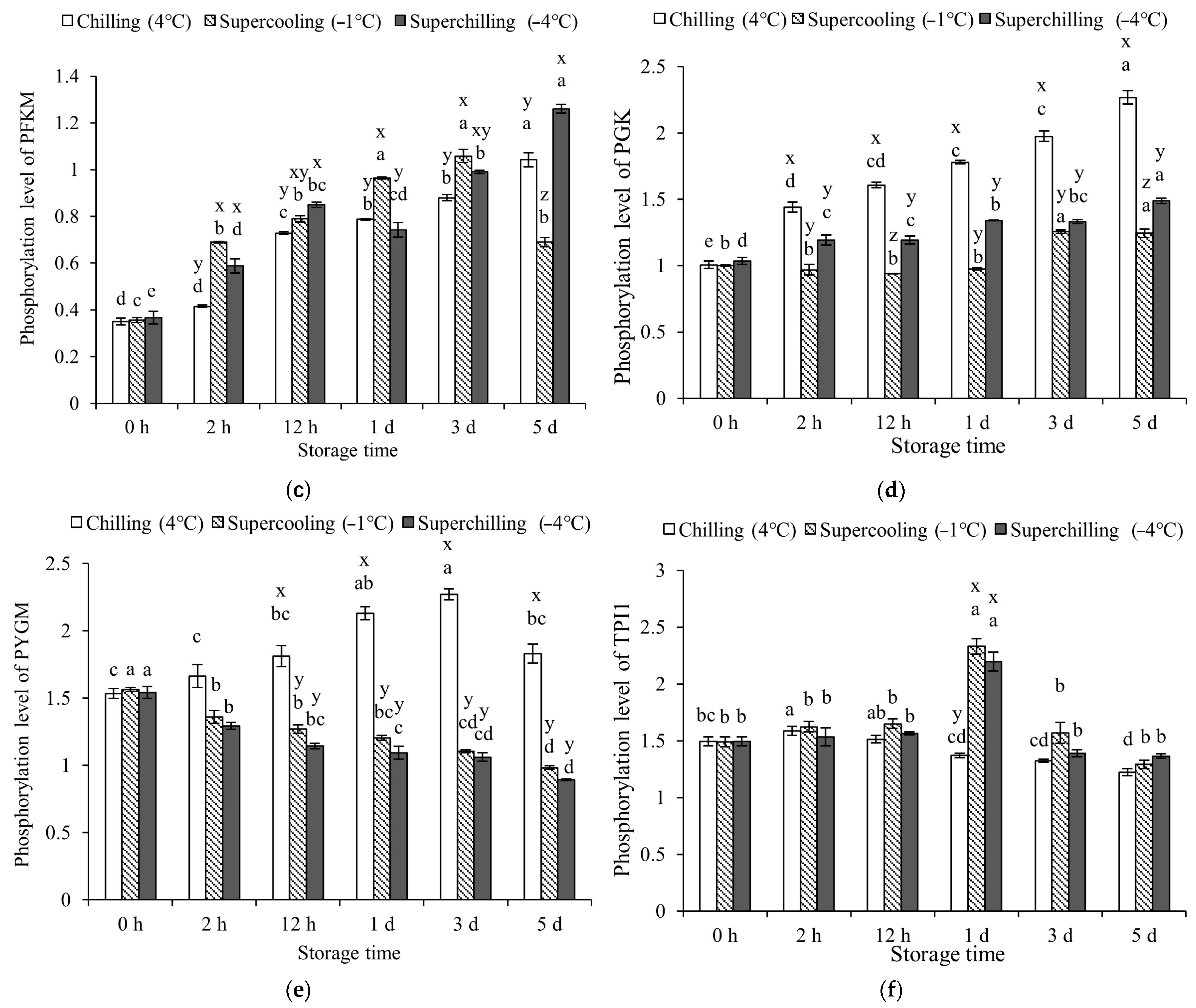

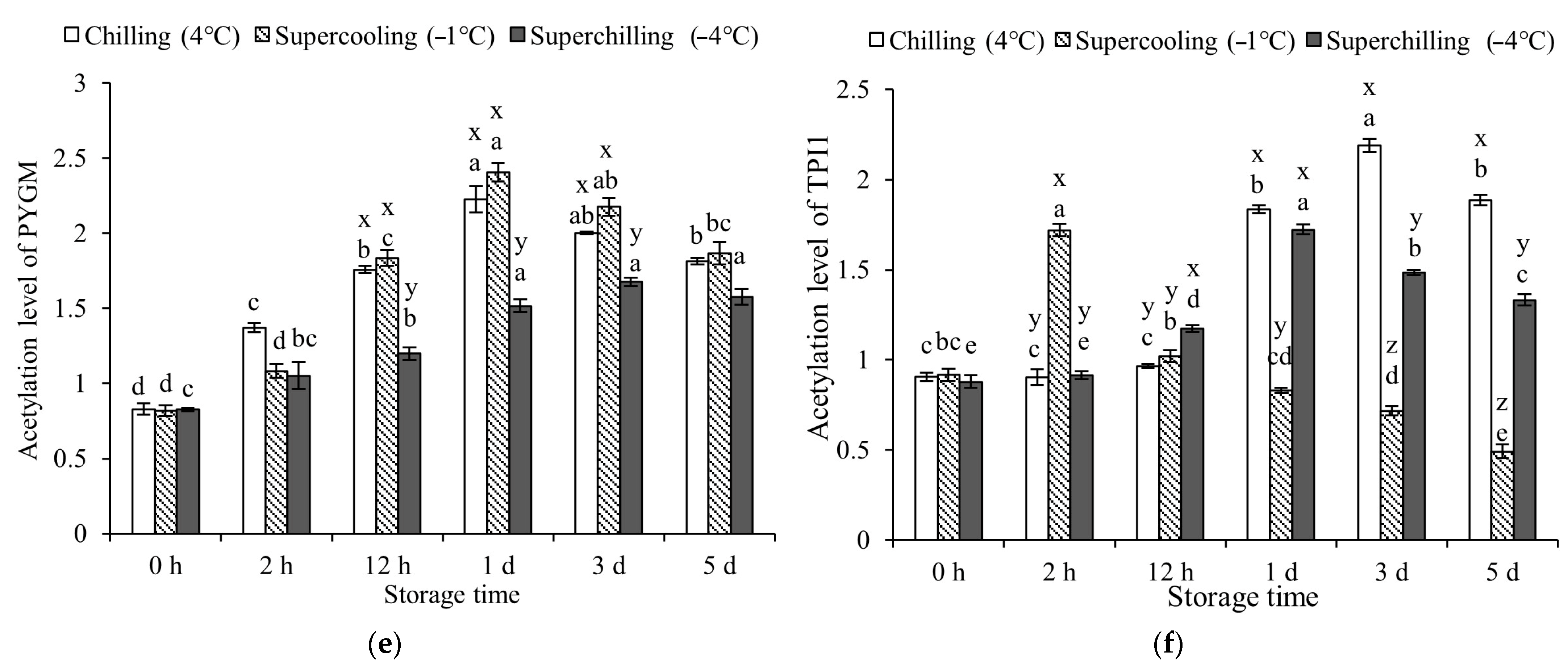
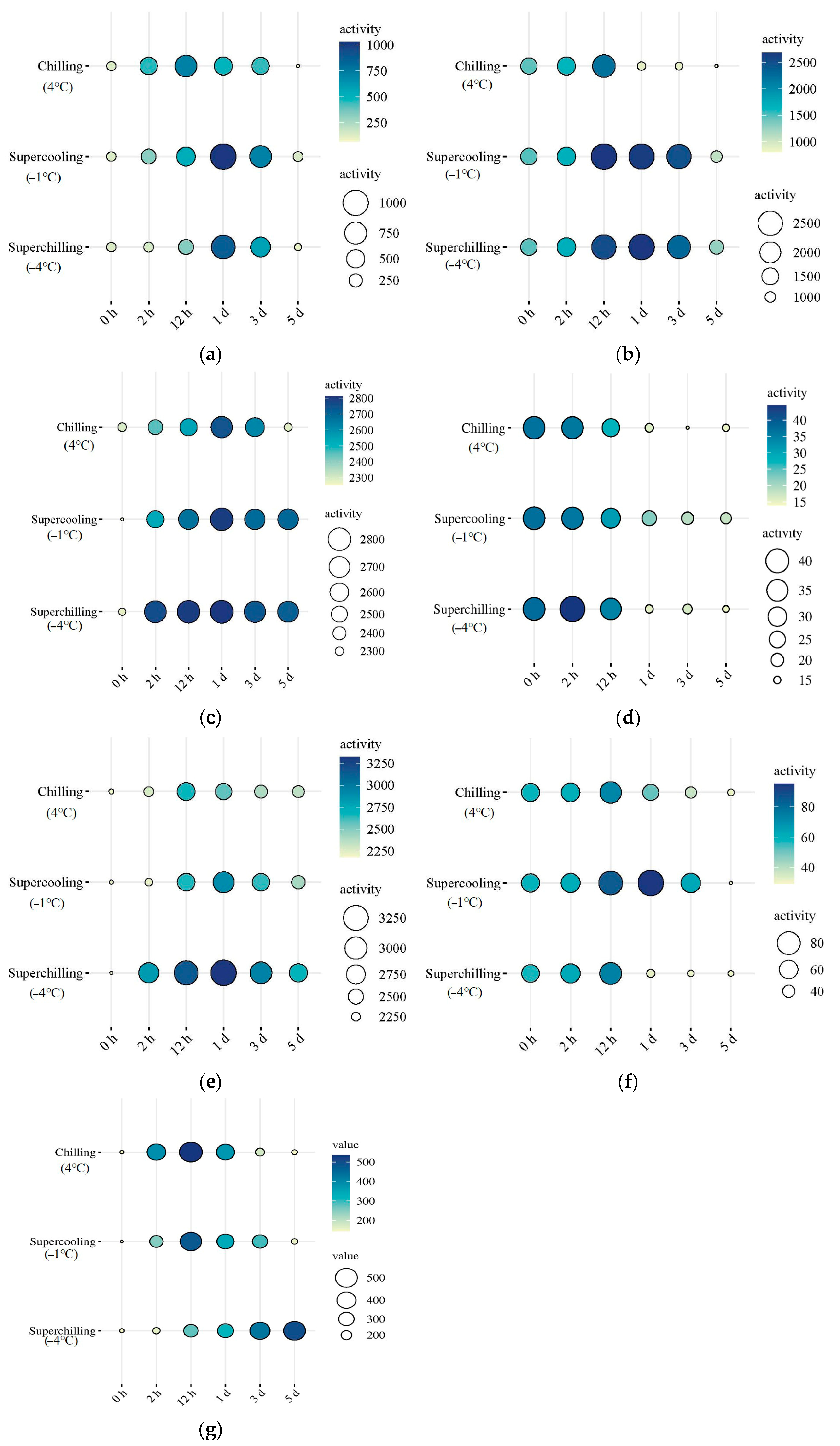
| 0 h | 2 h | 12 h | 1 d | 3 d | 5 d | ||
|---|---|---|---|---|---|---|---|
| pH | Chilling (4 °C) | 6.05 ± 0.24 a | 5.79 ± 0.1 5ya | 5.20 ± 0.03 zb | 5.14 ± 0.02 yc | 5.10 ± 0.05 c | 5.20 ± 0.09 c |
| Supercooling (−1 °C) | 6.18 ± 0.13 a | 6.08 ± 0.18 xa | 5.34 ± 0.05 yb | 5.17 ± 0.06 yc | 5.18 ± 0.07 c | 5.16 ± 0.04 c | |
| Superchilling (−4 °C) | 6.06 ± 0.12 a | 6.27 ± 0.19 xa | 5.62 ± 0.02 xb | 5.36 ± 0.06 xc | 5.04 ± 0.09 d | 5.13 ± 0.07 d | |
| ATP (μmol/g) | Chilling (4 °C) | 45.07 ± 1.63 a | 43.09 ± 2.11 a | 41.83 ± 1.55 xyab | 37.54 ± 1.74 yc | 33.60 ± 1.03 yd | 32.31 ± 1.66 d |
| Supercooling (−1 °C) | 44.87 ± 1.51 a | 42.65 ± 0.16 ab | 40.35 ± 2.06 ybc | 36.51 ± 2.58 yc | 33.48 ± 1.98 ycd | 31.54 ± 1.66 d | |
| Superchilling (−4 °C) | 45.62 ± 0.99 a | 44.88 ± 2.24 a | 43.91 ± 0.58 xa | 43.08 ± 0.32 xa | 37.57 ± 1.12 xb | 31.22 ± 1.12 c | |
| Glycogen (mg/g) | Chilling (4 °C) | 3.44 ± 0.06 a | 2.85 ± 0.11 yb | 2.17 ± 0.06 yc | 1.76 ± 0.12 yd | 1.39 ± 0.04 ye | 0.99 ± 0.06 yf |
| Supercooling (−1 °C) | 3.47 ± 0.05 a | 3.06 ± 0.01 xb | 2.43 ± 0.12 xc | 2.13 ± 0.11 xd | 1.88 ± 0.08 xe | 1.28 ± 0.01 xf | |
| Superchilling (−4 °C) | 3.47 ± 0.03 a | 2.65 ± 0.12 yb | 1.95 ± 0.03 zc | 1.36 ± 0.11 zd | 1.10 ± 0.02 ze | 0.85 ± 0.04 zf | |
| Lactate (mmol/g) | Chilling (4 °C) | 49.82 ± 0.30 c | 51.63 ± 0.94 c | 53.77 ± 0.55 zb | 56.14 ± 0.54 ya | 57.69 ± 0.63 ya | 60.06 ± 0.88 a |
| Supercooling (−1 °C) | 49.84 ± 1.13 e | 51.67 ± 0.71 d | 55.35 ± 1.12 ycd | 57.08 ± 0.29 ybc | 58.68 ± 0.17 yab | 61.21 ± 0.42 a | |
| Superchilling (−4 °C) | 49.80 ± 0.47 d | 51.53 ± 0.46 c | 59.34 ± 0.25 xb | 59.05 ± 0.46 xb | 63.88 ± 0.59 xa | 59.98 ± 0.47 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Ren, C.; Wu, S.; Hou, C.; Li, X.; Zhang, D. Co-Regulation of Very Fast Chilling Treatment and the Follow-Up Storage Temperature on Meat Tenderness Through Glycolysis. Foods 2025, 14, 2932. https://doi.org/10.3390/foods14172932
Bai Y, Ren C, Wu S, Hou C, Li X, Zhang D. Co-Regulation of Very Fast Chilling Treatment and the Follow-Up Storage Temperature on Meat Tenderness Through Glycolysis. Foods. 2025; 14(17):2932. https://doi.org/10.3390/foods14172932
Chicago/Turabian StyleBai, Yuqiang, Chi Ren, Saisai Wu, Chengli Hou, Xin Li, and Dequan Zhang. 2025. "Co-Regulation of Very Fast Chilling Treatment and the Follow-Up Storage Temperature on Meat Tenderness Through Glycolysis" Foods 14, no. 17: 2932. https://doi.org/10.3390/foods14172932
APA StyleBai, Y., Ren, C., Wu, S., Hou, C., Li, X., & Zhang, D. (2025). Co-Regulation of Very Fast Chilling Treatment and the Follow-Up Storage Temperature on Meat Tenderness Through Glycolysis. Foods, 14(17), 2932. https://doi.org/10.3390/foods14172932









