Abstract
Thyme oil (TO), an aromatic compound derived from Thymus species, exhibits potent antioxidant and antibacterial properties. To address its defects of high volatility and susceptibility to oxidation, TO was encapsulated in chitosan oligosaccharide (COS)-stabilized oil-in-water emulsions using a two-step emulsification method with ultrasound assistance. The droplet size of TO-in-water emulsions decreased significantly with increasing ultrasound power and treatment time, achieving sizes below 240 nm with an encapsulation efficiency exceeding 90%. The COS interface layer, combined with polyvinyl alcohol (PVA), effectively enhanced emulsion stability by preventing phase separation and maintaining droplet size and zeta potential during storage. Compared to its free form, the encapsulation of TO in the emulsion significantly improved the antioxidant activities, as evidenced by the enhanced ABTS (1.25-fold) and DPPH (1.33-fold) radical scavenging activities, at equivalent concentrations. Additionally, the TO emulsions exhibited superior antibacterial and antifungal properties, with minimum inhibitory concentration (MIC) values reduced by half and effective inhibition of Escherichia coli, Staphylococcus aureus, and Penicillium italicum growth. These findings highlight the potential of TO emulsions as an effective delivery system for improving the functionality and stability of TO in fresh food preservation applications.
1. Introduction
Thyme oil (TO), derived from Thymus species, is renowned for its potent antioxidant and antimicrobial properties, attributed to its bioactive compounds, such as thymol and carvacrol [1,2]. Despite its potential, the practical application of TO is hindered by its high volatility, hydrophobicity, and susceptibility to oxidation [3,4]. Encapsulation of TO has been widely reported to improve its functional performance compared to free oils, including enhanced bioavailability, sustained release, and better protection of active compounds during storage, ultimately leading to improved antimicrobial and antioxidant efficacy in practical applications [5,6,7]. Oil-in-water (O/W) emulsions are widely recognized as effective systems for encapsulating, protecting, and delivering lipophilic active ingredients, such as essential oils (EO) [7,8]. These systems improve the hydrophilicity and bacteriostatic activity of EO, enhancing their functionality in various applications [2,3]. However, one major challenge associated with EO-loaded emulsions is the phenomenon of Ostwald ripening, which compromises emulsion stability [9]. This instability arises from the higher solubility and permeability of smaller oil droplets, which merge into larger droplets, leading to phase separation and precipitation [10]. Conventional strategies to mitigate Ostwald ripening involve using water-insoluble triglycerides as ripening inhibitors. However, these inhibitors can diminish the antimicrobial potency of EO, necessitating alternative approaches to stabilize emulsions without compromising their functional properties [11]. Hence, we aimed to explore an alternative approach to prevent Ostwald ripening of EO without ripening inhibitors.
Chitosan oligosaccharides (COS) are low-molecular-weight derivatives of chitosan obtained through enzymatic or chemical hydrolysis of natural sources, like shrimp and crab shells [12]. COS has garnered significant attention due to its enhanced solubility and bioactivity compared to its parent polymer [13]. It exhibits a wide range of beneficial properties, including antimicrobial, antioxidant, and immunomodulatory activities, making it suitable for applications in pharmaceutical, agriculture, and food industries [14]. Additionally, COS retains the biocompatibility of chitosan but offers improved activity, allowing for versatile use in stabilizing emulsion systems. According to our previous works, chitosan can react with cinnamaldehyde (CA) at the oil–water interface to form a solid-like interface layer through the Schiff-base reaction to effectively inhibit Ostwald ripening and stabilize pure essential oil emulsions [11,15]. Given that COS has the same amino group as chitosan, COS is also expected to form a similar Schiff-base reaction with CA, resulting in a solid-like interfacial layer that could further stabilize oil droplets and inhibit TO diffusion, and the solid-like interfacial layer would stabilize oil droplets, potentially inhibiting the diffusion of TO molecules, thereby improving emulsion stability.
This study used the interfacial layer formed by COS and CA to stabilize TO emulsions (Scheme 1). Meanwhile, polyvinyl alcohol (PVA) was incorporated as a thickener to enhance the viscosity of the continuous phase and reduce gravitational separation. It was also used as a co-emulsifier to help stabilize the oil–water interface [11]. Additionally, ultrasound-assisted emulsification was used to achieve smaller droplet sizes and improve emulsion homogeneity, enhancing stability and functionality [7,16]. This approach is innovative in combining COS/CA interfacial chemistry with PVA co-emulsification, offering dual stabilization and improved functional performance, which has not been extensively reported. This study systematically investigated the effects of ultrasonic power, processing time, and TO concentration on emulsion droplet size and storage stability. Furthermore, the antioxidant and antimicrobial activities of encapsulated TO were evaluated and compared to those of free TO. Beyond its demonstrated efficacy in enhancing the stability and bioactivity of TO, the developed emulsion system holds promise for broader applications, including active food packaging, surface coatings for fresh produce, and potential use as a delivery vehicle for lipophilic bioactives in pharmaceutical formulations. By integrating these findings, the research aimed to provide a foundation for applying TO emulsions in food preservation and other relevant fields.
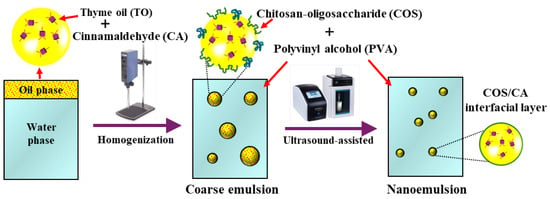
Scheme 1.
Schematic illustration of COS-stabilized thyme oil-in-water emulsion preparation by an ultrasonic-assisted method.
2. Materials and Methods
2.1. Materials
Chitosan-oligosaccharide (COS, MW 5 kDa, degree of deacetylation = 90%) was obtained from Zhejiang Golden-Shell Pharmaceutical Co., Ltd. (Yuhuan, China). Thyme oil (TO) and polyvinyl alcohol (PVA, 1799) were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Cinnamaldehyde (CA, purity ≥ 98%) was provided by Aladdin Reagent Co., Ltd. (Shanghai, China). Potato dextrose agar (PDA) and potato dextrose broth (PDB) were obtained from Bowei Biotechnology Co., Ltd. (Shanghai, China). Penicillium italicum (P. italicum) was isolated and purified by the Key Laboratory of Horticulture Science, Southwest University (Chongqing, China). Chemicals, other than those used in this study, were of analytical grade.
2.2. Preparation of the Thyme Oil Emulsion
The TO emulsion was prepared using a two-step emulsification method with some modifications [6,11]. The oil phase consisted of a mixture of TO and CA, where the concentration of TO was set to 1 wt%, 2 wt%, and 4 wt% in the emulsion (denoted as 1TO-E, 2TO-E, and 4TO-E), and the concentration of CA was 2.5 wt% of the oil phase. The aqueous phase was a mixed solution of COS and PVA at pH 6.5, with a COS concentration of 0.5 wt% and a PVA concentration of 1 wt%. The first step was to introduce the oil phase into the aqueous phase gradually, and the resulting mixture was homogenized using a high-speed disperser (FJ200-S; Lichen Instrument Technology Co., Ltd., Changsha, China) at a speed of 12,000 rpm for 3 min in an ice bath. Subsequently, the obtained coarse emulsion was subjected to ultrasonication using a 20 kHz ultrasonic processor (Scientz-II D, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) at a power output of 300–600 W for 5, 10, and 20 min in an ice bath, with a 5 s ultrasound pulse followed by a 5 s pause. The pH was then adjusted to 4 with 0.1 M hydrochloric acid solution to obtain the final emulsion. The pH was adjusted from 6.5 to 4 to enhance COS protonation, promoting stronger electrostatic interactions at the oil–water interface and improving emulsion stability. For storage stability tests, emulsions were stored in sealed glass containers at 25 °C in the light-avoiding environment and sampled at 1, 7, and 21 d for analysis.
2.3. Physicochemical Characterization of Thyme Oil Emulsion
2.3.1. Droplet Size and Zeta Potential Measurement
The droplet size, polydispersity index (PDI), and zeta potential of the emulsions were measured using a dynamic light scattering (DLS) instrument (Zetasizer Nano, Malvern Instruments Ltd., Malvern, Worcestershire, UK). To minimize the effects of multiple scattering, the original samples were diluted with deionized water before the measurement. All measurements were conducted at 25 °C and repeated three times.
2.3.2. Microscopy Observation
To observe the morphology of an emulsion using an optical microscope (BX53, OLYMPUS, Tokyo, Japan), samples were prepared by placing 5–10 μL of emulsion on a clean glass slide, diluting if necessary, and covering it with a coverslip. Using a bright-field microscope, the observation started with a low magnification (10×) to locate the sample and gradually increased to higher magnifications (40× or 100×). A digital camera was used to capture the microscopic images of emulsions. At least three independent samples were prepared to ensure reproducibility.
2.3.3. Encapsulation Efficiency Determination
The encapsulation efficiency (EE) of TO in the COS-stabilized emulsions was determined using a modified centrifugation method. Briefly, the freshly prepared emulsion (5 mL) was transferred into a centrifuge tube and centrifuged at 10,000 rpm for 30 min at 4 °C to separate the free (non-encapsulated) TO. The supernatant containing the free TO was carefully collected and extracted with an equal volume of ethanol under gentle agitation to ensure complete oil dissolution. The concentration of free TO was quantified spectrophotometrically at the characteristic absorption wavelength (274 nm), using a standard calibration curve. The EE of TO in the emulsion was calculated using the following formula:
where Ct is the total concentration of TO in the emulsion and C0 is the concentration of free TO in the aqueous phase. All measurements were performed in triplicate.
2.4. Antioxidant Quenching Activity of Thyme Oil Emulsion
The antioxidant activities of emulsions were evaluated by ABTS+ and DPPH radical scavenging methods with minor modifications [17,18]. The emulsion sample (0.1 g) was diluted with 5 mL of deionized water. Subsequently, the diluted emulsion and ABTS/DPPH assay solution were mixed at a volume ratio of 1:1 and incubated for 60 min at room temperature in a dark environment. Finally, a UV-vis spectrophotometer (UV-2550, Shimadzu Inc., Kyoto, Japan) was used to measure the absorbance at 734 nm and 517 nm. The radical scavenging activity was calculated using the following equation:
where A1 is the absorbance of the solutions treated with TO/TO emulsions. A2 is the absorbance of control solutions (without TO/TO emulsions, i.e., only DPPH or ABTS solutions).
2.5. Antimicrobial Activity of Thyme Oil Emulsion
2.5.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC and MBC of the emulsions were determined using a microdilution method with iodonitrotetrazolium chloride (INT) as a chromogen in sterile 96-well plates against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Penicillium italicum (P. italicum) with slight modifications [11,19]. Firstly, the samples were serially diluted two-fold in the wells. Then, 100 μL of bacterial or spore inoculum (104–105 CFU/mL) was added to each well, ensuring complete mixing to achieve final concentrations of TO emulsion between 10,000 μg/mL–78.125 μg/mL. For bacterial testing, the plates were incubated at 37 °C for 24 h, followed by adding 50 μL of 0.2 mg/mL iodonitrotetrazolium chloride (INT) chromogen. After 30 min incubation at 37 °C, the MIC was determined as the lowest concentration showing no color change or visible bacterial growth. The plates were incubated at 28 °C for 48 h for fungal testing, with fungal growth being observed. The MIC was defined as the lowest concentration without any precipitation. The non-discolored or non-precipitating wells were then transferred to fresh medium, with bacterial and fungal cultures incubated at 37 °C for 24 h and 28 °C for 48 h, respectively. The MBC was the lowest concentration at which no microbial growth was observed.
2.5.2. Determination of the Antibacterial Inhibition Zone of the TO Emulsion
The antibacterial activity of the TO or TO emulsions was evaluated using the inhibition zone method with an Oxford cup against E. coli and S. aureus. Bacterial strains were cultured in Luria–Bertani (LB) broth at 37 °C overnight to a concentration of approximately 1 × 107 CFU/mL. Sterile Mueller–Hinton agar (MHA) plates were prepared, and 100 µL of the bacterial suspension was evenly spread across the surface. After the agar was surface dried for 5 min, sterile Oxford cups were placed on the agar, and each cup was filled with 100 µL of the test samples. The plates were incubated at 37 °C for 24 h under aerobic conditions. Zones of inhibition around the Oxford cups were measured in millimeters using a digital caliper, with measurements averaged from three replicates.
2.5.3. Inhibitory Effect of the Emulsion on Mycelial Growth of P. italicum
The inhibitory effect of the TO emulsion on the mycelial growth of P. italicum was assessed following a previous method with minor modifications [20]. Briefly, sterilized and cooled potato dextrose agar (PDA) medium was thoroughly mixed with the free TO or TO emulsion and poured into several Petri dishes. Plates containing only PDA medium served as controls. Once the medium had solidified, a 7-day-old mycelial disk (5 mm in diameter) of P. italicum was placed at the center of each dish. All plates were incubated at 28 °C for one week. Colony diameters were measured and recorded after 3 d of incubation. The inhibition rate of mycelial growth was calculated using the following formula:
where d1 and d2 represent the average colony diameters of the control and treated groups, respectively, while d0 denotes the initial diameter of the mycelial disk, respectively.
2.6. Statistical Analysis
All experiments were conducted in triplicate, and the results are presented as mean ± standard deviation. Statistical analyses were performed using SPSS software (version 25.0, IBM, Armonk, NY, USA). Data were evaluated using analysis of variance (ANOVA), with statistical significance defined at p < 0.05.
3. Results and Discussion
3.1. Formation of Thyme Oil-in-Water Emulsions
A COS-stabilized TO-only oil phase emulsion was prepared using a two-step emulsification method, and the droplet sizes were effectively controlled using the ultrasound-assisted method. At lower ultrasound power (300 W), the size of TO-in-water emulsion was larger, with a droplet size range of 520.8 to 719.08 nm (Table 1). With the increase in ultrasonic power, the droplet size of the emulsion decreased significantly to less than 250 nm, which might be due to the exposure of more groups in ultrasonic-mediated unfolding and depolymerization of the COS chain, so it has more opportunities to react with CA to form a stable interfacial layer [15,21,22]. As ultrasound power increased, the number of generated cavitation bubbles also increased, resulting in enhanced energy around the bubbles, facilitating droplet dispersion, reducing droplet size, and improving emulsion stability [23,24]. This result is consistent with previous research reports, indicating that higher ultrasonic power dispersed oil droplets and helped to form emulsions with smaller droplet sizes [25,26]. Notably, although increasing the ultrasound power further resulted in a slight reduction in the droplet size of the emulsion, this change was deemed unnecessary from an energy-saving perspective [23]. Additionally, the ultrasonic treatment time also significantly impacted the size of emulsion droplets, because the droplet size decreased significantly from 404.75 nm to 224.27 nm as the treatment time increased from 5 min to 10 min. The cavitation effect of ultrasound facilitated the uniform dispersion of the water phase and the fragmentation of the oil phase, which increased the oil/water interface area and enhanced the interaction between the phases. As a result, COS rapidly accumulated with CA at the oil–water interface, which may form a solid-like interface layer similar to chitosan, exerting its emulsifying effect with PVA [11]. This method of using co-emulsifiers (COS/CA and PVA) in the interfacial phase is a very effective way to prevent Ostwald ripening [11,27]. As the processing time increased from 10 min to 20 min, there was no significant change in droplet size. Additionally, the lowest PDI values were observed at 10 min of processing under higher power conditions, indicating that this combination achieved the most uniform droplet size distribution (Table S1). Although the mean droplet size showed no significant difference between 10 and 20 min at higher powers, the slight increase in PDI at 20 min suggested the onset of destabilization, which might be due to the aggregation of COS and droplet coalescence, ultimately compromising the stability of the emulsion [25,26]. The encapsulation efficiency of TO in the emulsions was assessed, achieving approximately 94.74% at a 1% TO level and 91.73% at a 2% level (Figure S1), indicating the high payload and potential protection of TO. Finally, the optimized conditions (450 W, 10 min) were selected for all subsequent analyses based on achieving the smallest droplet size with a narrow size distribution and good stability.

Table 1.
The droplet size (nm) of emulsions prepared using different ultrasonic power levels and processing time.
3.2. Storage Stability of Thyme Oil-in-Water Emulsions
3.2.1. Appearance and Droplet Size Change of TO Emulsions
The appearance of TO-in-water emulsions was uniform and light brown, and there was no change in all TO emulsions within 7 d, which indicated that TO emulsions remained visually stable across varying TO concentrations (Figure 1A). However, the emulsion with high TO concentration (4TO-E) appeared to undergo slight creaming and phase separation at 21 d (red frame), and the droplets floated without demulsification and oil leakage, which was related to the lower density of TO and the influence of gravity on the oil droplets [28,29] This result also indicated that the stability of high-TO emulsions had relatively reduced. Notably, the absence of phase separation suggested effective stabilization by the COS/CA solid-like interface layer and PVA, and the presence of PVA also improved the viscosity of the emulsion and weakened the separation effect of gravity [11,30].
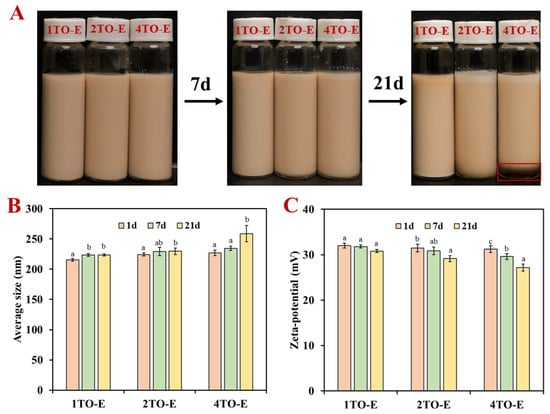
Figure 1.
(A) Appearance stability, (B) droplet size, and (C) zeta potential of TO-in-water emulsions under different TO concentrations. Different letters within the same TO concentration indicate statistically significant differences (p < 0.05). 1TO-E, 2TO-E, and 4TO-E refer to 1%, 2%, and 4% (wt) of TO in the emulsions, respectively.
The droplet size analysis, based on DLS measurements, showed a correlation between TO concentration and emulsion droplet size (Figure 1B). The initial droplet size did not differ significantly among TO concentrations, although a slight increasing trend was observed with higher oil phase content, where the average droplet size was approximately 215 nm at a TO concentration of 1%. Increasing the TO concentration to 2% and 4% slightly increased the droplet size to about 225 nm, likely due to the increased viscosity of the dispersed phase (Figure S2). A higher viscosity in the continuous phase can slow down droplet movement and reduce gravitational separation (creaming), thereby enhancing emulsion stability. This relationship between viscosity and droplet mobility is consistent with previous findings in essential oil-in-water emulsion stability studies [11]. The droplet size of TO-in-water emulsions had no noticeable change within one week and remained within 240 nm (Figure 1B). During the longer-term storage for 21 d, the droplet size of 1TO-E and 2TO-E emulsions remained stable and did not increase significantly, while that of 4TO-E emulsions increased to 258.66 nm. These minor changes might suggest minimal coalescence or Ostwald ripening, reflecting the effectiveness of the COS/CA solid-like interfacial layer and PVA stabilization mechanism [11,15]. Despite this increase, the droplet sizes remained within a range indicative of good emulsion stability. Zeta potential values revealed that the emulsions maintained high absolute values, exceeding 30 mV across all TO concentrations within 7 d of storage (Figure 1C). The consistent zeta potential across TO concentrations indicated that the COS/CA solid-like interface layer and PVA effectively stabilized the system regardless of oil loading [31]. After 21 days of storage and at a TO concentration of 1% and 2%, the zeta potential was around 29.13–32 mV (Figure 1C), ensuring strong electrostatic repulsion between droplets, which prevented aggregation and contributed to longer-term stability [27,32]. It should be noted that the zeta potential of 4TO-E emulsion decreased to 27.16 mV at 21 d, indicating the direct electrostatic repulsion of its droplets, which corresponds to the increase in its average size above. This phenomenon was similar to previous studies on sodium caseinate (self-assembled) and chitosan-stabilized TO emulsion [8,33].
3.2.2. Distribution of Droplets During Storage of TO Emulsions
The droplet size distribution over a 21-day storage period highlighted the emulsion’s stability under different TO concentrations. All emulsions showed a single peak distribution within 21 d, corresponding to their uniform droplet size (Figure 2A). However, the peak position of the 4TO-E emulsion showed the most obvious shift to the right with increasing storage time (Figure 2B), indicating the droplet size increase, which was also observed in the previous reports on chitosan-stabilized essential oil emulsions [11,27]. The droplets of emulsions presented a uniform spherical shape. The morphology of the 1TO-E and 2TO-E emulsions had no obvious change in 21 d, while the droplets of the 4TO-E emulsion appeared larger from 7 d to 21 d, indicating that coalescence and Ostwald ripening occurred [34,35]. Collectively, the stability mechanism of TO emulsions can be divided into the following two aspects: (i) The combination of the COS/CA solid-like interface layer and PVA as co-stabilizers can effectively adsorb at the oil–water interface to improve the stability of TO-in-water emulsions; (ii) PVA can be used as a thickener to increase the viscosity of the continuous phase, weaken the influence of gravity on the emulsion droplet, and enhance its stability. Meanwhile, TO concentration had a pronounced effect on droplet size, stability, and functional performance, underscoring the need to optimize oil content for targeted application requirements. Therefore, comparisons of functional properties were made between free TO (2%) and encapsulated emulsions at 1% and 2% TO, which exhibited stable physicochemical properties. In addition, ultrasonication significantly contributed to reducing droplet size and improving emulsion homogeneity. The ability of TO emulsions to maintain droplet size and distribution over time highlights their potential practical commercial use.
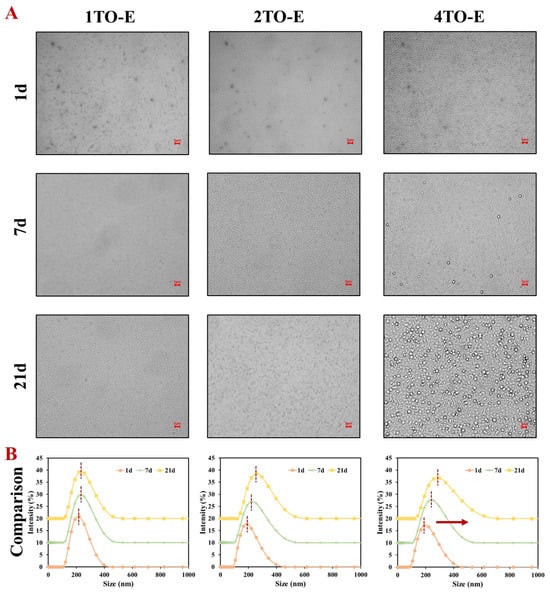
Figure 2.
(A) Microscopic images and (B) droplet size distribution of TO-in-water emulsions during 21-day storage under different TO concentrations. The scale bars were 5 μm. The red arrow indicates the rightward shift of the peak position. 1TO-E, 2TO-E, and 4TO-E refer to 1%, 2%, and 4% (wt) of TO in the emulsions, respectively.
3.3. Antioxidant Activity of Thyme Oil-in-Water Emulsions
The antioxidant activity of free and encapsulated TO was evaluated by ABTS and DPPH radical scavenging activity, which is depicted in Figure 3. Free TO had good antioxidant capacity, with its ability to scavenge ABTS and DPPH free radicals reaching 56.3% and 57.79%, respectively, at a concentration of 2%. Noticeably, encapsulation significantly enhanced the antioxidant potential compared to free TO across all tested concentrations. At a concentration of 1%, the scavenging activity of encapsulated TO against DPPH free radicals was approximately 62.1%, slightly higher than the scavenging activity of free TO at a concentration of 2%. Meanwhile, at a concentration of 2%, the ABTS and DPPH radical scavenging activities of encapsulated TO reached approximately 70.45% and 76.92%, respectively, which were 1.25- and 1.33-fold higher than the free TO at the same concentration, respectively. This improvement was likely due to the protective effect of the COS/CA solid-like interface layer and PVA matrix, which minimized oxidation and preserved the functional components of TO. Numerous studies have shown that encapsulation by natural polymer emulsions enhanced the scavenging abilities of essential oils, aligning with the findings of this research [18,36,37]. Interestingly, the emulsions demonstrated a more substantial free radical scavenging capacity against DPPH compared to ABTS, which could be attributed to the alcohol solubility of TO [17,38]. Generally, the DPPH radical scavenging activity of essential oils is often higher than that of ABTS due to the better solubility of lipophilic components in the organic solvents used in the DPPH assay. Moreover, the hydrogen atom transfer (HAT) mechanism of DPPH better matches the antioxidant behavior of terpenoids commonly found in essential oils. Browning and lipid oxidation are significant challenges in fresh food storage, with free radical scavenging being a key mechanism through which antioxidants inhibit lipid oxidation [39]. Therefore, due to its strong antioxidant capacity, using TO emulsion as a coating or packaging material for fresh food offers an effective strategy to slow down the quality deterioration during storage.
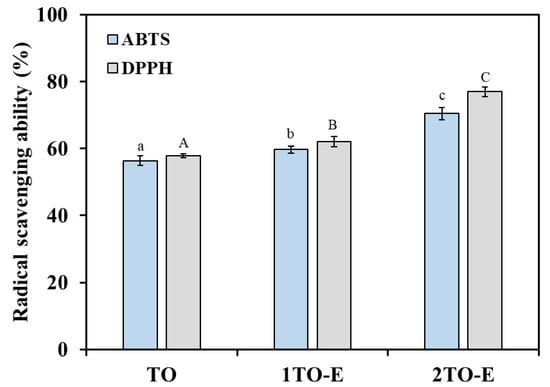
Figure 3.
Antioxidant activity of free and encapsulated TO under different concentrations. TO refers to 2% (wt) of TO in ethanol solution, and 1TO-E and 2TO-E refer to 1% and 2% (wt) of TO in the emulsions, respectively. Lowercase letters represent significant differences in ABTS assay results, whereas uppercase letters correspond to differences in DPPH assay (p < 0.05).
3.4. Antimicrobial Activity of Thyme Oil-in-Water Emulsions
3.4.1. MIC and MBC Evaluation of TO Emulsions
MIC and MBC serve as key indicators for evaluating the antimicrobial activity of essential oils. MIC refers to the minimum concentration required to inhibit pathogenic microorganisms’ growth or halt their survival, while MBC is the minimum concentration needed to eliminate 99.9% of pathogenic microorganisms. Lower MIC and MBC values indicate a more substantial antimicrobial effect [11]. TO can effectively suppress the growth and proliferation of bacteria and molds by causing cell membrane invagination, organelle disintegration, and compromising the integrity of cell structures [40]. For all tested strains, the MIC values for the TO emulsions (312.5 μg/mL) were half of the MIC value for free TO (625.0 μg/mL) at a 2% concentration, indicating that the emulsion formulation enhanced the antimicrobial efficiency by requiring lower concentrations to inhibit microbial growth (Table 2). Similarly, the MBC values demonstrated that the TO emulsion was more effective in achieving complete microbial eradication than free TO.

Table 2.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of free and emulsion (E)-encapsulated TO against E. coli, S. aureus, and P. italicum.
The enhanced antimicrobial activity of the COS emulsion systems could come from three primary mechanisms. Firstly, the improved performance could be attributed to the enhanced solubility and stability of TO within the emulsion system, which facilitated better interaction with microbial cells and more effective delivery of the active compounds [41]. Furthermore, the encapsulation likely enabled a sustained release of TO, maintaining an active concentration over time and preventing rapid evaporation or degradation [42]. Secondly, the positively charged emulsion droplets adhered to the negatively charged surfaces of microorganisms, promoting interactions with surface proteins. This interaction increased membrane permeability and disrupted cell wall integrity, thereby amplifying the antimicrobial effects of TO [18,43]. Thirdly, the reduction in emulsion droplet size and the increase in specific surface area, achieved via ultrasonic-assisted homogenization, improved the penetration efficiency of antimicrobial components into the cell membrane, which enhanced their overall effectiveness [37,44]. These results were consistent with previous studies that demonstrated the positive impact of emulsification on the antimicrobial activity of TO. Similar to the findings from a study on double-layer TO emulsions stabilized by whey protein and chitosan, which enhanced both stability and antimicrobial efficacy, an increase in antimicrobial activity was observed [31]. However, in contrast to this method, significant antimicrobial effects were achieved using a single-layer emulsion without ripening inhibitors. This approach offers an advantage over more complex formulations, as ripening inhibitors, such as corn oil or medium-chain long triglycerides, have been shown to reduce the antimicrobial efficacy of TO emulsions, as reported in another study [1]. Therefore, the simpler formulation not only maintains antimicrobial activity but also avoids the potential negative impact of complex structural designs or stabilizers on bioactivity.
3.4.2. Inhibition Zone Evaluation of TO Emulsions
The antibacterial ability of TO was evaluated in both free and emulsion-encapsulated forms against E. coli (Gram-negative) and S. aureus (Gram-positive) using the inhibition zone method. As shown in Figure 4, free TO at a concentration of 2% has a particular antibacterial effect, with inhibition zones of 6.38 mm and 6.77 mm against E. coli and S. aureus, respectively. Additionally, the inhibition zone diameters varied depending on the concentration of TO emulsions. For E. coli, 1TO-E and 2TO-E were 9.18 and 12.58 mm, respectively, and for S. aureus, the diameters were 10.94 and 13.15 mm, respectively. Generally, larger inhibition zones were observed with higher TO concentrations, indicating a dose-dependent antibacterial effect [45].
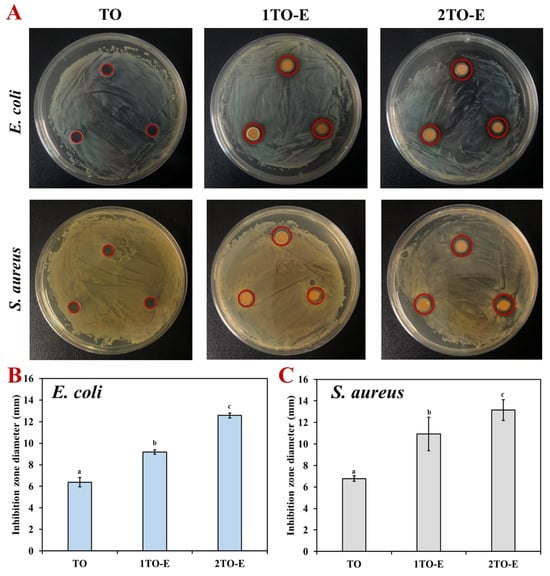
Figure 4.
Inhibition zone appearance (A) and diameters (B,C) of free and encapsulated TO against E. coli and S. aureus under different concentrations. The red circles highlight the diameter of the inhibition zones. Different letters within the same test indicate statistically significant differences (p < 0.05).
Regardless of the concentration, the encapsulated TO consistently exhibited larger inhibition zones than free TO. For example, at the same concentration of 2% TO, the inhibition diameters of emulsions were 1.97- and 1.94-fold larger than those of free TO, indicating a superior antibacterial effect (Figure 4B). This enhanced efficacy of encapsulated TO can be attributed to the emulsion system’s ability to improve the solubility and stability of TO, thereby facilitating better interaction with bacterial cells [8,33]. In addition, encapsulation likely aids in the controlled release of TO, maintaining a sustained antimicrobial action, and preventing rapid volatilization or degradation of active compounds. In contrast, free TO may lose potency due to its hydrophobic nature and higher susceptibility to environmental factors [28,31]. Interestingly, S. aureus exhibited larger inhibition zones than E. coli at corresponding concentrations, suggesting that Gram-positive bacteria might be more susceptible to TO emulsions. This difference in susceptibility could be attributed to the structural differences in bacterial cell walls: the thicker peptidoglycan layer of Gram-positive bacteria might allow more interactions with the active compounds in the TO emulsions [5,6,17]. These findings emphasized the critical role of emulsions in enhancing the antibacterial activity of TO, making them a promising delivery system for essential oil-based antimicrobial agents.
3.4.3. Inhibitory Effect of TO Emulsions on Mycelial Growth
The effects of free TO and TO emulsions on the growth of P. italicum mycelium were assessed by measuring the colony diameters under different concentrations, as shown in Figure 5. Both free and encapsulated TO exhibited inhibitory effects against P. italicum growth, with the inhibition increasing at higher concentrations (Figure 5A). The colony diameters of the 1TO-E and 2TO-E emulsions were 7.21 mm and 5.06 mm, respectively. Additionally, the TO emulsion demonstrated a significantly greater reduction in mycelium diameter compared to free TO across the same tested concentration (2%), where the colony diameter of TO was 9.58 mm, while that of the 2TO-E emulsion was 5.06 mm (Figure 5B).
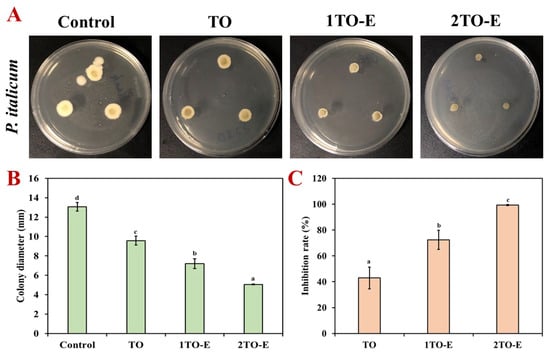
Figure 5.
Colony appearances (A), inhibition diameters (B), and inhibition rates (C) of free and encapsulated TO against P. italicum under different concentrations. Different letters within the same test indicate statistically significant differences (p < 0.05).
Meanwhile, the inhibition rate of free TO was only 42.92%, while the antifungal effect was significantly improved after emulsion encapsulation (Figure 5C). The inhibition rate of 1TO-E increased to 72.4%, while 2TO-E reached 99.31%, which almost completely inhibited the growth of P. italicum. This enhanced antifungal activity could be attributed to TO’s improved dispersion and stability within the emulsion system, which allowed for better penetration and sustained interaction with fungal cells [4,20]. The encapsulated TO likely disrupted the fungal cell membrane more effectively, potentially due to the uniform release of active compounds over time. In contrast, free TO’s hydrophobic nature and volatility might limit its availability and efficacy [6,40]. These results highlighted the superior performance of TO emulsions in controlling fungal growth, suggesting their potential application as an effective antifungal treatment in agricultural practices or food preservation.
4. Conclusions
This study demonstrated the efficacy of COS-stabilized TO-in-water emulsions as a novel delivery system for enhancing the stability, antioxidant capacity, and antimicrobial activity of TO. Ultrasonic-assisted emulsification achieved fine droplet sizes and high encapsulation efficiencies, while the COS/CA interface layer and PVA contributed to longer-term stability and resistance to coalescence and Ostwald ripening. Emulsion encapsulation markedly improved the antioxidant and antimicrobial properties of TO, as evidenced by enhanced free radical scavenging activities and more substantial antibacterial and antifungal effects compared to free TO. The emulsions’ ability to inhibit microbial and fungal growth, coupled with their stable physicochemical properties, underscores their potential as an effective solution for applications in food preservation. Future work could explore the combination of these emulsions with coatings or other active packaging, as well as their scalability and practical deployment in commercial settings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14172930/s1, Table S1: The polydispersity index (PDI) of emulsions prepared using different ultrasonic power and processing time; Figure S1: Encapsulation efficiency of thymol-in-water emulsions under different TO concentrations; Figure S2: Viscosity of thymol-in-water emulsions under different TO concentrations. Different letters within the same TO concentration indicate statistically significant differences (p < 0.05).
Author Contributions
Conceptualization, R.Z.; Methodology, H.W. and R.Z.; Software, H.W. and Q.D.; Formal analysis, W.W. (Wenyue Wang); Investigation, H.W. and J.C.; Re-sources, Q.D.; Data curation, H.W.; Writing—original draft, H.W.; Writing—review & editing, W.W. (Wenjun Wang), Z.F. and R.Z.; Supervision, R.Z.; Funding acquisition, W.W. (Wenjun Wang). All authors have read and agreed to the published version of the manuscript.
Funding
National Key Research and Development Program of China (2022YFD2100402).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. (Please specify the reason for restriction, e.g., the data are not publicly available due to privacy or ethical restrictions).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: Influence of ripening inhibitors. J. Agric. Food Chem. 2012, 60, 12056–12063. [Google Scholar] [CrossRef]
- Ribeiro, S.; Almeida, R.; Batista, L.; Lima, J.; Sarinho, A.; Nascimento, A.; Lisboa, H. Investigation of Guar Gum and Xanthan Gum Influence on Essential Thyme Oil Emulsion Properties and Encapsulation Release Using Modeling Tools. Foods 2024, 13, 816. [Google Scholar] [CrossRef] [PubMed]
- Romulo, A.; Anjani, V.S.; Wardana, A.A. Enhancing Antimicrobial Activity of Thyme Essential Oil Through Cellulose Nano Crystals-Stabilized Pickering Emulsions. Foods 2024, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.N.R.; Ferreira, C.D. Essential oil for the control of fungi, bacteria, yeasts and viruses in food: An overview. Crit. Rev. Food Sci. Nutr. 2023, 63, 8960–8974. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Qin, W.; Zhang, Q.; Luo, J.; Lin, D.; Chen, H. Essential-oil capsule preparation and its application in food preservation: A review. Food Rev. Int. 2022, 39, 4124–4158. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, J.; Yu, S.; Niu, R.; Yang, Z.; Wang, H.; Cheng, H.; Ye, X.; Liu, D.; Wang, W. Active chitosan/gum Arabic-based emulsion films reinforced with thyme oil encapsulating blood orange anthocyanins: Improving multi-functionality. Food Hydrocoll. 2023, 134, 108094. [Google Scholar] [CrossRef]
- Phyo, H.M.; Al-Maqtari, Q.A.; Mi, S.; Du, Y.; Khalid, M.U.; Yao, W. Ultrasound-assisted fabrication of chitosan-hydroxypropyl methylcellulose nanoemulsions loaded with thymol and cinnamaldehyde: Physicochemical properties, stability, and antifungal activity. Int. J. Biol. Macromol. 2024, 281, 136278. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of neutral nanoemulsions self-assembled from alkaline thyme oil and sodium caseinate mixtures. Int. J. Biol. Macromol. 2020, 148, 1046–1052. [Google Scholar] [CrossRef]
- Hanan, E.; Dar, A.H.; Shams, R.; Goksen, G. New insights into essential oil nano emulsions loaded natural biopolymers recent development, formulation, characterization and packaging applications: A comprehensive review. Int. J. Biol. Macromol. 2024, 280, 135751. [Google Scholar] [CrossRef]
- Garavand, F.; Jalai-Jivan, M.; Assadpour, E.; Jafari, S.M. Encapsulation of phenolic compounds within nano/microemulsion systems: A review. Food Chem. 2021, 364, 130376. [Google Scholar] [CrossRef]
- Zhao, R.; Song, R.; Sun, G.; Liu, S.; Li, B.; Cao, Y.; Li, Y. Cutoff Ostwald ripening stability of eugenol-in-water emulsion by co-stabilization method and antibacterial activity evaluation. Food Hydrocoll. 2020, 107, 105925. [Google Scholar] [CrossRef]
- Li, B.; Cui, J.; Xu, T.; Xu, Y.; Long, M.; Li, J.; Liu, M.; Yang, T.; Du, Y.; Xu, Q. Advances in the preparation, characterization, and biological functions of chitosan oligosaccharide derivatives: A review. Carbohydr. Polym. 2024, 332, 121914. [Google Scholar] [CrossRef]
- Milkova, V. Electrosteric stabilization of oil/water emulsions by adsorption of chitosan oligosaccharides—An electrokinetic study. Carbohydr. Polym. 2021, 265, 118072. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2022, 124, 107246. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Hu, J.; Wei, Z.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. One-Step Dynamic Imine Chemistry for Preparation of Chitosan-Stabilized Emulsions Using a Natural Aldehyde: Acid Trigger Mechanism and Regulation and Gastric Delivery. J. Agric. Food Chem. 2020, 68, 5412–5425. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Huang, M.; Zhu, Z.; Huang, T.; Huang, M. Effect of ultrasound assisted emulsification in the production of Pickering emulsion formulated with chitosan self-assembled particles: Stability, macro, and micro rheological properties. LWT 2022, 154, 112595. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, H.; Yan, T.; Li, J.; Xu, W.; Deng, Y.; Zhou, J.; Ye, X.; Liu, D.; Wang, W. Fabrication of multifunctional materials based on chitosan/gelatin incorporating curcumin-clove oil emulsion for meat freshness monitoring and shelf-life extension. Int. J. Biol. Macromol. 2022, 223, 837–850. [Google Scholar] [CrossRef]
- Niu, J.; Lin, Q.; Li, X.; McClements, D.J.; Ji, H.; Jin, Z.; Qiu, C. Pickering emulsions stabilized by essential oil-tannin-chitosan particles: Microstructure, stability, antibacterial activity, and antioxidant activity. Food Hydrocoll. 2024, 154, 110145. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Chen, H.; Chi, H.; Xiang, W.; Zhang, Q.; Tang, J.; Zhang, X. Fabrication and characterization of Zanthoxylum schinifolium essential oil Pickering emulsion stabilized by bacterial cellulose nanofibrils/whey protein isolate complexes and fortified with cinnamaldehyde. LWT 2024, 210, 116829. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Li, Y.; Zhou, Z. Limonin Enhances the Antifungal Activity of Eugenol Nanoemulsion against Penicillium italicum In Vitro and In Vivo Tests. Microorganisms 2021, 9, 969. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, V.K.; Amiri, H.; Pan, J.; Aghbashlo, M.; Tabatabaei, M.; Rajaei, A. Recent developments in improving the emulsifying properties of chitosan. Int. J. Biol. Macromol. 2023, 239, 124210. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Chen, G.; Xiao, H.; Wang, Y.; Tan, C. Sonochemical high internal phase emulsions stabilized solely by intermolecular hydrogen bonds of chitosan. Food Hydrocoll. 2025, 162, 110860. [Google Scholar] [CrossRef]
- Bai, L.; Geng, S.; Zhou, Y.; Ma, H.; Liu, B. Ultrasound-assisted fabrication and stability evaluation of okra seed protein stabilized nanoemulsion. Ultrason. Sonochem. 2024, 104, 106807. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, T.; Li, Y.; Chen, L.; Xu, Y.; Chi, X.; Yu, S.; Wang, W.; Liu, D.; Zhu, B.; et al. Biocompatible hydrophobic cross-linked cyclodextrin-based metal-organic framework as quercetin nanocarrier for enhancing stability and controlled release. Food Chem. 2024, 448, 139167. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xiao, J.; Li, L.; Wan, L.; Li, B.; Zhang, X. Ultrasound treatment of crystalline oil-in-water emulsions stabilized by sodium caseinate: Impact on emulsion stability through altered crystallization behavior in the oil globules. Ultrason. Sonochem. 2024, 106, 106897. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Wang, H.; Cao, W.; Lin, H.; Qin, X.; Chen, Z.; Gao, J.; Wu, L.; Zheng, H. Effect of ultrasonic power on the stability of low-molecular-weight oyster peptides functional-nutrition W(1)/O/W(2) double emulsion. Ultrason. Sonochem. 2023, 92, 106282. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Wang, D.; Tang, J.; Xu, M. Synergistic stabilization of garlic essential oil nanoemulsions by carboxymethyl chitosan/Tween 80 and application for coating preservation of chilled fresh pork. Int. J. Biol. Macromol. 2024, 266, 131370. [Google Scholar] [CrossRef]
- Jayari, A.; Donsi, F.; Ferrari, G.; Maaroufi, A. Nanoencapsulation of Thyme Essential Oils: Formulation, Characterization, Storage Stability, and Biological Activity. Foods 2022, 11, 1858. [Google Scholar] [CrossRef]
- Karsli, G.T.; Sahin, S.; Oztop, M.H. High-Pressure-Homogenized Clove and Thyme Oil Emulsions: Formulation, Stability, and Antioxidant Capacity. ACS Food Sci. Technol. 2022, 2, 1832–1839. [Google Scholar] [CrossRef]
- Tiang, S.S.L.; Low, L.E.; Ali, I.; Zhou, L.; Goh, B.-H.; Gew, L.T.; Tang, S.Y. Recent advances in ultrasonic cavitation technologies for emulsion preparation: A mini review. Curr. Opin. Chem. Eng. 2024, 45, 101046. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Yan, J.; Zhang, S.; Shi, C.; McClements, D.J.; Liu, X.; Liu, F. Development of antibacterial nanoemulsions incorporating thyme oil: Layer-by-layer self-assembly of whey protein isolate and chitosan hydrochloride. Food Chem. 2021, 339, 128016. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Han, S.; Hua, S.; Zhang, H.; Wang, J.; Xia, N.; Liu, Y.; Meng, D. Edible chitosan-based Pickering emulsion coatings: Preparation, characteristics, and application in strawberry preservation. Int. J. Biol. Macromol. 2024, 264, 130672. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L. Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. Int. J. Biol. Macromol. 2020, 162, 1509–1515. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Wang, X.; Zhang, L.; Xu, Z.; Sun, D. Nanoemulsions Stable against Ostwald Ripening. Langmuir 2024, 40, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Noh, Y.; McClements, D.J.; Choi, S.J. Impact of hydrophilic substances on Ostwald ripening in emulsions stabilized by varied hydrophilic group surfactants. NPJ Sci. Food 2024, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shi, Y.; Li, B.; Liu, C.; Zhang, Y.; Zhong, J. Characterization, Stability and Antioxidant Activity of Vanilla Nano-Emulsion and Its Complex Essential Oil. Foods 2024, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Feng, B.; Li, N.; Su, L.; Wang, Z.; Kong, F.; Bi, Y. Preparation, characterization, and biological activity of Cinnamomum cassia essential oil nano-emulsion. Ultrason. Sonochem. 2022, 86, 106009. [Google Scholar] [CrossRef]
- Rui, L.; Li, Y.; Wu, X.; Wang, Y.; Xia, X. Effect of clove essential oil nanoemulsion on physicochemical and antioxidant properties of chitosan film. Int. J. Biol. Macromol. 2024, 263, 130286. [Google Scholar] [CrossRef]
- Fu, H.; Huang, R.; Li, J.; Lin, Z.; Wei, F.; Lin, B. Multifunctional cinnamaldehyde-tannic acid nano-emulsion/chitosan composite film for mushroom preservation. Food Hydrocoll. 2023, 145, 109111. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.D.A.; Oliveira, M.S.D. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Zhong, W.; Li, D.; Li, L.; Yu, S.; Pang, J.; Zhi, Z.; Wu, C. pH-responsive Pickering emulsion containing citrus essential oil stabilized by zwitterionically charged chitin nanofibers: Physicochemical properties and antimicrobial activity. Food Chem. 2024, 433, 137388. [Google Scholar] [CrossRef]
- Hedayati, S.; Tarahi, M.; Iraji, A.; Hashempur, M.H. Recent developments in the encapsulation of lavender essential oil. Adv. Colloid Interface Sci. 2024, 331, 103229. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Zeng, L.; Bi, Y.; Kong, F.; Wang, Z.; Tan, S. Characterization, in-vitro digestion, antioxidant, anti-hyperlipidemic and antibacterial activities of Zanthoxylum bungeanum Maxim essential oil nano-emulsion. Food Biosci. 2023, 56, 103082. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Mukhamadiev, A.; Feng, J.; Gao, Y.; Zhuansun, X.; Han, R.; Chong, Y.; Jafari, S.M. Formulation optimization and characterization of carvacrol-loaded nanoemulsions: In vitro antibacterial activity/mechanism and safety evaluation. Ind. Crops. Prod. 2022, 181, 114816. [Google Scholar] [CrossRef]
- Yu, H.; Huang, G.; Ma, Y.; Liu, Y.; Huang, X.; Zheng, Q.; Yue, P.; Yang, M. Cellulose nanocrystals based clove oil Pickering emulsion for enhanced antibacterial activity. Int. J. Biol. Macromol. 2021, 170, 24–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).